Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761
Increased acetic acid removal from sugarcane bagasse hemicellulosic
hydrolysate by the yeast Issatchenckia occidentalis Y1’a in absence of
glucose
Aumento da remoção de ácido acético a partir do hidrolisado
hemicelulósico do bagaço de cana-de-açúcar pela levedura Issatchenckia
occidentalis Y1'a na ausência de glicose
DOI:10.34117/bjdv6n4-145
Recebimento dos originais:25/03/2020 Aceitação para publicação:09/04/2020
Bruna Caroline Marques Gonçalves
Doutora em Ciências
Escola de Engenharia de Lorena - Universidade de São Paulo
Estrada Municipal do Campinho, s/n - Ponte Nova, Lorena - SP. 12602-810 E-mail:brunacarolinebcmg@hmail.com
Messias Borges Silva
Doutor em Engenharia Química
Faculdade de Engenharia de Guaratinguetá - Universidade Estadual Paulista "Júlio de Mesquita Filho"
Escola de Engenharia de Lorena - Universidade de São Paulo
Estrada Municipal do Campinho, s/n - Ponte Nova, Lorena - SP. 12602-810 E-mail:messiasusp@gmail.com
Silvio Silvério da Silva
Doutor em Tecnologia Bioquímico-Farmacêutica Escola de Engenharia de Lorena - Universidade de São Paulo
Estrada Municipal do Campinho, s/n - Ponte Nova, Lorena - SP. 12602-810 E-mail:silviosilverio@icloud.com
ABSTRACT
Second generation ethanol is an attractive alternative to increase the worldwide ethanol production by utilizing agro-industrial waste crops. A pretreatment step is mandatory to expose sugars from vegetal biomass, which should be low cost and highly efficient. Even though dilute-acid pretreatment satisfies these prerequisites, metabolism inhibitors are generated during such process. In this study, I. occidentalis Y1’a was able to grow in no supplemented sugarcane bagasse hemicellulosic hydrolysate by using acetic acid as energy source. However acetic acid removal has been delayed in glucose-added medium. Given the fact that acetic acid is considered an inhibitor of microbial metabolism during ethanol production, this finding characterizes an efficient and low-cost hemicellulosic hydrolysate biodetoxification. Fermentation was successful with an ethanol yield (YP/S) of 0.26 g/g and
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 volumetric ethanol productivity (Qp) of 0.14 g/L.h, indicating that dilute-acid pretreatment
followed by biodetoxification and xylose fermentation by Candida shehatae UFMG52.2 are promising ways for 2G-bioethanol production.
Keywords: Second generation bioethanol, biodetoxification, box behnken design
RESUMO
O etanol de segunda geração é uma alternativa atraente para aumentar a produção mundial de etanol, utilizando resíduos agroindustriais. Uma etapa de pré-tratamento é obrigatória para expor os açúcares da biomassa vegetal, que devem ser de baixo custo e altamente eficientes. Embora o pré-tratamento com ácido diluído atenda a esses pré-requisitos, inibidores do metabolismo são gerados durante esse processo. Neste estudo, I. occidentalis Y1'a foi capaz de crescer em nenhum hidrolisado hemicelulósico de bagaço de cana suplementado usando ácido acético como fonte de energia. Contudo, a remoção do ácido acético foi atrasada no meio adicionado à glicose. Dado o fato de o ácido acético ser considerado um inibidor do metabolismo microbiano durante a produção de etanol, esse achado caracteriza uma biodetoxificação de hidrolisado hemicelulósico eficiente e de baixo custo. A fermentação foi bem sucedida com um rendimento de etanol (YP / S) de 0,26 g / g e produtividade volumétrica de etanol (Qp) de 0,14 g / Lh, indicando que o pré-tratamento com ácido diluído seguido de biodetoxificação e fermentação de xilose por Candida shehatae UFMG52.2 é promissor maneiras para a produção de 2G-bioetanol.
Palavras-chave: bioetanol de segunda geração, biodetoxificação, design de caixas behnken.
