Bowman-Birk inhibitors, proteasome peptidase activities and colorectal
pre neoplasias induced by 1,2-dimethylhydrazine in
Swiss
mice
Alessandra de Paula Carli
a,d, Paula Melo de Abreu Vieira
a, Karina Taciana Santos Silva
a,
Renata Guerra de Sá Cota
a,b, Cláudia Martins Carneiro
a,c, William Castro-Borges
a,b, Milton Hércules
Guerra de Andrade
a,b,⇑
aNúcleo de Pesquisas em Ciências Biológicas, Universidade Federal de Ouro Preto, Ouro Preto, Brazil bDepartamento de Ciências Biológicas, Universidade Federal de Ouro Preto, Ouro Preto, Brazil cDepartamento de Análises Clínicas, Escola de Farmácia, Universidade Federal de Ouro Preto, Brazil dCentro Universitário de Caratinga, Caratinga, Brazil
a r t i c l e
i n f o
Article history:
Received 30 May 2011 Accepted 19 January 2012 Available online 2 February 2012
Keywords:
Colon cancer
Bowman-Birk inhibitors Proteasome activity Dimethylhydrazine CD44
a b s t r a c t
Bowman-Birk inhibitors (BBIs) are protein molecules containing two inhibitory domains for enzymes similar to trypsin and chymotrypsin. Interest in these inhibitors arose from their properties against the cancer chemically induced by 1,2-dimethylhydrazine (DMH). In this study the effect of two BBI prepara-tions (fromGlycine maxandMacrotyloma axillare) were evaluated for the prevention of colorectal neopla-sia induced by intraperitoneal injections of DMH, given at a dose of 30 mg/kg, during 12 weeks. Mice treated with DMH presented histopathological alterations consistent with tumor development, aug-mented CD44 expression and increased proteasome peptidase activities. Lysosomal fractions, obtained from the intestines, were chromatographed in a Sepharose-BBI column and increased activity for trypsin and chymotrypsin-like proteases recovered from DMH-treated animals. In parallel, mice treated for eight weeks with BBIs showed a decrease in the chymotrypsin and trypsin-like proteasome activities compared to animals fed on normal diet. For the groups receiving simultaneous treatment with DMH and BBIs, dysplasic lesions were not observed and proteasome peptidase activities were similar to the control group after the 24th week. These results suggest that the mechanism by which BBIs could prevent the appearance of pre neoplastic lesions is associated with inhibition of both the lysosomal and protea-some-dependent proteolytic pathways.
Ó2012 Elsevier Ltd. All rights reserved.
1. Introduction
Advances in industrialization generate an increasing frequency
of exposure to new chemicals that are potentially harmful to cells
and hence capable of inducing cancer. Active substances known to
be important for the prevention of colorectal cancer are present in
a variety of plants (
Forman et al., 2004
). In this regard, soybean has
been considered an important food source containing
chemopre-ventive compounds (
Losso, 2008
). Earlier studies indicated the
Bowman-Birk inhibitor (BBI/
M
r8 kDa) which contains two
dis-tinct inhibitory domains for enzymes similar to trypsin and
chy-motrypsin as the main soy component endowed with anticancer
activity (
Deshimaru et al., 2004; Odani and Ikenaka, 1973
). In
particular, the antichymotrypsin inhibitory activity of BBIs has
been correlated to their anticarcinogenic activity (
Kennedy et al.,
2008
). Nevertheless, it has been demonstrated that other soy
constituents are known to contribute to this effect such as lunasin
and a variety of small organic components (
Gomes et al., 2005;
Hernandez-Ledesma et al., 2009; Kris-Etherton et al., 2002
).
The 20/26S proteasome complex is currently considered an
important intracellular target for antitumor drugs (
Montagut
et al., 2006
). Although the proteasome is classified as a threonine
protease, its proteolytic core contains catalytic subunits exhibiting
caspase (
b
1), trypsin (
b
2) and chymotrypsin (
b
5)-like activities
(
Tanaka, 2009
). Bortezomib is the first proteasome inhibitor
ap-proved by the FDA (Food and Drug Administration), known to be
effective in some hematological malignancies for treatment of
0278-6915/$ - see front matterÓ2012 Elsevier Ltd. All rights reserved. doi:10.1016/j.fct.2012.01.036
Abbreviations:BBIs, Bowman-Birk inhibitors; DMH, N,N0-dimethylhydrazine;
MCA, 7-amide-4-methylcoumarin.
⇑
Corresponding author. Address: Instituto de Ciências Exatas e Biológicas, Departamento de Ciências Biológicas, Núcleo de Pesquisas em Ciências Biológicas, Universidade Federal de Ouro Preto, Campus Morro do Cruzeiro, s/n, 35400000 Ouro Preto, MG, Brazil. Tel.: +55 31 3559 1705; fax: +55 31 3559 1680.E-mail addresses:alessandrapcarli@hotmail.com(A. de Paula Carli),paula@nu peb.ufop.br(P.M. de Abreu Vieira), karinasilva353@yahoo.com.br (K.T.S. Silva), rguerrasa@gmail.com(R.G. de Sá Cota),carneirocm@gmail.com(C.M. Carneiro), williamcborges@hotmail.com(W. Castro-Borges),mguerra@nupeb.ufop.br(M.H.G. de Andrade).
