JOURNAL OF BIOSCIENCE AND BIOENGINEERING Vol. 94, No. 4,304-308. 2002
Purification and Characterization of an Extracellular Trypsin-Like
Protease of
Fusarium oxysporum
var.
zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
Mini
RICARDO
ANDRADE BARATA,’ MILTON HERCULES GUERRA ANDRADE,2
ROBERTA DIAS RODRIGUES,’
ANDIESO MIRANDA CASTRO’*
Laboratdrio de Bioquimica e Fisiologia de Microorganismos, Nticleo de Pesquisa em Ci&cias Biolbgicas, Universidade Federal de Ouro Preto, CEP 35.400.000, Ouro Preto, MC? Brazil’ and Laboratdrio
de Enzimologia e Planejamento de Fcirmacos, Nhcleo de Pesquisa em Cihcias Bioldgicas, Universidade Federal de Ouro Preto, CEP 35.400.000, Ouro Preto, MG Brazil2
Received 23 January 2002/Accepted 3 July 2002
An alkaline serineprotease, capable of hydrolyzing Nu-benzoyl-DL arginine p-nitroanilide, was secreted by
Fusurium oxysporum
var.hi
grown in the presence of gelatin as the sole nitrogen and carbon source. The protease was purified 65-fold to electrophoretic homogenity from the culture supernatant in a three-step procedure comprising QSepharose chromatography, aMnity chro- matography, and FPLC on a MonoQ column. SDS-PAGE analysis of the purified protein indi- cated an estimated molecular mass of 41 kDa. The protease had optimum activity at a reaction temperature of 45OC and showed a rapid decrease of activity at 48OC. The optimum pH was around 8.0. Characterization of the protease showed that Ca*+ and MgZ+ cations increased the activity, which was not inhibited by EDTA or l,lO-phenanthroline. The enzyme activity on Nu- benzoyl-DL arginine p-nitroanilide was inhibited by 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride,p-aminobenzamidine dihydrochloride, aprotinin, 3-4 dichloroisocoumarin, and IV- tosyl-L-lysine chloromethyl ketone. The enzyme is also inhibited by substrate concentrations higher than 2.5x lo-4 M. The protease had a Michaelis-Menten constant of 0.16 mM and a V,, of 0.60 pm01 released product .min-‘.mg’ enzyme when assayed in a non-inhibiting substrate con- centration. The activity on Nu-benzoyl-DL arginine p-nitroanilide was competitively inhibited by p-aminobenzamidine dihydrochoride. A Ki value of 0.04 mM was obtained.[Key words: trypsin-like protease, Fusarium, inhibitor effects]
Fusarium is a non-dermatophytic mold, widespread in nature both in the soil and on many plants. The genus in- cludes more than 60 species, of which at least 10 are agents of infection in humans. I;: oxysporum, F moniliforme, and F solani are the species most frequently isolated from human lesions (1). However, the main interest in these fungi arises because of their ability to cause diseases in economically important plant hosts (2).
It has been suggested that K oxysporum may be a pro- ducer of a single-cell protein whose nutritive quality is high (3-6). In Brazil, studies on the nutritive value of the protein produced using vinasse (waste from sugar-cane distillation) as a raw material have indicated that a good quality food might be produced when I? oxysporum var. hi is grown in this substrate (5, 6). These studies led us to investigate the ability of F: oxysporum to grow in glycerol (7), lactic acid
* Corresponding author. e-mail: imcastro@cpd.ufop.br phone: +55-31-35591722 fax: +55-31-35591680
Abbreviations: BApNA, Na-benzoyl-DL arginine p-nitroanilide; BToNA. N-benzovl-L-tvrosine n-nitroanilide: DABB. 4.4’-diazoamino- bis~ben&unidine;~DCI,~3-4 dichloroisocoum&n; E-~~,‘(L-Mwzs-~-c~~- boxyoxiran-2-carbonyl+leucylagmatine; pAB, p-aminobenzamidine dihydrochoride; PEFABLOC (AEBSF), 4-(2 aminoethyl)-benzene- sulfonyl fluoride hydrochloride; TLCK, N-tosyl-L-lysine chloromethyl ketone; TPCK, N-tosyl-L-phenylalanyl chloromethyl ketone.
