REVISTA
BRASILEIRA
DE
REUMATOLOGIA
www . r e u m a t o l o g i a . c o m . b r
Original
article
Evaluation
of
performance
of
BASDAI
(Bath
Ankylosing
Spondylitis
Disease
Activity
Index)
in
a
Brazilian
cohort
of
1492
patients
with
spondyloarthritis:
data
from
the
Brazilian
Registry
of
Spondyloarthritides
(RBE)
Izaias
Pereira
da
Costa
a,∗,
Adriana
B.
Bortoluzzo
b,
Célio
R.
Gonc¸alves
c,
José
Antonio
Braga
da
Silva
d,
Antonio
Carlos
Ximenes
e,
Manoel
B.
Bértolo
f,
Sandra
L.E.
Ribeiro
g,
Mauro
Keiserman
h,
Rita
Menin
i,
Thelma
L.
Skare
j,
Sueli
Carneiro
k,
Valderílio
F.
Azevedo
l,
Walber
P.
Vieira
m,
Elisa
N.
Albuquerque
n,
Washington
A.
Bianchi
o,
Rubens
Bonfiglioli
p,
Cristiano
Campanholo
q,
Hellen
M.S.
Carvalho
r,
Angela
L.B.
Pinto
Duarte
s,
Charles
L.
Kohem
t,
Nocy
H.
Leite
u,
Sonia
A.L.
Lima
v,
Eduardo
S.
Meirelles
w,
Ivânio
A.
Pereira
x,
Marcelo
M.
Pinheiro
y,
Elizandra
Polito
z,
Gustavo
G.
Resende
aa,
Francisco
Airton
C.
Rocha
bb,
Mittermayer
B.
Santiago
cc,
Maria
de
Fátima
L.C.
Sauma
dd,
Valéria
Valim
ee,
Percival
D.
Sampaio-Barros
caUniversidadeFederaldoMatoGrossodoSul,CampoGrande,MS,Brazil
bInstitutoInsperdeEducac¸ãoePesquisa,SãoPaulo,SP,Brazil
cRheumatologyDiscipline,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
dUniversidadedeBrasília,Brasília,DF,Brazil
eHospitalGeraldeGoiânia,Goiânia,GO,Brazil
fUniversidadedeCampinas,Campinas,SP,Brazil
gUniversidadeFederaldoAmazonas,Manaus,AM,Brazil
hPontifíciaUniversidadeCatólica,PortoAlegre,RS,Brazil
iFaculdadedeMedicinadeSãoJosédoRioPreto,SãoJosédoRioPreto,SP,Brazil
jHospitalEvangélicodeCuritiba,Curitiba,PR,Brazil
kUniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
lUniversidadeFederaldoParaná,Curitiba,PR,Brazil
mHospitalGeraldeFortaleza,Fortaleza,CE,Brazil
nUniversidadeEstadualdoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
∗ Correspondingauthor.
E-mail:izapec@hotmail.com(I.P.d.Costa). http://dx.doi.org/10.1016/j.rbre.2014.05.005
oSantaCasadoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
pPontifíciaUniversidadeCatólica,Campinas,SP,Brazil
qSantaCasadeSãoPaulo,SãoPaulo,SP,Brazil
rHospitaldeBase,Brasília,DF,Brazil
sUniversidadeFederaldePernambuco,Recife,PE,Brazil
tUniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
uFaculdadedeMedicinaSouzaMarques,RiodeJaneiro,RJ,Brazil
vHospitaldoServidorPúblicoEstadual,SãoPaulo,SP,Brazil
wInstituteofOrthopedyandTraumatology,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
xUniversidadeFederaldeSantaCatarina,Florianópolis,SC,Brazil
yUniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil
zSantaCasadeBeloHorizonte,BeloHorizonte,MG,Brazil
aaUniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil
bbUniversidadeFederaldoCeará,Fortaleza,CE,Brazil
ccEscoladeMedicinaeSaúdePública,Salvador,BA,Brazil
ddUniversidadeFederaldoPará,Belém,PA,Brazil
eeUniversidadeFederaldoEspíritoSanto,Vitória,ES,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received13February2013 Accepted19May2014
Availableonline20December2014
Keywords:
Spondyloarthritis Diseaseactivity BASDAI Epidemiology
a
b
s
t
r
a
c
t
Objective:ToanalyzetheresultsoftheapplicationoftheBathAnkylosingSpondylitis
Dis-easeActivityIndex(BASDAI)inalargeseriesofBrazilianpatientswiththediagnosisofSpA andestablishitscorrelationswithspecificvariablesintothegroup.