1 INTRODUCTION
Novel transportation fuels are strictly necessary to reduce fossil fuel usage and, consequently, petrol dependence. Petrol-derived fuel combustion releases large amounts of gases, mainly CO2, that contribute to the greenhouse effect and climate changes. Fuels
obtained from vegetal biomass are known as biofuels. 2G-biofuels are obtained from sugars extracted from lignocellulosic biomass, such as agricultural, urban and industrial waste, whose composition is based on cellulose, hemicellulose and lignin (Prasad, Singh, & Joshi, 2007). Once cellulose is a glucose polymer and hemicellulose is composed of different types of sugars, they can be used as source of fermentable sugars. However, sugar extraction from lignocellulosic biomass requires previous stages of pretreatment in order to break down the lignocellulosic structure. Several physical, physico-chemical and chemical pretreatments methods have been reported in literature (Agbor, Cicek, Sparling, Berlin, & Levin, 2011; Alvira, Tomás-Pejó, Ballesteros, & Negro, 2010; Hendriks & Zeeman, 2009; Kumar et al., 2009a; Mosier et al., 2005), among which the dilute sulfuric acid pretreatment, a chemical pretreatment, has been proved one of the most promising technologies to disrupt
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 lignocellulosic biomasses, due to its high yield of sugar recovery (80-90%). Moreover, a little portion of lignin is dissolved in the reaction mixture during the pretreatment, thus increasing cellulose susceptibility to enzymes during saccharification (Martin, Alriksson, Sjode, Nilvebrant, & Jonson, 2007; Yang & Wyman, 2004).
A solid and a liquid fraction are generated after the dilute-acid pretreatment. The solid fraction is mainly composed of cellulose and lignin (cellulignin). A small amount of hemicellulose can be found, regardless of whether acid hydrolyses have been completely effective or not (Sun & Cheng, 2005). The liquid fraction corresponds to hemicellulosic hydrolysate which is rich in pentose sugars, i.e. xylose, arabinose, among others, that were released from the hemicellulose fraction. In addition to pentose sugars, hemicellulosic hydrolysate also contains a small amount of glucose, weak acids, furanic compounds and phenolic compounds (Martin et al., 2007; Sun & Cheng, 2005). Depending on their concentration, weak acids, furanic compounds and phenolic compounds are toxic to fermenting microorganisms, due to inhibiting their metabolism (Canilha et al., 2011; Larsson et al., 1999). Therefore, these byproducts must be removed from hemicellulosic hydrolysate before fermentation. Therefore, a detoxification step is essential to overcome the metabolism of sugars by the fermenting microorganism (Yu, Feng, Xu, Liu, & Li, 2011).
Partial or total removal of inhibitors from hemicellulosic hydrolysate is fundamental to increase ethanol productivity by microorganisms. Several physical and physico-chemical methods have been developed and applied in order to remove these degradation products from lignocellulose, as reported by (Mussatto & Roberto, 2004). The method selection depends on both the type of hemicellulose hydrolysate and the microorganism species to be used for fermenting the sugars, since each microorganism has a different degree of tolerance to inhibitors (Larsson et al., 1999). The major drawback of many detoxification methods is that they generate additional waste. A biological detoxification method is an alternative to physical and physico-chemical pretreatments as regards toxic compounds removal from hemicellulosic hydrolysate. It is a green method in which microorganisms (Cao, Ximenes, Nichols, Zhang, & Ladisch, 2013; Zhang, Ong, Li, & Wu, 2013) or their enzymes (Vithanage, Barbosa, Borsato, & Dekker, 2015) are utilized with minimum waste generation and no need for effluent treatment, though it requires longer periods of time. In addition, there is the possibility of sugar consumption by microorganism besides inhibitor conversion. Even though genetically modified microorganisms that are tolerant to inhibitor compounds have been studied (Hasunuma et al., 2014), it is a grey area due to an uncertainty of these strains behavior when
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 released into the environment. Thus, alternative microorganisms and experimental conditions must be explored.
The composition of SCB-derived hemicellulosic hydrolysate and the consumption of potential inhibitors during biological detoxification by I. occidentalis Y1’a have been evaluated herein. Hydrolysate was prepared by utilizing sulfuric acid and supplemented according to Box and Behken 23 design to assess the influence of a carbon source (glucose) and two different nitrogen sources (yeast extract and peptone). Moreover, the efficiency of hemicellulosic ethanol production by the yeast C. shehatae UFMG HM 52.2 has also been reported. It is known that this is the first study that reports acid acetic removal from SCB-hemicellulosic hydrolysate and influence of glucose on acetic acid consumption by I. occidentalis Y1’a.
2 MATERIALS AND METHODS
2.1 MATERIAL
Raw SCB samples were obtained from Fazenda Invernada, Bioserv, Unica (Vale do Rosario, Brazil). The yeasts I. occidentalis Y1’a and C. shehatae UFMG 52.2 were kindly provided by the Laboratory of Microbiology from Centre for Study of Social Insects from the São Paulo State University. The software Statistica (version 12) was used for data regression and statistical analyses. The statistical significance of regression coefficients was 95%.