Contents lists available at
SciVerse ScienceDirect
Food and Chemical Toxicology
multiple myeloma (
Cavo, 2006; Chen et al., 2011
). It was
demon-strated that the use of proteasome inhibitors results in multiple
cellular effects, including promotion of inflammatory,
anti-carcinogenic, anti-proliferative and apoptotic effects in cells
exhib-iting abnormal development (
Mitsiades et al., 2002; Myung et al.,
2001
). Other studies demonstrated that BBIs specifically inhibited
the chymotrypsin-like proteasomal activity in MCF7 tumor cells
causing accumulation of ubiquitinated substrates and a decrease
in the levels of regulatory cyclins (
Chen et al., 2005
).
The present study refers to an evaluation of the protective effects
of BBIs isolated from
Glycine max
and
Macrotyloma axillare
during
development of colorectal tumors caused by administration of the
carcinogen 1,2-dimethylhydrazine to mice. In addition to display
trypsin and chymotrypsin inhibitory properties
M. axillare
BBIs
share relevant primary and 3D structural features with soybean
BBI, such as the conserved cysteine residues involved in disulfide
bond formation (
Kumar et al., 2002
). However, specific differences
observed in amino acid composition may account for some
particu-lar pharmacokinetic properties of
M. axillare
BBIs, including a higher
distribution to stomach (
Santana et al., 2011
), most likely due to a
higher interaction followed by enhanced internalization in the
gas-tric tissue. In the present study the protective effects of these two
BBI preparations were assessed through histopathological
examina-tions, evaluation of the proteasome peptidase activities, CD44
pro-tein expression by
Western blotting
and evaluation of the enzyme
activities retained in a Sepharose-BBI affinity column.
2. Materials and methods
2.1. Ethics statement, experimental animals and reagents
Swiss male mice aged 120 days were obtained from the Center for Animal Science, Universidade Federal de Ouro Preto. All procedures involving animals were carried out in accordance with the national guidelines provided by the local ethics committee. The animals were maintained under a light/dark cycle of 12 h and given food and waterad libitum.N,N0-Dimethylhydrazine (DMH) 99% and fluorogenic
sub-strates Boc-Val-Pro-Arg-MCA (amide-4-methylcoumarin) and Suc-Ala-Ala-Pro-Phe-MCA were from Sigma (Sigma–Aldrich, St. Louis, USA). Purified anti-mouse CD44 was purchased from BD Biosciences Pharmingen™ (BD Biosciences PharMingen, New Jersey, USA). The fluorogenic substrates, Cbz-Gly-Gly-Arg-MCA and Suc-Leu-Leu-Val-Tyr-MCA were purchased from Biomol International (Biomol, Exeter, UK).
2.2. Experimental groups
The group of animals named BBIS received a diet supplemented with 0.1% w/w enriched soybean (G. max) BBI, produced according to methods previously de-scribed (Yavelow et al., 1985). BBIM group refers to animals treated with a prepa-ration of enriched BBIs from the leguminousM. axillare, produced according to our patented isolation protocol available at http://www.patentesonline.com.br/pro- cesso-de-preparacao-de-extrato-ativado-de-bowman-birk-de-macrotyloma-axil-lare-115536.html. In the last case, the dose added to the diet showed antitrypsin activity equivalent to that contained in 0.1% w/w soybean BBI. The homogeneity of the two BBI preparations was assessed using 15% SDS–PAGE. For the induction pre neoplastic lesions and neoplasia, mice were subjected to intraperitoneal injec-tions of DMH weekly, at a dose of 30 mg/kg in citrate buffer/EDTA (1 mM EDTA, 10 mM sodium citrate, 0.9% NaCl, pH 8.0) during 12 weeks. Injection volume was 150
l
L. Animals were maintained for a latency period of 12 additional weeks. Overall, mice were divided into six treatment groups (n= 18 animals/group) as fol-lows – Control group (citrate/EDTA), DMH group (DMH), DMHS group (DMH and diet supplemented with BBI fromG. max) and DMHM group (DMH and diet supple-mented with BBI fromM. axillare). Two additional control groups were given a diet supplemented with either 0.1%G. maxBBI (BBIS) orM. axillareBBIs (BBIM) for 8 weeks.2.3. Histopathological analyses
Representative animals of the various treatment groups had their colon and intestine removed during necropsy for histopathological analyses. The organs were fixed in buffered formalin and embedded in paraffin. Histological sections of 5
l
m were obtained using a microtome. These were fixed on slides followed by staining with a Hematoxylin Eosin preparation. Qualitatively evaluated parameters were:presence/absence of atypical cells either present in the mucous, middle or serosa layer; mucosa inflammation; necrosis; atypical structures and hyperplasia of the lymphoid nodules.