Methods:Acommonprotocolofinvestigationwasprospectivelyappliedto1492Brazilian
patientsclassifiedasSpAaccordingtotheEuropeanSpondyoarthropathiesStudyGroup (ESSG),attendedat29referralcentersofRheumatologyinBrazil.Clinicalanddemographic variables,anddiseaseindices(BASDAI,Basfi,Basri,Mases,ASQol)wereapplied.Thetotal valuesofBASDAIwerecomparedtothepresenceofthedifferentvariables.
Results:ThemeanscoreofBASDAIwas4.20±2.38.ThemeanscoresofBASDAIwerehigher
inpatientswiththecombined(axial+peripheral+entheseal)(4.54±2.38)clinical presen-tation, compared to the pure axial(3.78±2.27) or pure peripheral (4.00±2.38) clinical presentations(P<0.001).BASDAIalsopresentedhigherscoresassociatedwiththefemale gender(P<0.001)andpatientswhodidnotpracticeexercises(P<0.001).Regardingtheaxial component,highervaluesofBASDAIweresignificantlyassociatedwithinflammatorylow back pain(P<0.049),alternatingbuttockpain(P<0.001),cervicalpain(P<0.001)andhip involvement(P<0.001).TherewasalsostatisticalassociationbetweenBASDAIscoresand theperipheralinvolvement,relatedtothelower(P=0.004)andupperlimbs(P=0.025).The presenceofenthesitiswasalsoassociatedtohigherscoresofBASDAI(P=0.040).Positive HLA-B27andthepresenceofcutaneouspsoriasis,inflammatoryboweldisease,uveitisand urethritiswerenotcorrelatedwiththemeanscoresofBASDAI.LowerscoresofBASDAIwere associatedwiththeuseofbiologicagents(P<0.001).
Conclusion: In thisheterogeneous Brazilian seriesof SpA patients, BASDAIwas ableto
demonstrate“diseaseactivity”inpatientswithaxialaswellasperipheraldisease. ©2014ElsevierEditoraLtda.Allrightsreserved.
Avaliac¸ão
do
desempenho
do
BASDAI
(Bath
Ankylosing
Spondylitis
Disease
Activity
Index)
numa
coorte
brasileira
de
1.492
pacientes
com
espondiloartrites:
dados
do
Registro
Brasileiro
de
Espondiloartrites
(RBE)
Palavras-chave:
Espondiloartrites Atividadededoenc¸a
a
b
s
t
r
a
c
t
Objetivo:Avaliarosresultadosdaaplicac¸ãodoÍndicedeAtividadedeDoenc¸adaEspondilite
BASDAI Epidemiologia
Métodos: Umprotocolocomumdeinvestigac¸ãofoiprospectivamenteaplicadoem1.492
pacientesbrasileirosclassificadoscomoEpApeloscritériosdoGrupoEuropeudeEstudodas Espondiloartropatias(ESSG),acompanhadosem29centrosdereferênciaemreumatologia noBrasil.Variáveisclínicas,demográficaseíndicesdedoenc¸aforamcolhidos.Osvalores totaisdoBASDAIforamcomparadoscomapresenc¸adasdiferentesvariáveis.