(8), and protein (9), since they are the main constituents of vinasse and the main sources of energy and carbon for the production of a single-cell protein in the waste.
Like many heterotrophic organisms, l? oxysporum uti- lizes proteins and peptides as growth substrates and, in addi- tion, produces proteolytic-type enzymes, which are assumed to generate small peptides from the protein-based growth substrates. The effects of different culture conditions on the production of extracellular proteolytic enzymes in microor- ganisms are well documented (10-12). It has been shown that the activity of extracellular proteases in E oxysporum var. lini also involves the well-known induction by exoge- nous protein balanced by metabolite repression (9, 13). So far, only one trypsin-like protease, which is synthesized as a preproenzyme, has been characterized from F: oxysporum (14). Sequence alignment revealed equally close homology to both bovine trypsin and bacterial trypsins.
Microbial proteases are particulary important because they have different substrate specifities, making them useful in several areas of biochemistry and biotechnology (for a review, see Rao et al. [ 151). In this paper, we report the find- ing of an extracellular protease produced by F oxysporum var. Zini, grown in the presence of gelatin as the sole nitro- gen and carbon source. It was purified and biochemically characterized.
VOL.94,2002 EXTRACELLULAR TRYPSIN-LIKE PROTEASE OF FUSARIUM 305
MATERIALS AND METHODS M Tris-HCI buffer, pH 8.0, containing 1 and 10.0 mM ion con- centrations. Afterwards, the residual activities were assayed with 2.5 x 10m4 M BApNA as the substrate at 45°C for 4 h.
Organism and growth conditions Fusarium oxysporum var. hi ATCC 10960 was maintained on potato dextrose agar at 4”C, after growth for 5 d at 3O’C. A spore suspension for inoculation was prepared by adding water to potato dextrose agar in tubes. The fungus was grown in Treschow’s medium containing 2% gelatin (16). For growth, 750 ml medium was used in 2-l shake flasks, which were placed in an orbital incubator operated at 28°C. Cul- ture supernatants were obtained by filtration on glass fiber mem- branes (type GF/C; Whatman, UK) after 72-h growth.
Enzyme purification Culture supematants were used as the enzyme source. (i) Four hundred milliliters of culture supematant from E oxysporum cell cultures was loaded onto an ion-exchange column of QSepharose (2.5 x 7.5 cm) previously equilibrated with 0.01 M NH,HCO, buffer, pH 8.0, at a flow rate of 1 ml/min. The fraction that did not interact with the column was able to hydrolyse BApNA (Sigma, St. Louis, MO, USA). The column was then washed with NH,HCO, buffer containing 1 M NaCl to elute bound proteins, fusarin, fusaric acid, and other compounds. (ii) The ac- tive material was applied onto an affinity column of Sepharose- p-aminobenzamidine (2.0 x 10.0 cm) equilibrated with 0.05 M NH,HCO, buffer, pH 8.0. Bound fractions were eluted at a flow rate of 1 .O ml/min with 1 mM pAB in bicarbonate buffer. Fractions (2 ml) with activity on BApNA were pooled, dialyzed overnight against 0.01 M NH,HCO, buffer, pH 8.0, and lyophilized. (iii) The lyophilized fraction from the affinity chromatography was resus- pended in 1.5 ml of 0.01 M borate buffer, pH 8.5, and further puri- tied by ion-exchange chromatography on a MonoQ HR 515 col- umn (Amersham Biosciences, Uppsala, Sweeden) using an FPLC and a linear gradient of NaCl from 0.05 to 1 M in the same borate buffer, at a flow rate of 1 ml/min. The protein content in the column effluent was monitored by determining the absorbance at 280nm (A,,,). All the fractions were monitored by enzymatic assay and SDS-PAGE.
