ORIGINAL ARTICLE
The anxiolytic-like effect of 6-styryl-2-pyrone in mice involves
GABAergic mechanism of action
Edna Maria Camelo Chaves1,2&Jose Eduardo Ribeiro Honório-Júnior1,3&
Caren Nádia Soares Sousa1&Valdécio Silveira Monteiro4&
Dayanne Terra Tenório Nonato2&Leonardo Pimentel Dantas1&
Ana Silvia Suassuna Carneiro Lúcio5&José Maria Barbosa-Filho5&
Manoel Cláudio Azevedo Patrocínio3&Glauce Socorro Barros Viana1&
Silvânia Maria Mendes Vasconcelos1
Received: 22 August 2016 / Accepted: 23 October 2017 / Published online: 30 October 2017 #Springer Science+Business Media, LLC 2017
Abstract The present work aims to investigate the anxiolytic activity of 6-styryl-2-pyrone (STY), obtained from Aniba panurensis, in behavioral tests and amino acids dosage on maleSwissmice. The animals were treated with STY (1, 10 or 20 mg), diazepam (DZP 1 or 2 mg/kg) or imipramine (IMI 30 mg/kg). Some groups were administered with flumazenil, 30 min before administration of the STYor DZP. The behav-ioral tests performed were open field, rota rod, elevated plus maze (EPM), hole-board (HB) and tail suspension test (TST). After behavioral tests, these animals were sacrificed and had their prefrontal cortex (PFC), hippocampus (HC) and striatum (ST) dissected for assaying amino acids (aspartate- ASP, glutamate- GLU, glycine- GLY, taurine- TAU and Gamma-aminobutyric acid- GABA). In EPM test, STY or DZP in-creased the number of entries and the time of permanence in the open arms, but these effects were reverted by flumazenil. In the HB test, STY increased the number of head dips how-ever this effect was blocked by flumazenil. The effects of the
STY on amino acid concentration in PFC showed increased GLU, GABA and TAU concentrations. In hippocampus, STY increased the concentrations of all amino acids studied. In striatum, STY administration at lowest dose reduced GLU concentrations, while the highest dosage caused the opposite effect. GLI, TAU and GABA concentrations increased with STY administration at highest doses. In conclusion, this study showed that STY presents an anxiolytic-like effect in behav-ioral tests that probably is related to GABAergic mechanism of action.
Keywords 6-styryl-2-pyrone .Aniba panurensis. Anxiety . Behavior . Amino acids
Introduction
Aniba panurensisis a common plant in Northeast Brazil, and belongs to the family Lauraceae, in which the genus has 52 species (Barbosa-Filho et al. 1987). This plant is popularly known asBlouro amarelo^, and has economic importance in the culinary arts, carpentry, construction, papermaking, per-fume industry, in folk medicine and chemical industry (Marques2001).
In traditional medicine, leaves fromA. panurensisare com-monly used as an antispasmodic, digestive and astringent (Di Stasi and Hiruma-Lima2003). The 6-styryl-2-pyrone (STY) is extracted from leaves and fruits of this plant,also known as dihydrogoniothalamin (Rezende et al.2015). This substance has showed anticarcinogenic action and vasodilator effect de-pendent of nitric oxide in vitro (Rezende et al. 2015). However, studies in the effects of STY on the central nervous system are still insipient.
* Silvânia Maria Mendes Vasconcelos
silvania_vasconcelos@yahoo.com.br; silvania@pq.cnpq.br
1
Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Street Cel. Nunes de Melo 1127, CEP 60431-270 Fortaleza, Brazil
2 Institute of Biomedical Sciences, State University of Ceará,
Fortaleza, CE, Brazil
3
Health Science Center, School of Medicine, University Centre Christus, Fortaleza, CE, Brazil
4
Department of Biochemistry and Molecular Biology, Federal University of Ceará, Fortaleza, CE, Brazil
5 Laboratory of Pharmaceutics Technology, Federal University of
It is noteworthy that a large number of substances derived from plants have action in the central nervous system (CNS). It is worth to point that the kavalactonas also presents struc-tures similar to the 6- styryl-2-pyrone (STY) found in the speciesAnibathat have tranquilizing and anxiolytic properties (Smith et al.2001) and riparian II and III fromAniba riparia have anxiolytic and antidepressant actions (Sousa et al.2004). Anxiety is the most common type of psychiatric disorder and became a very important area of research interest in psy-chopharmacology (Muris et al.2017). Drugs that act on the GABAergic system, like benzodiazepines, are currently used as anxiolytics (Sirdifield et al. 2017; Griffin et al. 2013). However, these drugs are associated with multiple side effects including dependence (Griffin et al.2013). Thus, search for new compounds as therapeutic alternatives has progressed constantly (Rabbani et al.2008) including studies carried out by our group (Silva et al.2014).
Preliminary studies in our laboratory have shown action of the STY isolated from the fruits ofA. panurensison CNS (Vasconcelos et al.2012). The present work aimed to verify the anxiolytic activity of STY on behavioral tests and its un-derlying mechanism in amino acids on brain areas.
Material and methods
Plant material
Green fruits ofAniba panurensis (Meissn) Mez were col-lected by Dr. Hipolito P. Ferreira Filho, near Humaitá, Amazonas State, and identified by Prof. Klaus Kubitzki, which maintains herbarium vouchers in the Royal Botanic Gardens, Kew (K), K000601914. The fruits (1000g) were ground and extracted with ethanol (room temp.) and then the filtered solution was evaporated and the residue (48 g) was redissolved in 60% aq. EtOH. The solution was ex-tracted first with hexane and next with chloroform. The solvents were evaporated and CHCl3 extract (8.5g) was submitted to column chromatography (silica gel, 70g). Elution with solvent mixtures (CHCl3, CHCl3-EtOAc, CHCl3-MeOH) of increasing polarity gave nine crude fractions, each elaborated further by TLC chromatogra-phy. The compound 6-styryl-2-pyrone was obtained as yellow crystals resulting in a yield of 2.6 g. The chemical purity of 6-styryl-2-pyrone (more than 98%) was deter-mined by high-performance liquid chromatography. The chemical structure of 6-styryl-2-pyrone (Fig.1) was iden-tified on the basis of spectroscopic data (1H and 13C NMR) and by comparison with values reported in the literature (Bittencourt et al. 1971). The 6-styryl-2-pyrone (synonym dihydrogoniothalamin) was previously isolated fromAniba parviflora(Bittencourt et al. 1971).