2.4. In gel proteolytic digestion and peptidase activities of the fraction purified from a Sepharose-BBI column
Protein samples from the small intestine and colon of each experimental group were subjected to affinity chromatography using a Sepharose column containing immobilizedM. axillareBBI. Coupling of purifiedM. axillareBBI to the Sepharose matrix was performed as previously described (Matsumoto et al., 1981). Homoge-nates obtained from 100 mg of colon and intestine tissues were prepared in PBS pH 7.4 containing 1 mM MgCl2using aBranson 250sonifier. After centrifugation at 1000gfor 10 min, to eliminate cell/tissue debris, the supernatant was removed and recentrifuged at 10,000gfor 20 min to obtain a soluble and a pellet fraction. Lysosomes were further enriched from the pellet fraction following the protocol as described elsewhere (Billings et al., 1988). The lysosome-enriched pellet fraction was resuspended in 50 mM phosphate buffer pH 7.0 containing 1 mM MgCl2and 0.1% Triton X-100. Lysosomal proteins were extracted by five cycles of sonication for 30 s with 1 min intervals on ice. After centrifugation at 10,000gfor 5 min, the supernatant containing lysosomal proteins was obtained. The lysosomal and solu-ble fractions were separately loaded onto the Sepharose-BBI column. The column was extensively washed with 50 mM Tris–HCl pH 7.0, 1 mM MgCl2, 500 mM NaCl and the bound fraction obtained by decreasing the pH to 1 using 100 mM HCl. Pro-teolytic activity present in this fraction was demonstrated by 12% PAGE with the gel matrix containing 0.1% gelatin as described by (Billings et al., 1991). In parallel, pep-tidase activities related to trypsin and chymotrypsin were evaluated by hydrolysis of the fluorogenic substrates Boc-Val-Pro-Arg-MCA and Suc-Ala-Ala-Pro-Phe-MCA, respectively. The assays were conducted using an aliquot of 40
l
L of each fraction and 1.6 mM of the substrates in a final volume of 240l
L. The hydrolysis reaction occurred for 30 min at 37°C and stopped by addition of 3 mL 50 mM sodium phos-phate, pH 8.0. Fluorometric readings were obtained at the wavelengths of 380 nm (excitation) and 460 nm (emission) using the RF-5301PC fluorometer (Shimadzu, Japan). Results were expressed as fluorescence arbitrary units/l
g protein applied to the column.2.5. Assessment of proteasome peptidase activities
Approximately 100 mg of the small intestine and colon of animals from the dif-ferent experimental groups were homogenized in 3 mL of 20S buffer (25 mM Tris– HCl pH 7.5, 1 mM DTT, 10% glycerol). Protein extraction was first performed using a tissue homogenizer (Potter Elvejhem) followed by sonication under three cycles of 40 and 15 s intervals in an ice bath. Samples were centrifuged for 30 min at 10,000gand the supernatant submitted to ultracentrifugation for 6 h at 100,000g. One mL fractions taken from the bottom of the tubes were used to assess the pro-teasome’s trypsin- and chymotrypsin-like peptidase activities, using the respective fluorogenic substrates Cbz-Gly-Gly-Arg-7-amide-4-methylcoumarin and Suc-Leu-Leu-Val-Tyr-7-amide-4-methylcoumarin. The reactions were performed using 15
l
g total protein and 13l
M of fluorogenic substrates for a final volume of 240l
L, in 50 mM Tris–HCl pH 8.0 containing 10 mM MgCl2. The chymotrypsin-like assay was also performed in the presence of 20l
M MG132, a proteasome inhibitor, to control for proteasome-specific activity. Fluorometric readings were performed at wavelengths of 380 nm (excitation) and 460 nm (emission), and results expressed as fluorescence arbitrary units/l
g protein.2.6. Analysis of CD44 expression by Western blotting
2.7. Statistical analysis
Statistical analysis was performed by means of thePrism software, using analy-sis of variance between groups (ANOVA), which guarantees normal distribution of data and the complementary Tukey’s test, considering a significance level of
p60.05.
3. Results
3.1. BBIs from M. axillare and G. max protect against the development
of pre neoplastic lesions induced by DMH
The percentage of animals that presented macroscopic and
microscopic alterations in the colon and small intestine from the
different experimental groups is indicated in
Table 1
. It was
ob-served that 45% of the mice that received DMH presented
macro-scopic alterations in the colon and small intestine. With respect to
microscopic alterations, dysplastic and neoplastic lesions were
ob-served in 56% and 33% of the colon and small intestine, respectively,
only in animals under DMH-treatment.
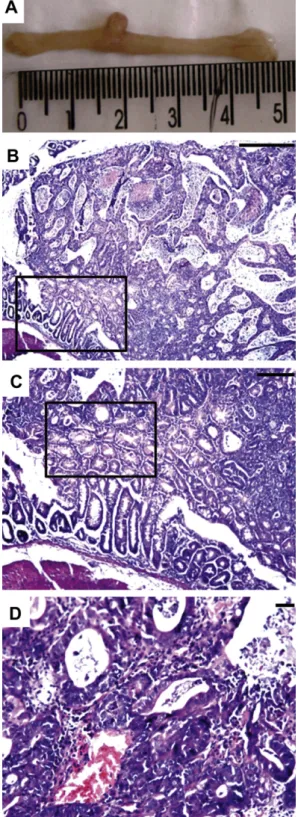
Fig. 1
A shows a macroscopic
lesion corresponding to a polyp formation in the colon of
DMH-trea-ted animals. These were found distribuDMH-trea-ted throughout the intestine.
