Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
Reproductive performance of non-ablated Penaeus vannamei females in a
Brazilian commercial hatchery/
Desempenho reprodutivo de fêmeas não abladas de Penaeus vannamei em
uma larvicultura comercial brasileira
DOI:10.34117/bjdv5n12-388
Recebimento dos originais: 30/11/2019 Aceitação para publicação: 27 / 12 /2019
Thiago Bastos Bezerra de Menezes
Mestre pela Universidade Federal do Ceará
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: thiagobezerra@gmail.com
Igor Gabriel Rodrigues Ferreira Gomes
Mestre pela Universidade Federal do Ceará
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: gabrielrfgomes@gmail.com
Hudson Makson Rocha Lucena
Graduado pela Universidade Federal do Ceará Celm Aquicultura S/A, 62.800-000, Aracati, Ceará, Brasil
E-mail: hudson@maris.com.br
Kamar Porto do Nascimento Filho
Mestre pela Universidade Federal do Ceará
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: kamarfilho_12@hotmail.com
Ana Luzia Assunção Cláudio de Araújo
Mestre pela Universidade Federal do Ceará
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: analuzia_aca@hotmail.com
Gabriel de Mesquita Facundo
Mestre pela Universidade Federal do Ceará
Programa de Pós-Graduação em Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
Rommel Rocha de Sousa
Doutor pela Universidade Federal do Ceará
Programa de Pós-Graduação em Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: rommelpesca@gmail.com
Elenise Gonçalves de Oliveira
Doutora pela Universidade Estadual de São Paulo
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: elenisego@yahoo.com.br
José Renato de Oliveira César
Doutor pela University of Hawaii
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: renatocesarufc@gmail.com
Francisco Hiran Farias Costa
Doutor pela Universidade Federal do Ceará
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: hiranfcosta@gmail.com
ABSTRACT
In Brazil, Penaeus vannamei breeding is performed in specialized hatcheries. In the reproduction phase of larviculture, most of the time, it is used broodstock that undergo a maturation induction by eyestalk ablation. However, the use of this technique may present different disadvantages, as other physiological and metabolic processes are also affected by the removal of the eyestalk. This study aimed to perform a comparative analysis between ablation and non-ablation effects on the reproductive process of P. vannamei females. It was observed that the NAF showed higher values (p<0.05) for mating frequency (16.5 ± 4.7 days), spawning frequency (17.8 ± 4.8 days), number of eggs/female (297,208 ± 24,827), number of nauplii/female (210,625 ± 21,681). On the other hand, the AF group presented higher values for mortality rate (39.1 ± 0.3%) and daily mating rate (11.7 ± 2.8%). Spawning rate (92.7 ± 5.3%) and hatch rate (70.8 ± 2.7%) were not affected by non-ablation. This study has shown that the use of NAF results in a superior reproductive performance when compared to the AF performance, and that the use of NAF does not compromise the zootechnical performance in the larval stage.
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
RESUMO
No Brasil, a reprodução de Penaeus vannamei é realizada em larviculturas especializadas. Na fase de reprodução da larvicultura, na maioria das vezes, são usados reprodutores que sofrem indução à maturação por ablação do pedúnculo ocular. No entanto, o uso dessa técnica pode apresentar diferentes desvantagens, pois outros processos fisiológicos e metabólicos também são afetados pela retirada do pedúnculo ocular. O objetivo deste estudo foi realizar uma análise comparativa dos efeitos da ablação e não ablação no processo reprodutivo de fêmeas de P.
vannamei. Foi verificado que o grupo FNA apresentou valores superiores (p<0,05) para
frequência de cópula (16,5% ± 4,7 dias), frequência de desova (17,8 ± 4,8 dias), número de ovos/fêmea (297.208 ± 24.827) e número de nauplios/fêmea (210.625 ± 21.681). Por outro lado, o grupo FA apresentou valores superiores para a taxa de mortalidade (39,1 ± 0,3%) e a taxa de cópula diária (11,7 ± 2,8%). A taxa de desova (92,7 ± 5,3%) e a taxa de eclosão (70,8 ± 2,7%) não foram afetadas pela não ablação. O presente estudo mostrou que o uso de FNA resulta em um desempenho reprodutivo superior ao de FA, e o uso de FNA não compromete o desempenho zootécnico na fase larval.
Palavras-chave: Penaeus, Ablação, Reprodução.
