Universidade de Trás-os-Montes e Alto Douro
Functional characterization of Hippo pathway in
planarians
Dissertação de Mestrado em Genética Molecular, Comparativa e
Tecnológica
Nídia de Sousa
Orientador: Emili Saló Boix
Co-orientador: Maria Teresa Adell Creixell
II
Universidade de Trás-os-Montes e Alto Douro
Functional characterization of Hippo pathway in
planarians
Dissertação de Mestrado em Genética Molecular, Comparativa e
Tecnológica
Nídia de Sousa
Orientador: Emili Saló Boix
Co-orientador: Maria Teresa Adell Creixell
Coordenador interno: Paula Filomena Martins Lopes
III
Universidade de Trás-os-Montes e Alto Douro
Functional characterization of Hippo pathway in
planarians
Dissertação de Mestrado em Genética Molecular, Comparativa e
Tecnológica
Nídia de Sousa
Orientador: Emili Saló Boix
Co-orientador: Maria Teresa Adell Creixell
Coordenador interno: Paula Filomena Martins Lopes
Composição do Júri:
____________________________________________________________
____________________________________________________________
____________________________________________________________
IV
“Não se pode pretender que alguém conheça tudo, mas sim que, conhecendo alguma coisa, tenha conhecimento de tudo”.
V
Agradecimentos
Ao magnífico Reitor da Universidade de Trás-os-Montes e Alto Douro.
Ao programa ERASMUS que me permitiu realizar a minha dissertação de mestrado fora de Portugal.
Ao Professor Doutor Emili Saló pela oportunidade que me deu aceitando-me no seu laboratório para realizar este trabalho e por toda a disponiilidade demostrada, fosse para discutir o trabalho ou para apenas conversar.
À Professora Doutora Teresa Adell por tudo o que me ensinou nestes nove meses. Por toda a dedicação a este trabalho e pela disponibilidade sempre demonstrada mesmo para as menores dúvidas.
À Professora Doutora Paula Lopes, que mesmo estando longe sempre tinha uma palavra de insentivo a dizer e pela disponibilidade em esclarecer as minhas dúvidas sempre que necessário.
Aos membros que compõem os grupos de investigação do E. Saló, F. Cebrià e J. Garcia pelo acolhimento. O vosso apoio, ajuda, disponibilidade e companheirismo foram muito para além do que eu esperava.
Ao Octávio Serra por ser o meu pilar nesta aventura e em muitos outros momentos. Sem ele todo este percurso seria muito mais difícil.
Aos meus amigos que sempre me deram força para continuar e jamais deixaram que as saudades me abalassem.
Finalmente, mas não menos importante, aos meus pais, irmão e cunhada que me proporcionaram esta experiência na esperança de que o meu futuro seja mais risonho. Obrigada por serem o meu porto de abrigo quando o mundo à minha volta se desmorona.
VI
Resumo
A via Hippo tem vindo a emergir como uma via de sinalização conservada essencial para coordenar a proliferação e a morte celular de forma a limitar o tamanho dos orgãos. Descobertas recentes sugerem que a via Hippo pode ainda desempenhar um importante papel na regulação das células estaminais ao nível da sua auto-renovação e expansão.
Com o objetivo de compreender e caraterizar a via Hippo em planárias procedeu-se à identificação e caraterização funcional dos elementos que compõem a via Hippo em Schmidtea mediterramea.
Os resultados preliminares demonstraram que a estádios iniciais de regeneração a proliferação aumenta e a apoptose diminui na ausência dos genes Smed-sav, Smed-hpo e Smed-wts. Para, além disso, observou-se que a regeneração de tecidos diferenciados como o cérebro, sistemas visual e digestivo era afetada. A inibição do gene Smed-yki não altera significativamente a proliferação, contudo é possível observar o aumento de células apoptóticas e a sua deslocalização. Além disso, os animais iniciam um processo de avolumação.
Em homeostase os animais submetidos a RNAi, sav, hpo and
Smed-wts mantêem o seu nível de proliferação e é observada uma redução ao nível da
apoptose originando o aparecimento de sobre-crescimentos e à não reposição celular dos tecidos diferenciados. Por outro lado a inibição do Smed-yki origina um aumento da proliferação enquanto que a apoptose mantêm-se. Durante a homeostase, estes animais apresentam uma forte desorganização ao nível dos tecidos e tal como nos regenerantes exibem um fenótipo avolumado.
Estes resultados sugerem que a via Hippo desempenha um importante papel tanto no processo de regeneração como durante a homeostase em planárias. Contudo, um estudo mais aprofundado necessita de ser realizado para caraterizar esta via em planárias.
VII
Abstract
The Hippo pathway has emerged as a conserved signaling pathway essential to coordinate both cell proliferation and death in order to limit organ size. Recent evidences suggest that the Hippo pathway may regulate stem cell and progenitor cell self-renewal and expansion.
In order to understand and characterize the Hippo pathway in planarians – flatworms with striking regenerative abilities – we identified and performed a functional analysis of the Hippo pathway elements in the planarian specie Schmidtea
mediterranea.
The preliminary results show that at early stages of regeneration the proliferation increases and the apoptosis is reduced in the absence of sav, hpo and
Smed-wts. Furthermore was observed that the regeneration of differentiated tissues as the
brain, visual and digestive systems is affected. In the absence of Smed-yki, proliferation and apoptotic rates are not severally altered, although animals show a dramatic blotted phenotype.
During homeostasis RNAi animals for Smed-sav, Smed-hpo and Smed-wts maintained the proliferation rate and the apoptosis was reduced, which leads to the generation of overgrowths and improper cell replacement of differentiated tissues. On the other hand, the proliferation is highly increased in Smed-yki RNAi animals whereas the apoptosis is maintained. During homeostasis Smed-yki RNAi planarians are also blotted and show severe affectation of the tissue organization.
These results suggest that the Hippo pathway plays an important role in both regeneration and homeostasis processes in planarians. However, deeper studies need to be performed in order to further characterize this pathway in planarians.
Key words: Hippo pathway, planarians, S. mediterranea, proliferation, apoptosis.
VIII
Índex
Agradecimentos ... V Resumo ... VI Abstract ... VII Índex ... VIII Figures Índex ... X Table Índex ... XI Abbreviations list ... XII1. PLANARIAN ... 2
1.1 Planarian anatomy ... 2
1.2 Schmidtea mediterranea ... 4
1.3 Mechanisms underlying planarian regeneration ... 4
1.4 Growth control in planarians ... 6
2. THE HIPPO PATHWAY ... 7
2.1 Hippo pathway core ... 7
2.2 Hippo pathway upstream regulators ... 7
2.3 Negative regulators of the Hippo signaling ... 10
2.4 Yki specific regulators ... 10
2.5 Yki DNA-binding patterns ... 11
2.6 Hippo pathway targets ... 12