Fig. 1
B reveals the histologic aspects of the polipomatous lesion and
its insertion in the mucosa, squared area.
Fig. 1
D demonstrates
architectural details of the proliferative lesion from DMH-treated
animals and its associated-inflammatory response. No tissue
alter-ations were found for animals in the remaining groups (DMHS and
DMHM), attesting for the protective effect of diet supplementation
with BBIs. At the employed dosage regimen BBI from the two
sources were equally effective for the prevention of pre neoplastic
lesions with no significant differences in the parameters used to
evaluate the small intestine and colon. Nevertheless, specific
inhib-itory activity assays comparing the properties of the main BBI
iso-forms present in these two preparations and isolated according to
(
César et al., 2009
) revealed BBI from
M. axillare
being 33% and
69% more active against bovine trypsin and chymotrypsin,
respec-tively, compared to
G. max
BBI – Supplementary Table 1.
It’s worth emphasizing that a severe inflammatory response
was observed in the colon and small intestine of animals in the
DMH-treated group. A representative result from the colon is
pre-sented in
Fig. 2
showing the inflammatory infiltrate in the muscle
layer in animals of the DMH group (
Fig. 2
B) and the absence of
such response in animals from DMHS and DMHM groups (
Figs.
2
C and D). Altogether these results revealed the protective effects
of our two BBI preparations for DMH-induced inflammation and
development of pre neoplastic lesions.
3.2. DMH-treatment induces increase in trypsin- and
chymotrypsin-like activities in the lysosomal fraction
Lysosomal fractions from intestine and colon of the various
ani-mal groups were used to evaluate their ability to hydrolyze
fluoro-genic substrates to trypsin and chymotrypsin-like enzymes, after
the 24th week of experimentation. Prior to the enzymatic assays,
the lysosomal fractions were submitted to affinity chromatography
using a Sepharose-BBI column, to recover proteolytic activity
related to trypsin and chymotrypsin enzymes.
Fig. 3
reveals that
both activities are increased in the intestine and colon of animals
from the DMH group. The low trypsin- and chymotrypsin-like
activities in the lysosomal fractions derived from the control group
allowed us to establish a correlation between the increase in
pro-teolytic activity and the presence of neoplastic lesions. Animals
from DMHS and DMHM groups exhibited proteolytic activities
similar to the untreated control group.
3.3. CD44 protein expression increases in neoplastic lesions from
DMH-treated animals
Total membrane fractions were obtained from the small
intes-tines of control and DMH-treated groups for evaluation of CD44
expression levels by
Western blotting
. Protein samples from three
representative animals in each group were separated by SDS–PAGE
and the gel image used for quantification of total protein loading.
Densitometric analysis revealed no significant difference between
the amounts of protein transferred to the PVDF membrane
com-paring control and DMH-treated animals. When the membrane
was immunoblotted with anti-CD44, it was clearly observed
in-creased levels of CD44 expression in the fractions corresponding
to membrane extracts from the intestinal polyps induced by
treat-ment with DMH,
Fig. 4
A. The appearance of CD44 positive bands
unique to DMH-treated animals is also indicated by arrows.
Fig. 4
B corresponds to the densitometric analysis of the blotted
membrane shown in
Fig. 4
A. Diet supplementation with both BBI
preparations revealed no significant difference in the expression
pattern of CD44, compared to control groups (data not shown).
This result provided molecular evidence for the development of
pre neoplastic lesions induced by DMH.
3.4. Treatment with BBIs inhibits the proteasome’s chymotrypsin and
trypsin-like activities
Treatment with either 0.1% w/w
G. max
BBI or
M. axillare
BBIs
(the latter given at a dose equivalent to the inhibitory activity
pres-ent on 0.1%
G.max
BBI) added to the animal diet for 8 weeks
pro-moted decrease in the chymotrypsin- and trypsin-like activities
of the proteasome extracted from the colon. The assays revealed
a reduction of approximately 50% in both activities for animals
treated with both BBI preparations,
Fig. 5
. Apparently BBI from
soy-bean seem to be more effective at inhibiting both the trypsin and
chymotrypsin-like proteasome activities but the observed
differ-ences were not statistically significant. In addition, aiming to
eval-uate the proteasome activities from mice simultaneously treated
with these inhibitors plus DMH, colon protein extracts from the
various groups were subjected to proteasome’s activity assays.
The results presented in
Fig. 6
clearly show a remarkable increase
in the trypsin- and chymotrypsin-like activities of the proteasome
for the DMH group. Proteasome peptidase activities were similar to
the control for DMHS and DMHM groups. Use of MG132, a classical
proteasome
b
5 subunit inhibitor, revealed approximately 80%
reduction in the chymotrypsin-like activity; meaning this enzyme
activity was major attributed to proteasome function,
Fig. 6
.
Table 1
Percentage and number of animals that presented macroscopic and microscopic alterations in the colon and small intestine from the different experimental groups.
