Salmonella
Gallinarum: Clinical,
Anatomopathological and Haematological
Studies
Mail Address
Keywords Author(s)
Arrived: M ay / 2007 Approved: August / 2007
ABSTRACT
This study aimed at evaluating the susceptibility of commercial laying hens to Salmonella Gallinarum (SG). Tw o experiments w ere carried using a m ut ant st rain of Salm onella Gallinarum resist ant t o nalidix acid (SGNALr). In the first trial, the resistance of birds w as evaluated based on clinical signs, faecal shedding, and mortality. It w as carried out w ith six lines of commercial layers being three light w hite layers, considered t o be resist ant t o SG (W 1, W 2, W 3), and t hree semi-heavy brow n varieties (B1, B2, B3), considered susceptible to SG. Each group contained 15 one-day-old birds. Hens w ere inoculated in the crop at 5 days of age w ith 0.2 mL of SGNALr neat culture. In addition, to each brow n variety, a new group of 15 birds w as challenged w ith 0.2mL of the same SGNALr culture diluted at 10-3. At the end of the first experiment, the surviving birds w ere sacrificed, and microbiological culture of liver and spleen w as performed. In the second experiment, w hite and brow n birds w ere inoculated w ith neat culture at five days of age. Samples w ere collected for evaluation of blood parameters and histopathology assessment at 1, 3, 5, 7, 9, 12, and 14 days post-infection. The results of the first experiment show ed higher resistance of w hite birds (p<0.05), although there w as no uniformity in the responses against fow l typhoid among the birds w ithin these groups. In the second experiment, there w ere differences betw een w hite and brow n birds both in blood parameters and in organ lesion intensity.
INTRODUCTION
Fow l typhoid is caused by Salmonella Gallinarum, and it is a serious systemic disease that affects birds. According to Shivaprasad (2000), fow l typhoid causes clinical signs in young and adult birds, including anorexia, diarrhea, dehydrat ion, w eakness, as w ell as gross and microscopic lesions in organs, such as spleen, liver, ovary, heart etc.
Fow l typhoid has a very complex epidemiology. Birds are mainly contaminated by horizontal route. The contact betw een healthy and sick birds, cannibalism, and the presence of dead birds, w ild birds, and w orkers contribute for dissemination of Salmonella Gallinarum in flocks (Berchieri, 2000a).
Therefore, Salmonella Gallinarum affects production parameters, resulting in high mortality and low er laying rates, causing economic losses (Pomeroy, 1987; Berchieri, 2000a; Shivaprasad, 2000). Although vaccination programs against this disease have been adopted, fow l typhoid is still reported in M exico, Central America, South America, Africa, India, and South Korea (Berchieri, 2000a; Shivaprasad, 2000; Ji-Dong et al., 2006).
The know ledge on the resistance mechanisms of birds to this infection is limited. The resistance of some birds to salmonellosis may be related
Freitas Neto OC1
Arroyave W2
Alessi AC3
Fagliari JJ4
Berchieri A3
1 Po st - Gr ad u at e st u d en t , Facu ld ad e d e
Ciên cias A g r ar ias e Vet er in ar ias, Universidade Estadual Paulista, Jaboticabal, São Paulo, Brazil.
2 Professor, Universidad de La Paz, Colombia. 3 Pr o f esso r , Dep ar t am en t o d e Pat o lo g ia
Animal, Faculdade de Ciencias Agrárias e Veterinárias, Universidade Estadual Paulista, Jaboticabal, São Paulo, Brazil.
4 Pr o f esso r , Dep ar t am en t o d e Clín ica e
Cirurgia, Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista, Jaboticabal, São Paulo, Brazil.
OC Freitas Neto
Rua Colombo Berlingieri, 127 Vila Industrial
14.870-000 . Jaboticabal, SP, Brasil E-mail: oliveirocaet ano@yahoo.com.br
Fo w l t yp h o id , h em at o lo g y, layin g h en s, Salmonella Gallinarum, susceptibility.
The aut hors t hank t he helpf ul assist ance of Aparecida Rodrigues, Antonio José dos Santos, M aria Yamazaky, and Francisca Ardisson, from the Pathology Laboratory (UNESP); and of M r. Eugenio Campos, for assistance w ith the clinical analyses. Funding f rom FA PESP and CNPq enabled the study.
t o t heir abilit y t o host t he bact eria, and t o cont rol b act er ial g r o w t h in sid e t h e cells o f t h e reticuloendothelial system, and also fecal shedding, w ithout eliciting clinical signs. Some studies indicate that resistance to salmonellosis occurs due to a better abilit y of t he phagocyt ic m ononuclear syst em in restraining infection onset (Qureshi, 1998; Wigley et al., 2002). Nevertheless, doubts still exist w hether such efficacy is due to the presence of a high number of phagocytic cells at infection onset, or if the number of cells involved, more than the type of cells, is w hat really det erm ines t he success in clearing up t he disease (Qureshi, 1998).
