w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Sedative
and
muscle
relaxant
activities
of
diterpenoids
from
Phlomidoschema
parviflorum
Abdur
Rauf
a,∗,
Umar
Farooq
b,∗,
Ajmal
Khan
b,∗,
Taibi
Ben
Hadda
c,
Sadia
Naz
b,
Aliya
Ibrar
b,
Noor
Jehan
a,
José
P.
Cerón-Carrasco
d,
Helena
den
Haan
d,
Jorge
Pe ˜na-García
d,
Horacio
Pérez-Sánchez
d,
Haroon
Khan
e,
Mohamed
Fawzy
Ramadan
f,
Tareq
Abu-Izneid
g,
Saud
Bawazeer
gaDepartmentofChemistry,UniversityofSwabi,KhyberPakhtunkhwa,Pakistan
bDepartmentofChemistry,Comsat,InstituteofInformationTechnology,Abbotabad,Pakistan
cMaterialsChemistryLaboratory,DepartmentofChemistry,MohammedFirstUniversity,Oujda,Morocco
dBioinformaticsandHighPerformanceComputingResearchGroup,UniversidadCatólicaSanAntoniodeMurcia,Murcia,Spain eDepartmentofPharmacy,AbdulWaliKhanUniversity,Mardan,Pakistan
fDepartmentofBiochemistry,FacultyofAgriculture,ZagazigUniversity,Zagazig,Egypt
gDepartmentofPharmaceuticalChemistry,FacultyofPharmacy,UmmAl-QuraUniversity,Makkah,SaudiArabia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received3March2017 Accepted25July2017 Availableonline17August2017
Keywords:
Rosanediterpenoids Stachysrosane Sedative
Skeletonmusclerelaxantactivity Moleculardocking
a
b
s
t
r
a
c
t
Phlomidoschemaparviflorum(Benth.)Vved.(Basionym:StachysparvifloraBenth.)Lamiaceae,have sig-nificancemedicinalimportanceasitisusedinnumberofhealthdisordersincludingdiarrhea,fever, soremouthandthroat,internalbleeding,weaknessesoftheliverandheartgenital tumors, sclero-sisofthespleen,inflammatorytumorsandcancerousulcers.Thepresentcontributiondealswiththe sedativeandmusclerelaxantlikeeffectsofditerpenoidstriviallynamedstachysrosaneand stachys-rosane,isolatedfromtheethylacetatesolublefractionofP.parviflorum.Bothcompounds(at5,10 and15mg/kg,i.p)wereassessedfortheirinvivosedativeandmusclerelaxantactivityinopenfield andinclinedplanetest,respectively.Thegeometriesofbothcompoundswereoptimizedwithdensity functionaltheory.Themoleculardockingofbothcompoundswereperformedwithreceptorgamma aminobutyricacid.Bothcompoundsshowedmarkedactivityinadosedependentmanner.The dock-ingstudiesshowedthatbothcompoundsinteractstronglywithimportantresiduesinreceptorgamma aminobutyricacid.Thereporteddatademonstratethatbothcompoundsexhibitedsignificant seda-tive andmusclerelaxant-like effectsinanimal models, whichopensa doorfornovel therapeutic applications.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Phlomidoschemaparviflorum(Benth.)Vved.(Basionym:Stachys parvifloraBenth.)belongstotheLamiaceae(mint)familythat dis-tributedinthetropicalandtemperateregionsofPakistan(Ahmad etal.,2006).Plantsofthisgenushavebeenextensivelyapplied totreatmentofwidepanelofhealthdisordersincludingdiarrhea, fever,soremouthandthroat,internalbleeding,weaknessesofthe liverandheart(Holimanetal.,1996),genitaltumors,sclerosisof
∗ Correspondingauthors.
E-mails:abdurrauf@uoswabi.edu.pk(A.Rauf),umarf@ciit.net.pk(U.Farooq),
ajmalkhan@ciit.net.pk(A.Khan).
thespleen,inflammatoryandcancerousulcers(Skaltsaetal.,1999). ThespeciesofthegenusStachyslavandulifoliashowedsedativeand anxiolyticlikeeffectsinanimalstudies(Rabbanietal.,2003,2005). PhytochemicalinvestigationsofStachysspecieshaveshownthe occurrenceofflavonoids,diterpenes,phenylethanoidglycosides, alkaloids,andsaponins(Khanavietal.,2005;Ahmadetal.,2006).