Groups Macroscopic alterations Dysplasia and pre neoplastic lesions Inflammation
Small intestine (%) Colon (%) Small intestine (%) Colon (%) Small intestine (%) Colon (%)
Control 0(0/6) 0(0/6) 0(0/6) 0(0/6) 0(0/6) 0(0/6)
DMH 45(4/9) 45(4/9) 56(5/9) 33(3/9) 45(4/9) 56(5/9)
DMHS 0(0/6) 0(0/6) 0(0/6) 0(0/6) 0(0/6) 0(0/6)
DMHM 0(0/6) 0(0/6) 0(0/6) 0(0/6) 0(0/6) 0(0/6)
4. Discussion
Considering that pre neoplastic lesions precede malignant
transformation our study confirms previous results demonstrating
the ability of soybean BBIs to inhibit carcinogenesis induced by
DMH, (
Kennedy, 1998; Kennedy et al., 1996; Kennedy et al.,
2002
). Experiments aimed at elucidating the mechanism of action
of BBIs, as cancer preventive agents, showed an increased
expres-sion of proteolytic enzymes exhibiting trypsin- and
chymotryp-sin-like activities. These could be recovered from the lysosomal
fraction after affinity chromatography on a Sepharose-BBI column
(
Billings et al., 1990; St Clair et al., 1990
). Therefore, the cell
molec-ular targets of BBIs have been primarily centered on lysosomal
pro-teases. Despite their unknown nature, zymogram analyses and
peptidase activities demonstrated that BBI protease ligands were
in the mass range of 45–66 kDa, as determined by SDS–PAGE. More
recently, it was demonstrated that purified soybean BBIs inhibited
the 20S proteasome’s chymotrypsin-like in breast cancer cells
(
Chen et al., 2005
). Reinforcing the proteasome’s inhibition by BBIs,
it was reported reduced degradation of ubiquitinated connexin 43
in tumor cells treated with BBIs, leading to enhanced cell adhesion
(
Saito et al., 2007
). In addition, specific inhibitors of the
protea-some’s chymotrypsin-like activity have been shown to induce
apoptosis of cancer cells and suppress tumor growth in several
experimental models (
Chen et al., 2005; Kisselev et al., 2006
).
Until now, few studies have demonstrated the use of BBIs as
potential inhibitors of proteasome activity. The present data reveals
for the first time the
in vivo
effects of two BBI preparations, given as
a diet supplement to healthy animals, upon proteasome
capabilities. The eight week’s treatment with either
G. max
BBI or
M. axillare
BBIs incorporated in the diet, resulted in a decrease of
approximately 50% in the trypsin- and chymotrypsin-like activities
of the proteasome isolated from the colon of mice. This
in vivo
effect
is consistent with both a considerable absorption of these inhibitors
given orally, reaching 50% bioavailability (
Billings et al., 1991
) and
the need for cellular internalization of BBI to gain access to their
po-tential targets (
Chen et al., 2005
). No histopathologic alterations
were observed in the small intestine and colon from animals
receiv-ing a diet supplemented with BBI from the two sources.
Further-more, the protective effects of these two BBI preparations were
correlated with maintenance of normal proteasome-dependent
proteolytic activity, during simultaneous treatment with DMH.
Altogether, our results provide evidence for the suppressive effect
of BBIs being correlated with inhibition of at least two intracellular
proteolytic systems: the lysosomal and the proteasome-dependent
pathway.
Histopathological analyses attested for the protective effect of
BBIs based on the absence of tissue alterations indicative of tumor
development. At the molecular level, control levels of CD44 protein
expression was observed in DMHS and DMHM groups, as opposed
to the clearly increased levels of CD44 isoforms in the colon
in-duced solely by DMH-treatment. It is important to emphasize that
increased CD44 protein expression and particularly the appearance
of aberrant CD44 isoforms, due to alternative splicing, is a hallmark
of the cell proteome associated to the development of colon
tumors (
Hynes et al., 2009
). Moreover, considering that CD44 are
proteoglycans their molecular diversity is also explained by
differ-entially attached glycans, adding complexity to their
electropho-retic pattern (
Arcinas et al., 2009
). Particularly, as our primary
antibody does not discriminate among CD44 isoforms we believe,
judged by the electrophoretic pattern, we have detected the
stan-dard CD44 (CD44s) at approx. 80 kDa and high molecular mass
CD44 molecules, which should account for other CD44 splice
vari-ants (
Brown et al., 2011; Godge and Poonja, 2011; Hanley et al.,
2006
). In addition to the appearance of high mass CD44 positive
bands, unique to the DMH-treated group, we have also observed
a marked increase in the amount of detected CD44 at the mass
range 25–30 kDa. As this mass range is not compatible with the full
length CD44, we hypothesized that it corresponds to CD44
cleav-age products previously shown to accumulate at the neoplastic
tis-sue and known to contribute to tumor progression in various
animal cell lines (
Sugahara et al., 2008; Wang et al., 2009
).