2.7 The interplay between the Hippo pathway and other signaling pathways ... 13
2.8 Hippo pathway and regeneration ... 13
3. OBJECTIVES ... 16
4. MATERIALS AND METHODS ... 18
4.1 Animals ... 18
4.2 Isolation, Cloning, and Sequencing of S. mediterranea genes ... 18
4.3 RNA interference analysis ... 18
4.4 Whole-mount in situ hybridization ... 19
4.5 Whole-mount Immunostaining ... 20
4.6 TUNEL assay ... 21
4.7 Imaging capture ... 21
4.8 Cell counting and statistical analysis ... 21
IX
5.1 Hippo pathway core genes are conserved in S. mediterranea ... 23
5.2 Hippo pathway genes are expressed throughout the animals ... 23
5.3 Hippo pathway genes are required for proper differentiation and regeneration of missing structures ... 23
5.4 Hippo pathway genes regulate stem cell proliferation and cell death in S. mediterranea during regeneration ... 25
5.5 The Hippo pathway is involved in the maintenance of differentiated structures during homeostasis in S. mediterranea ... 28
5.6 The Hippo pathway regulates cell proliferation and apoptosis during planarian homeostasis ... 33
6. DISCUSSION ... 37
6.1 The Hippo pathway plays an important role during S. mediterranea regeneration. ... 37
6.2 The Hippo pathway is essential during planarian homeostasis ... 39
7. CONCLUSIONS AND FUTURE PERSPECTIVES ... 44
8. BIBLIOGRAPHY ... 47 9. ANNEXES ... XIII
9.1 Annex 1 ... XIII 9.2 Annex 2 ... XIII
X
Figures Índex
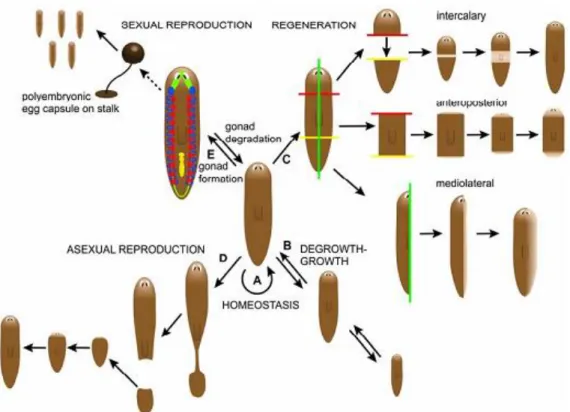
Figure 1 – Planarians regenerative, reproductive and homeostasis capacities. ... 3
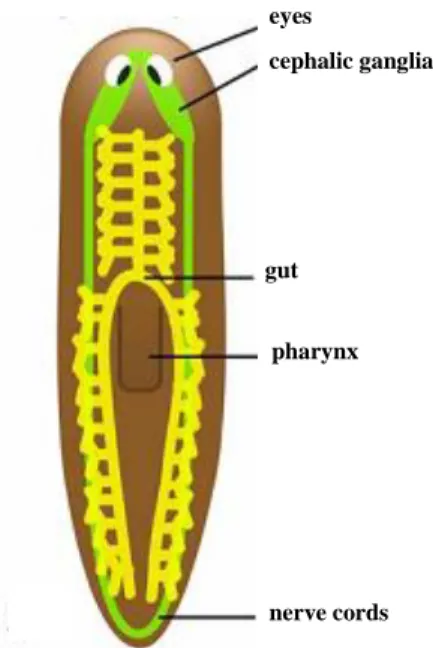
Figure 2 – Planarian anatomy. ... 4
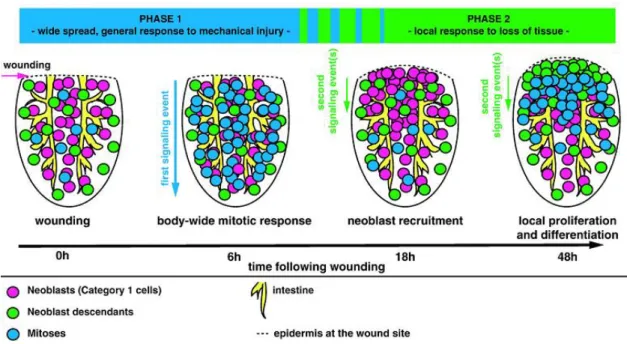
Figure 3 – Model of planarian neoblasts wound response. ... 5
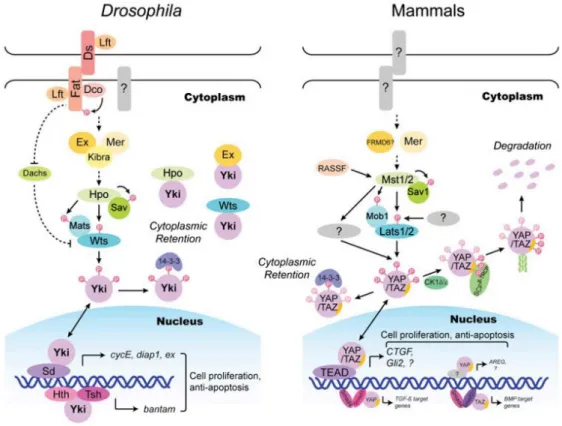
Figure 4 – Hippo pathway signaling in Drosophila and mammals. ... 8
Figure 5 – dsRNA injection procedure. ... 20
Figure 6 – Hippo pathway gene pattern expression... 24
Figure 7 – Hippo pathway inhibition phenotypes in regenerating animals. ... 24
Figure 8 – Effects of Hippo pathway genes inhibition in regenerating animals. ... 26
Figure 9 – Effects of Hippo pathway genes silencing in stem cell proliferation. ... 27
Figure 10 – Influence of Hippo pathway knocked down genes in cell death. ... 28
Figure 11 - Hippo pathway knocked down genes phenotypes. ... 29
Figure 12 – Outgrowths characterization. ... 30
Figure 13 – Excretory system of Crt, hpo, sav, wts and yki RNAi animals. ... 31
Figure 14 – Effects of Hippo pathway genes absence in the central nervous, the visual and the digestive systems. ... 32
Figure 15 – Hippo pathway silencing effects the pharynx size and its organization. ... 33
Figure 16 – Effects of Hippo pathway genes silencing in proliferation during homeostasis. ... 34
Figure 17 – Influence of Hippo pathway genes silencing in cell death during homeostasis. ... 35
Figure 18 – Regenerating phenotypes. ... 39
Figure 19 – Intact phenotype. ... 43 Figure A1 – Salvador protein alignment. ... XIII Figure A2 - Hippo protein alignment ... XIV Figure A3 – Warts protein alignment. ... XV Figure A4 – Yorkie protein alignment... XVI
XI
Table Índex
Table 1 – Sequence, melting and annealing temperature of the primers ... XIII Table 2 – PCR cycle ... XIII
XII
Abbreviations list
aPKC – Atypical protein kinase BCN – BarcelonaBMP – Bone morphogenetic proteins
cDNA – Complementary deoxyribonucleic acid CNS – Central nervous system
cRNA – Complementary ribonucleic acid DAPI – 4',6-diamidino-2-phenylindole Diap1 – Drosophila inhibitor of apoptosis 1 dJub – Drosophila LIM protein Ajuba DNA – Deoxyribonucleic acid dR – Days of regeneration
dRASSF – Drosophila Ras association family dsRNA – double strand RNA
dSTRIPAK – Drosophila striatin-interacting phosphatase and kinase DVL – Dishevelled Ex – Expander HCl – Hydrochloric acid Hpo – Hippo Hth – Homothorax JNK – Jun kinase
Lats1/2 – Large Tumor Suppressor ½ Lgl – Lethal giant larvae
Mad – Mother against Dpp
Mask – Multiple ankyrin repeats single KH domain Mer – Merlin
miRNA – microRNA Mob – Mps One Binder Mop – Myopic
Mst1/2 – Mammalian STE20-Like protein kinase ½ NDR - Nuclear Dbf2-Related
PBST – Phosphate Buffered Saline with Triton PH3 – phosphorylate histone 3
PTGS – post-transcriptional gene silencing rasiRNA – repeat-associated short interfering RNA Rb – Retinoblastoma
RISC – RNA-induced silencing complex RNA – Ribonucleic acid
RNAi – RNA interference Sav – Salvador
SD – Scalloped Shh – Sonic hedgehong Sik2/3 – Salt-inducible kinases siRNA – short interfering RNA
Smed-hpo – Schmidtea mediterranea hippo Smed-sav – Schmidtea mediterranea salvador Smed-wts – Schmidtea mediterranea warts Smed-yki – Schmidtea mediterranea yorkie
Taz – Tafazzin
TGF-ß – Transforming growth factor, Beta Receptor 1 TOR – Target of rapamycin
Tsh – Tashirt
TUNEL - Terminal deoxynucleotidyl transferase dUTP nick end labeling WISH – Whole-mount in situ hybridization
Wnt – Wingless-type
Wpb2 – WW domain-binding protein-2 Wts – Warts
Yap - Yes-Associated Protein 1 Yki – Yorkie
2
1. PLANARIAN
In the last few years, and because of the blooming of the Regenerative Medicine field, the interest in understanding how the regenerative process occurs has increased considerably. However, due to their extraordinary regenerative capacities planarians have attracted scientists since centuries ago, and they are one of the classical models to study regeneration (Reddien & Alvarado, 2004). Nowadays the planarian genome sequencing as well as the improvement of molecular techniques such as immunohistochemistry, in situ hybridization and the genetic silencing through RNAi, promotes it as an established model organism to study regeneration and stem cell biology (Saló, 2006; Robb et al., 2008).
Independently, of whether planarians are cut in small or big pieces, transversally, longitudinally or obliquely, each of the resulting fragments is able to regenerate a new organism within a few days (Figure 1). This remarkable regenerative ability of planarians is due to the presence of adult pluripotent stem cells, called neoblasts (Morgan, 1898; Saló & Beguñà, 1984; Saló, 2006). After amputation, a regenerative tissue is formed – the blastema – through the migration and accumulation of differentiating cells (Wagner et al., 2011).
Planarian great morphological plasticity is not only seen through their regenerative capacity: they also show continuous growth or reduction in size (degrowth) depending on food availability and temperature. Despite the changes in size, planarians maintain the normal function of its organs (Bowen et al., 1982; Saló, 2006).
1.1 Planarian anatomy
Freshwater planarians, are flatworms, because of their dorsoventrally flattened body, and belong to the phylum Platyhelmintes, included within the Lophotrocozoan clade of the Protostomes (review by Saló & Beguñà, 2002; Saló, 2006).