VariouschemicalconstituentslikestachyssaponinA, stachys-saponinB,parviflorosidesAaswellasparviflorosidesBhasbeen isolated fromP.parviflorum (Ahmad et al.,2006, 2008). Anew rosane-type diterpenoid from P. parviflorum has been isolated (Farooqetal.,2014).
Unfortunately,literaturesurveyrevealedthatlimitedworkhas beendonesofaronP.parviflorum.Consideringitsmedicinal impor-tance, the present study is based on the sedative and muscle
http://dx.doi.org/10.1016/j.bjp.2017.07.003
relaxanteffectsof compounds1and 2isolatedfromP. parviflo-rumintwoanimalprotocols.Thecomputationalstudiesofthese compoundswerecarriedout,inordertoknowthemolecular mech-anismatatomiclevel.
Materialsandmethods
Generalexperimentalprocedure
Column chromatography was performed using silica gel (70–230mesh,E-Merck)andsilicagel(230–400mesh,E-Merck). AnalyticalTLCwasperformedonprecoatedplates(silicagel60 F254). Optical rotations were recorded using a Jasco-DIP-360-digitalpolarimeter.UVandIRspectrawererecordedwithUV-240 and JASCO-320-A spectrophotometers, respectively. EI-MS and HR-EI-MSwere recordedona double-focusingvarian MAT-312 spectrometer. The 1H and 13C NMR spectra were recorded on a Bruker AMX-400 spectrometer, while 2D-NMR spectra were recorded on Bruker AMX-500MHz NMR spectrometer. Chemi-cal shifts in parts per million (ı) relative to tetramethylsilane as aninternal standard, and scalar couplings (J)werereported inHz.
Plantmaterial
The whole plant Phlomidoschema parviflorum (Benth.) Vved. (Basionym: Stachys parviflora Benth.), Lamiaceae, was collected fromAbbottabad(Pakistan)inMarch2012.Theplantwasidentified byDr.ManzoorAhmad(Taxonomist,DepartmentofBotany, Post-GraduateCollege,Abbottabad).Avoucherspecimen(No.14230) hasalsobeensubmittedintheherbariumofthesamedepartment.
Extractionandisolation
Theair-driedwholeplant(9kg)wasgrindedandextractedwith methanolatroomtemperature.Theextractwasevaporatedona rotaryevaporatorunderreducedpressuretoobtainacrudeextract. Theresultingmethanolextract(2500g)wassuspendedinwater andsuccessivelypartitionedtoprovidehexane(110g),chloroform (80g),ethylacetate(35g),n-butanol(95g),andaqueousextract (150g).
The ethyl acetate fraction was further subjected to column chromatographyoverasilicagelcolumnusinghexanewitha gra-dientof ethyl acetate upto 100% followed by methanol. Eight sub-fractions were collected through column chromatography. Sub-fraction7–8wassubjectedtocolumnchromatography and elutedwithEtOAc/hexane35:65topurifycompound1(20mg). Thecombinedlesspolarfractions5–6ofthisseparationwere re-subjectedtocolumnchromatography,whichresultedcompound
2 (17mg) at EtOAc/hexane 30:70 polarity. The purity of com-poundswascheckedonTLCandHPTLCplates. Thestructureof thesecompoundswasrecentlyreportedbyourgroup(Farooqetal., 2015).
11 12
17 15
16 16
15 17 12
13 14 8 9 11 1 2 3
4 10
20 5
6
19 18
7 14
13 8 9 10 1 2 3
4 5 20 7
6 18 19CO
2H CO2Me
H H
H
H
1 2
Animals
NMRImice(20–25g)wereusedinvariousexperiments.Animals werefedwithstandardlaboratoryfoodandwateradlibitum. Ani-malswerekeptunderstandardconditionoftemperatureandlight. Beforethestartofexperimentanimalswereacclimatizedwith lab-oratoryconditions.Allexperimentalprotocolswereapprovedfrom ethicalcommittee foranimal studiesof University ofPeshawar underreferencenumber14009(PUP)keepinginmindthe inter-nationalguidelineofanimalstudies.