Fig. 1.Colon photomicrographs of DMH-treated animals. (A) Macroscopic lesions inThe use of two BBI preparations aimed the comparison of their
tumor-preventive activities. The dose to be incorporated in the diet
was standardized based on their inhibitory activity over bovine
trypsin. The results revealed that both preparations were equally
effective at preventing the appearance of tissue biomarkers
associ-ated with tumor development. Nevertheless, a comparative analysis
of the primary sequences of BBIs isolated from
G. max
and
M.
axil-lare
, in particular at the regions corresponding to their inhibitory
Fig. 2.Colon photomicrographs of control animals or DMH-treated associated or not with diet supplementation withG.maxandM. axillareBBIs. (A) Normal histological aspect of the colon. (B) Inflammatory infiltrate (arrowed) in the muscle layer in animals from the DMH-treated group. Absence of inflammatory infiltrates from animals in DMHS (C) and DMHM (D) groups. Hematoxylin Eosin staining. Bar = 50l
m.Fig. 3.Chymotrypsin and trypsin-like activities recovered from lysosomal fractions after Sepharose-BBI affinity chromatography. Enzyme assays were performed using samples from six animals in each treatment group and the results were expressed as means through fluorescence arbitrary units/
l
g protein. Bars represent the standard errors of the means. Note the increased chymotrypsin- and trypsin-like activities found in the colon and intestine in the DMH-group and similar to control levels for both activities in animals receiving DMH and diet supplemented with eitherG.maxBBI (DMHS) orM. axillareBBIs (DMHM). Values significantly different from the control were asterisked consideringp60.05.heads, permitted observation of critical amino acid differences. In
this regard, the active sequences of
G. max
BBI are CTKS
N
PPQC
and CALSYPAQC for the inhibitory heads of trypsin and
chymotryp-sin, respectively.
M. axillare
BBIs are composed of two types of
mol-ecules named DE3 and DE4, displaying identical amino acid
sequences for the trypsin-inhibitory head (CTKS
I
PPQC) and distinct
sequences (CTFSIPAQC and CALSEPAQC) for the
chymotrypsin-inhibitory head, respectively, (
Joubert et al., 1979
). Given that the
amino acid found in the P2’ position of
M. axillare
native BBI
inhib-itor is
I
le and the best observed modification in P2
0position of a
cyc-lic synthetic peptide was found to be associated with the same
amino acid, resulting in a change of
K
ifrom 2.5 to 0.009
l
M (
Gariani
et al., 1999
), we suggest that
M. axillare
BBIs and/or their byproducts
generated once given orally, have the potential to act as potent
pro-tease inhibitors. This argument is strengthened considering that
during germination of
M. axillare
there is proteolytic processing of
the native inhibitor, giving rise to short molecules known to be 5
times more potent than the full length inhibitor found in dormant
seeds (
Kumar et al., 2002
). In addition, as for the chymotrypsin
inhibitory head, particularly between the P2 e P2
0positions,
signif-icant amino acid differences in DE3 and DE4 isoforms exist
com-pared to the respective sequence found in soybean BBI.
Altogether, these findings provided our rationale for proposing the
utilization of an alternative BBI source (
M. axillare
BBI) to prevent
DMH-induced lesions.
Given the structural differences and the potential therapeutic
role of BBIs, detailed investigations are necessary for elucidating
the structure/activity relationship of such inhibitors. This would
al-low exploration of their activity upon intracellular protein
turn-over, either mediated by the lysosome or the 20S/26S
Fig. 4.CD44 expression pattern in the colon from control and DMH-treated animals. (A) Extracted membrane proteins from the colon of three control and three DMH-treated animals were separated using 12% SDS–PAGE, transferred to a PVDF membrane and immunoblotted using an anti-CD44 antibody. Note the increased expression of CD44s in DMH-treated animals and the appearance of high mass CD44 isoforms unique to this group (arrowed). Detection of CD44 cleavage products at the mass range 25–30 kDa was also particularly prominent in the DMH group (arrowed). (B) Densitometric analysis of the blotted membrane shown in (A). Significantly different values were observed from the control using ANOVA/Tukey’s test atp60.05.Fig. 5.Animals fed a diet containing eitherG. maxBBI (BBIS) orM. axillareBBIs (BBIM) during 8 weeks displayed decreased proteasome activity. Proteasome’s trypsin (T) and chymotrypsin (Q)-like activities were assayed for control and BBIS/ BBIM groups. Q-like activity was also performed in the presence of MG132 to control for proteasome’s specific activity. Assays were performed using samples from six animals in each treatment group and the results were expressed as means through fluorescence arbitrary units/
l
g protein. Bars represent the standard errors of the means. Values significantly different from the control were observed atp60.05 using ANOVA/Tukey’ test.
Fig. 6.DMH-treatment induces increase in the colon proteasome’s trypsin- and chymotrypsin-like activities whereas DMH plus BBIs keep them equivalent to control levels. Q-like activity was also performed in the presence of MG132 to control for proteasome’s specific activity. Assays were performed using samples from six animals in each treatment group and the results were expressed as means through fluorescence arbitrary units/
l
g protein. Bars represent the standard errors of the means. Values significantly different from the control were observed atproteasome, towards prevention of various diseases including
cancer.