Proteolytic assay Proteolytic activity was assayed using BApNA as a substrate. The reaction mixture contained 0.2 ml culture filtrate (or fractions from the columns), 10 mM MgCl,, 2.5 x lOa M BApNA, and 0.01 M Tris-HCl buffer, pH 8.0. The mixture was incubated at 45°C for 4 h. The reaction was linear for 6 h. The absorbance at 410 nm (A,,,) was measured using a spec- trophotometer (DU68; Beckman, Fullerton, CA, USA). One unit of enzyme activity was defined as the amount of enzyme that could produce 1 pmol of product.min-’ under the described conditions. The specific activity was expressed as the number of units of ac- tivity per mg of protein.
Characterization of the enzyme The activity was assayed at different pH values ranging from 4 to 11. The following buffers were used: for pH 4-5, 0.01 M acetate; for pH 6-7, 0.0 1 M phos- phate; for pH 8-9, 0.01 M Tris-HCl; for pHs 10 and 11, bicar- bonate. The temperature dependence of the protease was deter- mined in the temperature range 25-70°C as described above. For thermostability testing, the purified protein was incubated at 55- 65°C for different lenghts of time (O-60 min) in Tris-HCI buffer, pH 8.0, containing 10 mM MgCl, and then assayed for 4 h. The effects of different ions (Ca2+, Mg2+, and Zn2+) on protease activ- ity were studied by incubating the purified protein at 45°C in 0.0 1
For inhibition studies, pure protease was preincubated with various compounds (1 ,I 0-phenanthroline, EDTA, PEFABLOC, PMSF, 3-4 DCI, TPCK, TLCK, pAB, E-64, pepstatin, iodaceta- mide, and aprotinin) for 60 min at room temperature, in 0.01 M Tris-HCl buffer, pH 8.0 (without MgCl, in the cases of EDTA and
1 ,10-phenanthroline), and the BApNA activity was assayed. Electrophoresis Protein fractions were analysed by SDS- PAGE by the method of Laemmli (17) with a separation gel of 10% polyacrilamide in Tris-glycine buffer, pH 8.2. Gels were stained with silver according to Wray et al. (18).
Protein determination The protein content of samples was estimated by the method of Bradford (19) using bovine serum albumin as the standard.
RESULTS AND DISCUSSION
In previous work, the extracellular protease production by E oxysporum was analyzed in several media containing different carbon and nitrogen sources (9). Findings sug- gested that protease production is induced by protein sources and that synthesis of the enzymes is, in part, regulated by catabolite repression.
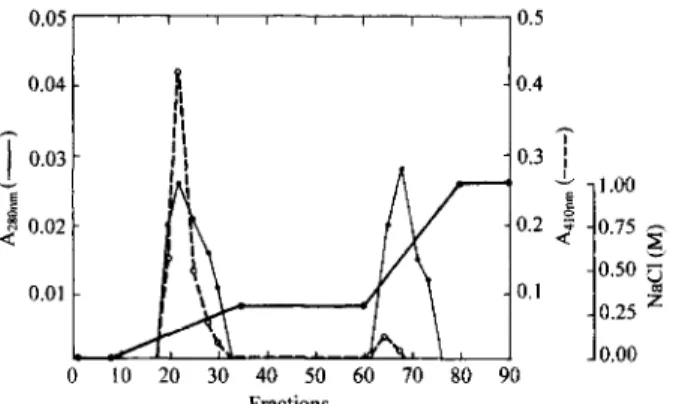
In the present study, the crude enzyme preparation from the culture supernatant of l? oxysporum var. lini, cultivated in gelatin as a carbon and nitrogen source, was loaded onto an ion-exchange QSepharose column. In this step of the purification, portions of the contaminating proteins and col- ored compounds were retained. Further purification of the fraction that did not bind to the QSepharose column (96% of the activity; Table 1) was achieved by an affinity column of Sepharose-p-aminobenzamidine (yield, 3%; Table 1). Our results indicated the probability that, more than one enzyme was acting on BApNA, since inhibition by PMSF was observed in the culture broth but not in the active frac- tion eluted in this step. Only a minor component was re- covered using the affinity column. Since increasing the pAB concentration did not result in elution of the major enzyme, most of the protease active on BApNA stayed bound to the