Morphologically they lack circulatory, respiratory and skeletal structures, but show a well-defined bilateral symmetry. They present anteroposterior polarity, with cephalization (bi-lobed brain ganglia) and two ventral nerve cords that run longitudinally and are interconnected by commissural neurons. Sensory organs, as the eyes and chemoreceptors are present in the head area. Planarian eyes are formed by two cell types: pigment cells that constitute the eye cups, which are visible as two dark spots
3 in the planarian head, and photosensitive cells, bipolar neurons that cluster around the pigment cups and send rhabdomeric projections towards them. The digestive system is composed of three main gut branches: one anterior and two posterior, which are highly diverticulated to facilitate the nutrient distribution all along the animal. These branches converge in the central region where they connect to the pharynx, a highly innervated muscular organ. Planarian digestive system is blind, they lack an anus, so food ingestion and waste expulsion takes place through the pharynx, which evaginates to the exterior through a mouth opening placed in the ventral surface (Figure 2). Regarding the body wall musculature, this is formed by circular, longitudinal, diagonal and transverse muscle fibers. The parenchyma consists of several non-proliferating differentiated cell types mixed with the stem-cell population. Diverse subepithelial gland cells produce a range of mucus secretions important for locomotion, protection and adhesion (Baguñà
Figure 1 – Planarians regenerative, reproductive and homeostasis capacities.
(A) Homeostasis, process of renewal of all planarian cells by the equilibrium of both cell death and proliferation. (B) Degrowth-growth, due to its plastic, planaria could reduce the total body cell number (degrowth) when is in starvation through the increase of cell death. After feeding, the planarian will grow in size and cell number will increase again (growth) through a shift in the equilibrium to favor mitosis over cell death. (C) Regeneration, practically any imaginable amputation of the planarian can give rise to two individual animals. (D) Asexual reproduction, takes place by fission in the posterior part of the planarian. Both pieces will regenerate and origin two planarians. (E) Sexual reproduction, takes place by cross-fertilization between two hermaphroditic planarians and a polyembryonic egg capsule is produced. Egg hatching occurs after 10 days to several weeks depending on the planarian species (Adapted from Handber-Thorsager, 2008).
4 & Ballester, 1978; Cebrià et al., 1996; Cebrià et
al., 1997; Cebrià et al., 2002; Newmark &
Alvarado, 2002; Saló, 2006).
1.2 Schmidtea mediterranea
Although several planarian species are known, only a few are currently used in regenerative studies, namely: Dugesia japonica,
Girardia tigrina and Schmidtea mediterranea. The
specie studied in the present work is Schmidtea
mediterranea (S. mediterranea). S. mediterranea is
the most-amenable organism for conducting molecular and genetic analyses given its small, stable diploid genome (4.8 x 108 bp), which is free of highly repetitive transposable elements, and its great regenerative and reproductive capacity. S.
mediterranea shows sexual and asexual strains.
Asexual strain reproduces by spontaneous tail fission and this small tail piece will become a feeding worm in one week (Saló, 2006).
1.3 Mechanisms underlying planarian regeneration
Planarian adult pluripotent stem cells, the neoblasts, underlie planarian remarkable regenerative abilities, and their exceptional characteristics have been studied from years ago. Neoblasts are classical defined as cells that proliferate, have a large nucleus and small cytoplasm, with 5-8 µm in diameter, and are presumed to be capable of producing both multiple cell types and to renewal (Baguñà et al., 1989; Reddien & Alvarado, 2004; Handberg-Thorsagerand & Saló, 2007).
In S. mediterranea, neoblasts are distributed throughout the parenchyma of the animal with the exception of the area in front of the photoreceptors and the pharynx (Morgan, 1898; Baguñà, 1981).
After healing it is observed the formation of an unpigmented epithelial/mesenchymal ledge from the wound known as a regenerative blastema. In the blastema there are not mitosis so, the blastema formation occurs by the early
eyes
cephalic ganglia
gut
pharynx
nerve cords
Figure 2 – Planarian anatomy.
The ventrally located nervous system is indicated in green with the cephalic ganglia connected to two lateral nerve cords. A pair of eyes can be found in the head region. The blind-ended three-branched digestive system (in yellow, gut) is connected to the pharynx, which is situated on the ventral side of the planarian and is used for eating and for expelling waste material (Adapted from Handber-Thorsager, 2008).
5 accumulation of cells at a distal end of the wound followed by the migration of undifferentiated cells from stump area to the base of blastema (Saló & Baguñà, 1984; Saló & Baguñà, 1989). Therefore the formation and maintenance of the regenerative blastema requires both cell division and migration, to target postmitotic cells to areas of active regeneration (Reddien & Alvarado, 2004).
Amputation triggers two peaks in neoblast mitoses early in regeneration. The first mitotic peak occurs within 6 hours after amputation and it is a general response, since the mitotic cells are observed throughout the entire animal fragment. This peak is followed by a general decrease in mitotic numbers, reaching a minimum by 18 hours following amputation. The second mitotic peak is observed at 48-72 hours in the wounding area (Figure 3). So, the mitotic response after a wound consists in a first, immediate, and widespread mitotic peak and a second, localized mitotic peak (Wenesomer & Reddien, 2010).
Furthermore, amputation causes two peaks of apoptotic cell death, one at 4 hours after injury that is localized in the wound region, and a second peak at 3 days after amputation that spreads throughout the animal (Pellettieri et al., 2010).
Figure 3 – Model of planarian neoblasts wound response.
Two distinct phases of neoblast responses occur during regeneration initiation. The first phase represents a generic response to injury that spreads quickly from the wound site and induces increased mitotic entry of neoblasts body-wide (6h). The second phase induces the recruitment of neoblasts followed by local proliferation and differentiation at the wound site (48h). Depicted are posterior (tail) fragments (Adapted from Wenesomer & Reddien, 2010).
6 The replacement of missing structures does not only require proliferation and growth but also correct differentiation and spatial organization of cells to re-establish the correct pattern of an adult animal. For example, the BMP and the Wnt/ß-catenin pathways are essential for the body axis establishment during planarian regeneration. BMP is responsible to establish the dorso-ventral axis of planarian while Wnt/ß-catenin establishes the antero-posterior axis (Cebrià et al., 2010).
1.4 Growth control in planarians
Planarians have an important mechanism to maintain the equilibrium between cell death and rebirth which is essential to promote the regeneration process and the homeostasis. However, the exact molecular pathways by which planarians maintain a precise population of adult stem cells and the balance between the proliferation and cell death remain to be elucidated. In the last years several studies have been performed in order to identify the pathways that controls these processes, some of them have been identified: (1) TOR, (2) Rb (3) Smg-1, (4) p53 and (5)
Smed-Jnk.
Smed-TOR and Smed-Rb have been recently associated to the maintenance of
cell proliferation in planarians. Their absence produces the restriction of cell proliferation and abolishment of cell renewal (Peiris et al., 2012; Zhu & Pearson, 2013). In contrast, Smed-p53 and Smed-JNK absence produces an increase in stem cell number and proliferation and an acceleration of the dynamics of neoblasts proliferation (Pearson & Alvarado, 2010; Almuedo-Castillo et al., submitted). On the other hand, Smed-Smg1 silencing leads to the formation of regenerative blastemas which remain undifferentiated and leads to the ectopic outgrowths formation (González-Estévez et al., 2012).
Several pathways are involved in the coordination between cell proliferation and cell death. To understand what lies behind these mechanism and the pathways involved is quite important in order to realize the most exciting planarians features.
7
2. THE HIPPO PATHWAY
The Hippo pathway has emerged as an evolutionary conserved signaling pathway essential to coordinate both proliferation and cell death in order to maintain homeostasis in several organisms. Commonly referred to as “Hippo signaling” it is also named as Fat signaling, Warts signaling, and Salvador-Warts-Hippo (SWH) signaling (Oh & Irvine, 2010).
2.1 Hippo pathway core
The activation of the Hippo pathway comprises a series of phosphorylation events that lead to the inhibition of the pathway target, the transcriptional activator Yorkie (Yki), in Drosophila, or the Yes-associated protein 1 (Yap) and Tafazzin (TAZ), in mammals (Pan, 2010; Badouel & McNeill, 2011). Protein kinase Hippo (Hpo) in
Drosophila, or Mst1/2 in mammals forms a complex with the WW domain scaffolding
protein Salvador (Sav) in order to phosphorylate and activate the NDR family kinase Warts (Wts). Once activated, Wts – in Drosophila – or Lats1/2 – in mammals – in association with Mats/Mob, from Drosophila and mammals, respectively, to phosphorylate and inactivate Yki or YAP/TAZ. Wts can phosphorylate Yki in three different sites – S111, S168 and S250. The phosphorylation site S168 is the most important and creates a 14-3-3 binding site which restricts Yki in cytoplasm. When Wts is inactive, it cannot phosphorylate Yki, which translocates to the nucleus. Once in nucleus Yki activates several genes involved in the activation of cell proliferation and inhibition of the apoptosis (Figure 4) (Dong et al., 2007; Pan, 2007; Badouel & McNeill, 2011).