Resultados: OvalormédiodoBASDAIfoide4,20±2,38.OsescoresmédiosdoBASDAIforam
maiselevadosnospacientescomformaclínicacombinada,comparadoàsformasaxiais eperiféricasisoladas,nospacientesdosexofemininoenossedentários.Comrelac¸ãoao componenteaxial,valoresmaisaltosdoBASDAIestiveramsignificativamenteassociados àlombalgiainflamatória,àdoralternanteemnádegas,àdorcervicaleaoacometimento decoxofemorais.Houveassociac¸ãoestatísticaentreosvaloresdoBASDAIeo comprome-timentoperiférico,relacionadoaonúmerodearticulac¸õesinflamadas,tantodosmembros inferioresquantodosmembrossuperiores,eàsentesites.ApositividadedoHLA-B27ea presenc¸ademanifestac¸õesextra-articularesnãoestiveramcorrelacionadascomosvalores médiosdoBASDAI.ValoresmaisbaixosdoBASDAIestiveramassociadosaousodeagentes biológicos(p<0,001).
Conclusão: NestasérieheterogêneadepacientesbrasileiroscomEpA,oBASDAIconseguiu
demonstrar“atividadededoenc¸a”tantonospacientescomacometimentoaxialquanto naquelescomenvolvimentoperiférico.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Thedenominationspondyloarthritis(SpA)definesa hetero-geneous group of diseases that share genetic and clinical characteristics,aswellasstructuralchangesinimaging stud-ies.ThepositivityofHLA-B27andtheabsenceofrheumatoid factorare common tothis groupofdiseases,and thehigh frequencyofinflammatoryprocessesofthespine,sacroiliac jointsandenthesisisconsideredthemainclinicalcriteriafor thediagnosisofSpA.1Otherclinicalmanifestationsoccurin
varyingdegrees ofinvolvementin thegroup ofSpA; more often, skin, ocular, intestinal and urogenital injuries and, less frequently, pulmonary, cardiac, renal and neurological involvement.1
Thisgroupofdiseasesconsistsofankylosingspondylitis (AS),psoriatic arthritis (PA),reactive arthritis(ReA), arthri-tisassociatedwithinflammatoryboweldiseases(alsoknown asenterophaticarthritis–EA)andundifferentiated spondy-loarthritis(USpA).1
With the advent of new therapeutic modalities for the groupofSpA,theelaborationofmeasuresofdisease activ-itythatcouldbeusedinlong-termfollow-upwasnecessary.2
Currently,toevaluateandmonitorclinicaldiseaseactivityin SpA,theBathAnkylosingSpondylitisDiseaseActivityIndex (BASDAI)hasbeenused.Thisindexisobtainedbysumming thevaluesofavisualanalog scale(VAS) thatevaluatessix items,namely:fatigue,axialpain, peripheralpain, enthesi-tis,durationandintensityofmorningstiffness.3BASDAIdoes
notuseany markerofinflammatoryactivity inits calcula-tion,sincethesetestswerenotstandardizedatthetimeof itsproposition.3BASDAIvaluesareinternationallyused
(BAS-DAI≥4isdeemedas“highdiseaseactivity”)fortheindication ofbiologicalagentsinthetreatmentofSpA,whentherewas noresponsetoconventionaltreatmentwithnonsteroidal anti-inflammatorydrugsorwithconventionalremissivedrugs.4,5
When the patient reaches BASDAI 50(improvement in the BASDAI scoreof ≥50%)in the first 12 weeksof treatment, thiscanbeconsideredasaverygoodclinicalresponse.6Due
toitsimportance,BASDAIhasbeentranslatedandvalidated inseverallanguages,includingFrench,7Swedish,8German,9
Spanish,10Turkish,11Arabic12andPortuguese.13Recently,the
Ankylosing SpondylitisDisease Activity Score(ASDAS) was created14;thisindexassociatesthepresenceofamarkerof
inflammatory activity,erythrocytesedimentation rate (ESR) or C-reactive protein (CRP); nowadays, ASDAS has its cut-offscorestodifferentiate“moderate”,“high”and“veryhigh” activity.15
ThispaperanalyzestheapplicationofBASDAIina hetero-geneousBraziliancohortof1492patientswithSpA.