So m e st u d ies d escrib e t h e d if f eren ces in b ird resist ance t o t he inf ect ion caused by S. Gallinarum (Oliveira et al., 2005; Prince, 1966; Wigley et al., 2002). How ever, the information provided by such sources are not useful to currently produced birds . The modern poultry industry demands better animal performance, w hich in turn compromises the immune system of birds, adversely af f ect ing t heir resist ance t o diseases. The objectives of the present study w ere to evaluate layer genetic lines considered to be resistant and susceptible to fow l typhoid, taking into account mortality, fecal shedding, clinical signs, macroscopic and microscopic lesions, as w ell as to monitor changes in hematological parameters of birds experimentally challenged w ith S. Gallinarum.
MATERIAL AND METHODS
Inoculum
In both experiments, birds w ere challenged using a Salmonella enterica serovar Gallinarum strain resistant to nalidixic acid (SGNalr). The strain w as prepared, and k ep t at t h e Dep ar t m en t o f A n im al Pat h o lo g y, Facu ld ad e d e Ciên cias A g r ár ias e Vet er in ár ias, Un iver sid ad e Est ad u al Pau list a (FCA VJ- UNESP), according t o t he met hodology described by Smit h (1955) and Berchieri et al. (2001).
Bact er ia w er e g r o w n in n u t r ien t b r o t h , an d incubated w ith shaking at 37°C for 24 h. The culture contained approximately 3.3 x 108 colony forming units per milliliter (CFU/mL).
Experiment 1
On e h u n d r ed an d t h ir t y- f ive f em ales o f six commercial layer genetic lines w ere obtained at 1 day of age from commercial hatcheries. Three light w hite (considered t o be resist ant ) and t hree sem i-heavy brow n lines (considered t o be suscept ible t o f ow l
typhoid) w ere used. Chicks w ere housed in battery cages w ith electrical heating, located at the isolating units of the Laboratory of Animal Pathology of the Depart ment of Animal Pat hology of FCAVJ-UNESP. Food and w at er w ere provided ad libit um. A f ast seroagglut inat ion t est w as perf orm ed, and cloacal sw abs w ere taken immediately after the birds arrived in order to confirm that they w ere free from Salmonella. Birds w ere randomly distributed into nine groups. Fifteen birds from light lines (W1, W2, and W3) w ere inoculated in the crop at five days of age w ith 0.2 mL of SGNalr neat culture (3.3 x 108 CFU /mL). Each semi-heavy variet y (B1, B2, and B3) w as divided int o t w o groups of 15 birds each. The birds of first one group w ere inoculat ed w it h t he neat cult ure of SGNALr, w hereas t he birds of t he second one group w ere inoculated w ith 0.2 mL of the culture diluted at 10-3 (approximately 3.3 x 105 CFU SGNalr/mL). Bacteriology t est s w ere perf orm ed t w ice a w eek using cloacal sw abs. The surviving birds w ere sacrificed at 23 days post-infection, and sw ab samples w ere taken from the liver and t he spleen. Bact eriological examinat ion of these samples w as carried out according to a previously described methodology (Berchieri et al., 2001). Briefly, the sw abs w ere placed in tubes w ith 2.0 mL of Selenite-Novobiocin broth (SN), and then streaked onto brilliant green agar w ith nalidixic acid (25µg/mL) and Novobiocin (1µg/mL) (BG Nal Nov). Both the plate and the tube w ere incubated at 37°C for 24 hours. In the absence of grow t h, new plat ing w as perf orm ed f rom t he incubat ed sw ab. During t he experim ent al period, clinical signs and mortality w ere daily recorded.
Experiment 2
In this experiment, 160 one-day-old male birds w ere u sed , b elo n g in g t o W1 (w h it e) an d B3 (b r o w n ) commercial genetic lines. Birds w ere housed in battery cages w ith electrical heating, located at the isolation units of the Laboratory of Pathology of FCAVJ-UNESP, and received w ater and food ad libitum. The absence of Salmonella w as confirmed as described above.
spleen, thymus, bursa of Fabricius, duodenum, and cecal tonsils. The first sampling w as performed one day after infection, and then every 48 hours, w ith a total of seven samplings for light birds and five samplings for semi-heavy birds. Such difference w as due to higher mortality presented by the semi-heavy birds. Blood for hematological analyses w as also collected 1 day before experimental inoculation.