Sedativepropertiesinopenfieldtest
Theapparatususedforthis activitywasconsistedofanarea ofwhitewood(150-cmdiameter)enclosedbystainlesssteelwalls anddividedinfoursquaresbyblacklines.Theopenfieldwasplaced insidealightandsound-attenuatedroom.Animalswere acclima-tizedunderredlight (40Wredbulb)60minbeforethestartof experimentinlaboratorywithfoodandwateravailableadlibitum. Animalsweredividedinvariousgroups(n=6)andwere admin-istered withnormal saline(negativecontrol,10ml/kg,i.p.), test compounds(5,10and15mg/kg,i.p)andbromazepam(0.5mg/kg,
i.p.).After30min,eachanimalwasplacedinthecenterofthebox andthenumbersoflinescrossedwerecountedforeachmouse (Raufetal.,2017a,b).Toassessprocessofadjustmenttothe nov-eltyoftheground,micewereexposedtotheapparatusfor5min ontwosuccessivedays.
Inclinedplanetest
Theplaneusedinthisprocedurewasconsistingoftwo play-woodboards.Bothboardswereconnectedwitheachotherinsuch awaythatoneboardformthebaseandotherisfixwiththebase at 65◦.Different groups (n=6) weretreated withbromazepam
(1mg/kg,i.p.),normalsaline(10ml/kg,i.p.)andtestcompounds (5,10and15mg/kg,i.p).After30,60and90minoftreatmentthe animalswereplacedontheupperpartoftheinclinedplanefor30s tohangorfall(Raufetal.,2017a,b).
Statisticalanalysis
Resultsareexpressedasmean±S.E.M.One-wayANOVAwas usedforcomparisontestofsignificantdifferencesamonggroups followedbyDunnet’smultiplecomparisonposttest.Alevelof sig-nificance(p<0.05or0.01)wasconsideredforeachtest.
Molecularmodeling
Molecularmodelingexperimentshavebeenperformedonboth compounds.Theirgeometrieswerefullyoptimizedwithinthe den-sityfunctionaltheory(DFT)frameworkattheB3LYP/6-31G(d)level (NewmanandCragg,2012;Malamietal.,2014).Vibrational cal-culations weresubsequently performed toascertainthe nature ofeverylocalizedstructure:astablestructure correspondstoa minimuminthepotentialenergysurfaceandconsequentlyall fre-quenciesarereal.Partialatomicchargeswerenextcomputedby usingtheMerz-Singh-KollmanESPprotocol(NewmanandCragg, 2012),tobeusedduringdockingsimulations.Requiredquantum chemicalcalculationswereperformedwiththeGaussian09suite ofprograms(Malamietal.,2014).
100
30 min 60 min 90 min
30 min 60 min 90 min 75
50
25
0
100
75
50
25
0
5 10
Bromazepam
mg/kg
%animal falling in 30 s %animal falling in 30 s
mg/kg
Bromazepam
15 5 10 15
*
* *
* * *
* *
* * * * *
*
* * *
* *
* *
* * * * *
* * *
1
2
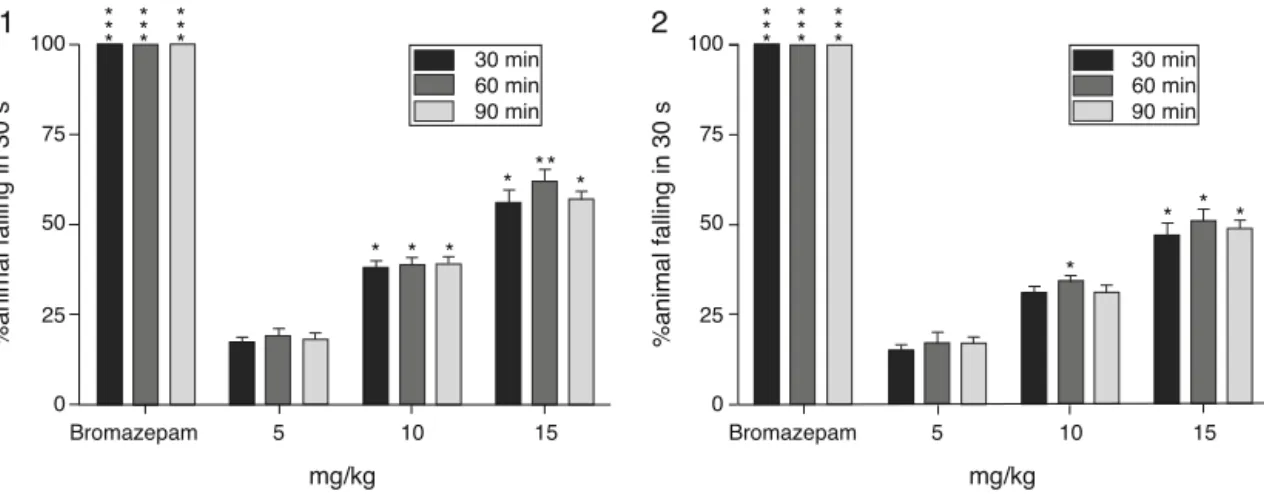
Fig.1. Effectofisolatedcompounds1and2fromP.parviflorumonmusclecoordinationininclinedplane,barrepresentsthepercenttimespentinsecondsbywhichmice slideofftheinclinedplane,30,60and90minaftertreatmentwithnormalsaline(10ml/kg),compounds(5,10and15mg/kg)orbromazepam(1mg/kg).*p<0.05,***p<0.001, allwithrespecttocontrol.