5. Conclusions
In our experiments mice simultaneously treated with DMH and
either
G.max
or
M. axillare
seed BBIs, the induction of neoplastic
le-sions could be prevented. Since their effects correlated with their
inhibitory potential over bovine trypsin and given the increased
activity of
M. axillare
BBIs over this enzyme, we suggest that the
latter inhibitors could be used in doses much lower than that
re-quired for soybean BBI. Histopathological analyses revealed that
both BBI preparations protected against the onset of severe
inflam-matory processes and ultimately the appearance of pre-malignant
lesions, observed in DMH-treated animals. The two investigated
biomarkers (expression of CD44 and lysosomal protease activities
associated with a BBI-affinity column) showed consistent results
under the different experimental conditions, demonstrating their
reliability and usefulness in this experimental model. Finally, this
study demonstrated a significant increase in the trypsin and
chy-motrypsin-like activities of the proteasome in pre neoplastic
le-sions of the colon from DMH-treated animals. In contrast, normal
levels were observed for proteasome activity found in animals
treated simultaneously with BBIs and DMH.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the financial support
provided by FAPEMIG (Fundação de Amparo à Pesquisa do Estado
de Minas Gerais), CNPq (Conselho Nacional de Desenvolvimento
Científico e Tecnológico), Centro Universitário de Caratinga and
Universidade Federal de Ouro Preto. The authors are also greatful
to José H.B. Fortes for his technical assistance.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in
the online version, at
doi:10.1016/j.fct.2012.01.036
.
References
Arcinas, A., Yen, T.Y., Kebebew, E., Macher, B.A., 2009. Cell surface and secreted protein profiles of human thyroid cancer cell lines reveal distinct glycoprotein patterns. J. Proteome Res. 8 (8), 3958–3968.
Billings, P.C., Brandon, D.L., Habres, J.M., 1991. Internalisation of the Bowman-Birk protease inhibitor by intestinal epithelial cells. Eur. J. Cancer 27 (7), 903–908. Billings, P.C., Newberne, P.M., Kennedy, A.R., 1990. Protease inhibitor suppression of
colon and anal gland carcinogenesis induced by dimethylhydrazine. Carcinogenesis 11 (7), 1083–1086.
Billings, P.C., St Clair, W., Owen, A.J., Kennedy, A.R., 1988. Potential intracellular target proteins of the anticarcinogenic Bowman Birk protease inhibitor identified by affinity chromatography. Cancer Res. 48 (7), 1798–1802. Brown, R.L., Reinke, L.M., Damerow, M.S., Perez, D., Chodosh, L.A., Yang, J., Cheng, C.,
2011. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Invest. 121 (3), 1064–1074.
Cavo, M., 2006. Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia 20 (8), 1341–1352.
César, J., de Santana, M., de Oliveira, M., Santos, A., de Miranda, A., Santoro, M., de Andrade, M., 2009. A New Extraction and Purification Methodology of Bowman-Birk Inhibitors from Seeds and Germinated Seeds of<i> Macrotyloma axillare</i>.Chromatographia69 (3), 357–360.
Chen, D., Frezza, M., Schmitt, S., Kanwar, J., 2011. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr. Cancer Drug Targets 11 (3), 239–253.
Chen, Y.W., Huang, S.C., Lin-Shiau, S.Y., Lin, J.K., 2005. Bowman-Birk inhibitor abates proteasome function and suppresses the proliferation of MCF7 breast cancer cells through accumulation of MAP kinase phosphatase-1. Carcinogenesis 26 (7), 1296–1306.
Deshimaru, M., Yoshimi, S., Shioi, S., Terada, S., 2004. Multigene family for Bowman-Birk type proteinase inhibitors of wild soja and soybean: the presence of two BBI-A genes and pseudogenes. Biosci. Biotechnol. Biochem. 68 (6), 1279–1286. Forman, M.R., Hursting, S.D., Umar, A., Barrett, J.C., 2004. Nutrition and cancer prevention: a multidisciplinary perspective on human trials. Annu. Rev. Nutr. 24, 223–254.
Gariani, T., McBride, J.D., Leatherbarrow, R.J., 1999. The role of the P20position of
Bowman-Birk proteinase inhibitor in the inhibition of trypsin. Studies on P2’ variation in cyclic peptides encompassing the reactive site loop. Biochim. Biophys. Acta 1431 (1), 232–237.
Godge, P.Y., Poonja, L.S., 2011. Quantitative assessment of expression of cell adhesion molecule (CD44) splice variants: CD44 standard (CD44s) and v5, v6 isoforms in oral leukoplakias: An immunohistochemical study. Indian J. Dent. Res. 22 (3), 493–494.
Gomes, C.E., Barbosa, A.E., Macedo, L.L., Pitanga, J.C., Moura, F.T., Oliveira, A.S., Moura, R.M., Queiroz, A.F., Macedo, F.P., Andrade, L.B., Vidal, M.S., Sales, M.P., 2005. Effect of trypsin inhibitor from Crotalaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (fruit fly). Plant Physiol. Biochem. 43 (12), 1095–1102.
Hanley, W.D., Napier, S.L., Burdick, M.M., Schnaar, R.L., Sackstein, R., Konstantopoulos, K., 2006. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J. 20 (2), 337–339.
Hernandez-Ledesma, B., Hsieh, C.C., de Lumen, B.O., 2009. Lunasin and Bowman-Birk protease inhibitor (BBI) in US commercial soy foods. Food Chem. 115 (2), 574–580.