Blood samples w ere collected using anticoagulant (0.1 mL heparin/ mL blood). Globular volume (GV) w as determined using microhematocrit method (Jain, 1986), h em o g lo b in (Hb ), acco r d in g t o t h e cyanomethemoglobin technique (Jain, 1986), and red cell an d w h it e cell co u n t s u sin g a Neu b au er hemocyt omet er (Nat t & Herrick, 1952). Dif f erent ial leukocyte count w as performed using blood smears stained according to the Rosenfeld method (Lucas & Jamroz, 1961). M ean corpuscular hemoglobin (M CH), mean corpuscular hemoglobin concentration (CHCM ), and m ean corpuscular volum e (M CV) w ere t hen calculated (Jain, 1986; Lucas & Jamroz 1961). Albumin concent rat ion (Alb) and t he act ivit y of t he enzyme aspart at e aminot ransf erase (AST) w ere det ermined u sin g co m m er cial k it s an d a sem i- au t o m at ic spectrophotometer*, according to bromocresol green and Reitman-Frankel methods, respectively.
Organs w ere f ixed f or at least 24 hours in 4% buf f ered f ormaldehyde, pH 7.2, at a rat io of 20:1 (buf f er:organ f ragm ent ). Fragm ent s w ere f urt her processed at the Histopathology Laboratory of FCAVJ-UNESP according to routine methods (Behmer et al., 1976); 4-µm sect ions w ere t aken and st ained w it h hematoxylin-eosin. The stained sections w ere analyzed under a light microscope equipped w ith a digital camera (NICKON ECLIPSE E 200 x COOLPIX 5400)
Statistical analysis
Chi-square w as used to determine differences in
mortality among the groups in the first experiment. In the second experiment, the Tukey Studentized Range t est (HSD) w as used t o det ermine mean, st andard deviat ion, analysis of variance, and t he signif icant differences among groups. In both experiments, the St at ist ical Analysis Syst em (SAS) sof t w are w as used t o p erf o rm t h e an alyses, an d d if f eren ces w ere considered significant at a 5% level.
RESULTS
In t he f irst experiment , serum agglut inat ion t est evidenced that the birds w ere free from Salmonella w hen t hey arrived. Higher m ort alit y (p< 0.05) w as observed in the semi-heavy bird group as compared to t he light birds w hen t he neat cult ure w as used as inoculum (3.3 x108 CFU SGNalr/ml). On the other hand, there w ere no significant differences betw een groups (p>0.05) w hen the diluted inoculum w as used. M ortality w as different (p<0.05) betw een W1 and W3 (light lines); mortality rate w as 40% in W3 and 7% in W1. There w as no statistical difference in mortality among semi-heavy lines inoculat ed w it h neat cult ure (p>0.05), neither there w ere differences w hen diluted culture w as used as inoculum. M ortality rate varied betw een 7% and 40% in light birds, and betw een 40 and 100% in semi-heavy birds (Table 1).
Bact eriological assessm ent of f eces show ed t he presence of Salmonella in one semi-heavy bird (B3) 7 days post-inoculation; sampling w as performed just bef ore t he bird died. At 23 days post -inoculat ion, SGNalr w as isolated from the liver of 5/12, 4/9, 2/14 of the resistant W2, W3 and W1 lines, respectively, w hereas only one semi-heavy bird (B2) w as positive for SGNalr w hen neat culture w as used (3.3 x 108 CFU/mL).
Considering the clinical evaluation, light W2 and W3 birds show ed signs of disease, such as depression, w eakness, loss of appetite, ruffled feathers, dropped
Table 1 - M ortality of experimental birds inoculated w ith Salmonella Gallinarum Nalr:
Variety Number Days after SG inoculation M ortality
of Birds 11111 22222 33333 44444 55555 66666 77777 88888 99999 1010101010 1111111111 1212121212 1313131313 1414141414 1515151515 1616161616 1717171717 1818181818 1919191919 2020202020 (% )
Number of dead birds in each day (Tot )
B1 15 0 0 0 1 3 4 4 1 - - - 13 87 B2 15 0 0 0 1 3 2 4 2 0 0 1 - - - 13 87 B3 15 0 0 0 0 1 8 1 5 - - - 15 100 B1* 15 0 0 0 0 2 0 2 2 1 - - - 7 47 B2* 15 0 0 0 0 3 1 2 1 1 - - - 8 53 B3* 15 0 0 0 0 3 2 3 1 1 - - - 10 67 W1 15 0 0 0 0 0 0 0 1 - - - 1 7 W2 15 0 0 0 0 0 1 0 1 1 - - - 3 20 W3 15 0 0 0 0 0 0 2 1 1 0 1 0 0 0 0 0 0 1 - - 6 40 B1, B2, and B3= semi-heavy brow n birds; W1, W2 and W3 = light w hite birds: Groups B1, B2, B3, W1, W2 and W3 received 0.2 mL of culture w ith 3.3 x 108 CFU SG Nalr /mL, w hile B1* , B2* and B3* received 0.2mL of the same culture diluted at 10-3.