1 INTRODUCTION
Marine shrimp production has strongly contributed to meet the worldwide food demand. Shrimp rearing is based and characterized in three stages: reproduction, larviculture and grow-out. Thus, the production of offspring is the base of the productive chain, assuming great importance for the continuous growth of the activity. Currently, the commercial hatchery depends, precipitously, on the broodstock management in captivity, where the reproductive efficiency is directly related to the economic success (TREERATTRAKOOL; PANYIM; UDOMKIT, 2011; WEN et al., 2015; VENTURA-LÓPEZ, 2017; FAO, 2018).
The implementation of strategies that assure a high reproductive performance is essential for the increase of postlarvae production and the economic success of the activity. Commercial hatcheries commonly use the process of unilateral eyestalk extirpation of female adults as a way of accelerating sexual maturity, reproduction and spawning. This procedure is named ablation, which can be performed by three methods: extirpation, crushing and enucleation (CARDONA et al., 2016; ALFARO-MONTOYA et al., 2019; DAS et al., 2015).
This technique is based on the removal of the terminal system X of the neurohemal organ, which is placed in the base of both eyestalks and is responsible forthe releasing of gonad inhibiting hormone (GIH), hormone that interferes in the sexual maturation process. Therefore, the unilateral ablation reduces partially the function of the organ X, decreasing the GIH concentration in the hemolymph and inducing ovarian maturation (BROWDY;
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 SAMOCHA, 1985; CUNHA; OSHIRO, 2010; SHAILENDER et al., 2013; KANG et al., 2014).
In the eyestalk of shrimp, there are also different organs that are responsible for the production of different hormones that control a diverse range of metabolic processes. As a consequence, ablation leads the animals to a physiological breakdown, producing deleterious effects, affecting functions such as molting, osmoregulation, glucose modulation, phenoloxidase activity, total hemocyte count, hemolymph agglutination, which points to a general depletion of the immune status of animals and may even cause an increase in the capacity for replication of pathogens such as the Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV), which indicates a greater susceptibility to pathogens. In addition, a succession of behavioral changes can also be attributed to the ablation process, such as decreased feeding activity, erratic swimming and bewilderment. Another relevant factor is the reduction in the quantity and quality of eggs produced by females that are submitted to the ablation process, besides, early reproductive exhaustion and high mortality rate (BENZIE, 1998; MOTTE et al., 2003; CHAN et al., 2003; MAGGIONI et al., 2004; TAYLOR et al., 2004; HSU et al., 2006; SAINZ-HERNÁNDE et al., 2008; HOPKINS, 2012; LEE et al., 2017). It is important to highlight the growing concern with the welfare and ethics in animal production by consumers, demanding the cultivated shrimp industry to promote improvements in the rearing conditions (LITTLE et al., 2018; ZACARIAS et al., 2019).
Taking into account all aspects of eyestalk unilateral ablation of females for the reproduction of P. vannamei, the present study aimed to perform a comparative analysis of reproductive parameters of ablated and non-ablated females through data related to the mortality rate, mating rate, mating frequency, spawning rate, spawning frequency, hatching rate, number of nauplii per female and number of eggs per female in a commercial hatchery (Aracati, Ceará, Brazil).
2 MATERIALS AND METHODS
The present study was conducted in the commercial hatchery CELM Aquicultura S.A. located in the city of Aracati (Ceará, Brazil). The experiment was performed between August 1st and September 29th, 2017, for a total of 60 days.
The broodstock used in the experiment came from the breeding sector, which belonged to the same company. Males and females, weighing between 33 and 39g, were separated by
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 sex and sent to the hatchery quarantine sector. The transport was made at a density of 300 animals/m³ in 1,000L tanks, containing seawater (35 ppt) with oxygen levels around 8 mg/L, pH 7.5 and temperature 27 °C. Prior to entering the quarantine sector, the broodstock were subjected to prophylactic treatment, using 50 ppm formalin for 30 seconds, followed by intensive renewal of the water in the transport tanks before the animals were transferred (FAO, 2003).
In the quarantine sector, the broodstock were placed in 3 tanks of 36 m³ (30 animals/m³), where they remained for 30 days of conditioning. During this phase, the animals were fed a diet consisting of fresh squid with a feed rate of 2.5% of the total biomass (per time) at 02:00 pm and 09:00 pm, adult artemia with a feed rate of 2.5% of total biomass (per time) at 03:00 am and 06:00 pm, commercial feed for broodstock with a feed rate of 0.5% of total biomass (per time) at 08:00 am (40% crude protein, Zeigler, USA), 11:00 am (40% crude protein, Skretting, Norway) and 06:00 pm (59% crude protein, INVE, Belgium). In these tanks, which were installed in a closed environment, the daily water renewal rate was up to 150%, with the tanks being subjected to siphoning daily.