3 METHODS
3.1 INOCULUM PREPARATION
Strains were grown in Agar Sabouraud 2% (w/w) medium by the agar plate method and incubated at 30oC for 48h. The I. occidentalis Y1’a inoculum was grown in YPD medium containing (g/L): yeast extract, 10; peptone, 20; glucose, 20 (López, Moreno, Nichols, Dien, & Bothast, 2004), while C. shehatae UFMG 52.2 inoculum was grown in a medium containing (g/L): yeast extract, 10; peptone, 20; xylose, 30. Distilled water and solutions of peptone, yeast extract and sugar were autoclaved separately at 110oC for 15 min and mixed up aseptically in a 1000 mL conical flask with final medium volume of 400 mL for each strain. As regards I. occidentalis Y1’a, cultivations were carried out at 30oC and 200 rpm for 48 h in a rotatory shaker. C. shehatae UFMG 52.2 was incubated under the same conditions for 24h. Cultivations were centrifuged at 2600 x g for 20 min. The supernatant was discarded and cells
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 were washed out and resuspended with NaCl 0.9% (w/v). Cell concentration was determined by OD (ʎ = 600 nm) and the inoculum was standardized in 0.5 g/L of cells for both strains.
3.2 HEMICELLULOSIC HYDROLYSATE
SCB was sundried until reaching 10% moisture content, which was then measured in an infra-red weightier at 100oC. The acid pre-treatment was carried out in a 50 L vertical rotatory reactor at 121oC for 15 min with 10% H2SO4. At the end of the reaction, its content
was filtered in order to separate the solid fraction (cellulignin) from the hemicellulosic hydrolysate, which was 5-fold concentrated by a vacuum evaporator apparatus at 70oC due to high total sugar content and stored at 10oC. Natural and pretreated SCB were chemically characterized according to NREL/TP-510-42618 protocol (Sluiter et al., 2004).
3.3 EXPERIMENTAL DESIGN
A Box Behnken 23 design in triplicate at central point was applied in order to evaluate whether I. occidentalis Y1’a is able to grow in SCB hemicellulosic hydrolysate. The impact of independent variables of yeast extract (g/L), peptone (g/L) and glucose (g/L) concentrations was evaluated during acetic acid removal. Total phenol concentration was also evaluated along the time-course experiment. The hemicellulosic hydrolysate pH was adjusted with NaOH to 4.0 in accordance with previous studies (Soares et al., 2016), autoclaved at 110oC for 15 min
and centrifuged (2600 x g) for 20 min so as to remove suspended solids. A volume of 55 mL of sterile hemicellulosic hydrolysate was poured into each 125 mL conical flask and supplemented according to Box and Behnken matrix condition with glucose, yeast extract and peptone. The experiment has been conducted at 30o C, 300 rpm for 96h and samples were taken every 24h within a period of 96h. The best nutritional condition for biological detoxification has been validated by an experimental run which was performed in triplicate and tested for ethanol production by C. shehatae UFMG 52.2.
3.4 SUPPLEMENTATION OF BIODETOXIFIED HEMICELLULOSIC HYDROLYSATE Biodetoxified hemicellulosic hydrolysate was supplemented with (g/L): yeast extract, 3; malt extract, 3 and ammonium sulfate, 5; and incubated in shaker at 30oC, 200 rpm for 48h. Samples were taken every 24h and 48h in order to evaluate sugar consumption, ethanol production, YP/S and Qp.
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 3.5 ANALYTICAL METHODS
Total phenolic compounds were quantified according to the Folin-Ciocalteu method (Singleton, Orthofer, & Lamuela-Raventos, 1999). Xylose, glucose, arabinose, acetic acid and ethanol concentrations were determined by High-Performance Liquid Chromatography (HPLC) equipped with a refractive index detector (Waters 410; Milford, MA, USA), and a BIO-RAD AMINEX HPX-87H column (7.8 x 300 mm) (Bio-Rad, Hecules, CA, USA) at 45°C. It was used 0.005 M sulfuric acid at 0.6 mL/min flow rate as eluent. Samples were previously diluted (1:10) and filtered with a Sep Pak C18 filter. Furfural and HMF concentration were determined by HPLC equipped with a UV detector (Waters 2487/USA) and Eclipse XDB-C18 5μm (4.6 x 150 mm) column at 25°C. Acetonitrile was used as eluent was: water (1:8) with 1% acetic acid and pH was adjusted to 3.0 with phosphoric acid. Flow rate was adjusted to 0.9 mL/min. Samples were previously filtered using a 0.45 μm Schleicher & Schuell membrane.