S
u
sc
e
p
ti
b
le
R
e
s
is
ta
n
w in g s, an d clo sed eyes. In ad d it io n , t h ese b ird s presented mortality five days post-inoculation. On the other hand, semi-heavy birds died suddenly at five to seven days post-inoculation, and show ed no clinical signs.
In the second experiment, there w as no evidence of bacterial infection by serology. As to mortality, no birds died in t he cont rol (non-inoculat ed) and light groups. The number of dead birds in the semi-heavy groups w as higher than in the other groups, being nine, nine, and six at days 5, 6, and 7 post -inoculat ion, respect ively.
Gross changes w ere observed in the organs of semi-heavy birds, mainly in the liver, w hich w as larger, w ith green-yellow ish color, and f riable. There w as also spleen congestion and splenomegaly. There w ere no evident anat omopat hological changes in light birds (Figure 1).
Figure 1 - Gross changes in w hite (W) and brow n birds (B) ex-perimentally infected w ith SG NaLr.
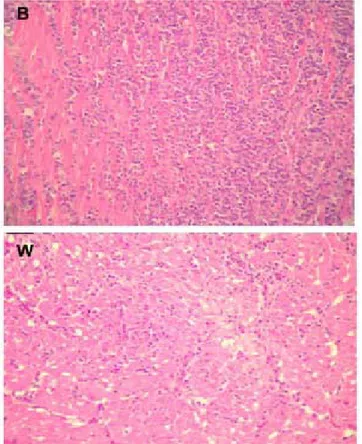
The m ost im port ant m icroscopic changes w ere observed in the liver, heart, and kidney in both genetic lines (Figures 2, 3, and 4). In semi-heavy birds, the liver present ed m ult if ocal necrosis, w hereas light birds show ed vacuolar degenerat ion. The heart of semi-h eavy b ir d s semi-h ad in f lam m at o r y in f ilt r at es w it semi-h predominantly heterophils; light birds had inflammatory in f ilt r at e w it h n o p r ed o m in an ce o f p o lym o r p h populations. There w as an inflammatory reaction w ith polymorph infiltration in the kidney of semi-heavy birds, but rare polymorphs w ere seen in the kidney of light birds. The lungs of birds of both lines presented mild inflammatory infiltrate.
As for hematological changes (Table 2), the group of semi-heavy birds presented significant reduction in t o t al red cell co u n t at f ive an d n in e d ays p o st -inoculation as compared to the same group of birds before being inoculated (p<0.05). In light birds, there
w ere n o d if f eren ces in m ean valu es d u rin g t h e experimental period (p>0.05). For both strains, there w ere no significant differences in mean hemoglobin concentration values. There w ere also no significant dif f erences in globular volum e, m ean corpuscular h em o g lo b in , m ean co r p u scu lar h em o g lo b in concentration, or in mean corpuscular in either bird strain.
Figure 2 - Photomicrography (40x) of the heart of brow n (B) and w hite birds (W) experimentally challenged w ith SGNaLr (second
exp er im en t ). Ob ser ved t h at in t h e u p p er p ict u r e, t h e inflammatory reaction is more intensive than in the low er picture.
Serum albumin levels of semi-heavy birds presented no statistical differences during the experiment, but t h er e w as a m ar k ed d ecr ease f ive d ays p o st -inoculat ion. In light birds, t here w as a signif icant increase in albumin values 12 days post-inoculation as compared to the values reported before inoculation (Table 3).
Figure 3 - Photomicrography (40 x) of the kidney of brow n (B) and w hite birds (W) experimentally challenged w ith SG Nalr.
Observe in the upper picture, the intensive inflammatory reac-tion. In the low er picture, there is less inflammareac-tion.
Figure 4 - Photomicrography (40 x) of the liver of brow n (B) and w hite birds (W) experimentally challenged w ith SG NaLr. See in
the upper picture multifocal necrosis of hepatocytes (irrevers-ible lesion). In the low er picture, there is a vacuolar degenera-tion of hepatocytes (reversible lesion).
Table 2 - M eans of red blood cell counts and some hematological parameters of birds of brow n (B) and w hite (W) lines experimentally challenged w ith S. Gallinarum Nalr.