Essential to an intrinsic size “checkpoint” that stops growth when a given organ reaches its characteristic size, the loss of Hippo pathway in Drosophila leads to an excessive cell proliferation and a reduction of cell death (Huang et al., 2005; Kango-Singh & Kango-Singh, 2009).
2.2 Hippo pathway upstream regulators
Although the knowledge about how the Hippo pathway is regulated is scarce, a singular feature is that it integrates multiple upstream inputs. So far, several upstream regulators of the Hippo pathway have been reported in Drosophila: (1) Kibra-Expanded-Merlin complex, (2) Fat signaling, (3) Lgl pathway, (4) Jnk and (5) dRASSF.
8 An overview of the Hippo pathway upstream regulators can be seen in Figure 4.
Kibra-Expanded-Merlin complex is composed by three proteins, two FERM domain-containing proteins Merlin (Mer), Expanded (Ex) and a WW-domain-containing protein Kibra. To promote the Hippo pathway activation, Kibra associates with Mer and Ex and together they recruit the Hippo pathway core to the apical membrane of the cell. There Hpo and Wts are phosphorylated at sites associated with their activation (Grusche et al., 2010). Mutations of Mer and Ex individually results in increased of Yki activation and tissue growth. Ex can also bind directly to Yki causing it entrapment on the cytoplasm, providing an alternative Yki regulation mechanism. Other interactions between Kibra-Mer-Ex complex and the Hippo pathway core were identified: Mer can bound to Sav, Kibra bounds to Sav and Wts and Ex bounds to Hpo, but the mechanism by how they interact remains unclear (Chen et al., 2010; Grusche et
al., 2010; Ling et al., 2010; Staley & Irvine, 2012).
Figure 4 – Hippo pathway signaling in Drosophila and mammals.
Drosophila and mammals proteins are indicated by matching colors and shapes. Direct interactions
are indicated by solid lines or drawn as proteins in direct contact. Dashed lines indicate indirect genetic interactions for which do not exist direct protein-protein contact (adapted from Zhao et al., 2010).
9 Fat is a large transmembrane protein whose tumor suppressor activity has been linked to Hippo signaling through several observations, including regulation of shared downstream target genes, genetic interactions and influences of the levels and localization of Hippo pathway components such as Wts and Yki (Staley & Irvine, 2012). Two distinct mechanisms by which Fat can influence Yki activity have been described: (1) Fat is required to Wts stability, as revealed by the finding that Wts levels are greatly reduced in fat mutant tissue and (2) Fat regulates the levels and subcellular localization of Ex, given that Ex protein levels are reduced and/or shifted to a more sub-apical region in fat-deficient tissue (Bennett & Harvey, 2006; Grusche et al., 2010; Staley & Irvine, 2012).
The Drosophila Ras association family (dRASSF) protein negatively regulates Hippo pathway by competing with Sav for binding to Hpo via carboxy-terminal Sav/Ras/Hpo (SARAH) domain, which is common to all three protein. When dRASSF is bound to Hpo it phosphorylation levels are low, indicating that Hpo is inactive. Sav and dRASSF not only compete to Hpo but they negatively regulate each other at the protein level. rassf clones have increase Sav levels and when Sav is overexpressed dRASSF levels are lowered. However, is still unclear how this reciprocal regulation of protein levels occurs (Grusche et al., 2010).
Lethal giant larvae (lgl) silencing was associated with the activation of Yki promoting the proliferation of lgl mutant cells. Is known that Jun Kinase (JNK) have an essential role in the Yki activation in lgl-depleted cells, but the mechanism by which JNK activates Yki remains unclear (Sun & Irvine, 2011). Atypical protein kinase (aPKC) overexpression or lgl decrease leads to a relocation of Hpo and dRASSF, which could be the main reason to prevents Hpo association with Sav and as a consequence prevent the activation of Hpo (Grusche et al., 2010).
In 2011, Sun & Irvine suggested that Jnk signaling could promote Yki activation. The mechanism by which Jnk activation induces Yki activation remains unclear. The observation that it could be suppressed by over-expression of Wts or Hpo suggests that it might impinge on Hippo signaling. However, the possibility that Jnk-dependent Yki regulation occurs in parallel to these Hippo pathway components cannot be excluded.
10
2.3 Negative regulators of the Hippo signaling
Despite swift progress in recent years, key steps of the Hippo pathway remain poorly understood. The regulation of the Hippo signaling as well as its inhibitors have been extensively studied in the last years, namely in Drosophila and at least four direct inhibitors of Hippo pathway are known (1) Drosophila striatin-interacting phosphatase and kinase (dSTRIPAK), (2) Drosophila LIM protein Ajuba (dJub), (3) Drosophila
Zyxin family gene (Zyx) and (4) Salt-inducible kinases (Sik2 and Sik3).
In 2010, Ribeiro and colleagues identified dSTRIPAK as a phosphatase that acts as an inhibitor of Hippo pathway. dSTRIPAK complex controls Hpo activity by reverting the phosphorylation of Hpo, resulting in the Hippo pathway silencing and Yki activation. This regulation could be due to a direct effect on Hpo or through a regulation of an upstream component that regulates Hpo phosphorylation (Ribeiro et al., 2010). In the same year, Da Thakur and others found that dJub specifically associates with Sav and Wts and negatively regulates them, but how this interaction occurs is still unclear (Da Thakur et al. 2010).
Zyx, a protein which is associated with cytoskeletal and transcriptional regulation was recently included as a Hippo pathway component. Zyx positively regulates Yki and its loss results in a reduction of Yki activity and organ growth (Rauskolb et al., 2011).
In addition, Wehr and colleagues (2012) identified Sik2 and Ski3 as Hippo pathway upstream regulators. Their results show that activated Sik2 can induce the overgrowth and activation of Yki transcription activity, whereas the decrease of Sik2/3 leads to undergrowth. They proposed that the effect of Sik2/3 in Hippo signaling is mediated, at least in part by Sav phosphorylation in Ser 143. Once phosphorylated, Sav reduces its ability to efficiently scaffold Hpo/Wts core kinase complex, reducing Yki inhibitory phosphorylation.
2.4 Yki specific regulators
Yki regulation has been devoted to processes that impinge on Wts-mediated phosphorylation of Yki. However, there has also been evidence that, in Drosophila, Yki can be regulated by direct binding to upstream components of the Hippo pathway, including Ex, Wts, and Hpo (Gilbert et al., 2011). This binding is mediated through Yki’s WW domains, a protein-protein interaction domain that associates with short
11 Proline-rich sequences, often within a PPXY motif (where X is any amino acid). Recently, two additional PPXY-motif containing proteins, WW domain-binding protein-2 (Wpb2) and Myopic (Mop) have been identified as proteins directly interacting with Yki through its WW domains (Gilbert et al., 2011; Zhang et al., 2011).
Gilbert and colleagues (2011) suggest that Mop could be a regulator of the Hippo pathway. They demonstrate that Mop regulates Yki activity via a mechanism involving direct binding and modulation of Yki endossomal modulation. The Mop:Yki interaction involves a WW:PPxY interaction mechanism shared by the Hippo pathway components – Ex, Wts and Hpo – that can directly bind to Yki and inhibit its activity independent of S168 phosphorylation status. In contrast, Wbp2 enhances Yki intrinsic transcriptional activity and that the ability of Wbp2 to bind and activate Yki was dependent on Yki’s WW domains and Wbp2’s PPxY motifs. The precise mechanism by which Wbp2 activates Yki is still unclear (Zhang et al., 2011).
Myc is a transcriptional target of Drosophila Hippo pathway and functions as a critical cellular growth effector of this pathway. In 2010, Neto-Silva and others found evidences that, in a negative feedback loop, dMyc modulates Yki expression and activity though both transcriptional and post-transcriptional mechanisms suggesting that the level of dMyc in a cell regulates the cellular quantity of Yki. This negative feedback loop between dMyc and Hippo pathway provides a stabilizing homeostatic mechanism that contributes for regulation of organ size.
Recently, a novel regulator of Yki activity have been identified, the protein Mask (Multiple ankyrin repeats single KH domain). It was shown that Drosophila Mask is required downstream of the Hippo pathway forming a complex with Yki and its binding partner, Scalloped, and together they induces the transcription of several Yki targerts (Sansores-Garcia et al., 2013; Sidor et al., 2013).