Sepharose-p-aminobenzamidine column. To adress this problem, we will try to elute the enzyme still bound to the resin with a new inhibitor that has a diglycidyl ether group bound to the p-NH, group of pAB, simulating the spacer arm of the afftnity resin. Only the fractions containing ac- tivity, eluted with 1 mM pAB, were pooled, dialyzed, and concentrated. The protein mixture eluted from Sepharose- p-aminobenzamidine was separated by FPL chromatogra- phy on a MonoQ HR 5/5 column. The first peak, eluted in 0.22 M NaCl, contained a protease that was able to hydro- lyse BApNA (Fig. 1). About 87% of the protease activity bound to Sepharose-p-aminobenzamidine was eluted in this
TABLE 1. Purification of F: oxysporum trypsin-like protease
Purification step Culture suuematant
Total protein (mg) 50.4
Specific activity (U/mg) x 1 O2
0.097
Total activity (U)x 102
4.92
Purification (fold)
1.0
Yield (%) 100.0
QSepharose (fraction not bound) 38.0 0.124 4.72 1.3 95.9
Sepharose-p-aminobenzamidine 0.096 1.562 0.15 16.1 3.0
306 BARATA ET AL. J.Broscl.
zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
BIOENG.,- 0.4
0 IO 20 30 40 50 60 70 80 90 Fractions
FIG. 1. Representative elution profile on MonoQ HR 5/5 ion ex- change chromatography of an enzyme preparation deriving from a Sepharose-p-aminobenzamidine column: thin line, A,,,,; dotted line, A 4,,,nmr activity on BapNA; thick line, NaCl gradient.
peak. Probably, this enzyme, active on BApNA, does not bind to QSepharose (the first step in the purification proc- ess) because its isoeletric point is higher than 8.0. Indeed, in the first step of the purification process we observed prefer- ential ligation of other compounds and proteins excreted by E oxysporum.
After the FPLC, the extracellular protease eluted in the first peak was purified 65 fold (Table 1). There was little decrease in the yield in this purification step, which can be explained by the fact that the activity on BApNA is concen- trated in the peak I. The enzyme purification was followed by SDS-PAGE, under non-reducing and reducing condi- tions. The electrophoretic pattern was the same under both conditions. In Fig. 2, the protein composition of the enzyme preparation isolated by Sepharose-p-aminobenzamidine chromatography (lane 2) is compared to that obtained by FPLC (lane 3). The material eluted from Sepharose-p- aminobenzamidine column (lane 2) gave two outstanding protein bands, with molecular masses of 41 and 23 kDa. After the last purification step, a single band, corresponding to the first peak, was observed after gel electrophoresis of the sample. The molecular mass of the corresponding pro- tein, determined by SDS-PAGE, was 41,000 Da. The E oxysporum trypsin reported by Rypniewski et al. (14) is homologous to trypsins from Streptomyces griseus, S. cry- thraeus and to bovine trypsin. The enzyme consists of 224 amino acid residues and has a molecular mass of 22,190 Da. The authors mention two other proteinases isolated from l? oxysporum: a subtilisin-like enzyme and an aspartic pro- teinase ( 14).
Detailed studies were carried out in order to characterize the purified enzyme. The optimum pH within the range from 4.0 to 11.0 was determined using BApNA as a sub- strate. The enzyme showed maximum activity at pH 8.0, indicating that it is an alkaline protease. The trypsin de- scribed by Rypniewski et al. (14) had a completely different pH activity profile.