In the quarantine tanks, the animals remained for a period of 30 days, where they were selected for the establishment of the sex ratio (1 male: 1.28 female), for the unilateral eyestalk ablation of a portion of the females, and for the transport to the maturation sector. In the case of females subjected to unilateral eyestalk ablation, the region of the animals that suffered injury was exposed to an immersion bath in a 20 ppm PVP-iodine solution for 30 seconds (FAO, 2003). After the quarantine period, the broodstock were evaluated for morphological, physiological and sanitary integrity, subsequently they were sent to the maturation sector.
The maturation sector consists of 27 circular tanks, with a volume of 10 m³ each, distributed in 4 blocks, installed in a closed environment using an inverted photoperiod (14 hours of light and 10 hours of dark). The daily rate of water renewal in the tanks varied from 100 to 200% of the total volume with siphoning procedures. The broodstock were randomly distributed in two blocks (B-01 and B-02), each consisting of 7 tanks, where they were identified by sex, weight, date and origin of arrival. Females subjected to unilateral eyestalk ablation (AF) were stored in B-01, while females that were not subjected to eyestalk ablation (NAF) were stored in B-02. In each tank of both blocks, 70 males and 90 females were stored (sex ratio of 1 male: 1.28 female). Feeding management was identical to that used for the quarantine sector.
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 It was performed a daily monitoring of the number of dead females since the realization of the eyestalk ablation process until the last day of collection of females with spermatophore to calculate the mortality rate (%), which is expressed in percentage and calculated according to the formula below.
𝐌 = 𝐍𝐃𝐅𝐱𝟏𝟎𝟎 𝐍𝐓𝐅
(1)
Where: M = Mortality rate (%); NDF = Number of dead females; NTF = Total number of
females.
To calculate the mating rate (MR), females were checked daily for spermatophores
impregnation. To perform this procedure, at 14:00h the aeration system was turned off and the water renewal reduced, facilitating the decantation of suspended material and, therefore, mating. The capture of the females that had mated was performed between 17:00 and 19:00h, and if they had achieved gonadal maturity, they were transferred to the spawning sector. Daily, the mating rate (MR) and the mating frequency (MF) were determined using the formulas
below.
𝐌𝐑= 𝐍𝐅𝐓 𝐱 𝟏𝟎𝟎
𝐍𝐅𝐌 (2)
Where: MR = Mating rate; NFT = Number of females transferred daily to the spawning
sector; NFM = Number of females in the maturation sector.
𝐌𝐅 =
𝟏𝟎𝟎 𝐌𝐑
(3)
Where: MF = Mating frequency (days); MR = Mating rate.
The spawning sector consists of 4 collective tanks, with a usable volume of 10 m³ each. To avoid the risk of egg infection, the water in the spawning tanks was prepared with 15g of disodium EDTA, 1 mL of Premerlin® 600 EC (Adama) and 30 mL of calcium hypochlorite, and it was waited an interval of 8 hours after the treatment for the reception of the females.
After spawning, the females were transferred again to the maturation sector after immersion bath in PVP-iodine solution at 20 ppm for 30 seconds (FAO, 2003). During the procedure of returning the females to the maturation and mating sector, a daily assessment was performed regarding the effective spawning, making it possible to calculate the spawning rate (SR) and spawning frequency (SF), using the formulas below.
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 𝐒𝐑 = 𝐍𝐅𝐌 𝐱 𝟏𝟎𝟎
𝐍𝐅𝐓
(4)
Where: SR = Spawning rate (%); NFS = Number of females that spawned; NFT = Number
of females transferred daily to the spawning sector. 𝐒𝐅 = 𝟏𝟎𝟎
𝐒𝐑 (5)
Where: SF = Spawning frequency (%); SR = Spawning rate.
After removing all the females, the spawning tanks were drained by gravity for carboys equipped with a filter ring, where the eggs were washed through an intense water exchange to eliminate possible residues. After this procedure, they were transferred to the hatching sector with the help of 3L beakers.
In the hatching sector, twelve 1,000 L carboys were used to hatch the eggs. The water in these tanks was maintained with 30 ppt salinity; pH 7.5; temperature 30 °C; oxygen 8 mg/L. Two hours before receiving the eggs, 1.5g of disodium EDTA, 0.1 ml of Premerlin® 600 EC (Adama) was added to the water of the carboys to avoid the risk of infection of the eggs. The density adopted in these tanks varied between 3,000 and 4,000 eggs/L.
For egg counting, 5 samples of 20 mL of water were taken from the carboys using a 50 mL beaker. Then, the arithmetic mean of the samples and the number of eggs obtained by extrapolation were calculated using a simple rule of three. After determining the number of eggs, the calculation was performed to obtain the number of eggs per female (NEF), according to the formula below.