Animals
Male Swiss mice (25 - 30g) were used in each experiment. Animals were maintained at a controlled temperature (23 ±1°C) with a 12-h dark/light cycle and free access to water and food. The animals were treated in accordance with the current law and the NIH Guide for Care and Use of Laboratory Animals. The study was performed under the con-sent and surveillance of the Committee of Ethics in Animal Research, Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Brazil, un-der the protocol number 10/08.
Drugs
STY was emulsified with 4% Tween 80 (Sigma- USA) and dissolved in saline solution. Controls animals received vehicle (saline with 4% Tween 80) at the same volume of 0.1 mL/10g. Diazepam (DZP), flumazenil (FLU), and Imipramine (IMI) were purchased from Pharmaceutical Laboratory União Química®, Brazil. Diazepam was emulsified with 4% Tween 80 and dissolved in distilled water. Flumazenil and imipramine were diluted with distilled water.
Experimental protocol
The animals were treated with STY at doses of 1, 10 or 20 mg/ kg, intraperitonealy (i.p.) or Diazepam (DZP), 1 or 2 mg/kg, as a positive control to anxiolytic and sedative effects, respective-ly, 30 min before the experiments. Imipramine (IMI-30mg/kg) was used for a positive control to antidepressive effect. Controls received vehicle at the same volume and were admin-istered by the same route as the treated groups. The animals were treated with flumazenil (FLU- 2,5 mg/kg, i.p.) 15 min before the treatment with STY or DZP (1 mg/kg) to elucidate the possible action mechanism of the substance (Fig.2).
Behavioral assessment
Open field test
The open field area was made of acrylic (transparent walls and black floor, 30 × 30 × 15 cm) divided into nine squares of equal area. The open field was used to investigate the explor-atory activity of the animal (Archer 1973). The numbers of
O O
squares crossed with the four paws (locomotor activity) and the numbers of grooming (stereotypes) and rearing (explor-atory behavior) were evaluated.
Rota rod test
Animals were selected for the rota rod test 24h before the pharmacological test. Mice were placed with the four paws on a 2.5 cm diameter bar, 25 cm above the floor and the time of permanence on the bar was measured for 1 min, for each animal. The rotating speed was of 12 rpm (Dunham and Miya
1957).
Elevated plus-maze test
The elevated plus maze consists of two perpendicular open arms (30 × 5 cm) and two closed arms (30 × 5 × 25 cm) also in the perpendicular position. The open and closed arms were connected by a central platform (5 × 5 cm). The platform and the lateral walls of the closed arms were made of trans-parent acrylic (Lister1987). After treatment, the animal was placed at the center of the plus maze with its nose in the direction of one of the closed arms, and observed for 5 min, according to the following parameters: the number of entries in the open arms (NEOA), and the time of permanence in the open arms (TPOA). The ratios‘number of entries into open arms/number of entries into all (i.e. open and closed) arms’ and‘time spent in the open arms/time spent in all arms’were calculated and multiplied by 100 to yield the percentages of entries into open arms (PEOA) and the percentage of time spent in the open arms (PTOA).
Hole-board test
The hole-board test for exploratory behavior of mice was used as described previously (Clark et al.1971). The ap-paratus used was an Ugo Basile of 60 cm × 30 cm with 16
evenly spaced holes with inbuilt infrared sensors. For each animal, the number of head dips into the holes was counted for 5 min.
Tail suspension test
The tail suspension test has been described for the evaluation of antidepressive activity (Steru et al. 1985). Animals were transported from the housing room to the testing area in their own cages and allowed to adapt to the new environment for 1 h before testing. For the test, the animals were suspended on the edge of a shelf 58 cm above a table top by adhesive tape placed approximately 1 cm from the tip of the tail. The dura-tion of immobility (seconds) was recorded for a period of 6 min.
Amino acids concentration measurement
Immediately after experimental behavior tests, the animals were sacrificed by rapid decapitation and the brains were quickly removed and placed on aluminum foil in a Petri dish on ice. Prefrontal cortex (PFC), hippocampus (HC) and stria-tum (ST) were dissected for the measurement of aminoacids. After dissection, the areas were weighed and stored at -70 ° C for amino acids determinations.
Analyses of the amino acids aspartate (ASP), glutamate (GLU), glycine (GLY), taurine (TAU) and gamma-aminobutyric Acid (GABA) were carried out with a high-performance liquid chromatography (HPLC, Shimadzu, Japan) apparatus. A fluorimetric detection method was used, as previously described (Lindroth and Mopper1979). The area of each peak was determined with Shimadzu software and compared to the peak area of the corresponding external standard. Amino acid concentrations were expressed as μmol/g wet tissue.
Statistical analysis
Statistical analysis was performed with Graph Pad Prism 5.0 for Windows, Graph Pad Software (SanDiego, CA, USA). The behavioral results were evaluated by one-way ANOVA followed by Student-Newman-Keuls’spost hoctest. Amino acids results was evaluated by two-way ANOVA followed by Student-Newman-Keuls’s post hoc test. All results are expressed as means ± S.E.M (standard errors of the mean).