2.5 Yki DNA-binding patterns
Yki is a transcription co-activator and lacks its own DNA-binding domain. So, depended of the cellular context, Hippo pathway can recruit different DNA-binding transcription factors. Until now, several DNA-binding Yki partners have been related in
Drosophila such as: (1) Scalloped (SD), (2) Mother against Dpp (Mad) and (3)
Homothorax (Hth) – Tashirt (Tsh).
In 2008, Gouleu and colleagues identified SD as a binding partner of Yki in
12 domain binds to Yki through their C terminus to the Yki N terminus. SD:Yki interaction increases SD transcription activity and promotes Yki nuclear localization. It was described that the transcription factor dE2F1 directly binds to the SD:Yki complex in order to promote the expression of shared target genes. Furthermore, these complex SD:Yki:dE2F1 was associated to the expression of a specific transcriptional program necessary to bypass the cell cycle exit, promoting out of control cell proliferation (Gouleu et al., 2008; Nicolay et al., 2011).
Oh and Irvine (2011) establish Mad as a DNA-binding Yki partner. They found several observations that support the Mad:Yki connection: (1) the ability of each of them to promote growth is partially dependent of each other; (2) they bind directly to each other, and a mutation that decreases binding, decreases their transcription activity, while directly fusion between them dramatically enhances it. Mad:Yki complex regulates the micro RNA gene batam which has ability to promotes the growth.
Yki can regulate target genes in conjunction with Hth and Tsh, two DNA-binding transcription factors expressed in the uncommitted progenitor cells of the
Drosophila eyes. Hth-Tsh-Yki complex perform several functions namely promoting
cell proliferation and blocking apoptosis, at least in part by up-regulating the levels of micro RNA bantam (Peng et al., 2009).
2.6 Hippo pathway targets
Several downstream targets of Yki that are important for its role in growth control have been identified, such as (1) promoters of growth like bantam and Myc, (2) promoters of cell cycle progression like E2F1 and cyclins B, D and E and (3) inhibitors of apoptosis like Diap1.
Myc and bantam are essential for Drosophila Yki induced over-proliferation
driving tissue growth through activation of numerous target genes and by regulation of apoptosis (Thompson & Cohen, 2006; Neto-Silva et al., 2010; Ziosi et al., 2010).
Drosophila Hippo kinase cassete – Hpo, Sav, and Wts – appears to be capable to
regulate both cell cycle exit and apoptosis by virtue of the ability to modulate the levels of the two key regulators – Cyclin E and Diap1. Loss of these proteins leads to an increment of Cyclin E transcriptionally levels and a Diap1 levels by post-transcriptional mechanism (Tanpon et al., 2002; Wu et al., 2003; Silva et al., 2006).
13 Recently a novel target of Yki was discovered, the growth factor BMP4. Lai and Yang (2013) revealed that in mammal cells TAZ induce BMP4 transcription and then they formed a complex to promote the cell migration in breast cancer.
2.7 The interplay between the Hippo pathway and other signaling pathways
Organ development is a coordinated process of increase in organ size and morphogenesis that is mediated by extensive crosstalk between signaling pathways. The Hippo pathway is not an exception and it interlays with other established signaling cascades such as: (1) TGF-ß signaling, (2) Notch signaling, (3) Wnt signaling and (4) Hedgehog signaling.
In 2009, Alarcon and colleagues found a link between the Hippo and the TGF-ß pathway for the self-renewal capability of mouse embryonic stem cells, which is promoted by interaction of Smad1 with the WW domains of YAP. In de same year (2009), Genetev and others found an increment of Notch receptors in the apical membrane of Drosophila eyes when Hippo pathway components are depleted. However, Notch signaling activity is decreased when the Hippo pathway is absent.
Regarding the Hippo/Wnt signaling, Varelas and others (2010) showed that, in mice, citoplasmatic TAZ bind to Dishevelled (DVL) – an integral member of Wnt pathway – and negatively regulated Wnt signaling. Furthermore they prove that the loss of TAZ increased DVL phosphorylation and Wnt-dependent ß-catenin activation, resulting in a ß-catenin nucleus accumulation. In contrast, Fernandez et al. (2009) demonstrated that in human medulloblastomas, Sonic hedgehong (Shh) signaling pathway promotes Yap1 nuclear localization reducing the phosphorylation levels of Lats1.
2.8 Hippo pathway and regeneration
While the Hippo pathway is well known for its contributions to growth control during normal development, recent studies in Drosophila melanogaster and
Macrostomum lignano have emphasized that the Hippo pathway also plays an important
role during regenerative growth.
Recent data showed that Yki is hyperactivated in Drosophila imaginal discs in response to tissue damage, whether induced surgically, genetically, or by irradiation. Indeed, higher levels of Yki activation are required for normal regeneration. Fat
14 signaling could be, at least in part, responsible to this Yki hyperactivation by Hippo signaling inhibition (Grusche et al., 2011; Sun & Irvine, 2011).
Hippo pathway knocked down in the flatworm Macrostomum lignano leads to disruption of allometric scaling during regeneration and increased size of regenerated parts (Demircan & Berezikov, 2013).
The Hippo pathway has been associated with the growth control and increment in the capacity of regeneration in several organisms (Grusche et al., 2011; Sun & Irvine, 2011; Demircan & Berezikov, 2013). Accordingly, planarians are a great model organism to study this pathway, since they have incredible features which allow them to regenerate missing parts and to growth and degrowth during homeostasis.
16
3. OBJECTIVES
The main objective of this work was to characterize the function of Hippo pathway core components, namely Hippo, Salvador, Warts and Yorkie during planarian regeneration and homeostasis. The specific aims were the following:
Identification and sequence analysis of the Hippo pathway elements in the S. mediterranea genome.
Functional characterization of Hippo, Salvador, Warts and Yorkie during planarians regeneration.
Functional characterization of Hippo, Salvador, Warts and Yorkie during planarians homeostasis.
18
4. MATERIALS AND METHODS
4.1 Animals
Schmidtea mediterranea from the asexual clonal line BCN-10 were used for all
experiments (Fernàndez-Taboada et al., 2010). Planarians were kept at 20oC in a 1:1 (v/v) mixture of distilled water and tap water treated with AguaSafe (TetraAqua). Animals were feed once a week with beef liver and starved for at least one week before the experiments. To study regeneration, planarians were cut pre- and post-pharyngeal in order to analyze the anterior and posterior regeneration of the trunk, head and tail fragments. All the experiments were performed using animals of the same size.
4.2 Isolation, Cloning, and Sequencing of S. mediterranea genes
Planarian homologs of the Hippo pathway were identified from the S.
mediterranea genomic database (Washington University, St. Louis, MO) through a
BLAST search. In order to amplify corresponding transcripts, cDNA was obtained through RNA using Superscript III (Invitrogen). Planarian RNA was extracted using TRIzol®Reagent (Invitrogen).
Specific primers were designed to amplified Smed-Hpo, Smed-Sav, Smed-Wts and Smed-Yki from cDNA (Annex 1). A PCR was performed with these primers in order to amplify the cDNA fragments. After the amplified fragments were inserted in the pGEM-T (promega) vector and then inserted in E.coli DH5α competent cells. These were growth O/N at 37oC and white colonies were separated. The plasmids with the interest fragment were purified using QIAprep Spin Miniprep Kit de QIAGEN. To confirm the sequence of the fragments a capillary sequencing was performed by the Genomic service of Serveis Cienticotècnics, Universitat de Barcelona.
4.3 RNA interference analysis
RNA silencing or RNA interference (RNAi) is a mechanism by which genes are silenced through alterations of mRNAs level. It is triggered by double strand RNAs (dsRNA): short interfering RNAs (siRNAs), repeat-associated short interfering RNAs (rasiRNAs) and microRNAs (miRNAs). The artificial introduction of long dsRNAs or siRNAs has been adopted as a tool to inactivate gene expression, both in cultured cells and in living organisms (Meister & Tuschl, 2004; Arraiano & Fialho, 2007; Kim & Rossi, 2007).
19 Exogenous siRNAs target complementary mRNAs for transcript cleavage and degradation in a process known as post-transcriptional gene silencing (PTGS). Effective PTGS requires perfect or near-perfect Watson–Crick base pairing between the mRNA transcript and the antisense or guide strand of the siRNA resulting in the cleavage of the mRNA by the RNA-induced silencing complex (RISC). The RNAi mechanism mediated by siRNA is aptly described in the followed reviews: Meister & Tuschl, 2004; Collins & Cheng, 2005 and Kim & Rossi, 2007.