thepotentialenergy surface withthe geneticalgorithm imple-mentedintheLeadFinderdockingprogram.Fortherecords,the full-atommodelofGABAAusedindockingcalculationswas pre-paredfromtherawPDBstructure 4COF.Watermoleculeswere removedandtheionizationstatesoftheaminoacidswereassessed usingProtonate3DfunctionoftheMOEsoftwarepackage (Chemi-calComputingGroup,LLC).Thesameprotocolwasusedforadding missinghydrogenatomsinthePDBfiles,whereallchargedside chainswereconsideredintheirdefaultprotonationstatesat neu-tralpH.Proteinatomicpartialchargeswerederivedthroughthe AMBER99 forcefield (Newman and Cragg, 2012), implemented inMOE. The sizeof thegridbox forliganddocking wassetto 120 ˚AineachdirectionfromthegeometriccenteroftheAquaporin model.ThedGscoreproducedbyLeadFinderwastakenasthe pre-dictedvalueoftheligandbindingenergy.Scoringfunctionfrom LeadFinderconsidersLennard–Jonesterm(LJ),metalinteractions, solvationterm,hydrogenbonds(H-bonds),andelectrostatic inter-actions,internalenergyoftheligand,contributionstoentropydue toligandtorsionsandasolvationpenaltyterm.Inthisblinddocking approachmultipledockingrunsstartedaroundgeometriccenters ofallresidueswithintheselectedthreshold.
Results
Sedativeeffectoftestcompounds(1and2)
Thecompoundscauseddose-dependenteffectonmobilityat varioustestdoses.Pretreatmentofbothcompoundshad signifi-cantreductioninmobilityat10and15mg/kg.Theoveralleffectof compound1and2wasalmostequallysignificant.Thelocomotion decreasedbybromazepamwasmostsignificantthanthetestdoses ofisolatedcompounds(Table1).
Musclerelaxanteffectoftestcompounds
Theresultsofmusclerelaxanteffectoftestcompounds(1and
2)ininclinedplanetestarepresentedinFig.1.Bothcompounds elicitedsignificantskeletonmusclerelaxantlikeeffectinadose dependentmanner.Themaximumactivitywasnotedat15mg/kg after60minoftreatment.Compounds1and2producedalmost similarrelaxationofmiceskeleton musclebutless pronounced thenstandarddrug(bromazepam).
Table1
Effectofisolatedcompounds1and2fromP.parvifloruminopenfieldtest (locomo-tiveactivity).
Sample Dose No.oflinescrossed Control 5ml/kg 130±3.95 Bromazepam 0.5mg/kg 9±0.55c
Compound1
5mg/kg 100±5.03
10mg/kg 82±4.34a
15mg/kg 74±3.60b
Compound2
5mg/kg 101±5.67
10mg/kg 88±4.67a
15mg/kg 79±4.45a
Valuesrepresentthenumberoflinescrossedbyanimalinbox,30minafter treat-mentwithnormalsaline(10ml/kg,control),compounds(5,10and15mg/kg)or bromazepam(0.5mg/kg).Datapresentedasmean±S.E.M.(n=6).
ap<0.05. bp<0.01.
c p<0.001,allcomparedwithcontrol.