Methods
This is a prospective, observational, multicenter study, conductedwith 1492patientsfrom 29 referral centers par-ticipating in the Brazilian Registry of Spondyloarthritides
(Registro Brasileiro de Espondiloartrites – RBE). All patients
fulfilled the criteria of the European Spondyloarthropathy StudyGroup(ESSG).16DatawerecollectedfromJune2006to
December 2009. RBE participates in the RESPONDIA group
(RegistroIberoamericanodeEspondiloartrites),consistingofnine
LatinAmericancountries(Argentina,Brazil,CostaRica,Chile, Ecuador,Mexico,Peru,UruguayandVenezuela)andthetwo countriesoftheIberianpeninsula(SpainandPortugal).
C-reactive protein – CRP), as well as the treatment (nons-teroidanti-inflammatorydrugs–NSAIDs,corticosteroidsand conventionalandbiologicalremissivedrugs).
ForthediagnosisofAS,theNewYorkcriteriawereused17;
forpsoriaticarthritis,theparticipantshadtomeetthe crite-riaofMollandWright.18 Thediagnosis ofreactive arthritis
wasconsideredifasymmetricoligoarthritisofthelowerlimbs were present,with enthesopathy and/or inflammatorylow backpainarousingafteranentericorurogenitalinfection,19
andspondyloarthritis/arthritisassociatedwithinflammatory boweldisease,ifthepatienthadaninflammatoryaxial dis-order and/or peripheraljoint involvement, associated with confirmedCrohn’sdiseaseorulcerativecolitis.
BASDAI is a tool for evaluation of disease activity that contains six questions.3 The responses were marked on a
horizontallinemeasuring10cm(from0to10cm),wherethe patientevaluateshowhefeelsinrelationtoeachiteminthe lastweek,markingonthescale:ifthepatientisfine(“very well”),he/shemarkszerocm,graduallyincreasinguntil“very poor”,correspondingtoa10-cmmark.Thesixquestions com-prisingBASDAIareasfollows:(1)Howwouldyoudescribethe degreeoffatigueortirednessyouhavehad?;(2)Howwould youdescribetheoveralllevelofneck,backandhippainrelated toyourillness?;(3)Howwouldyoudescribetheoveralllevel ofpainandedema(swelling)inotherjoints,apartfromneck, backandhip?;(4)Howwouldyoudescribetheoveralllevelof discomfortyoufelttothetouchorcompressioninthepainful regionsofyourbody?;(5)Howwouldyoudescribethe inten-sityofmorningstiffnessyouhavehad,fromthetimeyouwake up?;(6)Howlongdoesyourmorningstiffnesstake,fromthe timeyouwakeup?
Statisticalanalysis
Thevariable categories were comparedusing2 and Fisher’s
exacttests, andcontinuousvariables werecomparedusing ANOVA.Avalue ofP<0.05was considered significant;and 0.05>P>0.10wasconsideredasastatisticaltrend.
Results
ThemeanscoreoftotalBASDAIwas4.20±2.38.Amongthe sixitemsthatmakeupthefinalvalueofBASDAI,theitem2 (relatedtoaxialpain;5.05±3.20)hadthehighestmean,and theitem3(relatedtoperipheralcomponent;3.28±3.18)was thatwiththelowestvalue.ThemeanvaluesofBASDAItoall thequestionsthatmakeuptheindexaredescribedinTable1.
Table1–ResultsofBASDAIperitem.