Days post M eans of red blood cell counts and some hematological parameters
-inoculat ion Rc x 105/ µL GV (% ) Hb (g/ dL) M CV M CH (pg) M CHC (g/ dL)
(Dpi) B W B W B W B W B W B W
- 1 Dpi 2074B 2000A 28.00A 28.4A 13.07A 14.47A 135.88A 144.93A 64.20A 72.78A 47.00A 51.42A
1º Dpi 1700AB 1955A 26.25A 29.5A 13.98A 14.77A 149.15A 157.25A 82.05A 79.55A 53.45A 50.15A
3º Dpi 1938AB 1824A 28.40A 32.8A 15.40A 15.28A 147.98A 194.07A 79.40A 91.70A 55.29A 46.74A
5º Dpi 1300A 1856A 23.00A 30.0A * * 186.07A 161.71A * * * *
7º Dpi 1722AB 2436A 28.80A 30.4A 12.50A * 169.62A 125.88A 71.57A * 41.94A *
9º Dpi 1233A 1858A 22.33A 30.8A 12.81A 12.55A 182.72A 167.85A 112.61A 68.68A 61.35A 68.68A
12º Dpi * 2976B * 28.6A * 15.71A * 96.11A * 52.91A * 54.96A
14º Dpi * 2024A * 30.8A * 9.18A * 139.74A * 41.88A * 30.11A
Rc = Red blood cell count s; GV = globular volume; Hb = hemoglobin; * M CH = M ean corpuscular hemoglobin; M CHC = mean corpuscular hamoglobin Concentration; M CV = mean corpuscular volume; * = lost sample. M eans follow ed by different letters in the same column indicate significant differences by Tukey’s test (p < 0.05).
Table 3 - Albumin concentration (Alb) and of aspartate aminotransferase enzyme activity (AST) of birds of brow n (B) and w hite (W) lines experimentally challenged w ith S. Gallinarum Nalr.
Days post-inoculation (Dpi)
Days post-inoculation (Dpi)Days post-inoculation (Dpi)
Days post-inoculation (Dpi)
Days post-inoculation (Dpi) Albumin (g/ dL)Albumin (g/ dL)Albumin (g/ dL)Albumin (g/ dL)Albumin (g/ dL) Aspartate aminotransferase (U/ L)Aspartate aminotransferase (U/ L)Aspartate aminotransferase (U/ L)Aspartate aminotransferase (U/ L)Aspartate aminotransferase (U/ L)
Brow n Brow n Brow n Brow n
Brow n W hiteW hiteW hiteW hiteW hite Brow nBrow nBrow nBrow nBrow n W hiteW hiteW hiteW hiteW hite
- 1 Dpi 1.59A 1.60A 214.4A 180.72A
1º Dpi 1.46A 1.42A 228.73A 183.35A
3º Dpi * * * *
5º Dpi 1.10A 1.27A 394.14B 309.32B
7º Dpi 1.25A 1.41A 208.56A 276.40B
9º Dpi 1.16A 0.99A 211.23A 223.52A
12º Dpi * 2.32B * 299.70B
14º Dpi * 1.73A * 267.40B
M ean t ot al leukocyt e count of semi-heavy birds present ed a signif icant decrease (p<0.05) f ive days post -inf ect ion as com pared t o t he count s bef ore infection, follow ed by a significant increase (p<0.05) at 7 and 9 days after infection (Table 4). Light birds show ed a marked reduction in this parameter on the f if t h d ay p o st - in o cu lat io n , b u t w h ich w as n o t statistically significant (p>0.05), follow ed by a significant increase (p<0.05) at 12 and 15 days post-infection. Therefore, the behavior of total leukocyte count curves w as similar for both strains (Figure 5).
Lym p h o cyt es o f sem i-h eavy b ird s sig n if ican t ly decreased (p<0.05) seven days post -inoculat ion as compared to the values seen in the birds of the same group before infection. In light w hite birds, there w as no significant difference (p>0.05) in this parameter t hroughout t he experiment al period (Table 4). The ot her w hit e cell count s (het erophils, eosinophils, monocyt es, and basophils) present ed no signif icant differences (p>0.05) in both varieties.
DISCUSSION
Experiment 1
The comparatively higher percentage of mortality of semi-heavy birds indicates higher resistance of light birds to fow l typhoid, w hich is consistent w ith previous studies (Pomeroy and Nagaraja, 1991; Berchieri et al., 2001). Nevertheless, there w as a variation in resistance among w hite birds, w hich are generally considered as resistant to this disease. W3 line w as more susceptible t h an t h e o t h er lin es, an d p resen t ed t h e h ig h est mortality. The behavior show n in the present study may be due to organic variations resulting from the genetic selection pressure to w hich the birds are submitted in order to achieve better performance results, w hich in t he end adversely af f ect s im m une responses and resistance to diseases (Sw aggerty et al., 2003).
Figure 5 - Graph of leukocyte kinetics of brow n (B) and w hite birds (W) experimentally challenged w ith SG Nalr.
As to the mortality rate of semi-heavy birds, there w as a significant dose-dependent response. The results corroborate an earlier study that show ed that disease depends on inf ect ing dose and bird genet ic line (Oliveira et al., 2005). On the other hand, Solomon (1968) considers that bird resistance to fow l typhoid depends on the age of the bird at the time of infection. Fecal shedding w as observed seven days post -in f ect io n -in o n e sem i-h eavy b ird t h at w as sick . According to Oliveira et al. (2005), although birds do not constantly shed SG, birds that survive the disease may do it.