3.6 CALCULATIONS
Sugars (xylose, glucose and arabinoses) and acetic acid consumption (%) during fermentation were determined according to equation (01):
equation (01)
where X is the consumption (%), Xi, the initial sugar/acetic acid concentration (g/L) and Xf, the final sugar/acetic acid concentration (g/L).
Xylose conversion into ethanol was determined by the substrate – product conversion factor (YP/S), according to equation (02):
equation (02)
where Pf is the final concentration of ethanol (g/L), Pi, the initial concentration of ethanol (g/L), Sf, the final concentration of substrate (g/L), Si, the initial concentration of substrate (g/L).
Volumetric productivity (QP) (g/L.h) of ethanol was calculated according to equation
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761
equation (03)
where Pf the is the final concentration of ethanol (g/L), Pi, the initial concentration of ethanol (g/L) and t the fermentation time (h).
4 RESULTS
4.1 CHEMICAL COMPOSITION OF SUGARCANE BAGASSE
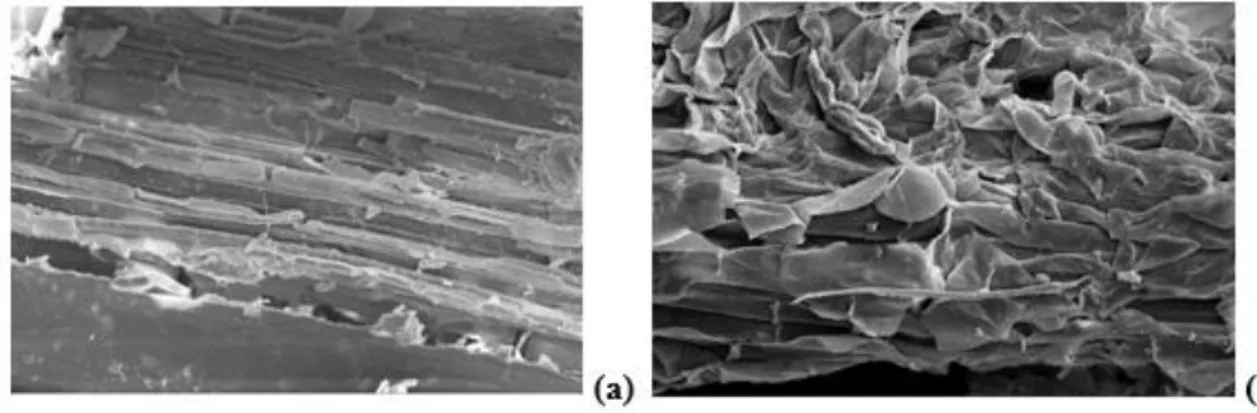
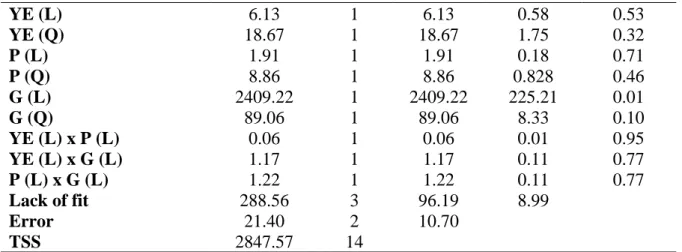
SCB composition and its compact structure before and after the acid hydrolysis pretreatment are shown in Table 1 and Figures 1a and 1b. A cellulose and lignin proportion increase in acid-pretreated SCB samples was observed (Table 1). On the other hand, the proportion of hemicellulose decreased after the acid pretreatment due to its depolymerization into sugars in the aqueous fraction, which can be explained by the physical structure of each polymer. Component (% w/w) Natural Pre-treated S uga rc ane b aga ss e Cellulose 37.44 47.25 Hemicellulose 28.31 23.35 Xylose 21.69 18.45 Arabinose 2.68 1.48 Acetyl radicals 2.49 2.33 % Sugars 65.75 70.60 Total Lignin 22.22 26.47 Ashes 1.48 1.49 Extractives 3.19 - Total 92.64 98.56 Component (g/L) Hydrolysate Raw Concentrated (CF = 5) He mi ce ll ulosi c Hydr olysate D-xylose 15.10 86.38 D-glucose 2.23 10.54 L-arabinose 2.41 11.11 Acetic Acid 3.28 1.23 Furfural 0.20 0.25 HMF 0.02 0.02 pH 0.67 0.11 CF: concentration factor
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 Total cellulose (37.44%), hemicellulose (28.31%) and lignin (22.22%) content in SCB was approximately 88% (Table 1). The acid hydrolysis pretreatment promoted SCB fiber disorganization after a partial removal of the hemicellulose fraction (Figure 1b). The physical structure of the solid residue, which is composed of cellulose (47.25%), lignin (26.47%) and hemicellulose (23.35%), is shown in Table 1. The same acetyl radical proportion in both unpretreated and pretreated SCB indicates that acetyl radicals were released in the same way as acetic acid during hemicellulose depolymerization. There was no change in ash content, which indicates that the acid pretreatment has not altered the mineral composition of SCB. However, a complete removal of extractives during the pretreatment was observed (Table 1).