Results
Effects of STY, DZP and FLU on locomotor activity of mice in the open field test
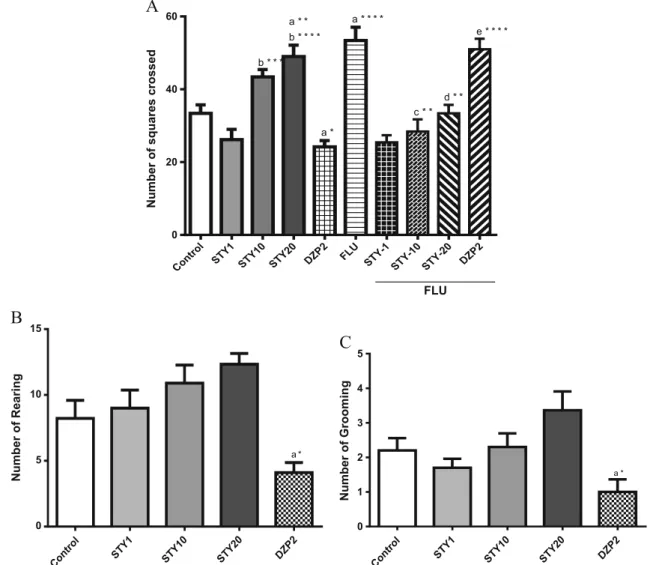
After intraperitoneally injections of STY, the behavior of the mice was assessed using the open field test. The results of this test, which are presented in Fig.3, reveal that only the groups treated with high dose of STY increased the number of cross-ing (STY 10mg/kg: 43.40 ± 2.05; STY 20mg/kg: 49.00 ± 3.10) as compared to control (33.44 ± 2.33) [F(9,88)=17.55; p<0.0001]. As expected, DZP 2 mg/kg (24.20 ± 1.70) de-creased this parameter and this effect was reverted by FLU (51.00 ± 2.90). Flumazenil also reversed the action of STY10 (28.40 ± 3.34) and STY20 (33.40 ± 2.33).
No effect was observed with STY in rearing and grooming as compared to control. On the other hand, the positive control (DZP) decreased the number of rearing (4.10 ± 0.71) and grooming (1.00 ± 0.30).
Effects of STY, DZP and FLU on motor coordination of mice in the rota rod test
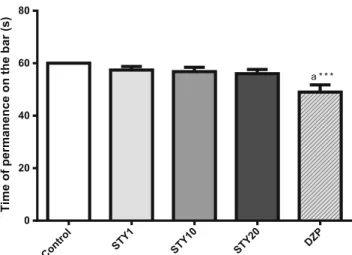
No change was observed in the rota rod test (Fig.4) at 12 rpm after treatment with STY1, 10 or 20 mg/kg, compared to con-trol (60.00 ± 00.00), while diazepam 2 (49.00 ± 2.76) mg/kg, as expected, decreased the parameter time permanence of an-imals in the bar [F (4, 45) = 5.598; p<0.001] when compared to control group.
Effects of STY, DZP and FLU on anxiety-like behavior of mice in the elevated plus-maze test
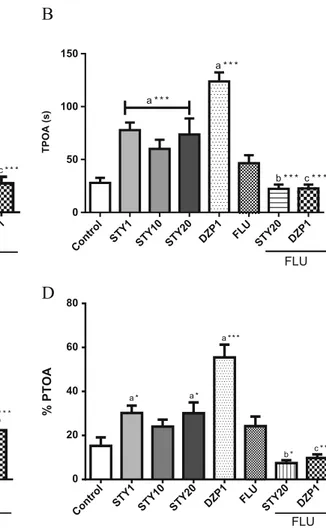
Elevated plus-maze test (Fig.5) was conducted to investigate anxiety-like behavior of the animals after administration of STY, FLU and DZP. In this evaluation, STY (1, 10 or 20 m g / k g ) a n d D Z P ( 1 m / k g ) i n c r e a s e d t h e N E O A [F(7,81)=20,40; p<0.0001] and PEOA [F(8,85)=17.95; p<0.0001] compared to control (NEOA: 1.88 ± 0.20; PEOA: 23.16±2.32). Similar effect was observed in TPOA [F(7,78)=17.44; p<0.0001] and PTOA [F(7,76)=16.05; p<0.0001] compared to control (TPOA: 27.90 ± 4.74;
PTOA: 15.24 ± 3.86) . Flumazenil blocked these effects ob-served with STY20.
Effects of STY, DZP and FLU on anxiety-like behavior of mice in the hole-board test
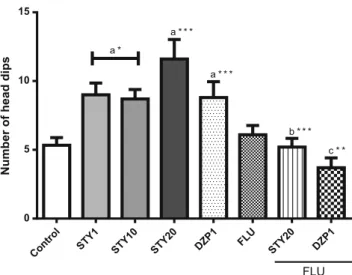
Another method used to measure the anxiety-like behavior of the animals was the hole-board test. In this test(Fig.6), STY 1mg/kg (9.00 ± 0.85); STY 10 mg/kg (8.70 ± 0.68) and STY 20mg/kg (11.60 ± 1.42) increased the number of head dips when compared to control (5.50 ± 0.59). Similar effects were observed with administration of DZP 1mg/kg (10.00 ± 1.052). As expected, these effects were reverted by pre-treatment with flumazenil (FLU+DZP 1mg/kg: 3,70 ± 0.71; FLU+STY 20 mg/kg: 5,20 ± 0.26) [F(11,121)=8.344; p<0.0001].
Effects of STY and IMI on depressive-like behavior of mice in the tail suspension test
In the TST(Fig.7), STY in all doses (1mg/kg: 74.40±11.07, 10 mg/kg: 80.70±9,84 or 20mg/kg: 60.89±9.75) did not change the immobility time when compared to control (88.70±4.37). On the other hand, imipramine administration decrease this parameter (47.30±6.71) [F(4,44)=3.640; p=0.0120].