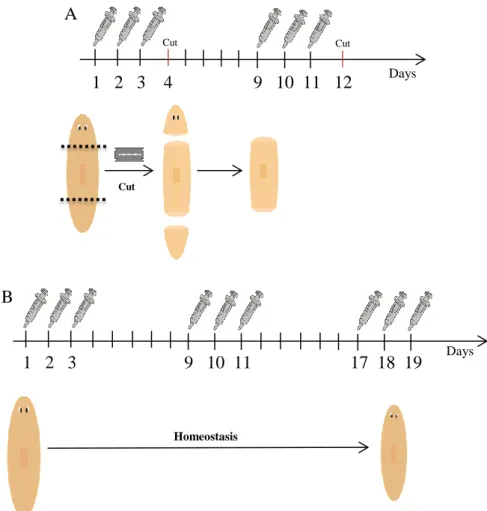
dsRNAs were synthesized by in vitro transcription (Roche) using the primers T7 and Sp6 whose correspond to the plasmid where the fragments were inserted. The dsRNA fragments have 30 to 65 bp. The dsRNA were micro-injected into planarians as previously described (Alvarado & Newmark, 1999), using a Drummond Scientific Nanoject. For regeneration experiments, animals were injected during two weeks. They were injected for three days and then amputated at the fourth day. In the following week the trunk pieces of these animals were submitted to the same procedure. The animals were fixed at different times of regeneration (Figure 5A). For the study of intact, non-regenerating, animals they were injected during three weeks with three injections every week, without amputation, with the exception of yki RNAi animals, which were injected only for two weeks (Figure 5B). Control animals were injected with water miliQ.
4.4 Whole-mount in situ hybridization
In situ hybridization provides invaluable information regarding the localization
of gene expression in heterogeneous tissues. The technique is extremely sensitive and can detect the amount of mRNA contained in a single cell. There are many different protocols to perform in situ hybridization, including probing with cDNAs, cRNAs, or synthetic oligonucleotides (Wilconx, 1993).
In order to analyze gene expression, planarias were fixed and then processed in an InsituPro hybridization robot (Abimed/Intavis) as previously described (Umesono et
al., 1997; Pearson et al., 2009; Molina et al., 2011). Digoxigenin-labeled riboprobes for Smed-hpo, Smed-sav, Smed-wts, Smed-yki and Smed-Pantothenate kinase 1 (kindly
provided by Francesc Cebrià group, Genetic Department, University of Barcelona) and
Smed-H.14.9d (Alvarado et al., 2002) were synthetized through cDNA using an RNA in vitro transcription kit (Roche) with the primers T7 and Sp6 whose correspond to the
20 bp. Samples were observed through a Leica MZ16F stereomicroscope and images were captured with a ProgRes C3 camera from Jenoptik.
4.5 Whole-mount Immunostaining
Immunohistochemistry consist in the identification of a certain antigen in a histological tissue section, cytological preparation or whole organism (whole-mount) via an antibody specific to the antigen. The localization of the primary antibody (and therefore the target antigen) is then visualized microscopically via an appropriate enzymatic or fluorescent detection system (Renshaw, 2006).
In order to perform this technique, the animals were killed in 2% HCl for four minutes on ice and then fixed in Carnoy solution for two hours at 4oC. After fixation, samples were processed as described (Cebrià & Newmark, 2005) in order to increase the permeability. The following primary antibodies were used: anti-arrestin (1:15,000;
Figure 5 – dsRNA injection procedure.
(A) – Regenerating animals. Animals are injected during three days and cut at the fourth day. In the following week the trunks of the animals were submitted to the same process. (B) – Intact animals. Animals were injected three times a week during three weeks.
A 1 2 3 4 9 10 11 12 Cut Cut Cut B 1 2 3 9 10 11 17 18 19 Days Homeostasis Days
21 kindly provided by Hidefumi Orii, Himeji Institute of Technology, Hyogo, Japan), anti-synapsin (anti-SYNORF1, 1:50; Developmental Studies Hybridoma Bank), anti–α-tubulin (1:20; Developmental Studies Hybridoma Bank), anti–Smed–β-catenin 2 (1:2,000; Chai et al., 2010) and anti-phospho-histone H3 (anti-PH3) (Ser10) (D2C8) (1:500; Cell Signaling Technology). Alexa Fluor 488-conjugated goat anti-mouse IgG or Alexa Fluor 568-conjugated goat anti-rabbit IgG secondary antibodies (Molecular Probes) were used at dilutions of 1/400 and 1/1000, respectively. Samples were mounted in SlowFade®Gold antifade reagent (Invitrogen) and/or glycerol at 70%.
4.6 TUNEL assay
The TUNEL method utilizes exogenous terminal transferase to label strand breaks by incorporating modified nucleotides. This method primarily produces end labeling. For the detection of DNA strand breaks, there is a requirement for pre-fixing cells with a crosslinking agent such as formaldehyde (Lawry, 2003).
The procedure used to detect apoptotic cells during this study was the previously described (Pellettieri et al., 2010) with some modifications to increase permeability: between the fixation step with 4% formaldehyde in PBST, samples were incubated with Proteinase K (20 μg/mL) in PBST (PBS with 0.3% Triton X-100) for 10 minutes at 37ºC in a water bath while agitating by hand, and an additional reduction step was added after fixation (Pearson et al., 2009). Finally, samples were incubated overnight in terminal transferase enzyme at 37ºC, and again with anti-dioxigenin-rhodamine (overnight at 4ºC).
4.7 Imaging capture
Samples from immunostaining and TUNEL analysis were imaged using a confocal laser scanning microscopy Leica SP2, and then images were treated using ImageJ and Photoshop CS3 software. Furthermore samples from immunostaining and in situ hybridization were observed in the microscope Zeiss Stemi SV6 and the Leica MZ16F stereomicroscope. The images were captured by ProgRes C3 camera.
4.8 Cell counting and statistical analysis
In order to perform a further analysis, the number of proliferative cells stained with anti-PH3 anti-body and with the Smed-H.14.9d probe were counted using ImageJ
22 program. The data was treated in the Excel and normalized, removing the data which did not follow a normal distribution.
23
5. RESULTS
5.1 Hippo pathway core genes are conserved in S. mediterranea
A single representative of the core genes of the Hippo pathway was identified in the S. mediterranea genome (hippo, salvador, warts and
Smed-yorkie) available on the NCBI platforms. Since there are some differences in the Hippo
pathway gene names between mammals and Drosophila melanogaster, S. mediterranea orthologs were named following the more frequently used abbreviated names: hpo, sav,
wts and yki. For simplicity, in the following sections the ‘Smed’ prefix will not be used
when referring to the S. mediterranea Hippo pathway genes.
A comparative analysis of the corresponding protein of those planarian genes with the orthologs of other animal species such as Macrostomum lignano, Mus
musculus and Xenopus laevis, demonstrate the conservation of the functional domains
in the planarian sequences (Annex 2).
5.2 Hippo pathway genes are expressed throughout the animals
Whole mount in situ hybridization (WISH) analysis revealed that hpo, sav, wts and yki genes show a similar pattern expression in intact animals, through all the parenchyma, although Hpo mRNA presents higher levels. yki expression was found in the parenchyma, but also in the pharynx (Figure 6A). During regeneration (at 3 and 5 days of regeneration) hpo and yki showed a higher expression in regenerating blastemas, and yki appeared to be expressed also in the regenerating pharynx (Figure 6B and 6C). The ubiquitous expression of those genes was expected, since they have a function in basic cell behaviors, however, it must be stressed, that their function is regulated by phosphorylation, and the mRNA levels could not really represent functional activity.
5.3 Hippo pathway genes are required for proper differentiation and regeneration of missing structures
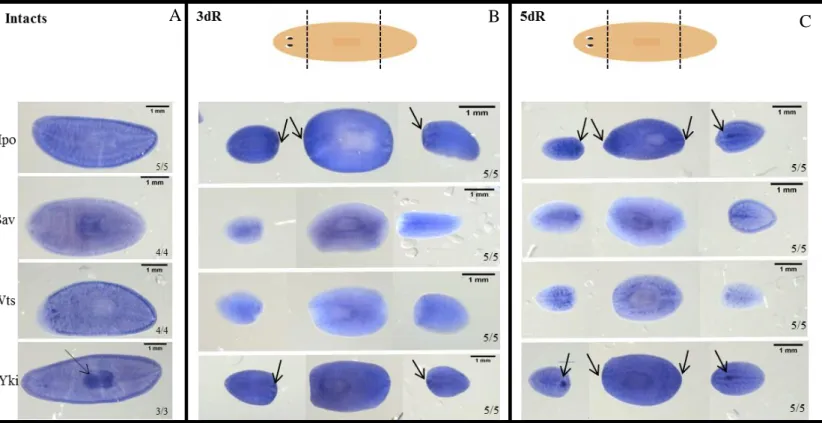
In order to characterize the Hippo pathway function in S. mediterranea during regeneration, the silencing of hpo, sav, wts and yki was performed through RNAi mechanism.