Figure 1: Scanning electron microscopy (SEM) with 500 x magnification of (a) natural SCB and (a) sulfuric acid pretreated.
4.2 PHYSICO-CHEMICAL CHARACTERIZATION OF HEMICELLULOSIC HYDROLYSATE
The composition of crude and concentrated hemicellulosic hydrolysate is shown in Table 1. The hydrolysis conditions yielded hydrolysate with 19.7 g/Lof sugars, mainly xylose (77%), followed by arabinose (12%) and glucose (11%). Xylose concentration (15.10 g/L) in hemicellulosic hydrolysate which was not concentrated was about 7-fold higher than glucose concentration (2.23 g/L), thus confirming, as expected, that hemicellulose was more susceptible to acid hydrolysis than cellulose. Lignocellulosic biomass acid hydrolysis generates fermentation inhibitors, i.e. furfural, acetic acid and HMF, which adversely affect microorganism growth, sugar consumption, and cell metabolism (Kim, Park, Song, Wee, & Jeong, 2013). After SCB acid hydrolysis, a small concentration of acetic acid (3.28 g/L) was observed in the hemicellulosic hydrolysate (Table 1). Acetic acid is formed after acetyl groups
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 present in hemicellulose are released during the acid pretreatment (Ulbricht, Northup, & Thomas, 1984). A low concentration of sugar degradation products, such as furfural (0.20 g/L) and HMF (0.02 g/L), was observed (Table 1).
Crude hydrolysate was concentrated through a concentration factor (CF) of five in order to facilitate storage. Although hemicellulosic hydrolysate sugar concentration increased proportionally to CF, acetic acid concentration was reduced due to its volatilization and high temperatures (70oC) under vacuum. A slightly increase (1.25 times) in furfural concentration was observed, whereas no variation in HMF concentration occurred (Table 1). Once acetic acid, furfural and HMF are volatile compounds, this fact can be explained by the fact that they are easily dragged by vapors, and also by a drop in their boiling point due to vacuum conditions, regardless of process temperature (Rodrigues, Felipe, Silva, Vitolo, & Gómez, 2001).
4.3 EVALUATION OF HEMICELLULOSIC HYDROLYSATE SUPPLEMENTATION
USING BOX AND BEHNKEN 23 DESIGN IN BIODETOXIFICATION PROCESS
The earliest total consumption of acetic acid was observed in runs 5 and 6 after 48h, followed by runs 9 and 10 (Table 2). By taking into account industrial processes and considering a reduction of cost and time, 48h was the time chosen for the statistical analysis. The coefficient of determination (R2) was 0.89. Table 3 shows the ANOVA, in which only the
linear term of the variable glucose concentration was significant at 5% (p = 0.01) with a negative effect (-34.71). The impact of variables and their interaction are shown in Figure 2a. Thus, glucose concentration showed a strong influence on acetic acid removal. Moreover, high concentrations of glucose in hemicellulosic hydrolysate might inhibit or retard acetic acid consumption, regardless of yeast extract (Figure 2b) or peptone (Figure 2c).
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 Table 2: Supplementation of hemicellulosic hydrolysate and acetic acid removal (%) after 48h of biodetoxification with I. occidentalis Y1'a.
Run
Independent Variables Dependent Variable
X1 X2 X3 Acetic Acid Removal
(%) 1 -1 -1 0 84.38 2 +1 -1 0 80.05 3 -1 +1 0 86.80 4 +1 +1 0 81.97 5 -1 0 -1 100.00 6 +1 0 -1 100.00 7 -1 0 +1 52.60 8 +1 0 +1 54.76 9 0 -1 -1 91.74 10 0 +1 -1 92.63 11 0 -1 +1 69.75 12 0 +1 +1 68.43 13 (CP*) 0 0 0 80.69 14 (CP*) 0 0 0 84.08 15 (CP*) 0 0 0 87.23 Variables (g/L) Levels -1 0 +1
X1 yeast extract concentration 10 20 30
X2 peptone concentration 10 20 30
X3 glucose concentration 0 10 20
*CP: central point
Table 3: Analysis of variance (ANOVA) for the effect of yeast extract, peptone and glucose concentration on acetic acid removal from the hemicellulosic hydrolysate by I. occidentalis Y1'a after a 48 h-detoxification.