Under the conditions described in Materials and Methods, the purified protease exhibited optimum activity at 45°C. The enzyme remained active in the range from 20°C to 55°C at which temperatures the relative activity was ap- proximately 29% and 19%, respectively. The enzyme be-
kDa
FIG. 2. SDS-PAGE under reducing conditions of fractions eluted at different steps in the purification of the trypsin-like protease from Fusarium oayporum var. hi. Lane 1, Molecular weight; lane 2, frac- tion eluted from p-aminobenzamidine chromatography; lane 3, peak 1
from MonoQ column.
came unstable at about 48°C as deduced from an Arrhenius plot (not shown). Total enzyme activity was lost after incu- bation of the protein in 0.01 M Tris-HCl buffer, pH 8.0, for 24 h at 55°C. The rate of heat denaturation of the enzyme was 0.62%.min-’ at 55°C 0.65% at 60°C and 1.15% at 65°C (k values: 0.0062,0.0065, and 0.0115, respectively) as deduced by the equation
In AIA 0 =emk’
The effects of different protease inhibitors and cations on the enzyme activity were also investigated (Tables 2 and 3). The purified enzyme was strongly inhibited by 4- (2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (PEFABLOC), an irreversible serine protease inhibitor, pAB, and aprotinin. Significant inhibition was also ob- served with TLCK. Pepstatin, a potent inhibitor of the as- partic proteases, did not affect the activity. The lack of in- hibition by iodacetamide and the small inhibitory effect of E-64 excluded the enzyme from the cysteine proteinase class. The protease has trypsin-like characteristics as in- ferred by the inhibitor effects (Tables 2 and 3), activity on BApNA, and lack of activity on BTpNA. Subtilisins are not inhibited by pAB (Table 4) and DABB (20). The chelating
TABLE 2. Effects of different inhibitors on the activity of the trypsin-like protease from Fusarium oxysporum var. lini Compound Final concentration Percentage inhibition
None 0
PEFABLOC ImM 86.1
Aprotinin 1 tLg/ml 79.8
zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
P A
B
ImM 76.1DC1 1mM 28.4
TLCK 1mM 42.8
E-64 1mM 23.6
Pepstatin 1 &ml 0
Iodacetamide 1mM 0
VOL. 94,2002
TABLE 3. Effects of divalent ions and chelating agents on activity of the protease purified from Fusarium oxysporum var. lini
Compound Relative activity (%)
None 100
EDTA (1 mM) 100
1 , 1
zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
0-Phenanthroline (1 mM) 100M&l, (1 mM) 127
MgCl, (10 mM) 128
CaCI, (1 mM) 129
CaCl, (IOmM) 127
ZnCl, (1 mM) 107
ZnCl, (10 mM) 33
TABLE 4. Enzymatic activity (U x 1 02) of subtilisins and F: oxysporum purified enzyme
Enzymes
Substrate Subtilisin Subtilisin Purified enzyme (BPN) (Carlsberg) (F. oxysporum)
BApNA 0.004 0.010 6.501
BTpNA 0.106 0.043 0.108
BApNA+pAB 0.004 0.009 1.544
BTpNA+pAB 0.109 0.042 0.081
The enzymatic activities were determined in 0.01 M Tris-HCI, pH 8.0, 1OmM MgCl,, at 45”C, in the presence of 1 mM BTpNA or BApNA for subtilisins and 1 mM BTpNA or 0.25 mM BApNA for the E oxysporum purified enzyme. The absorbance was measured at 410 nm.
agents EDTA and l,lO-phenanthroline did not affect the proteolytic activity. On the other hand, protease activity was slightly enhanced when CaCl, or MgCl, was added to the reaction mixture. On the contrary, ZnCl, had an inhibitory effect at a high concentration. Most probably, the ions Ca2’ and Mg*+ are involved in stabilizing the active structure, but they are not needed for the catalytic function or for the protein chain folding process to form the active structure. Calcium ions are known to protect proteinase against de- naturation and proteolytic degradation (21, 22). There is no evidence of any divalent cation binding sites in the structure of l? oxysporum trypsin as described by Rypniewski et al. (14).