The result s of liver sw abs t aken 23 days post -infection indicated that SG persisted more markedly in light birds, although there w ere no clinical signs of the disease. Nevertheless, the presence of bacteria in semi-heavy birds w as insignificant. These findings are consistent w ith a previous study, w hich suggested that bird lines genetically resistant to fow l typhoid may carry the agent for w eeks w ithout show ing clinical signs (Berchieri et al., 2000a). This indicates that, although
Table 4 - M eans of total leucocytes counts and differential leukocyte counts of birds of brow n (B) and w hite (W) lines experimentally challenged w ith S. Gallinarum Nalr.
Days post- W hite cell counts
Inoculat ion (Dpi) Leucocytes / µL Heterophils (% ) Lymphocyt es (% ) M onocytes (% ) Eosinphils (% ) Basophils (% )
B W B W B W B W B W B W
- 1 Dpi 8800B 10000A 35.8A 44.4A 59.2B 51.2A 2.0A 2.6A 1.8A 0.4A 1.2A 1.4A
1º Dpi 7833AB 10375A 33.0A 53.0A 61.0B 44.0A 3.3A 1.5A 0.7A 0.25A 2.0A 1.25A
3º Dpi 13900BC 7400A 55.4A 49.2A 39.2B 47.2A 5.0A 2.2A 0.2A 0.8A 0.2A 0.6A
5º Dpi 3000A 4833A 56.0A 43.7A 40.05B 51.3A 2.25A 3.0A 0.5A 1.0A 0.75 1.0A
7º Dpi 19600C 9700A 58.0A 44.0A 34.4A 50A 4.0A 3.4A 1.8A 0.8A 1.8A 1.8A
9º Dpi 19833C 8400A 40.3A 47.8A 56.0B 47.2A 3.3A 3.6A 0.3A 0.6A 0.0A 0.8A
12º Dpi * 13700B * 47.0A * 48.8A * 2.2A * 1.4A * 0.6A
14º Dpi * 16100B * 40.8A * 55.0A * 3.0A * 0.6A * 0.6A
the birds do not constantly shed SG, in the presence of cannibalism they may become sources of infection of other birds.
In terms of clinical evaluation, light birds show ed characteristic signs of fow l typhoid before dying, such as depression, prostration, anorexia, dropped w ings, ruffled feathers, and dirty vents. On the other hand, semi-heavy birds died suddenly w ith no clinical signs. Smith (1955) and Assoku et al. (1970) described similar signs in young broilers three to four days after oral inoculation w ith S. Gallinarum; some inoculated birds developed the acute form of the disease, w hile others developed the sub-acute form of the disease.
Experiment 2
In this experiment, no light birds died, w hereas semi-heavy birds show ed considerable mortality. There w ere no clinical signs of t he disease, and t he birds died suddenly. The results are similar to those reported by Assoku et al. (1970), Allan and Duffus (1971), Hall (1991), and Christ ensen et al. (1996), all of w hich reported high mortality rates during the development of the disease, w hich w as up to 50% among birds affected betw een five and seven days post-infection. Interestingly, the mortality rate among light birds varied betw een the first and the second experiment, although the same inoculum concentration w as used in bot h experim ent s. W hereas m ort alit y w as high among these birds in the first experiment, in the second there w ere no dead birds. This may be explained by the fact that females w ere used in the first experiment, w h er eas m ales w er e u sed in t h e seco n d o n e. Physiologically, males of domest ic birds are usually heavier at hatching as compared to females. Therefore, it may be inferred that the development of the disease w ould be dose-dependent, under the conditions of this experiment , as previously described (Oliveira et al., 2005).
Gross lesions w ere restricted to the liver and the spleen of sem i-heavy birds. They w ere larger, and some presented w hite spots five days after inoculation. The liver w as green-yellow ish and friable, similar to the lesions described w hen birds w ere inoculated w ith Salmonella Gallinarum (Smith, 1955; Assoku et al., 1970; Pomeroy, 1987; Berchieri, 2000b). There w as no evidence of anatomical changes in light birds.
M icroscopically, the liver and the heart w ere the organs t hat suf f erred t he great er dam age in t heir cellular structure, considering both bird lines. On the ot her hand, t he kidneys show ed a mild degree of lesion, as w ell as the lungs . Liver lesions w ere more
int ense in sem i-heavy birds, w hich present ed an irreversible pathological change (hepatocyte necrosis). Th ese lesio n s w ere m o re evid en t f ive d ays af t er inoculation. Histological liver lesions in light birds w ere less intense (vacuolar degeneration of hepatocytes). Hall (1991) described histopathological lesions in the liver after experimental infection of birds w ith SG, such as hepatocyte degeneration around the centrilobular veins that w ould eventually result in substitution of the parenchyma for conjunctive tissue and an interstitial fibrosis. Later, a process of inflammatory infiltration develops, w hich is macroscopically evidenced as w hite spots on the surface of the organ. In the analysis of the histological sections, the heart of both semi-heavy and light birds presented inflammatory infiltration; in the former, it w as characterized by the presence of h et ero p h ils. Sim ilarly, Sm it h (1 9 5 5 ) d escrib ed a proliferative cell reaction in this organ, w ith nodular w hite masses disseminated in the myocardium.