YE (L) 6.13 1 6.13 0.58 0.53 YE (Q) 18.67 1 18.67 1.75 0.32 P (L) 1.91 1 1.91 0.18 0.71 P (Q) 8.86 1 8.86 0.828 0.46 G (L) 2409.22 1 2409.22 225.21 0.01 G (Q) 89.06 1 89.06 8.33 0.10 YE (L) x P (L) 0.06 1 0.06 0.01 0.95 YE (L) x G (L) 1.17 1 1.17 0.11 0.77 P (L) x G (L) 1.22 1 1.22 0.11 0.77 Lack of fit 288.56 3 96.19 8.99 Error 21.40 2 10.70 TSS 2847.57 14
SS: sum of squares; DF: degree of freedom; MS: mean square; TSS: total sum of squares; YE: yeast extract; P: peptone; G: glucose; (L): linear term; (Q): quadratic term.
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 Figure 2: Pareto chart indicating the variables effect and interactions between the variables after 48 h of hemicellulosic hydrolysate detoxification (a). Response of surface related to acetic acid consumption and (b) glucose versus yeast extract concentration (c) glucose versus peptone concentration, after 48h of hemicellulosic hydrolysate detoxification by I. occidentalis Y1'a.
(a)
(b) (c)
YE: yeast extract; P: peptone; G: glucose; (L): linear term; (Q): quadratic term
4.4 EVALUATION OF SUGARS CONSUMPTION AND TOXIC COMPOUNDS
REMOVAL FROM HEMICELLULOSIC HYDROLYSATE DURING THE
BIODETOXIFICATION PROCESS
Glucose assimilation by yeast was followed by ethanol production after 24 h of cultivation in hemicellulosic hydrolysate supplemented with 10g/L glucose. In addition, the amount of ethanol was proportional to the amount of glucose in the culture medium. In the absence of glucose, ethanol was slightly consumed during the biodetoxification process as carbon source. Xylitol production was also observed, accompanied by xylose metabolization.
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 No xylitol consumption was observed throughout the experiment, which is most likely due to xylose being still in abundance in the cultivation medium as an alternative carbon source.
Xylose consumption by I. occidentalis Y1’a ranged from 14.79 to 36.02% and was influenced by hemicellulosic hydrolysate supplementation. The highest consumption of such sugar was combined to higher yields of xylitol (9.10 g/L), whose production has been initiated after 48 h of cultivation. Ethanol consumption started after all glucose had been depleted from the culture medium. Therefore, xylose was not the most appropriate carbon source for such yeast, which is capable of synthesizing the enzyme apparatus necessary for xylose-into-xylitol conversion. No arabinose consumption was observed, which reveals that this sugar is not metabolized by I. occidentalis Y1’a.
No glucose supplemented medium showed higher acetic acid consumption, which ranged between 91.7% and 100% (runs 5, 6, 9 and 10 in Table 2). Moreover, acetic acid was almost totally consumed during the first 24 h of biodetoxification (up to 89.2%), which was accompanied by culture media acidity reduction, thence resulting in a slight pH increase (data not shown). However, glucose was metabolized prior to acetic acid in the glucose-added medium. In the culture medium supplemented with 10 g/L glucose, a complete consumption of acetic acid was observed after 72h of detoxification, whereas acetic acid consumption was observed only after 96h in the media supplemented with 20 g/L glucose. These results show that, besides ethanol and xylose, acetic acid was a second-plan nutritional source when compared to glucose. Those results evince the influence of glucose on acetic acid metabolism by I. occidentalis Y1’a. Therefore, for a rapid uptake of this acid, there should be a low initial glucose concentration in hemicellulosic hydrolysate.
In contrast with acetic acid removal, phenolic compound removal proved independent of glucose concentration in the culture medium. Phenolic compound concentration remained constant during the first 48h of incubation in the medium with no-glucose addition. On the other hand, it fluctuated when the medium was supplemented with 10 g/L glucose. A considerable increase in it was observed after 48h of hemicellulosic hydrolysate biodetoxification supplemented with 20 g/L glucose. Neither yeast extract nor peptone concentration showed actuation in the metabolism of those compounds. After 72h, it increased for all experiments, regardless of supplementation.