24 After amputation it was verified that the hpo, sav, wts and yki RNAi trunk pieces are unable to regenerate properly the blastemas at early stages of regeneration (5 days) (Figure 7B, 7C, 7D and 7E). Furthermore, at this stage, hpo, sav and wts RNAi trunk pieces do not correctly form the eyes (Figure 7B, 7C and 7D) and yki RNAi animals
Figure 7 – Hippo pathway inhibition phenotypes in regenerating animals.
(A) Control animals at 5 and 12 days of regeneration. (B, C, D and E) At early stages of regeneration it is possible to observe that hpo, sav, wts and yki RNAi animals are unable to form a proper blastema. Later, these animals appear to recuperate the capacity to form the missing structures. However, hpo and wts RNAi animals do not form properly the eyes and yki RNAi animals appear blotted and with an indented tail (C, D and E).
Figure 6 – Hippo pathway gene pattern expression.
(A) hpo, sav, wts and yki genes show a similar pattern expression in intact animals, through all the parenchyma. yki is also expressed in the pharynx (black arrow). (B) Gene pattern expression at 3 days of regeneration. hpo and yki showed a higher expression in regenerating blastemas, and yki appeared to be expressed also in the regenerating pharynx. sav and wts are lower expressed at this time of regeneration than hpo and yki. Black arrows point to labeled regenerating blastemas (C) Gene pattern expression at 5 days of regeneration. hpo and yki are highly expressed in the blastemas of trunk piece and widely expressed in head and tail pieces. These genes appear to be expressed at the pharynx in the head and tail pieces in this time of regeneration.
25 start to blot and became transparent (Figure 7E). At later stages (12 days) hpo, sav and
wts RNAi animals do regenerate the head and the tail, although the shape is not normal
and the eyes are not properly differentiated. yki RNAi animals never regenerate proper head or tail. The tail appears indented and the animals appear completely blotted (Figure 7E). yki RNAi animals die around the day 18 of regeneration.
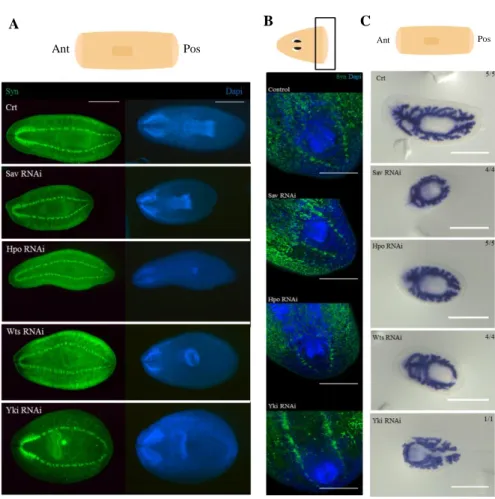
To clarify the influence of Hippo pathway in the regeneration and differentiation of missing structures, molecular techniques were applied. To analyze the formation of the central nervous system (CNS) an immunohistochemistry was performed using the anti-synapsin antibody in 7 and 12 days regenerating animals (Figure 8A and 8B). The samples were also stained with DAPI, which labels DNA, and allows the visualization of the nucleus. It was verified that the CNS, brain and ventral nerve cords formation is affected in hpo, sav and wts RNAi animals, since the brain is shorter and thinner. The brain of yki RNAi animals seems quite differentiated but it is not well organized, and the posterior closure of the ventral nerve cords is also affected.
To observe the digestive system a WISH was perform with the
Smed-pantothenate kinase 1 probe in animals with 10 days of regeneration (Figure 8C). The
results show that all the RNAi animals are unable to form a proper digestive system in both, anterior and posterior part, since it appears less branched and disrupted, namely in the case of yki RNAi.
Together these results suggest that the Hippo pathway is essential for the proper regeneration and differentiation of missing structures during planarians regeneration.
5.4 Hippo pathway genes regulate stem cell proliferation and cell death in S.
mediterranea during regeneration
To elucidate the Hippo pathway influence in both proliferation and cell death in planarians an immunohistochemistry with anti-PH3 and a TUNEL assay was performed, respectively.
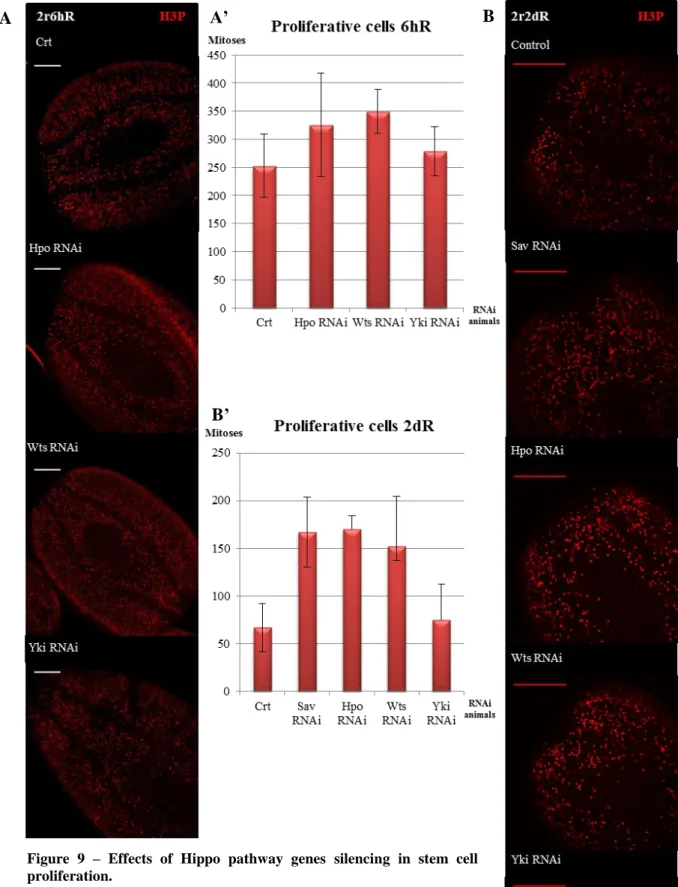
In planarians, amputation triggers a signaling that induces two peaks of neoblast proliferation, the first at 6 hours after injury and a second one at 2 days after amputation (Wenesomer & Reddien, 2010). The proliferative cells labeled with anti-PH3 in the anterior region near the blastema were counted in control and RNAi animals.
The results revealed that hpo and wts silencing increases the proliferation at 6 hours of regeneration. On the other hand, yki silencing does not affect the proliferation at early stages of regeneration (Figure 9A). After 2 days of regeneration the
26 proliferation highly increased in hpo, sav and wts RNAi planarians. However, in yki RNAi animals the proliferation was not affected (Figure 9B). Although these results are reliable, due to the low number of specimens analyzed, they do not show statistical significance.
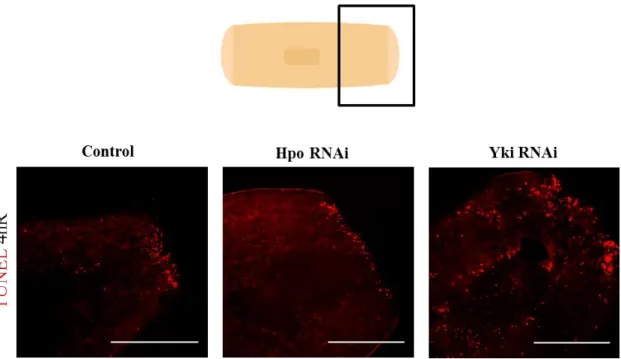
Amputation triggers two peaks of apoptotic cell death, one at 4 hours after injury that is localized in the wound region, and a second peak at 3 days after amputation that spreads throughout the animal (Pellettieri et al., 2010).
Preliminary results show that at early stages of regeneration (4 hours) hpo and
yki knocked down animals have the apoptotic processes modified, namely in the
posterior blastema and nearby region. hpo RNAi animals seem to exhibit a decrease in the number of apoptotic cells while yki RNAi animals show an increase and relocation
Figure 8 – Effects of Hippo pathway genes inhibition in regenerating animals.
(A) Central nervous system labeled with anti-synapsin antibody (green) and dapi which labels the cell nucleus (blue) in trunks of 12 days of regeneration n=6. It was observed that all the RNAi animals show alterations in the CNS, namely in the brain and the nerve cords. (B) Central nervous system labeled with anti-synapsin antibody (green) and DAPI which labels cell nucleus (blue) in heads of 7 days of regeneration n=4. The formation of the nerve cords in the posterior part of the animal is affected when the genes of the Hippo pathway are silenced. (C) WISH with pantothenate kinase 1 probe which marks the digestive system of planarians. All the RNAi animals are unable to form a proper digestive system. Ant – anterior part; Pos – posterior part. Scale bar: A: 1mm, B: 300µm and C: 0,5mm.