BASDAI Mean Standard
deviation
Total 4.20 2.38
Question1 4.21 2.99
Question2 5.05 3.20
Question3 3.28 3.18
Question4 4.28 3.34
Question5 4.59 3.29
Question6 3.85 3.36
Table2–ResultsofBASDAIaccordingtospecificSpA.
% Mean SD P
PrimaryAS 67.6 4.21 2.36 PsoriaticAS 4.6 4.38 2.32 EnteropathicAS 2.6 4.70 2.41
PsoriaticArthritis 14.2 4.13 2.47 0.305
USpA 6.8 4.35 2.28
ReactiveArthritis 3.4 4.12 2.57 EnteropathicArthritis 0.8 4.37 3.05
Therewasnosignificantdifferencebetweenthemean val-uesofBASDAIandanyspecificdiseasewithintheSpAgroup. ThemeanvaluesofBASDAIforeachdiseasewithinthegroup are shown in Table 2. As to the clinical presentation, the meanvaluesofBASDAIweresignificantlyhigherinenthesitic (4.96±2.22)and combined(axialand peripheral;4.50±2.38) forms,comparedtopureforms,bothaxial(3.78±2.28) and peripheral(3.87±2.23)(Table3).
Withregardtodemographicvariables,BASDAIwas signifi-cantlyhigherinfemales(P<0.001)andinthosepatientswho didnotexerciseregularly(P<0.001).Ethnicity,HLA-B27 and familyhistoryofSpAdidnotinfluencetheresultsofBASDAI (Table4).
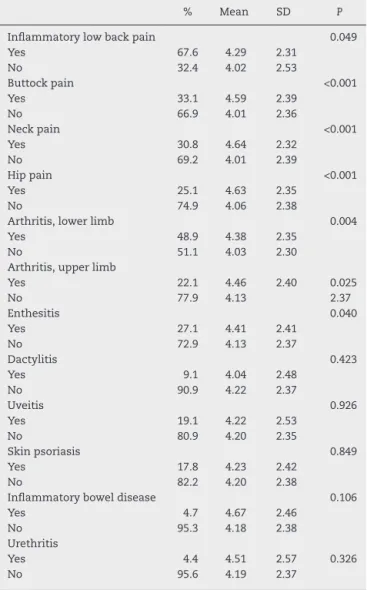
Numerous clinical variables influenced BASDAI scores. Inflammatorylowbackpain(P=0.039),buttockpain(P<0.001), neck pain (P<0.001), hip pain (P<0.001), arthritis oflower (P=0.004)andupper(P=0.025)limbs,andenthesitis(P=0.040) weresignificantlyassociatedwithhighermeanvaluesof BAS-DAI.Extra-articularmanifestationssuchasuveitis,psoriasis,
Table3–ResultsofBASDAIaccordingtoclinicalform.
% Mean SD P
Mixed 48.5 4.50 2.38
Axial 34.5 3.78 2.28 <0.001 Peripheral 11.0 3.87 2.23
Enthesitic 6.0 4.96 2.22
Table4–ResultsofBASDAIaccordingtoepidemiological data.
% Mean SD P
Gender
Male 72.3 3.97 2.95 <0.001 Female 27.7 4.84 3.05
Race
White 67.4 4.03 2.38 0.057 Non-white 32.6 4.30 2.40
Exercise
Yes 40.8 3.89 2.35 <0.001
No 59.2 4.42 2.38
Familyhistory
Yes 18.0 4.32 2.49 0.413
No 82.0 4.18 2.36
HLA-B27
Positive 69.0 4.16 2.33 0.391
Table5–ResultsofBASDAIaccordingtoclinicaldata.