Molecularmodeling
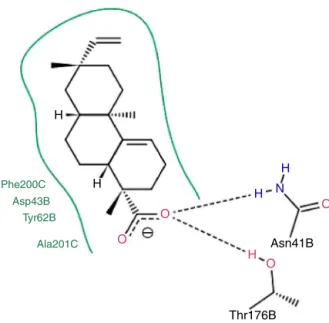
Thebromazepamand compounds1and 2werefirstdocked intotheGABAAreceptor,whichactsasthehostentity.Theposes withthehighestvaluefortheprotein–ligandinteractionenergy werenextselectedforaqualitativeanalysis.InFigs.2–4results obtainedfromblinddockingforbromazepam,compound 1and compound2areshown.Thesefigurespointedoutthatmain sta-bilizinginteractionforbromazepanareduetopi–piinteractions between Phe200C, Tyr62B and aromatic scaffold of the ligand, hydrophobicinteractionswithsameresidueTyr62Bandhydrogen bondwithGln64B.Suchlargenumberofprotein–ligand interac-tionsexplainswhybromazepaminteractssostronglywithGABAA. Regarding docking results for compound 1 and 2, we can see that in both cases they bind to the protein in the same area asbromazepam,throughhydrophobicinteractionswithresidues Phe200C,Asp43BandTyr62B,andhydrogenbondswithAsn41B and Thr176B (compound 1, Fig. 3) and Tyr205C (compound 2,
Phe200C
Tyr62B
Tyr62B
Gln64B H2B
HO
O
O NH
H H N
N
N
Fig.2. 2Drepresentationofmainprotein–ligandinteractionsestablishedbetween bromazepamandGABAA.Greenlinerepresentshydrophobicinteractions,black
dashedlineshowshydrogenbondsandgreendashedlinescorrespondtopi–pi interactions.
H
Phe200C Asp43B
Tyr62B
Ala201C Asn41B
Thr176B H
H
O
O O
O
H
H N
Fig.3. 2Drepresentationofmainprotein–ligandinteractionsestablishedbetween compound1andGABAA.Greenlinerepresentshydrophobicinteractions,andblack
dashedlinesshowhydrogenbondinteractions.
Asp43B Phe200C
Tyr62B
Tyr205C H
H
H O O
O
Fig.4. 2Drepresentationofmainprotein–ligandinteractionsestablishedbetween compound2andGABAA.Greenlinerepresentshydrophobicinteractions,while
blackdashedlineshowshydrogenbondinteractions.
Discussion
Thepresentstudydemonstratedamarkedsedativeand mus-clerelaxanteffectsofditerpenoidsisolatedfromP.parviflorumin twoanimalmodels.Theopenfieldtestisoneofthesimplestand commonlyusedscreeningtoolsfortheassessmentofsedativelike potential(Uddinetal.,2014;Raufetal.,2017a,b).Additionally,it canbeusedtoevaluateexplorationand anxietybehaviors.The numberofcentralsquareentriesandthedurationoftimespent in thecentral squarearemeasuresof exploratorybehaviorand anxiety.A highfrequency/duration ofthese behaviorsindicates highexploratorybehaviorandlowanxietylevelsandviceversa. Theisolatedcompounds(1and2)leadtoasignificantlyreduction ofthemovementofmiceintheopenfieldtestapparatus.These evidenceshintthesedativelikenatureofbothcompounds. Simi-larly,themusclerelaxantlikepropertyofcompounds(1and2)also wastestedintheinclinedplanetestthatiscommonlyusedassay (ElliotandWhite,2001).Itwasinitiallydesignedtodifferentiating betweenneurolepticsfromothercentrallyactivedrugs(Randall etal.,1961),butlateron,themethodwasusedformuscle relax-antlikeeffectoftestarticles(RivlinandTator,1977).Theresults showedmarkedskeletonmusclerelaxantlikeeffects ofthetest compounds.