Hynes, M.J., Huang, K.M., Huang, E.H., 2009. Review paper: Implications of the ‘‘cancer stem cell’’ hypothesis on murine models of colon cancer and colitis-associated cancer. Vet. Pathol. 46 (5), 819–835.
Joubert, F.J., Kruger, H., Townshend, G.S., Botes, D.P., 1979. Purification, some properties and the complete primary structures of two protease inhibitors (DE-3 and DE-4) from Macrotyloma axillare seed. Eur. J. Biochem. 97 (1), 85–91.
Kennedy, A.R., 1998. Chemopreventive agents: protease inhibitors. Pharmacol. Ther. 78 (3), 167–209.
Kennedy, A.R., Beazer-Barclay, Y., Kinzler, K.W., Newberne, P.M., 1996. Suppression of carcinogenesis in the intestines of min mice by the soybean-derived Bowman-Birk inhibitor. Cancer Res. 56 (4), 679–682.
Kennedy, A.R., Billings, P.C., Wan, X.S., Newberne, P.M., 2002. Effects of Bowman-Birk inhibitor on rat colon carcinogenesis. Nutr. Cancer 43 (2), 174–186. Kennedy, A.R., Davis, J.G., Carlton, W., Ware, J.H., 2008. Effects of dietary antioxidant
supplementation on the development of malignant lymphoma and other neoplastic lesions in mice exposed to proton or iron-ion radiation. Radiat. Res. 169 (6), 615–625.
Kisselev, A.F., Callard, A., Goldberg, A.L., 2006. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J. Biol. Chem. 281 (13), 8582–8590.
Kris-Etherton, P.M., Hecker, K.D., Bonanome, A., Coval, S.M., Binkoski, A.E., Hilpert, K.F., Griel, A.E., Etherton, T.D., 2002. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 113 (9B), 71S–88S.
Kumar, P., Sreerama, Y.N., Gowda, L.R., 2002. Formation of Bowman-Birk inhibitors during the germination of horsegram (Dolichos biflorus). Phytochemistry 60 (6), 581–588.
Losso, J.N., 2008. The biochemical and functional food properties of the bowman-birk inhibitor. Crit. Rev. Food Sci. Nutr. 48 (1), 94–118.
Matsumoto, I., Kitagaki, H., Akai, Y., Ito, Y., Seno, N., 1981. Derivatization of epoxy-activated agarose with various carbohydrates for the preparation of stable and high-capacity affinity adsorbents – their use for affinity-chromatography of carbohydrate-binding proteins. Anal. Biochem. 116 (1), 103–110.
Mitsiades, N., Mitsiades, C.S., Poulaki, V., Chauhan, D., Fanourakis, G., Gu, X., Bailey, C., Joseph, M., Libermann, T.A., Treon, S.P., Munshi, N.C., Richardson, P.G., Hideshima, T., Anderson, K.C., 2002. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc. Natl. Acad. Sci. USA 99 (22), 14374–14379.
Montagut, C., Rovira, A., Albanell, J., 2006. The proteasome: a novel target for anticancer therapy. Clin. Transl. Oncol. 8 (5), 313–317.
Myung, J., Kim, K.B., Crews, C.M., 2001. The ubiquitin-proteasome pathway and proteasome inhibitors. Med. Res. Rev. 21 (4), 245–273.
Odani, S., Ikenaka, T., 1973. Scission of soybean Bowman-Birk proteinase inhibitor into two small fragments having either trypsin or chymotrypsin inhibitory activity. J. Biochem. 74 (4), 857–860.
Saito, T., Sato, H., Virgona, N., Hagiwara, H., Kashiwagi, K., Suzuki, K., Asano, R., Yano, T., 2007. Negative growth control of osteosarcoma cell by Bowman-Birk protease inhibitor from soybean; involvement of connexin 43. Cancer Lett. 253 (2), 249–257.
Santana, M.A., Castro-Borges, W., Amorin, L.L., Santos, A.G., Cristine Leal, S., Andrade, M.H.G., 2011. Perennial Horse Gram (Macrotyloma axillare) Seeds: biotechnology applications of its peptide and protein content – Bowman-Birk inhibitors and lectin. In: Preedy, V.R., Watson, R.R., Patel, V.B. (Eds.), Nuts & Seeds in Health and Disease Prevention, 1st ed. Burlington, San Diego, London, pp. 899–907.
St Clair, W.H., Billings, P.C., Carew, J.A., Keller-McGandy, C., Newberne, P., Kennedy, A.R., 1990. Suppression of dimethylhydrazine-induced carcinogenesis in mice by dietary addition of the Bowman-Birk protease inhibitor. Cancer Res. 50 (3), 580–586.
Sugahara, K.N., Hirata, T., Tanaka, T., Ogino, S., Takeda, M., Terasawa, H., Shimada, I., Tamura, J., ten Dam, G.B., van Kuppevelt, T.H., Miyasaka, M., 2008. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 68 (17), 7191–7199.
Tanaka, K., 2009. The proteasome: overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 85 (1), 12–36.
Towbin, H., Staehelin, T., Gordon, J., 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76 (9), 4350–4354.
Wang, S.J., Wong, G., de Heer, A.M., Xia, W., Bourguignon, L.Y., 2009. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 119 (8), 1518–1530.