𝐍𝐄𝐅 =
𝐍𝐄
𝐍𝐅𝐒
(6)
Where: NEF = number of eggs per female; NE = Number of eggs; NFS = Number of
females that spawned.
Past 17 hours after hatching, the process of collecting and transferring nauplii (N3) with positive phototaxis was completed, and the individuals were transferred to the nauplii room. During the transfer, they were subjected to an immersion bath in a PVP-iodine solution at 20 ppm for 30 seconds (FAO, 2003). Subsequently, the number of nauplii was calculated by sampling and extrapolation using asimple rule of three, and then the hatching rate (HR) and
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 𝐇𝐑= 𝐍𝐍 𝐱 𝟏𝟎𝟎
𝐍𝐄
(7)
Where: HR = Hatching rate (%); NN = Number of nauplii; NE = Number of eggs.
𝐍𝐍𝐅 = 𝐍𝐍
𝐍𝐅 (8)
Where: NNF = Number of nauplii per female; NN =Number of nauplii; NF = Number of
females.
After the counting procedure, nauplii were transferred to the larviculture sector. A larviculture trialwas performed during Phase 1, with a duration of 10 days, which means that it was conducted since the nauplii (N3) stocking until the postlarvae stage (PL3), using the
protocols described in FAO (2003) and FAO (2007) with adaptations. For this test, 8 rectangular tanks (25,000 L) were used, each stored with 6,250,000 nauplii, with an initial density of 250 N3/L. For each treatment (NAF and AF), the tests were conducted with 4
repetitions and randomly distributed in 8 tanks.
Microalgae were added daily to the tanks at concentrations of 90,000-100,000 (Chaetoceros sp.) and 20,000-30,000 cells/mL (Thalassiosira sp.) until the postlarvae stage (PL3), when the experiment was completed. From the Zoea stage (Z3), it was used nauplii of
artemia (from Mysis 1 to PL3) and artificial liquid and dry feeds from different manufacturers
(Frippak - InveAquaculture S.A.; Nutrimia - Irca S.A; Liqualife ZM and MPL - Cargil SA; liquid feed - Epicore S.A from Zoea 1 to PL3), and the feeding management for each feed was followed using the manufacturers recommendations and the nutritional protocol of company. The development and survival of the larval stage were monitored daily. From three 100 mL samples from each tank, the arithmetic mean of the samples was calculated and survival was determined through extrapolation by simple rule of three. Using three 1g samples of the postlarvae of each tank, the average weight of the samples was calculated by dividing the weight of the sample by the number of postlarvae of each tank. The water temperature, salinity, dissolved oxygen and pH were maintained at 32.0 ± 0.5 °C; 33.0 ± 0.2 ppt; 5.5 ± 0.1 mg / L; 8.0 ± 0.5, respectively.
All data were expressed as mean±SD. One-way analysis of variance (ANOVA) was used to determine the effects of stocking density on the water quality and growth performance and Tukey test was used for post hoc comparisons.
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
3 RESULTS
In this experiment, Penaeus vannamei broodstock with average weight for males and females of 34.5 ± 2.5 and 43.0 ± 3.0 g, respectively, were used in the maturation and mating sector, with no significant difference (p>0.05) between treatments.
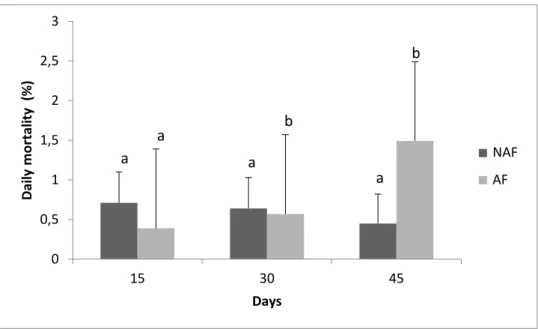
The reproductive performance of non-ablated (NAF) and ablated (AF) females is shown in Table 1. At the end of the experiment, there was a significant difference (p<0.05) in the mortality rate between NAF and AF. The evolution of the mortality rate during the experimental period can be seen in Figure 1. For the first 30 days after ablation, there was no significant difference (p>0.05) in the daily mortality rate between NAF and AF. However, in the last 15 days of evaluation, there was a significant difference (p<0.05) between the groups, and AF presented a higher daily mortality rate when compared to NAF.
Regarding the daily mating rate, a significant difference (p<0.05) was observed between treatments, in which NAF presented a lower value compared to FA (Table 1). Consequently, mating and spawning frequencies were higher in NAF compared to AF, indicating a longer gonadal development period, and therefore there was a significant difference (p<0.05) between treatments.