Effects of STYon amino acid concentrations in brain areas of mice
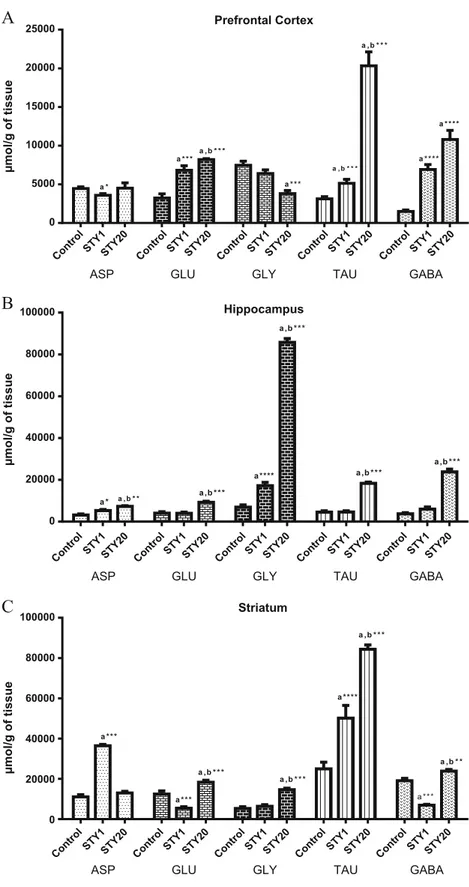
Figure8a shows the concentration of amino acids in prefrontal cortex of mice after STY treatment. The administration of STY reduced ASP concentrations only at 1mg/kg (3596 ± 225.1), compared to control (4427 ± 193) [F(3,22)=4.705; p<0.0001]. On the other hand, STY increased GLU concentrations at 1 and 20 mg/kg, (STY1: 6822 ± 573.6; STY20: 8203 ± 127.3), after compared to vehicle group (3235 ± 524) [F(3,23)=31.83; p<0.0001]. While regard to the inhibitory amino acids, GLY concentrations reduced after the administration of STY at 20 mg/kg (3805 ± 395.8) when compared with animals treated with vehicle (7463 ± 536.2) [F(3,23)=16.30; p<0.0001]. A great increase was observed in TAU concentrations after ad-ministered STY in the high doses (20 mg/kg) (20330 ± 178), when compare d to control group (3120 ± 294.7) [F(3,20)=74.12; p<0.0001]. Besides that GABA concentra-tions increased after STY 1 and 20 mg/kg administration (STY1: 6915 ± 641.6; STY20: 10801 ± 1173) compared to control (1495 ± 194.3) [F(3,23)=35.91; p<0.0001].
Whilst respect to inhibitory amino acids GLY concentrations demonstrated a great increase at dose STY20 (8573 ± 180) as compared to control (609 ± 110) [F(3,23)=776.6; p<0.0001]. Besides that TAU and GABA concentrations increased at the highest dose (TAU: 18295 ± 6567; GABA: 23758 ± 133), when compared with control (TAU: 4230 ± 837.4; GABA: 3843 ± 569.6), TAU [F(3,19)=134; p<0.0001] and GABA [F(3,23)=115.1; p<0.0001].
In striatum (Fig.8c), the administration of STY increased ASP concentration at the lowest administration dose (STY1: 36415 ± 7356) when compared to control (11036 ± 1085) [F(3,23)= 251.2; p<0.0001]. On the other hand, the STY ad-ministration at the lowest dose reduced GLU concentrations (GLU: 5399 ± 655.8), while the highest dosage caused the opposite effect (GLU: 18195 ± 8452) [F(3,23)=40.70; p<0.0001] when compared with control group. With regard to
the inhibitory amino acids, Fig.7c shows that GLI, TAU and GABA concentrations increased with STY administration at the highest doses (GLI: 14639 ±749.8; TAU: 84325 ± 1884; GABA: 23838 ± 803.6), when compared to control (GLI: 5281 ± 881.4; TAU: 24954 ± 3327; GABA: 19094 ± 1160). GLI [F(3,23)=40.35; p<0.0001], TAU: [F(3,24)=53.26; p<0.0001], GABA: [F(3,23)=102.9; p<0.0001]. Besides that, the lowest doses also demonstrated similar effect on TAU concentrations. On the other hand, STY 1 mg/kg caused a reduction in GABA concentrations when compared to control group.
Discussion
In this work, the effects of STY fromAniba panurensiswere studied in animal behavioral models such as open field, rota-Control STY1 STY10 STY20 DZP2
FLU
STY-1 STY-10 STY-20 DZP2 0
20 40 60
Number of squares crossed
a * *
b * * * *
a * * * *
b * * *
c * * d * *
FLU
e * * * *
a *
Control STY1 STY10 STY20 DZP2 Control STY1 STY10 STY20 DZP2
0 5 10 15
Number of Rearing
a *
0 1 2 3 4 5
Number of Grooming
a *
A
B
C
Fig. 3 Effects of STY, diazepam and flumazenil in Open Field Test. Mice (n=10-12 animals) received vehicle, 6-styryl-2-pyrone (STY 1, 10 or 20mg/kg), diazepam (DZP-2mg/kg) or flumazenil (FLU- 2.5 mg/kg) by intraperitoneally injection. The behavioral tests were carried out 30 minutes after the last injection. Were analyzed number of squares crossed
rod, elevated plus maze, hole-board and tail suspension test, aiming to evaluate its possible activities on the central nervous system. The tests cited above are classical animal models for screening of activities on the central nervous system, and pro-vide information about anxiety and psychomotor performance (Prut and Belzung2003). Also it was investigated the concen-tration of excitatory (ASP and GLU) e inhibitory (GLY, TAU and GABA) amino acids after administration of STY in pre-frontal cortex, hippocampus and striatum of mice.