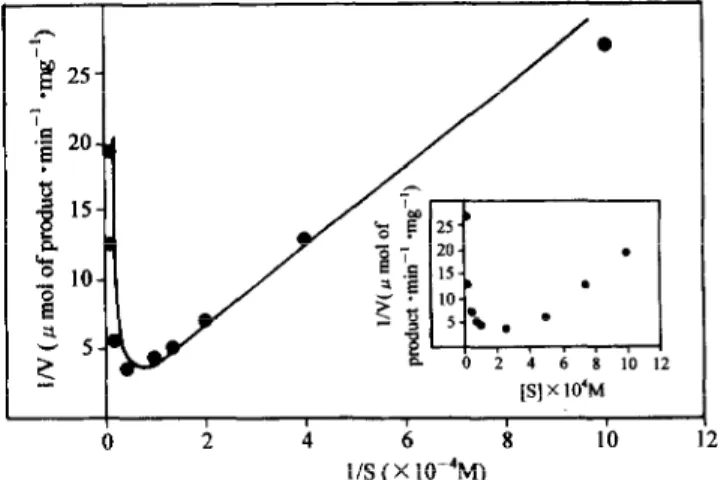
Figure 3 shows a plot of l/V against l/S. It can be seen that inhibition arose at substrate concentrations higher than 2.5 x 10“ M. There are a number of causes of this effect. The formation of ineffective complexes with two substrate molecules combined at an active site was discussed by Haldane (23). Our data (Fig. 3, insert) do not fit the model described by Haldane. However, inhibition by a high sub- strate concentration can be also due to substrate molecules binding to the enzyme in an incorrect orientation or to other factors. Analysis of the kinetic data at lower substrate con- centrations (0.25 to 1 .O x 10e4 M) by the graphical method of Lineweaver-Burk indicates a half-saturation constant (K,) of 0.16kO.02 mM and a V,, of 0.60 umol released prod- uct . min. mg-’ enzyme.
The profile of enzyme inhibition by pAB (Fig. 4) indi- cates a competitive type of inhibition within the inhibitive concentration range studied. The slopes of reciprocal plots made at a series of different inhibitor concentrations were plotted against the inhibitor concentrations to determine the
EXTRACELLULAR TRYPSIN-LIKE PROTEASE OF FUSARIUM 307
[S] X
zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
IO’M6
i
i
6
i
IO
l/S (X 10-4M)
2
FIG. 3. Effect of substrate concentration on me reaction velocity: Lineweaver-Burk plot of initial velocity of reaction on BApNA. In- sert: l/V versus S.
[pAB]XlO’M FIG. 4. Determination of K,,
Ki (23). A Ki value of 0.04 mM was obtained, which is sug- gestive of strong inhibition.
The stability of the enzyme over wide pH and tempera- ture ranges suggests that it may play an important role in the growth and development of Fusarium. It has been proposed that in some fungus-plant interactions, trypsin-like pro- teases may function as pathogenicity factors (10). In con- clusion, our results showed that F: oxysporum var. lini, se- cretes a protease that has affinity to p-aminobenzamidine. The enzyme can be classified as a trypsin-like serine pro- tease.
ACKNOWLEDGMENT
R. A. Barata was supported by a grant from FundacBo de Amparo a Pesquisa de Minas Gerais (FAPEMIG), Minas Gerais, Brazil.
REFERENCES
Romano, C., Miracco, C., and Difonzo, E. M.: Skin and nail infections due to Fusarium oxysporum in Tuscany, Italy. Mycoses, 41,433-437 (1998).
Alves-Santos, F. M., Benito, E. P., Eslava, A. P., and Diaz- Minguez, J. M.: Genetic diversity of Fusarium oxysporum
strains from common bean fields in Spain. Appl. Environ. Microbial., 65,3335-3340 (1999).