It was found that there was no significant difference (p>0.05) in spawning and hatching rates (Table 1). However, there was a significant difference (p<0.05) between NAF and AF groups for number of eggs and nauplii per female, in which non-ablated females had a higher amount of eggs and nauplii compared to ablated females. The total number of eggs and nauplii produced by the two groups indicated a statistically significant difference (p<0.05), with NAF values higher than AF.
In the larviculture phase 1 experiment, there was no difference in larval development between treatments (unpublished data). Additionally, survival and final weight values showed no significant difference (p>0.05) (Table 2).
4 DISCUSSION
In the present study, unilateral ablation of the ocular peduncle was found to cause increased mortality. Similar performance was found by Zacarias et al. (2019) who found a daily mortality rate of 1.3% and 2.3% for NAF and AF, respectively. Chamberlain and Lawrence (1981) and Palacios et al. (1999) found mortality rates in their studies with Penaeus
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 females (AF), after 97 and 90 days, respectively. Similarly, Santiago-Junior (1977) observed that non-ablated females of P. monodom had mortality rates of 61%, while for unilaterally ablated females the rate was 73%, reaching 100% of mortality for bilaterally ablated females over a period of 265 days.
The increase in the daily mortality rate in AF explains the stressful and traumatic effect of using this technique. Different studies point to a general physiological imbalance, with effects on the immune defense response through changes in phenoloxidase and prophenoloxidase activity, total hemocyte count, glucose level, and protein concentration in the hemolymph, including disorders in nutrient transport (PERAZZOLO et al, 2002; MAGGIONI et al., 2004; SAINZ-HERNÁNDEZ et al., 2008; VERGHESE; RADHAKRISHNAN; PADHI, 2008; BAE et al., 2013; TREERATTRAKOOL et al., 2014; DAS et al., 2015; WANG et al., 2019).
The ablation process causes a hormonal imbalance, reducing the concentration of the gonadal inhibitor hormone, which limits the reproduction process, allowing females of peneids to reach sexual maturity early, as well as mating and spawning more frequently (BAE et al., 2013; TREERATTRAKOOL et al., 2014; DAS et al., 2015; AMANKWAH et al., 2019). As a result of the significant difference (p<0.05) for the daily mating rate between NAF and AF, mating and spawning frequencies were higher in NAF compared to AF, indicating a longer gonadal development period. Similar responses were found by Chamberlain and Lawrence (1981), Choy (1987), Palacios et al. (1999), Andriantahina et al. (2012), Zacarias et al. (2019). There was no significant difference (p>0.05) between NAF and AF females in relation to spawning and hatching rates. Zacarias et al. (2019) found similar results, but with values for hatching rate higher than those observed in this study. Spawning and hatching rates are directly linked to the nutritional composition of eggs. The levels of triglycerides, phospholipids and polyunsaturated fatty acids are directly associated with successful hatching and the quality of the resulting nauplii, influencing the results of the entire larviculture process (PALACIOS et al., 2000; RACOTTA et al., 2003).
In the present study, there was a significant difference (p<0.05) between NAF and AF groups for number of eggs and nauplii per female, in which non-ablated females had a higher number of eggs (297,208 ± 24,827) and nauplii (210,625 ± 21,681) compared to ablated females (179,120 ± 32,466 eggs and 124,028 ± 25,362 nauplii). Carvalho et al. (2015) and Zacarias et al. (2019) found similar results, considering both the relationship between NAF and AF and the amount of eggs and nauplii produced. On the other hand, Alfaro, Zúñiga and
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 Komen (2004) found a production of 92,667 eggs per ablated female of P. vannamei, which is below the values found in the present study. Palacios et al. (1999) found that after 45 days of the ablation process, females of P. vannamei used in the maturation and mating sector showed reproductive exhaustion, leading to a decrease in the quantity and quality of eggs, with the need for replacement of the animals.
Total egg production, and consequently total nauplii production, differed between treatments over the analysis period. The higher daily mortality rate for AF compared to NAF influenced considerably this outcome. These results differ from those found by Zacarias et al. (2019), despite very similar numbers for all other parameters analyzed compared to the present study.
In the larviculture phase 1, there was no significant difference (p>0.05) in larval development between treatments. Additionally, survival and final weight values were similar (Table 2). These results are similar to those found by Zacarias et al. (2019) who followed larviculture from nauplii (N5) until post larvae (PL12-14), during phases 1 and 2, in response to the saline stress test, survival and final weight, and no significant difference was observed between treatments (FNA and AF).