27
Figure 9 – Effects of Hippo pathway genes silencing in stem cell proliferation.
(A) Immunohistochemistry using anti-PH3 anti-body at 6 hours of regeneration. Inhibition of hpo and wts leads to an increase in proliferation while the silencing of yki leads to the maintenance of the prolideration n=3. (A’) Graph corresponding to the number of mitotic cells in the anterior blastema at 6 hours of regeneration in the different RNAi animals. (B) Immunohistochemistry using anti-PH3 anti-body at 2 days of regeneration. hpo, sav and wts RNAi exhibited a high increase in proliferation. The silencing of yki show a lightly increase n=5. (B’) Graph corresponding to the number of mitotic cells in the anterior blastemas at 2 days of regeneration in the different RNAi animals. Scale bar: A and B: 300µm.
A A’ B
28 of these cells (areas with high number of TUNEL positive cells, next to areas completely negative) (Figure 10).
Altogether, these results suggest that during planarian regeneration hpo, sav and
wts silencing promotes proliferation and reduce the apoptosis, while yki silencing does
not affect the proliferation and promotes the delocation of apoptotic cells.
5.5 The Hippo pathway is involved in the maintenance of differentiated structures during homeostasis in S. mediterranea
To understand the Hippo pathway function in S. mediterranea during homeostasis an RNAi experiment was performed to knockdown hpo, sav, wts and yki genes.
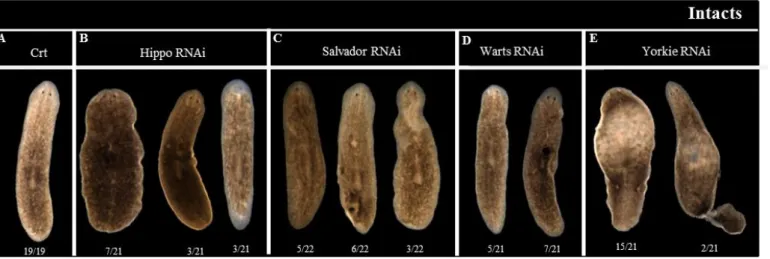
After two weeks of dsRNA injection hpo, sav and wts RNAi showed the formation of outgrowths, which disappeared 2 or 3 days after its appearance, leaving unpigmented regions (Figure 11B, 11C and 11D). With the time those animals also showed an apparent dedifferentiation of the eyes. In vivo yki RNAi animals showed a blotted phenotype and some of them (around 10%) showed tail loss caused by the constriction of the tail region, after two weeks of dsRNA injections (Figure 11E).
hpo, sav and wts RNAi animals were analyzed by immunohistochemistry using
anti-PH3 and anti-synapsin antibodies and WISH using the pantothenate kinase 1
Figure 10 – Influence of Hippo pathway knocked down genes in cell death.
TUNEL performed in trunk pieces with 4 hours of regeneration. All the animals show an accumulation of apoptotic cells in the blastema. However, yki RNAi animals show an increase and a delocalization of apoptotic cells n=3. Scale bar: 300µm.
29 probe. The results showed that the outgrowths regions show proliferation and cell accumulation, as observed by the anti-PH3 and the DAPI staining. It was further verified that knocked down animals for hpo, sav and wts causes the disappearance of several structures, as the pigmented or the digestive cells, probably due to the improper replacement of the dead cells. This phenomenon is observed mostly in the overgrowths regions (Fig. 12).
Taken together these results show that the silencing of hpo, sav and wts induces the formation of outgrowths, whose have proliferative activity, accumulation of cells and improper differentiation.
Since yki RNAi animals show a strong blotted phenotype, which could be related with a dysfunction of the excretory system, WISH analysis was performed using the
Smed-H.14.9.d probe, which labels excretory cells. In addition, an immunohistochemistry was performed with the antibodies anti-tubulin and anti-ß-catenin2, whose label the excretory and the digestive system. The data obtained revealed that the excretory system of yki RNAi animals is faulty and the number of excretory cells is reduced when compared with the control and the other knocked down animals (Figure 13A and 13B). Thus, the blotted phenotype of yki RNAi animals could be explained by the regression of its excretory system.
To a deeper comprehension of how the Hippo pathway genes silencing influence the maintenance of the visual and CNS an immunohistochemistry was performed using anti-VC1 and anti-synapsin anti-bodies, respectively. The CNS of hpo, sav and wts
Figure 11 - Hippo pathway knocked down genes phenotypes.
(A) Control phenotype after 3 week of water injection. (B, C and D) hpo, sav and wts RNAi phenotypes 3 weeks after dsRNA injection. All these animals show outgrowths formation, general deformation and depigmentation. (E) yki RNAi phenotype weeks after 3of dsRNA injection. These animals are blotted and present a tail loss.
30 RNAi animals showed asymmetries, appeared shorted and where located much closer to the end of the animal. yki RNAi animals showed CNS asymmetries and it appeared not well differentiated. Furthermore, the posterior nerve cords were disappearing in the constriction of the tail region (Figure 14A). In addition, it was observed that the hpo,
sav and wts knocked down leads to both malformation and size decrease of the visual
system in most animals. sav and yki knocked down animals present improper projection of some visual axons (Figure 14B).
These data demonstrates that the Hippo pathway is required for the proper maintenance of the CNS and visual system in S. mediterranea.
To analyze the digestive system an immunohistochemistry with anti-ß-catenin 2 was performed in Hippo pathway RNAi animals. The data obtained reveals that after
hpo, sav, wts and yki silencing, the digestive system appears weaker stained and less
branched. This result suggests that the digestive system is in regression, namely in the posterior region, when those genes are silenced (Figure 14C) and, thus, that the Hippo
Figure 12 – Outgrowths characterization.
(A) Detailed outgrowths. Anti-PH3 (red) accumulation, strong DAPI (blue) intensity and strength loss of anti-synapsin (green) under the outgrowth. These results suggest that under the overgrowths exist a cell accumulation, high proliferation and disappearance of differentiated structures. (B) WISH with pantothenate kinase 1 which shows improper differentiation of gut cells in the indicated areas. Scale bar: A: 100µm and B: 1mm.
A
31 pathway plays an important role in the maintenance of the digestive system during planarians homeostasis.
To elucidate the influence of the Hippo pathway in the maintenance of the pharynx during homeostasis an immunohistochemistry with the antibody anti-ß-catenin2 which labels the pharynx, in addition to the digestive system, was performed in the hpo, sav, wts and yki RNAi animals.
The results of ß-catenin 2 and DAPI staining show that the inhibition of hpo, sav and wts genes leads to a significant decrease of the pharynx size (Figure 15A). In some animals, the pharynx even totally disappeared. Moreover, we could observe that not
Figure 13 – Excretory system of Crt, hpo, sav, wts and yki RNAi animals.
(A) WISH with H.14.9d probe. Note the disorganized pattern of H.14.9d positive cells throughout the yki RNAi animals compared with controls. hpo, sav and wts RNAi animals do not show differences neither in the number of excretory cells neither in its organization while yki RNAi animals show a decrease in the number of excretory cells. (A’) Graph corresponding to the number of excretory cells labeled with the probe H.14.9d in all RNAi animals during planarian homeostasis. (B) Immunohistochemistry with tubulin and ß-catenin2 anti-bodies of control and yki RNAi animals. In yki silenced animals there are less tubular structures, which correspond to the excretory system, and the ones that remain appear collapsed (see red arrows). Scale bar: A: 1mm B: 100µm.
A B
32 only the size but also the organization of the pharynx was affected (Figure 15B). In contrast, yki RNAi animals did not present a decrease in the size of the pharynx, although it was also disorganized.
B
A C
Figure 14 – Effects of Hippo pathway genes absence in the central nervous, the visual and the digestive systems.
(A) Immunohistochemistry using anti-synapsin anti-body which labels the central nervous system. All the knocked down animals present a defective central nervous system namely in the shape of the brain and the nerve cords, n=7. (B) Immunohistochemistry using VC-1 anti-body that labels the visual system. hpo, sav and wts RNAi animals show small eyes and shorter visual axons compared to controls. Some sav and yki RNAi animals present defective projection of visual axons. (C) Immunohistochemistry with anti-ß-catenin2 anti-body which labels, showing the digestive system. All the RNAi animals show a faulty digestive system. The regression of the digestive system is more severe in the posterior part of the animal n=16. Scale bar: A, B and C: 300µm.