% Mean SD P
Inflammatorylowbackpain 0.049
Yes 67.6 4.29 2.31
No 32.4 4.02 2.53
Buttockpain <0.001
Yes 33.1 4.59 2.39
No 66.9 4.01 2.36
Neckpain <0.001
Yes 30.8 4.64 2.32
No 69.2 4.01 2.39
Hippain <0.001
Yes 25.1 4.63 2.35
No 74.9 4.06 2.38
Arthritis,lowerlimb 0.004
Yes 48.9 4.38 2.35
No 51.1 4.03 2.30
Arthritis,upperlimb
Yes 22.1 4.46 2.40 0.025
No 77.9 4.13 2.37
Enthesitis 0.040
Yes 27.1 4.41 2.41
No 72.9 4.13 2.37
Dactylitis 0.423
Yes 9.1 4.04 2.48
No 90.9 4.22 2.37
Uveitis 0.926
Yes 19.1 4.22 2.53
No 80.9 4.20 2.35
Skinpsoriasis 0.849
Yes 17.8 4.23 2.42
No 82.2 4.20 2.38
Inflammatoryboweldisease 0.106
Yes 4.7 4.67 2.46
No 95.3 4.18 2.38
Urethritis
Yes 4.4 4.51 2.57 0.326
No 95.6 4.19 2.37
inflammatoryboweldiseaseandurethritisdidnotinfluence BASDAIscores(Table5).
Withregardtotreatment,onlytheuseofanti-TNF biolog-icalagentswassignificantlyassociatedwithlowerscoresof BASDAI(P<0.001),whiletheuseofNSAIDs,corticosteroids, methotrexateandsulfasalazinedidnotinfluencethevalues ofBASDAI(Table6).
Discussion
ThisstudyaimedtoevaluatetheactivityofSpAusing BAS-DAI as a clinical activity index; this is a tool traditionally usedinpatientswithSpA.TheresultsshowedthatBASDAI coulddemonstrate “diseaseactivity”,bothinpatients with anaxialcomponentasinthosewithperipheralinvolvement, eveninpatients withSpA but withno diagnosis ofAS.In view of the fact that BASDAI evaluate “axial” (question 2) and“peripheral”(questions3and4)components,an impor-tant finding in this study was represented by the highest meanscoresinpatientswhohadaninvolvementdescribedas “combined”(wherethe“axial”and“peripheral”components areobservedinthesamepatient)–acommon characteris-ticofBrazilianpatients.20Similarly,anEuropeanmulticenter
Table6–ResultsofBASDAIaccordingtotreatment.
% Mean SD P
NSAID 0.888
Yes 67.6 4.21 2.35
No 32.4 4.19 2.45
NSAIDon-demand 0.713
Yes 24.9 4.16 2.40
No 75.1 4.22 2.38
Corticosteroid 0.297
Yes 35.3 4.29 2.37
No 64.7 4.16 2.39
Methotrexate 0.866
Yes 51.7 4.21 2.32
No 48.3 4.19 2.45
Sulfasalazine 0.934
Yes 44.7 4.21 2.44
No 55.3 4.19 2.34
Biologicals <0.001
Yes 20.4 3.73 2.47
No 79.6 4.32 2.35
Infliximab <0.001
Yes 15.3 3.58 2.50
No 84.7 4.32 2.35
Etanercept 0.104
Yes 2.8 4.78 2.30
No 97.2 4.19 2.38
Adalimumab
Yes 2.3 3.70 2.06 0.164
No 97.7 4.22 2.39
studyevaluating214patientswithSpAfoundhighervalues ofBASDAIinthoseparticipantswithaperipheralcomponent (4.4±2.3)comparedtopatientswithanisolatedaxial compo-nent(3.1±1.9)(P<0.001).21Alsoimportantwasthefactthat
themeanvaluesofBASDAIweresignificantlyelevated,bothin presenceofaxial(inflammatorylowbackpain,buttockpain, neckpainandhippain)andperipheral(lowerandupperjoints) clinicalvariables,inadditiontoenthesitis.