5 CONCLUSIONS
This was the first study in Brazil to evaluate the effect of non-ablation of Penaeus
vannamei females on reproductive parameters under commercial rearing conditions. The
present study demonstrated that the use of non-ablated females results in a reproductive performance similar to the procedure using the eyestalk ablation technique, and there is no impairment of zootechnical performance during the larval phase. These results demonstrate that it is possible to reproduce these animals in captivity without the surgical process of removing the complex organ X gland from the breast, providing a longer reproductive life to the animals and, consequently, reducing costs in the acquisition or development of the broodstock, in addition to acting in accordance with the latest concepts of animal welfare
ACKNOWLEDGEMENTS
The authors are grateful to the company CELM Aquicultura S.A. that allowed the development of this study in its facilities. We are indebted to the Coordination for the Improvement of Higher Education Personnel (CAPES) who provided M.Sc. T.B.B. de Menezes with a scholarship from masters.
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
REFERENCES
ALFARO, J.; ZÚÑIGA, G.; KOMEN, J. Induction of ovarian maturation and spawning by combined treatment of serotonin and a dopamine antagonist, spiperone in Litopenaeus
stylirostris and Litopenaeus vannamei. Aquaculture, v. 236, p. 511-522, 2004.
ALFARO-MONTOYA, J.; BRAGA, A.; UMAÑA-CASTRO, R. Research frontiers in penaeid shrimp reproduction: Future trends to improve commercial production. Aquaculture, v. 503, p. 70-87, 2019.
AMANKWAH, B. K.; WANG, C.; ZHOU, T.; LIU, J.; WANG.; CHAN, S. Eyestalk ablation, a prerequisite for crustacean reproduction: A review. The Israeli Journal of Aquaculture, v. 71, p. 1-14, 2019.
ANDRIANTAHINA, F.; LIU, X.; HUANG, H.; XIANG, J.; YANG, C. Comparison of reproductive performance and offspring quality of domesticated Pacific white shrimp,
Litopenaeus vannamei. Aquaculture, v. 324-325, p. 194-200, 2012.
BAE, S.; OKUTSU, T.; KANG, J. B.; WILDER, M. N. Alterations of pattern in immune response and vitellogenesis during induced ovarian development by unilateral and bilateral ablation in Litpenaeus vannamei. Fisheries Science, v. 79, n. 6, p. 895-903, 2013.
BENZIE, J. A. H. Penaeid genetics and biotechnology. Aquaculture, v. 164, p. 23–47, 1998. BROWDY, C. L.; SAMOCHA, T. M. The effect of eyestalk ablation on spawning, moulting and mating of Penaeus semisulcatus De Haan. Aquaculture, v. 49, p. 19-29, 1985.
CARDONA, E.; LORGEOUX, B.; CHIM, L.; GOGUENHEIM, J.; LE DELLIOU, H.; CACHU, C. Biofloc contribution to antioxidant defence status, lipid nutrition and reproductive performance of broodstock of the shrimp Litopenaeus stylirostris: consequences for the quality of eggs and larvae. Aquaculture, v. 452, p. 252-262, 2016.
CARVALHO, F. G.; MENDES, L. G.; SILVA, J. G.; ANDREATTA, E. R. Primeiro relato da inserção do sistema de bioflocos na maturação em cativeiro do camarão marinho
Litopenaeus vannamei. Boletim do Instituto de Pesca, v. 41, p. 441-448, 2015.
CHAMBERLAIN, G. W.; LAWRENCE, A. L. Effect of light intensity and male and female eyestalk ablation on reproduction of Penaeusstylirostris and P. vannamei. Journal of the
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761 CHAN, S. M.; GU, P. L.; CHU, K. H.; TOBE, S. S. Crustacean neuropeptide genes of the CHH/MIH/GIH family: implications from molecular studies. General and Comparative
Endocrinology, v. 134, p. 214-219, 2003.
CHOY, S. C. Growth and reproduction of eyestalk ablated Penaeus canaliculatus (Olivier, 1811)(Crustacea: Penaeidae). Journal of Experimental Marine Biology and Ecology, v. 112, p. 93-107, 1987.
CUNHA, C. H.; OSHIRO, L. M. Y. The influence of eyestalk ablation on the reproduction of the freshwater Macrobrachium acanthurus shrimp in captivity. Acta Scientiarum Biological
Sciences v. 32, n. 3, p. 217-221, 2010.
DAS, R; KRISHNA, G; PRIYADARSHI, H; BABU P, G; KUMAR, A, P; RAJENDRAN, K,V; REDDY, A, K; MAKES, M; CHAUDHARI, A. Captive maturation studies in Penaeus
monodon by GIH silencing using constitutively expressed long hairpin RNA. Aquaculture,
v. 448, p. 512-520, 2015.