Th e resu lt s o f t h e p resen t st u d y sh o w ed n o differences betw een lines in terms of globular volume (GV), hemoglobin (Hb), mean corpuscular hemoglobin (M CH), mean corpuscular hemoglobin concentration (M CHC), or mean corpuscular volume (M CM ) values caused by experimental infection w ith SG. There w as only a decrease in erythrocytes value in semi-heavy birds at five and nine days post-infection. These findings are different from a previous study, w hich reported changes in erythrocyte counts during the acute phase of the infection due to an increase in the destruction of these cells (Assoku et al., 1970). As fow l typhoid may develop as an acute, sub-acute, or chronic disease, h em at o lo g ical ch an g es w ill b e d if f eren t in each situation, and this may explain the differences found in the present study. In sub-acute fow l typhoid, there is no evidence of m arked anem ia or eryt hrocyt e fragility. There is only a slight reduction in globular volume, w hich also returns to normal values after the animal has recovered (Pomeroy, 1987).
by the cytolytic effect of Salmonella on leukocytes during the initial infection (Lam and M unn, 2002). It may also be due to the high susceptibility of these cells to the cytopathic effect of SG lipopolysaccharides (Buxton and Allan, 1963). It is interesting to note that the behavior of leukocyte kinetics show n during the initial infection w ith Salmonella Gallinarum in the present study, both in semi-heavy and light birds (Figure 5), has not been described in the retrieved studies w ith birds, despite being described in mammals (Santos et al., 2002).
Lymphocyte percentage values in semi-heavy birds decreased (lymphopenia) after the 7th day post-infection as compared to the same group of birds before being inoculated. Values increased after the 9th day post -infection up to normal values, similar to those before inoculation. In the light birds, leukocyte numbers varied w ithin normal ranges during the experiment. In a similar study, Allan and Duffus (1971) found no changes in lymphocyte counts during the course of fow l typhoid. On the other hand, Assoku et al. (1970) w orked w ith SG in birds, and found lymphocyte counts low er than norm al values. Lym phopenia is com m on in acut e in f lam m at o ry resp o n ses, b ecau se in f lam m at o ry mediators stimulate the migration of heterophils and lymphocytes from the blood and lymphoid tissues to the inflammation site (Jain, 1993).
The marked increase in heterophil counts observed af t er t he 5th day post -inf ect ion w as not st at ist ically signif icant . How ever, leukocyt osis w as previously reported caused by an increase in heterophils up to five times higher than before inoculation (Assoku et al., 1970). Leukocytosis is usually due to heterophilia, and common causes are general inf ect ions due t o sept icem ias caused by inf ect ious agent s, such as salmonella (M orgulis, 2002).
M onocyt e, eosinophil, and basophil percent age values in birds both susceptible and resistant to fow l t yphoid, in t he present st udy, show ed no relevant changes, and w ere consistent w ith previous reports (Allan and Duffus, 1971; Cardoso et al., 2003).
Although serum albumin levels w ere normal in both groups, they w ere low er in susceptible birds five days post-inoculation as compared to healthy birds of the same strain.
There w as an increase in the activity of aspartate aminotransferase (AST) in semi-heavy birds five days post-inoculation as compared to the mean value in birds of the same group, before being infected, and the values decreased af t er t he 7th day post-inoculation. Light birds show ed an increase in mean enzyme value at 5, 7, 12, and 14 days post-inoculation.
In this study, w hen the values of albumin and AST in semi-heavy birds are compared w ith the degree of liver lesion at five-days post-inoculation, a coincidence is observed among highest lesion score (mult if ocal necrosis), higher AST levels, and low er albumin levels in these birds. This may be interpreted as an incapacity of t he liver t o synt hesize prot ein due t o t he lesion intensity, microscopically evidenced by hepatomegaly and loss of protein in the affected kidney. Therefore, the damage in the glomerular filtration barrier may result in the presence of plasma proteins in the urine; in addition, inflammation of the renal parenchyma or epithelial damage of the tubules may cause loss of protein to the urine (Reldford and Lees, 1996).
In conclusion, the absence of a similar behavior in the response of the defense system of the birds in the presence of fow l typhoid makes it difficult to understand the relationship betw een Salmonella Gallinarum and affected birds. Similarly, the observations of the present study raise questions regarding the pathogenesis of fow l typhoid. Further studies are necessary to elucidate the relationship betw een birds and this bacterium.