Open field test is able to evaluate exploratory activity and locomotor effects of drugs in mice. The animal, in a new environment, has a tendency to explore it, despite the stress and conflict (Montgomery1955). The spontaneous locomotor activity test is used as a general parameter to study the central action of a drug. A decrease in locomotor activity means that the drug has a depressing effect on the CNS in the studied animal, i.e., there is a motor change that may interfere with other behavioral tests conducted in the same animal (Treit and Fundytus1988). In open field test, an increase in locomotor activity was observed with STY only with the highest dose. Diazepam as reference showed an opposite effect, in high doses, and both effects were reverted by FLU, a benzodiaze-pine receptor antagonist.
A possible anxiolytic action has been reported in other medicinal species carried out with extracts or natural sub-stances, such as 1,4-cineole monoterpene (Magnani et al.
2010), extracts from Gelsemium sempervirens (Melo et al.
2010), 5-isopropyl-2-methylphenol (Aragão et al.2006), and alpha-and-beta-amyrin molecules fromProtiu heptaphyllum (Maurmann et al. 2011), Valepotriate fraction from Valeriana glechomifolia(Pellow et al. 1985). The present
study shows that the administration of different doses of 6-styryl-2-pyrone in mice was able to induce anxiolytic effects, in plus maze and hole-board tests. Elevated plus maze (EPM) test is the most popular test to search for new benzodiazepine-like anxiolytic agents (Rodgers and Dalvi1997). This test is based on the observation that rodents tend to avoid elevated areas and, therefore, avoidance of the open arms in EPM test is interpreted as anxiety behavior.
Animals treated with STY in all doses on EPM test showed a significant reduction of aversion for open arms, indicating an anxiolytic effect. Flumazenil was used to iden-tify the probable mechanism of action of STY. The results showed that flumazenil was able to reverse the action of STY, suggesting that this compound may have a mechanism of action similar to benzodiazepines, on the GABAA inhib-itory complex. Benzodiazepines are the most commonly pre-scribed anxiolytics in clinical practice and cause depression of central nervous system. Benzodiazepines have selective activity on GABAAreceptor inhibitor complex, by increas-ing the frequency of chloride-channels opening and, there-fore, the flow through the GABAA receptor, enhancing the inhibitory effect of GABA (Crawley1985).
In order to confirm the findings of anxiolytic activity ob-served in the EPM test, we decided to use the hole-board test, where an increase of exploration of the space by animals was also observed. Similar to EPM, that test is also useful for testing anxiety. Because anxiolytic agents do increase the number of head dips (Siegel and Sanacora2012). Our results showed that the all doses of 6-styryl-2-pyrone increased the number of head dips, suggesting anxiolytic action.
For any dose of 6-styryl-2-pyrone, no loss of perfor-mance and motor coordination on rota rod test were ob-served, suggesting that this molecule has anxiolytic ef-fects without showing loss of reflex, a common collateral effect of benzodiazepines and observed with diazepam in this experiment.
Our results with of 6-styryl-2-pyrone showed an anxiolytic action in EPM and hole-board tests. Then these results suggest anxiolytic effects for STY, and it is very likely that this action is caused by a GABAergic mechanism of action, since it was blocked by flumazenil, a competitive antagonist in GABAA receptor. STY induced an increase in locomotor activity in open field test, without causing loss of reflex It is possible that STY has a selective action inα2 subunit from the GABAA receptor. This subunit is involved in anxiolytic action, while α1 subunit mediate sedation, amnesia, and ataxic effects of benzodiazepines (Trevor2015).
To exclude the possibility of a depressive-like effect we conducted the tail suspension test. In this test, STY did not present any effect, suggesting that it possibly has an anxiolytic action and no effect on behavior depressive-like.
As on the behavioral results STY indicated that its anxio-lytic action seems to be related to the GABAergic system, we
Time of permanence on the bar (s)
Control STY1 STY10 STY20
DZP
0 20 40 60 80
a * * *
decided to study the effect of this substance at the lowest and higher dose, on excitatory (ASP, GLU) and inhibitory (GLY, TAU GABA) amino acid concentrations in brain areas (PFC, HC and ST) related to anxiety disorders (Francisco and Guedes2015).
It is known that the PFC, HC and ST participates in anxiety-related behaviors (Kjelstrup et al. 2002; Zhang et al. 2014; Leggio et al. 2015; Motzkin et al. 2015). Researches point that prefrontal cortex is related to neural substrate of human social and affective function and is considered central to the pathophysiology of mood and anxiety disorders (Motzkin et al.2015). Besides that, syn-aptic and extrasynsyn-aptic GABAA receptors, are widely expressed in the hippocampus (Sperk et al.1997,1998), been closely related to negative emotions such as depres-sion, anxiety, catastrophizing and stress (Kjelstrup et al.
2002). While the striatum seems to be related to
modulation of anxiety behavior, influencing even in the responsivity of diazepam (Leggio et al.2015).
The acute administration of STY, in high dose, increased the concentration of GLU, TAU and GABA amino acids in all areas studied. This result suggests that STY has an impact in the two most important amino acid in the central nervous system that could mediate important function such as learning, and memory and even as anxiety disorders (Nuss2015).