FAO. Health management and biosecurity maintenance in White shrimp
(Penaeusvannamei) hatcheries in Latin America. FAO Fisheries Technical Paper. Roma,
FAO, n. 450, 2003. 62 p.
FAO. Improving Penaeus monodon hatchery practices. Manual based on experience in
India. FAO Fisheries Technical Paper. Roma, FAO, n. 446, 2007. 117p.
FAO. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Roma. 2018. 227 p.
HOPKINS, P. M. The eyes have it: a brief history of crustacean neuroendocrinology. General
and Comparative Endocrinology, v. 175, p, 357–366, 2012.
HSU, Y. W. A.; MESSINGER, D. I.; CHUNG, J. S.; WEBSTER, S. G.; HORACIO, O.; CHRISTIE, A. E. Members of the crustacean hyperglycemic hormone (CHH) peptide family are differentially distributed both between and within the neuroendocrine organs of Cancer crabs: implications for differential release and pleiotropic function. The Journal of
Experimental Biology, v. 209, p. 3241–3256, 2006.
KANG, B. J.; OKUTSU, T.; TSUTSUI, N.; SHINJI, J.; BAE, S.; WILDER, M. N. Dynamics of vitellogenin and vitellogenesis-inhibiting hormone levels in adult and subadult white leg shrimp, Litopenaeus vannamei: relation to molting and eyestalk ablation. Biology of
Reproduction, v. 90, p. 1-10, 2014.
LEE, J.; SURYANINGTYAS, I. T.; YOON, T.; SHIM, J. M.; PARK, H.; KIM, H. Transcriptomic analysis of the hepatopancreas induced by eyestalk ablation in shrimp,
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
Litopenaeus vannamei. Comparative Biochemistry and Physiology - Part D, v. 24, p.
99-110, 2017.
LITTLE, D.; YOUNG, J. A; ZHANG, W.; NEWTON, R. W.; MAMUN, A.; MURRAY., F. Sustainable intensification of aquaculture value chains between Asia and Europe: A framework for understanding impacts and challenges. Aquaculture, v. 493, p. 338-354. 2018.
MAGGIONI, D. S.; ANDREATTA, E. R.; HERMES, E. M.; BARRACCO, A. Evaluation of some hemato-immunological parameters in female shrimp Litopenaeus vannamei submitted to unilateral eyestalk ablation in association with a diet supplemented with super doses of ascorbic acid as a form of immunostimulation. Aquaculture, v. 241, p. 501-515, 2004. MOTTE, E.; YUGCHA, E.; LUZARDO, J.; CASTRO, F.; LECLERCQ, G.; RODRÍGUEZ, J.; MIRANDA, P.; BORJA, O.; SERRANO, J.; TERREROS, M.; MONTALVO, K.; NARVÁEZ, A.;TENORIO, N.; CEDEÑO, V.; MIALHE, E.; MONTALVO, K. Prevention of IHHNV vertical transmission in the White shrimp Litopenaeus vannamei. Aquaculture, v. 219, p. 57-70, 2003.
PALACIOS, E.; IBARRA, A. M.; RACOTTA, I. S.; Tissue biochemical composition in relation to multiples pawning in wild and pond-reared Penaeus vannamei broodstock.
Aquaculture, v. 185, p. 353–371. 2000.
PALACIOS, E.; PEREZ-ROSTRO, C. I.; RAMIREZ, J. L.; IBARRA, A. M.; RACOTTA, I. S. Reproductive exhaustion in shrimp (Penaeus vannamei) reflected in larval biochemical composition, survival and growth. Aquaculture, v. 171, p. 309-321, 1999.
PERAZZOLO, L. M.; GARGIONI, R.; OGLIARI, P.; BARRANCO, M. A. Evaluation of some hemato-immunological parameters in the shrimp Farfantepenaeus paulensis submitted to environmental and physiological stress. Aquaculture, v. 214, 19–33, 2002.
RACOTTA, I. S.; PALACIOS, ELENA.; INARRA, A. M. Shrimp larval quality in relation to broodstock condition. Aquaculture, v. 227, p. 107-130, 2003.
SAINZ-HERNÁNDEZ, J. C.; RACOTTA, I. S.; DUMAS, S.; HERNÁNDEZ-LÓPEZ, J. Effect of unilateral and bilateral eyestalk ablation in Litopenaeus vannamei male and female on several metabolic and immunologic variables. Aquaculture, v. 283, n. 1-4, p. 188-193, 2008.