308 BARATA ET AL. J. BIOSCI.
zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
BIOENG.,4. Kumar, K. and Misbra, S.: Mass and energy balance in glucose and lactose metabolism in Fusarium oxysporum. J. Ferment. Technol., 66,449-453 (1988).
5. Silva, M. E. S. T. and Nicoli, J. R.: Vinasse as substrate for production of microbial protein from Fusarium oxysporum var. Zini. Rev. Lat-amer. Microbial., 25, 125-130 (1983). 6. Silva, M. E. S. T. and Nicoli, J. R.: Production and nutritive
value of single cell protein from Fusarium oxysporum var. lini. J. Ferment. Technol., 63, 91-94 (1985).
7. Castro, I. M. and Loureiro-Dias, M. C.: Glycerol utilization in Fusarium oxysporum var. lini: regulation of transport and metabolism. J. Gen. Microbial., 137, 1497-1502 (1991). 8. Castro, I. M. and Loureiro-Dias, M. C.: Utilization of lactic
acid by Fusarium oxysporum var. lini: regulation of transport and metabolism. Appl. Environ. Microbial., 60, 102-105 (1994).
9. Castro, I. M., Lima, A. A., Paula, C. A., Nicoli, J. R., and Brandso, R. L.: Effect of substrate and pH on the activity of proteases from Fusarium oxysporum var. lini. J. Ferment. Bioeng., 72, 132-134 (1991).
10. Dobinson, K. F., Lecomte, N., and Lazarovits, G.: Produc- tion of an extracellular trypsin-like protease by the fungal plant pathogen Verticillium dahliae. Can. J. Microbial., 43, 227-233 (I 997).
11. Secades, P. and Guijarro, J. A.: Purification and character- ization of an extracellular protease from the fish pathogen Yersinia ruckeri and effect of culture conditions on produc- tion. Appl. Environ. Microbial., 65, 3969-3975 (1999). 12. Levisohn, S. and Aronson, A. I.: Regulation of extracellular
protease production in Bacillus cereus. J. Bacterial., 93, 1023-1030 (1967).
13. Urbanek, H. and Yirdaw, G.: Acid proteases produced by Fusarium species in cultures and in infected seedlings. Phys- iol. Plant Pathol., 12, 81-87 (1978).
14. Rypniewshi, W.R., Hastrup, S., Betzel, Ch., Dauter, M., Dauter, Z., Papendorf, G., Branner, S., and Wilson, K. S.: The sequence and X-ray structure of the trypsin from Fu- sarium oxysporum. Protein Eng., 6,341-348 (1993). 15. Rao, M. B., Tanksale, A. M., Ghate, M. S., and Deshpande,
V.V.: Molecular and biotechnological aspects of microbial proteases. Microbial. Mol. Biol. Rev., 62, 597635 (1998). 16. Treschow, C.: Nutrition of cultivated mushrooms. Dansk
Botanisk Arkiv, 11, l-80 (1944).
17. Laemmli, U. K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227,680- 685 (1970).
18. Wray, W., Boulikas, T., Wray, V. P., and Hancock, R.: Silver staining of proteins in polyacrilamide gels. Anal. Bio-
them., 118, 197-203 (1981).
19. Bradford, M. M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248- 254 (1976).
20. Andrade, M. H. G., Silva, E., and Mares-Guia, M.: A plau- sible identification of the secondary binding site in trypsin and trypsinogen. Brazilian J. Med. Biol. Res., 23, 1223-123 I (1990).
21. Voordouw, G., Milo, C., and Roche, R. S.: Role of bound calcium ions in thermostable proteolytic enzymes. Separation of intrinsic and calcium ion contributions to the kinetic ther- mal stability. Biochemistry, 15, 3716-3724 (1976).
22. Frommel, C. and Sander, C.: Thermitase, a thermostable subtilisin: comparison of predicted and experimental struc- tures and molecular cause of thermostability. Proteins, 5, 22- 37 (1989).