Drugs that increase GABA concentrations in brain regions such as amygdala, hippocampus and prefrontal cortex have dem-onstrated anxiolytic action (Etkin2009). Benzodiazepines, for example, act through modulation of the GABAAreceptor, creasing the affinity of the receptor for GABA leading to in-creased subsequent chloride conductance (Nielsen 2015). Through their action at the GABAAreceptor, benzodiazepines drugs enhance the activity of GABA, an inhibitory neurotrans-mitter amino acid, resulting in a slowing of neurotransmission
Control STY1 STY10 STY20 DZP1 FLU
STY20 DZP1
0 5 10 15
a * *
b * a * * *
c * * *
NEOA
FLU
Control STY1 STY10 STY20 DZP1 FLU
STY20 DZP1 FLU
Control STY1 STY10 STY20 DZP1 FLU
STY20 DZP1
FLU Control
STY1 STY10 STY20 DZP1 FLU STY20 DZP1 FLU 0
50 100 150
a * * *
a * * *
b * * * c * * *
TPOA (s)
0 20 40 60 80 100
a * * *
a * *
b * c * * *
% PEOA
0 20 40 60 80
b *
a * a *
a * * *
c * * *
% PTOA
A
C
B
D
Fig. 5 Effects of STY, diazepam and flumazenil in elevated plus-maze test. Mice (n=10-12 animals) received vehicle, 6-styryl-2-pyrone (STY 1, 10 or 20mg/kg), diazepam (DZP-1mg/kg) or flumazenil (2.5 mg/kg) by intraperitoneally injection. The behavioral tests were carried out 30 minutes after the last injection. Were analyzed number of entries in open arms (a), time of permanence in open arms (b), percentage of
and sedative and anxiolytic effects (Phan et al.2005). The in-crease in GABA concentrations after use of STY suggests that this molecule might have a similar effect found in anxiolytic drugs.
Glutamate is the main excitatory amino acid in CNS and an important precursor of GABA. His role in the pathophysiolo-gy of anxiety is still unclear. Clinical study demonstrated that, increased cortical glutamate concentrations were positively correlated with anxiety symptoms in patients, suggesting that hyperfunction of cortical glutamatergic neurons may be an important contributing factor for the etiology of anxiety and related disorders (Modi et al.2014).
D-aspartate has received significant attention due to its abundant expression during the embryonic phases of brain development. However, its share in the pathophysiology of anxiety is not yet clear (Errico et al.2008). STY showed the opposite effect in the concentration of ASP depending on the brain area and dose administered. In prefrontal cortex and striatum STY at low dose showed a decreased and increased concentration of ASP, respectively. In the hippocampus, STY increased the concentration of this amino acid.
Another interesting result observed in this study was a potent increase in GLY levels (1242%) in the HC. Glycine acts as a neuromodulator to regions rich in glutamatergic synapses, such as the forebrain, and antagonists of recep-tor GlyB has been show anxiolytic effect in mice (Trullas et al. 1989). In HC, glycine is stored in glutamatergic presynaptic terminals and plays a role in decreasing
neuronal excitability by opposing glutamatergic neuro-transmission in pathological states such as epilepsy or ischemia. Therefore, we would suggest that those glycine levels in the HC could result from a compensation mech-anism to counter a rise in glutamate levels, which was noticed after the administration of STY.
With respect to other inhibitory amino acids, our results show that STY increased the concentration of TAU in all brain areas studied. However, the greatest increased was observed in PCF (651.6%). Taurine (TAU) is an amino sulfonic acid with several functions in central nervous system. Mounting evidence suggests that it acts in osmoregulation, neuromodulation and as an inhibitory neurotransmitter (Wu et al. 2005; Junyent et al.2009; Oja and Saransaari1996). Previous reports showed that TAU acts as a ligand that acti-vates GABAAand as an inhibitory molecule of the NMDA receptor complex through multiple mechanisms (Chan et al.
2014). A recent study showed that TAU treatment exerts an anxiolytic-like effect in zebrafish using the novel tank and the light-dark tests (Mezzomo et al.2016). Thus, our suggests that anxiolytic-like effect observed with STY can be related with the increase of TAU concentrations in PCF, either by a direct effect or modulated by glutamatergic and GABAergic systems.
Previous studies in our laboratory with molecules of vege-table origin have shown that acute administration of coumarin led to a rise in levels of inhibitory amino acids GABA, GLY, TAU and of GLU, which is excitatory in the PFC and HC (Pereira et al.2009). Understanding that the mutual regulation
Control STY1 STY10 STY20 DZP1 FLU
STY20 DZP1
0 5 10 15
a *
a * * *
b * * * a * * *
c * *
Number of head dips
FLU
Fig. 6 Effects of STY, diazepam and flumazenil in Hole-board test. Mice (n=10-12 animals) received vehicle, 6-styryl-2-pyrone (STY 1, 10 or 20mg/kg), diazepam (DZP-2mg/kg) or flumazenil by intraperitoneally injection. The behavioral tests were carried out 30 minutes after the last injection. Results are expressed as the mean ± SEM. a Significant difference when compared with the control; b significant difference when compared with STY 20 mg/kg; c significant difference when compared with diazepam 1 mg/kg (DZP 1). (* p < 0.05, ** p<0.01, *** p<0.001). ANOVA and Student–Newman–Keuls’s as the post hoc test
Immobility time (s)
Control STY1 STY10 STY20 IMI30
0 20 40 60 80 100
a *
Fig. 7 Effects of STY, and imipramine (IMI) in tail suspension test (TST). Mice (n=9-10 animals) received vehicle, 6-styryl-2-pyrone (STY 1, 10 or 20 mg/kg), or IMI (30 mg/kg) by intraperitoneally injection. The behavioral tests were carried out 30 minutes after the last injection. Results are expressed as the mean ± SEM. a Significant difference when compared with the control (* p < 0.05). ANOVA and Student–
mechanism of amino acids is a complex task, since it often involves more than one system.
In conclusion, this study showed that STY presents an anxiolytic-like effect in behavioral tests that
probably is related to GABAergic mechanism of action and also influenced by the increase of GABA, GLY and TAU inhibitory amino acids in the PFC, HC and STY.
ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20 0
5000 10000 15000 20000 25000
a *
a * * * a , b * * *
a , b * * *
a * * *
a , b * * *
ASP GLU GLY TAU GABA
ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20
ASP GLU GLY TAU GABA
ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20 ControlSTY1STY20
ASP GLU GLY TAU GABA
Prefrontal Cortex
µmol/g of tissue
a * * * * a * * * *
Hippocampus
0 20000 40000 60000 80000 100000
a * a , b * *
a ,b * * * a ****
a ,b * * *
a ,b * * *
a ,b * * *
µmol/g of tissue
A
B
Striatum
0 20000 40000 60000 80000 100000
a * * *
a * * * a ,b * * *
a ,b * * *
a * * ** a ,b * * *
a * * * a ,b * *
µmol/g of tissue
C
Fig. 8 Effects of STY on aminoacids concentrations in prefrontal cortex (a), hippocampus (c) and striatum (c). Mice received vehicle or STY (1 or 20mg/kg) by intraperitoneally injection. Results are expressed as the mean ± SEM.aSignificant difference when compared with the control; bsignificant difference when compared with STY 1 mg/kg. (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). ANOVA and Student–Newman–
Keuls’s as the post hoc test. ASP–
Acknowledgements This work was supported by grants from the National Council for Scientific and Technological Development (CNPq), Brazilian Coordination for Professorship Improvement for Higher Education (CAPES), and Research Foundation of the State of Ceará (FUNCAP), all from Brazil.
References
Aragão GF, Carneiro LMV, Junior APF, Vieira LC, Bandeira PN, Lemos TLG, Viana GS (2006) A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protiu heptaphyllum (Aubl.) Pharmacol Biochem Behav 85(4):827–834 Archer J (1973) Tests for emotionality in rats and mice: a review. Anim
Behav 21(2):205–223
Barbosa-Filho JM, Yoshida M, Gottlieb OR, Barbosa RCSBC, Giesbrecht AM, Young MCM (1987) Benzoyl esters and amides, stryrylpyrones and neolignans from the fruits ofAniba riparia. Phytochemistry 26(9):2615–2617
Bittencourt AM, Gottlieb OR, Mors WB, Magalhaes MT, Mageswaran SM, Ollis WD, Sutherland IO (1971) The natural occurrence of 6-styryl-2-pyrones and their synthesis. Tetrahedron 27(5):1043–1048 Chan CY, Sun HS, Shah SM, Agovic MS, Friedman E, Banerjee SP (2014) Modes of direct modulation by taurine of the glutamate NMDA receptor in rat cortex. Eur J Pharmacol 728:167–175 Clark G, Koster AG, Person DW (1971) Exploratory behavior in chronic
disulfoton poisoning in mice. Psychopharmacology 20(2):169–171 Crawley JN (1985) Exploratory behaviour models of anxiety in mice.
Neurosci Biobehav Rev 9(1):37–44
Di Stasi LC, Hiruma-Lima CA (2003) Medicinal plants in the Amazon and Atlantic forest, 2nd edn. São Paulo, pp 139–143
Dunham NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficits in rats and mice. J Am Pharm Assoc Am Pharm Assoc 46(3):208–209
Errico F, Rossi S, Napolitano F, Catuogno V, Topo E, Fisone G, D'Aniello A, Centonze D, Usiello A (2008) D-Aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J Neurosci 28(41):10404–10414
Etkin A (2009) Functional neuroanatomy of anxiety: a neural circuit perspective. In: Stein MB, Steckler T (eds) Behavioral neurobiology of anxiety and its treatment. Springer Verlag, Berlin, pp 251–277 Francisco ES, Guedes RC (2015) Neonatal taurine and alanine modulate
anxiety-like behavior and decelerate cortical spreading depression in rats previously suckled under different litter sizes. Amino Acids 47(11):2437–2445
Griffin CE, Kaye AM, Bueno FR, Kaye AD (2013) Benzodiazepine pharmacology and central nervous system–mediated effects. Ochsner J 13(2):214–223
Junyent F, Utrera J, Camins A, Pallàs M, Romero R, Auladell C (2009) Synthesis, uptake and release of taurine in astrocytes treated with 8-Br-cAMP. Neurosci Lett 467(3):199–202
Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB (2002) Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A 99:10825–10830 Leggio GM, Torrisi SA, Castorina A, Platania CBM, Impellizzeri AAR,
Fidilio A, Caraci F, Bucolo C, Drago F (2015) Dopamine D3 receptor-dependent changes in alpha6 GABAA subunit expression in striatum modulate anxiety-like behaviour: responsiveness and tol-erance to diazepam. Eur Neuropsychopharmacol 25(9):1427–1436 Lindroth P, Mopper V (1979) High performance liquid chromatographic
determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal Chem 51(11):1667–1674
Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92(2):180–185
Magnani P, Conforti A, Zanolin E, Marzotto M, Bellavite P (2010) Dose-effect study of Gelsemium sempervirens in high dilutions on anxiety-related responses in mice. Psychopharmacology 210(4):533–545 Marques CA (2001) Economic importance of the family Lauraceae Lindl.