The bromazepam, which demonstrated outstandingsedative andmusclerelaxanteffects,wasusedhereasstandardcompound toprovideameaningfulbiologicalconclusions.Indeed,the ben-zodiazepines(BDZs)exhibitmarkedsedativeandmusclerelaxant effects mostlyattributedtointerferewiththeactionof gamma aminobutyricacid(GABAA)(DaillyandBourin,2008),whichmakes bromazepamanexcellentcompoundtoprovidearelativeactivity order.Thesignificantsedativeandmusclerelaxantlikeeffectsof compoundsinourstudycouldbeattributedtointerferewiththe actionofGABAA likeBDZ.However,furtherstudiesarerequired toascertainitsmechanismforsedativeandmusclerelaxant-like effectsofthesecompoundsforleadcompoundsinthetherapeutic classwithbetterefficacyandsafety.
Tyr62Bandaromaticscaffoldoftheligand,hydrophobic interac-tionswithsameresidueTyr62BandhydrogenbondwithGln64B. Such largenumber ofprotein–ligand interactions explainswhy bromazepaminteractssostronglywithGABAA.Regarding com-pounds1and2,ourmolecularmodelingapproachdoesnotfindso manyinteractionsforthesecompoundsaswithbromazepambut thereishighshapecomplementarityofthetwocompoundswith thebindingsite.Therefore,thehydrogenbonds,hydrophobicity andshapecomplementarityallowustodelineatethe experimen-tallyobservedaffinityofGABAAtothesecompounds.
Conclusion
We can conclude that compounds 1 and 2 isolated from
P.parviflorum possess significant sedativeand skeleton muscle relaxant-likeeffectsinanimalmodels.Ourstudyconfirmedthefolk usesoftheplantassedativebyprovidingpharmacologicalrationale ofisolated compoundsfrom theplant.Results arefully consis-tentwiththeperformedmolecularmodelingcalculations,which demonstratethemolecularmechanismatatomiclevel.According tothecomputationalsimulations,bothcompounds1and2 estab-lishedsimilarinteractionswithGABAAthanbromazepamthough withlessaffinitygiventhelowernumberofprotein–ligand inter-actions.The reported resultsnot only help in rationalizingthe observedselectivityofstachysrosaneand stachysrosanetoward GABAAbutalsocouldbeusedtoguidethedesignofmoreselective compoundsinfurtherexperimentaland/ortheoreticalstudies.
Author’scontribution
AR,UFandAKsupervisedthewholeproject.SZandAIinvolved intheisolationofcompounds,JP,HDH,JPandHPperformed com-putationalstudyandHKperformedbiologicalscreening.NJ,TBH, MFR,TAandSBmakefurthereditingandcorrectionsinthisMSand finalizeforsubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
WeacknowledgeHigherEducationCommission(HEC)of Pak-istanandCOMSATSforfinancialsupport.Thisworkwassupported bytheFundación Séneca–AgenciadeCienciayTecnologíadela RegióndeMurcia(Spain)underProject19419/PI/14-1andbythe CentrodeAltoRendimientodelaRegióndeMurcia(Spain)under projectCFE-CAR-23/15.
References
Ahmad,V.U.,Arshad,S.,Bader,S.,Ahmed,A.,Iqbal,S.,Tareen,R.B.,2006.New phenethylalcoholglycosidesfromStachysparviflora.J.AsianNat.Prod.Res.8, 105–111.
Ahmad,V.U.,Arshad,S.,Bader,S.,Iqbal,S.,Khan,A.,Khan,S.S.,Hussain,J.,Tareen,R.B., Ahmed,A.,2008.NewterpenoidsfromStachysparvifloraBenth.Magn.Reson. Chem.46,986–989.
Dailly,E.,Bourin,M.,2008.Theuseofbenzodiazepinesintheagedpatient:clinical andpharmacologicalconsiderations.Pak.J.Pharm.Sci.21,144–150.
Elliot,E.E.,White,J.M.,2001.Theacuteeffectsofzolpidemcomparedtodiazepam andlorazepamusingradiotelemetry.Neuropharmacology40,717–721.
Farooq,U.,Ayub,K.,Hashmi,M.A.,Sarwar,R.,Khan,A.,Ali,M.,Ahmad,M.,Khan, A.,2014.Anewrosane-typediterpenoidfromStachysparvifloraanditsdensity functionaltheorystudies.Nat.Prod.Res.29,813–819.