SHAILENDER, M.; AMARNATH, D.; KISHOR, B.; SURESH, B. C. H..Effect of unilateral eyestalk ablation (UEA) on the reproductive success of giant fresh water prawn,
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
Macrobrachium rosenbergii (De Man) in captivity. International Journal of Chemistry and Life Sciences, v. 2, p. 1112-1120, 2013.
TAYLOR, J.; VINATEA, L.; OZORIO, R.; SCHUWEITZER, R.; ANDREATTA, E. R. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture, v. 233, n. 1-4, p. 173-179, 2004. TREERATTRAKOOL, S.; PANYIM, S.; UDOMKIT, A. Induction of ovarian maturation and spawning in Penaeus monodon broodstock by double-stranded RNA. Marine biotechnology, v. 13, p. 163-169, 2011.
TREERATTRAKOOL, S.; BOONCHOY, C.; URTGAM, S.; PANYIM, S.; UDOMKIT, A. Functional characterization of recombinant gonad-inhibiting hormone (GIH) and implication of antibody neutralization on induction of ovarian maturation in marine shrimp. Aquaculture, v. 428-429, p. 166-173, 2014.
VENTURA-LÓPEZ, C.; TORRES, P. E.; ARCOS, F. G.; GALINDO-SÁNCHEZ, C.; RACOTTA, I. S.; ESCOBEDO-FREGOSO, C.; LLHERA-HERRERA, R.; IBARRA, A. M. Transcriptomic information from Pacific white shrimp (Litopenaeus
vannamei) ovary and eyestalk, and expression patterns for genes putatively involved in there
productive process. General and Comparative Endocrinology, v. 246, p. 164-182, 2017. VERGHESE, B.; RADHAKRISHNAN, E. V.; PADHI, A. Effect of moulting, eyestalk ablation, starvation and transportation on the immune response of the Indian spiny lobster,
Panulirus homarus. Aquaculture, v. 39, p. 1009-1013, 2008.
WANG, Z.; LUAN, S.; MENG, X.; CAO, B.; LUO, K.; KONG, J. Comparative transcriptomic characterization of the eyestalk in Pacific white shrimp (Litopenaeus vannamei) during ovarian maturation. General and Comparative Endocrinology, v. 274, p. 60-72, 2019. WEN, W. G.; YANG, Q. B.; MA, Z. H.; JIANG, S. G.; QIU, L. H.; HUANG, J. H.; ZHOU, F. L.; QIN, J. G. Comparison of ovarian maturation and spawning after unilateral eyestalk ablation of wild-caught and pond-reared Penaeus monodon. Spanish Journal of Agricultural
Research, v. 13, p. 1-6, 2015.
ZACARIAS, S.; CARBONI, S.; DAVIE, A.; LITTLE, D. C. Reproductive performance and offspring quality of non-ablated Pacific white shrimp (Litopenaeus vannamei) under intensive commercial scale conditions. Aquaculture, v. 503, p. 460-466, 2019.
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
ANNEXES
Table 1 Daily reproductive performance of non-ablated females (NAF) and ablated females (AF) of Penaeus
vannamei. Variable Treatment NAF AF Mortality rate (%) 28.5±0.9a 39.1± 0.3b Mating rate (%) 6.5 ± 1.7a 11.7 ± 2.8b
Mating frequency (day) 16.5 ± 4.7a 9.1 ± 2.3b
Spawning frequency (day) 17.8 ± 4.8a 10.2 ± 2.6b
Spawning rate (%) 92.7 ± 5.3 89.0 ± 3.8
Hatching rate (%) 70.8 ± 2.7 69.0 ± 3.5
Number of eggs/female 297,208 ± 24,827a 179,120 ± 32,466b Number of nauplii/female 210,625 ± 21,681a 124,028 ± 25,362b Total egg production (30 days) 340.474.426a 306.968.243b Total nauplii production (30 days) 241.286.998a 212.553.915b
Table 2 Zootechnical performance during larviculture of P. vannamei originated from the non-ablated females (NAF) and ablate females (AF).
Variable Treatment
NAF AF
Survival (%) 58.4 ± 3.5 61.1 ± 4.6
Braz. J. of Develop.,Curitiba, v. 5, n. 12,p.33454-33470 dec. 2019. ISSN 2525-8761
Figura 1Daily mortality variation (mean ± SD) of non-ablated females (NAF) and ablate females (AF) of P.
vannamei throughout the experimental period.
0 0,5 1 1,5 2 2,5 3 15 30 45 D ai ly m o rtali ty (% ) Days FNA FA a a a a b b NAF AF