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 4.5 CONFIRMATION EXPERIMENT
Confirmation experiment results have indicated that I. occidentalis Y1’a was capable of consuming acetic acid from unsupplemented hemicellulosic hydrolysate. After 24h, 55% of the acetic acid was removed and glucose resulting from the pretreatment was totally consumed. After 48h, around 81% acetic acid was metabolized. There was no consumption of arabinose and xylose after 96h of detoxification. In addition, a low concentration of ethanol (1 g/L) was observed after 24h of incubation, which was completely consumed after 96h.
4.6 ETHANOL PRODUCTION FROM BIOLOGICALLY DETOXIFIED
HEMICELLULOSIC HYDROLYSATE
After evaluating nutritional parameters, unsupplemented hemicellulosic hydrolysate has been chosen for biodetoxification by I. occidentalis Y1'a. Unsupplemented hemicellulosic hydrolysate has been biodetoxified for 48h and centrifuged in order to remove yeast cells and solid particles. The supernatant was supplemented according to nutrients required for C. shehatae UFMG 52.2 metabolism and inoculated with 0.5 g/L cells.
I. occidentalis Y1'a effectively removed around 79% acetic acid from the hemicellulosic hydrolysate after 48h of detoxification. Lower acetic acid removal was observed during fermentation, which suggests that I. occidentalis Y1'a cells may have not been completely removed during centrifugation and remained active in the hemicellulosic hydrolysate. Furthermore, this item of information indicates the possibility of future studies aimed at co-cultivation by using I. occidentalis Y1'a and C. shehatae UFMG 52.2 yeasts. Moreover, co-cultivation between I. occidentalis Y1'a and C. shehatae UFMG 52.2 yeasts can be successful, since I. occidentalis Y1'a did not metabolize xylose in the presence of C. shehatae UFMG 52.2.
5 CONCLUSION
These results indicate that I. occidentalis Y1'a is a promising microorganism for SCB hemicellulosic hydrolysate biological detoxification, since it was able to remove around 80% acetic acid within 48 h, thus providing the process with economic advantages. Moreover, I. occidentalis Y1'a have not shown a considerable consumption of xylose, which was converted into ethanol by C. shehatae UFMG 52.2 during fermentation. I. occidentalis Y1'a and C. shehatae UFMG 52.2 have worked synergically, thence suggesting the possibility of future studies involving detoxification and simultaneous fermentation in one pot.
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761
ACKNOWLEDGMENTS
This work has been supported by the funding agency CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). We would also like to thank Msc. Eduardo Henrique Breda for the SEM analysis.
REFERENCES
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., & Levin, D. B. (2011). Biomass pretreatment: Fundamentals toward application. Biotechnology Advances, 29(6), 675–685. doi:10.1016/j.biotechadv.2011.05.005
Alvira, P., Tomás-Pejó, E., Ballesteros, M., & Negro, M. J. (2010). Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresource Technology, 101(13), 4851–4861. doi: 10.1016/j.biortech.2009.11.093
Canilha, L., Santos, V. T. O., Rocha, G. J. M., Almeida E Silva, J. B., Giulietti, M., Silva, S. S., … Carvalho, W. (2011). A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. Journal of Industrial Microbiology and Biotechnology, 38(9), 1467–1475. doi: 10.1007/s10295-010-0931-2
Cao, G., Ximenes, E., Nichols, N. N., Zhang, L., & Ladisch, M. (2013). Biological abatement of cellulase inhibitors. Bioresource Technology, 146, 604–610. doi: 10.1016/j.biortech.2013.07.112
Hasunuma, T., Hori, Y., Sakamoto, T., Ochiai, M., Hatanaka, H., Kondo, A., … Ochiai, M. (2014). Development of a GIN11/FRT-based multiple-gene integration technique affording inhibitor-tolerant, hemicellulolytic, xylose-utilizing abilities to industrial Saccharomyces cerevisiae strains for ethanol production from undetoxified lignocellulosic hemicelluloses. Microbial Cell Factories, 13(1), 112–145. doi: 10.1186/s12934-014-0145-9
Hendriks, A. T. W. M., & Zeeman, G. (2009). Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresource Technology, 100(1), 10–18. doi: 10.1016/j.biortech.2008.05.027