For Environ 8(1):195–206
Maurmann N, Reolon GK, Rech SB, Fett-Neto AG, Roesler R (2011) Valepotriate fraction ofValeriana glechomifoliashows sedative and anxiolytic properties and impairs recognition but not aversive mem-ory in mice. Evid Based Complement Alternat Med 8:1–7 Melo FHC, Venâncio ET, Sousa DP, Fontele MMF, Vasconcelos SMM,
Viana SGB (2010) Sousa FC (2010) anxiolytic-like effert of carva-crol (5-isopropyl-2-methyphenol) in mice: involvement with GABAergic transmission. Fundam Clin Pharmacol 24(4):437–443 Mezzomo NJ, Silveira A, Giuliani GS, Quadros VA, Rosemberg DB (2016) The role of taurine on anxiety-like behaviors in zebrafish: a comparative study using the novel tank and the light-dark tasks. Neurosci Lett 613:19–24
Modi S, Rana P, Kaur P, Rani N, Khushu S (2014) Glutamate level in anterior cingulate predicts anxiety in healthy humans: a magnetic resonance spectroscopy study. Psychiatry Res 224(1):34–41 Montgomery KC (1955) The relation between fear induced by novel
stimulation and exploratory behavior. J Comp Physiol Psychol 48(4):254–260
Motzkin JC, Philippi CL, Wolf R, Baskaya MK, Koenigs M (2015) Ventromedial prefrontal cortex is critical for the regulation of amyg-dala activity in humans. Biol Psychiatry 77(3):276–284
Muris P, Simon E, Lijphart H, Bos A, Hale W, Schmeitz K, Wolters (2017) The youth anxiety measure for DSM-5 (YAM-5): develop-ment and first psychometric evidence of a new scale for assessing anxiety disorders symptoms of children and adolescents. Child Psychiatry Hum Dev 48(1):1–17
Nielsen S (2015) Benzodiazepines. In: Geyer MA, Ellenbroek BA, Marsden CA, Barnes ThRE (ed) Curr Top Behav Neurosci. Springer, pp 1–19
Nuss P (2015) Anxiety disorders and GABA neurotransmission: a distur-bance of modulation. Neuropsychiatr Dis Treat 11:165–175 Oja SS, Saransaari P (1996) Taurine as osmoregulator and
neuromodulator in the brain. Metab Brain Dis 11(2):153–164 Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed
arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167
Pereira EC, Lucetti DL, Barbosa-Filho JM, de Brito EM, Monteiro VS, Patrocínio MC, De Moura RR, Leal LK, Macedo DS, de Sousa FC, de Barros Viana GS, Vasconcelos SM (2009) Coumarin effects on amino acid levels in mice prefrontal cortex and hippocampus. Neurosci Lett 454(2):139–142
Phan KL, Fitzgerald DA, Cortese BM, Seraji-Bozorgzad N, Tancer ME, Moore GJ (2005) Anterior cingulate neurochemistry in social anxi-ety disorder: 1H-MRS at 4 Tesla. Neuroreport 16(2):183–186 Prut L, Belzung C (2003) The open field as a paradigm to measure the
effects of drugs an anxiety-like behavior: a review. Eur J Pharmacol 463(1-3):3–33
Rabbani M, Sajjadi SE, Mohammadi A (2008) Evaluation of the anxio-lytic effect of Nepeta persica Boiss. in mice. Evid Based Complement Alternat Med 5(2):181–186
Rezende BA, Silva GC, Corradi RG, Teles MM, Barbosa-Filho JM, Lemos VS, Cortes SF (2015) Dihydrogoniothalamin, an Endothelium and NO-Dependent Vasodilator Drug Isolated from
Aniba panurensis. Planta Med 81(15):1375–1381
Rodgers RJ, Dalvi A (1997) Anxiety, defense and the elevated plus-maze. Neurosci Biobehav Rev 21(6):801–810
Silva MCC, Sampaio LR, De Araújo DP, Araújo PV, Monte AS, Rodrigues FT, Woods DJ, de Sousa FC, Fonteles MM, Vasconcelos SM (2014) Central effects of lipoic acid associated with paroxetine in mice. Am J Ther 21(2):85–90
Sirdifield C, Chipchase SY, Owen S, Siriwardena AN (2017) A system-atic review and meta-synthesis of patients’experiences and percep-tions of seeking and using benzodiazepines and Z-drugs: towards safer prescribing. Patient 10:1
Smith KK, Dharmaratne HR, Feltenstein MW, Broom SL, Roach JT, Nanayakkara NP, Khan IA, Sufka KJ (2001) Anxiolytic effects of kava extract and kavalactones in the chick social separation-stress paradigm. Psychopharmacology 155(1):86–90
Sousa FCF, Melo CTV, Monteiro AP, Lima VTM, Guiterrez SJC, Pereira BA, Barbosa-Filho JM, Vasconcelos SM, Fonteles MF, Viana GS (2004) Antianxiety and antidepressant effects of riparin III from
Aniba riparia (Nees) Mez (Lauraceae) in mice. Pharmacol Biochem Behav 78(1):27–33
Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W (1997) GABA(A) receptor subunits in the rat hippocampus I: immunocy-tochemical distribution of 13 subunits. Neuroscience 80:987–1000 Sperk G, Schwarzer C, Tsunashima K, Kandlhofer S (1998) Expression
of GABA(A) receptor subunits in the hippocampus of the rat after kainic acid-induced seizures. Epilepsy Res 32(1-2):129–139
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharma-cology 85:367–370
Treit D, Fundytus M (1988) Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav 31(4):959–962
Trevor AJ (2015) Sedativo-hypnotic drugs. In: Katzung BG, Masters SB, Trevor AJ (eds) Basic and clinical pharmacology, 13rd edn. Mc Graw Hill, New York, pp 369–383
Trullas R, Jackson B, Skolnick P (1989) Anxiolytic properties of 1-aminocyclopropanecarboxylic acid, a ligand at strychnine-insensitive glycine receptors. Pharmacol Biochem Behav 34(2): 313–316
Vasconcelos SMM, Chaves EM, Cunha NL, Patrocinio MCA, Sampaio LRL, Cordeiro RC, MTV DS, Lucio ASSC, Barbosa-Filho JM, Macedo DS (2012) Effects of the natural 6-styryl-2-pyrone on amino-acid levels in the prefrontal cortex of mice after pentylenetetrazole-induced seizures. Eur Neuropsychopharmacol 22(2):S18
Wu H, Jin Y, Wei J, Jin H, Sha D, Wu J (2005) Mode of action of taurine as a neuroprotector. Brain Res 1038(2):123–131