REFERENCES
A llan D, Duf f us W . The im m unophat ology in f ow ls (Gallus domesticus) of acute and subacute Salmonella Gallinarum infection. Research in Veterinary Science 1971; 12:140-151.
Assoku R, Buxton A, Penhale W. Haematological changes in acute experimental Salmonella Gallinarum infection in chickens. Journal of Comparative Pathology 1970; 80:473-485
Behmer O, Tolosa E, Freitas A. Técnicas para histologia normal e patológica. São Paulo: Editora da Universidade de São Paulo; 1976.
Berchieri Jr A. Salmoneloses aviárias. In: Berchieri Junior A, M acari M .editors. Doenças das aves. Campinas: Facta; 2000. p. 185-196
Berchieri Jr A, M urphy A, M arston K, Barrow PA. Observations on the persistence and vertical transmission of Salmonella enterica sorovars Gallinarum and Pollorum in chickens: effect of bacterial and host genetic background. Avian Pathology 2001; 30(3):221–231.
Berchieri Jr A, Oliveira G, Pinheiro I, Barrow PA. Experiment al Salmonella Gallinarum infection in light laying hen lines. Brazilian Journal of M icrobiology 2000; 31:50-52.
Buxton A. Pathological changes in the blood of chickens infected w ith Salmonella Gallinarum. The Journal of Comparative Pathology and Therapeutics 1960; 70:308-325.
Cardoso A, Cast ro A, Tessari E. Est udo hemat ológico em aves inoculadas com Salm onella Gallinarum . Arquivos do Inst it ut o Biológico 2003; 70(1):35-42.
Christ ensen JP, Barrow PA , Olsen JE, Poulsen JS, Bisgaard. Correlation betw een viable counts of Salmonella Gallinarum in spleen and liver and the development of anaemia in chickens as seen in experimental fow l typhoid. Avian Pathology 1996; 25(4): 769-783.
Hall W. Fow l Typhoid. En: Biester HE, Schw arte HL editors. Diseases of poultry. Iow a: State University Press; 1991. p. 87-99.
Jain NC. Essentials of veterinary hematology. Philadelphia: Lea & Febiger; 1993. p. 365-372.
Ji-Dong JIN, Dong-Seok LEE, Eun-Kyng S HIN, Rose JUNG and Tae-W ook HAHH. M olecular Typing by Random Am plif icat ion of Polymorphic DNA (RAPD) and det ect ion of virulence genes of Salmonella enterica subspecies enterica serovar Gallinarum biovar Gallinarum. Journal Vet erinary M edicine Science 2006; 68(12): 1321-1326.
Lam KM , M unn R. The cytolytic effects of Salmonella enterica sorovar Typhimutium on chicken heterophils. Avian Pathology 2002; 31: 277-283.
M orgulis M . Imunologia aplicada. In: M acari M , Furlan RL, Gonzalez E editors. Fisiologia aviária aplicada a frangos de corte. Jaboticabal: FUNEP/ UNESP; 2002. p. 375-429.
Oliveira GH, Fernandes AC, Berchieri Jr A. Experimental infection of laying hen w it h Salm onella Gallinarum . Brazilian Journal of M icrobiology 2005; 36:51-56.
Pomeroy B. Fow l Typhoid. In: Hofstad M D, Calnek CF, Helmbolt WM , Reid HW, Yoder JR editors. Diseases of poultry. Ames: Iow a, State University Press; 1987. p. 100-116.
Prince WR, Garren HW. An investigation of the resistance of White leghorn chicks to Salmonella Gallinarum. Poultry Science 1966; 45(6):1149-1153.
Qureshi M A. Role of M acrophages in Avian Health and Disease. Poultry Science 1998; 77(7):978-982.
Relford RL, Lees GE. Nephrotic syndrome in dogs: diagnosis and treatment. Compendium Continuing Education Practice Veterinary 1996; 18:279-292.
Smith H. Observations on experimental fow l tiphoid. Journal of Comparative Pathology 1955; 65:37-57.
Solomon JB. Immunity to Salmonella Gallinarum during Ontogeny of chicken. I. Onset of resistance to infection; the minor roll of opsonins. Immunology 1968; 15:197-206.
Santos RL, Zhang S, Tsolis R, Baumher AJ, Adams G. Haematologic an d ser u m b io ch em ical ch an g es in Salm o n ella so r o var Typhimurium- inf ect ed calves. American Journal of Vet erinary Research 2002; 63:1145-1150.
Shivraprasad HL. Pullorum disease and f ow l t yphoid. Revue Scientifique et Technique Office International Epizooties 2000; 19: 405-424.
Sw aggert y CL, Pevzner IY, Low ry VK, Farnellm B, Kogut M H. Functional comparison of heterofils isolated from commercial broiler chickens. Avian Pathology 2003; 32:95-102.