Kim, S. K., Park, D. H., Song, S. H., Wee, Y. J., & Jeong, G. T. (2013). Effect of fermentation inhibitors in the presence and absence of activated charcoal on the growth of Saccharomyces
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 cerevisiae. Bioprocess and Biosystems Engineering, 36(6), 659–666. doi: 10.1007/s00449-013-0888-4
Kumar, P., Kumar, P., Barrett, D. M., Barrett, D. M., Delwiche, M. J., Delwiche, M. J., … Stroeve, P. (2009). Methods for pretreatment of lignocellulosic biomass for ef cient hydrolysis and biofuel production. Industrial and Engineering Chemistry (Analytical Edition), 3713– 3729. doi: 10.1021/ie801542g
Larsson, S., Palmqvist, E., Hahn-Hägerdal, B., Tengborg, C., Stenberg, K., Zacchi, G., & Nilvebrant, N. O. (1999). The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme and Microbial Technology, 24(3–4), 151–159. doi: 10.1016/S0141-0229(98)00101-X
López, M. J., Moreno, J., Nichols, N. N., Dien, B. S., & Bothast, R. J. (2004). Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Applied Microbiology and Biotechnology, 64(1), 125–131. doi: 10.1007/s00253-003-1401-9
Martin, C., Alriksson, B., Sjode, A., Nilvebrant, N. O., & Jonson, L. J. (2007). Dilute sulfuric acid pretreatment of agricultural and agro-industrial residues for ethanol production. Applied Biochemistry And Biotechnology, 136, 339–352.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96(6), 673–686. doi:10.1016/j.biortech.2004.06.025
Mussatto, S., & Roberto, I. C. (2004). Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresource Technology, 93(1), 1–10. doi:10.1016/j.biortech.2003.10.005
Prasad, S., Singh, A., & Joshi, H. C. (2007). Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resources, Conservation and Recycling, 50(1), 1–39. doi:10.1016/j.resconrec.2006.05.007
Rodrigues, R., Felipe, M., Silva, J. B. A. e, Vitolo, M., & Gómez, P. V. (2001). The influence of pH, temperature and hydrolyzate concentration on the removal of volatile and nonvolatile compounds from sugarcane bagasse hemicellulosic hydrolyzate treated with activated charcoal before or after vacuum evaporation. Brazilian Journal of Chemical Engineering, 18(3), 299–311. doi:10.1590/S0104-66322001000300009
Braz. J. of Develop.,Curitiba, v. 6, n.4,p.18621-18636 apr. 2020. ISSN 2525-8761 Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folic-Ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Sluiter, A., Hames, B., Ruiz, R. O., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2004). Determination of structural carbohydrates and lignin in biomass. Biomass Analysis Technology Team Laboratory Analytical Procedure, 2011(July), 1–14. doi: NREL/TP-510-42618
Soares, L. C. S. R., Chandel, A. K., Pagnocca, F. C., Gaikwad, S. C., Rai, M., & da Silva, S. S. (2016). Screening of yeasts for selection of potential strains and their utilization for in situ microbial detoxification (ISMD) of sugarcane bagasse hemicellulosic hydrolysate. Indian Journal of Microbiology, 56(2), 172–181. doi:10.1007/s12088-016-0573-9
SUN, Y., & CHENG, J. (2005). Dilute acid pretreatment of ryce straw and bermudagrass for
ethanol production. Bioresource Technology, 96(14), 1599–1606.
doi:10.1016/j.biortech.2004.12.022
Ulbricht, R. J., Northup, S. J., & Thomas, J. a. (1984). A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Toxicological Sciences, 4(5), 843–853. doi:10.1093/toxsci/4.5.843
Vithanage, L. N. G., Barbosa, A. M., Borsato, D., & Dekker, R. F. H. (2015). Value adding of poplar hemicellulosic prehydrolyzates: Laccase production by Botryosphaeria rhodina MAMB-05 and its application in the detoxification of rrehydrolyzates. BioEnergy Research, 8, 657–674. doi:10.1007/s12155-014-9547-0
Yang, B., & Wyman, C. E. (2004). Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnology and Bioengineering, 86(1), 88–95. doi:10.1002/bit.20043
Yu, Y., Feng, Y., Xu, C., Liu, J., & Li, D. (2011). Onsite bio-detoxification of steam-exploded corn stover for cellulosic ethanol production. Bioresource Technology, 102(8), 5123–5128. doi: 10.1016/j.biortech.2011.01.067
Zhang, D., Ong, Y. L., Li, Z., & Wu, J. C. (2013). Biological detoxification of furfural and 5-hydroxyl methyl furfural in hydrolysate of oil palm. doi: 10.1016/j.bej.2013.01.003