Farooq,U.,Naz,S.,Sarwar,R.,Khan,A.,Khan,A.,Rauf,A.,Khan,H.,Ahmad,M., Azhar-ul-HaqAliShah,A.-H.A.,Hameed,S.,2015.Isolationand characteriza-tionoftwonewditerpenoidsfromStachysparviflora:antidiarrhealpotentialin mice.Phytochem.Lett.14,198–202.
Holiman,P.C.H.,Hertog,M.G.L.,Katan,M.B.,1996.Analysisandhealtheffectsof flavonoids.FoodChem.57,43–46.
Khanavi,M.,Sharifzadeh,M.,Hadjiakhoondi,A.,Shafiee,A.,2005.Phytochemical investigationandanti-inflammatoryactivityofaerialpartsofStachysbyzanthina
C.Koch.J.Ethnopharmacol.97,463–468.
Malami,I.,Hassan,S.W.,Alhassan,A.M.,Shinkafi,T.S.,Umar,A.T.,Shehu,S.,2014.
Report:Anxiolytic,sedativeandtoxicologicaleffectofhydromethanolicstem barkextractofMaeruaangolensisDC.inWisterrats.Pak.J.Pharm.Sci.27, 1363–1370.
Newman,D.J.,Cragg,G.M.,2012.Naturalproductsassourcesofnewdrugsoverthe 30yearsfrom1981to2010.J.Nat.Prod.75,311–335.
Rabbani,M.,Sajjadi,S.E.,Jalali,A.,2005.HydroalcoholextractandfractionsofStachys lavandulifoliaVahl:effectsonspontaneousmotoractivityandelevatedplus mazebehaviour.Phytother.Res.19,854–858.
Rauf, A., Hadda, T.B., Uddin, G., Cerón-Carrasco, J.P., Pe ˜na-García, J., Pérez-Sánchez,H.,Khan,H.,Bawazeer,S.,Patel,S.,Mubarak,M.S.,Abu-Izneid,T., Mabkhot,Y.N.,2017a.Sedative-hypnotic-likeeffectandmoleculardockingof di-naphthodiospyrolfromDiospyroslotusinananimalmodel.Biomed. Pharma-cother.88,109–113.
Rauf,A.,Uysal,S.,Hadda,T.B.,Uddin,G.,Nawaz,M.A.,Khan,H.,Siddiqui,B.S.,Raza,M., Bawazeer,S.,Zengin,G.,2017b.Invivoandinsilicosedative-hypnoticlikeactivity of7-methyljugloneisolatedfromDiospyroslotusL.Biomed.Pharmacother.87, 678–682.
Rabbani,M.,Sajjadi,S.E.,Zarei,H.R.,2003.AnxiolyticeffectsofStachyslavandulifolia
Vahlontheelevatedplus-mazemodelofanxietyinmice.J.Ethnopharmacol. 89,271–276.
Randall,L.,Heise,G.,Schallek,W.,Bagdon,R.,Banziger,R.,Boris,A.,Moe,R.,Abrams, W.,1961.PharmacologicalandclinicalstudiesonValium(TM)anew psy-chotherapeuticagentofthebenzodiazepineclass.Curr.Ther.Res.Clin.Exp.3, 405–425.
Rivlin,A.,Tator,C.,1977.Objectiveclinicalassessmentofmotorfunctionafter exper-imentalspinalcordinjuryintherat.J.Neurosurg.47,577–581.
Skaltsa,H.D.,Lazari,D.M.,Chinou,I.B.,Loukis,A.E.,1999.Compositionand antibac-terialactivityoftheessentialoilsofStachyscandidaandS.chrysanthafrom southernGreece.PlantaMed.65,255–256.
Sussman,J.L.,Lin,D.,Jiang,J.,Manning,N.O.,Prilusky,J.,Ritter,O.,Abola,E.E.,1998.
ProteinDataBank(PDB):databaseofthree-dimensionalstructuralinformation ofbiologicalmacromolecules.ActaCrystallogr.D54,1078–1084.
Uddin,G.,Rauf,A.,Siddiqui,B.S.,Muhammad,N.,Khan,A.,Shah,S.U.A.,2014.