InthespectrumofSpA,psoriaticarthritisisthatdisease wheretheperipheralcomponentismoststriking.Ourstudy showedthatBASDAIcanalsobeeffectiveintheevaluationof patientswithPA,asshowninrecentstudies,22,23evenwhen
comparedtoASDAS.24
With the proposition of ASDAS as a valid method of assessingdiseaseactivityincasesofAS,14,15itwillbe
impor-tanttoapplythistooltopatientswithSpAinthesecondphase ofRBE,tocompareitseffectivenessversusBASDAI.Thereis noestablishedconsensusaboutwhatisthebestmethodfor assessmentofdiseaseactivityinpatientswithAS(ifASDAS isbetterthanBASDAI).Meanwhile,BASDAIhasbeenshown asanefficientindexinthetherapeuticfollow-upofpatients withAS.6,25,26ThecombinationofBASDAIwiththefunctional
index BASFI (Bath Ankylosing Spondylitis Disease Activity Index)27madeitpossibletoobtainimportantcharacteristics
ofpatientsintheBrazilianRegistryofSpondyloarthritides.28
Althoughrepresentingonly27.7%ofthepatients,women had higher mean BASDAI scores, when compared tomen. These results certainly may vary with the population evaluated.29
differenceinBASDAIscoresinrelationtoHLA-B27,family his-toryandextra-articularmanifestationswasfound.
In short, BASDAI showed to be an efficient method of assessingdiseaseactivityinaheterogeneouspopulationof BrazilianpatientswithSpA.
Conflicts
of
interest
Theelectronicversion ofthe Brazilian Registry of Spondy-loarthritidesissupportedbyagrantfromWyeth/PfizerBrazil, whichhasnoinfluenceonthecaptureandanalysisofdata, aswellasinthewritingandpublicationofarticles.Dr. Per-civalSampaio-BarrosreceivedaresearchgrantfromFederico Foundation.
r
e
f
e
r
e
n
c
e
s
1. SieperJ,RudwaleitM,BaraliakosX,BrandtJ,BraunJ, Burgos-VargasR,etal.TheAssessmentofSpondyloarthritis InternationalSociety(Asas)handbook:aguidetoassess spondyloarthritis.AnnRheumDis.2009;68Suppl.II:ii1–44. 2. VanderHeijdeD,LandewéR.Assessmentofdiseaseactivity,
functionandqualityoflife.In:WeismanMH,ReveilleJD,Van derHeijdeD,editors.Ankylosingspondylitisandthe spondyloarthropathies.MosbyElsevier,Filadélfia,1a.ed.;
2006.p.206–13.
3. GarrettS,JenkinsonT,KennedyLG,WhitelockH,GaisfordP, CalinA.Anewapproachtodefiningdiseasestatusin ankylosingspondylitis:theBathAnkylosingSpondylitis DiseaseActivityIndex.JRheumatol.1994;21:2286–91. 4. VanderHeijdeD,SieperJ,MaksymowychW,DougadosM,
Burgos-VargasR,LandewéR,etal.2010updateofthe internationalAsasrecommendationsfortheuseofanti-TNF agentsinpatientswithaxialspondyloarthritis.AnnRheum Dis.2011;70:905–8.
5. Sampaio-BarrosPD,PinheiroMM,XimenesAC,MeirellesES, KeisermanM,AzevedoVF,etal.Recomendac¸õessobre diagnósticoetratamentodaespondiliteanquilosante.Rev BrasReumatol.2013;53:242–57.
6. RudwaleitM,ListingJ,BrandtJ,BraunJ,SieperJ.Predictionof amajorclinicalresponse(Basdai50)totumournecrosis factoralphablockersinankylosingspondylitis.AnnRheum Dis.2004;63:665–70.
7. ClaudepierreP,SibiliaJ,GoupilleP,FlipoRM,WendlingD, EulryF,etal.EvaluationofaFrenchversionoftheBath AnkylosingSpondylitisDiseaseActivityIndexinpatients withspondyloarthropathy.JRheumatol.1997;24:1954–8. 8. WaldnerA,CronstedtH,StenstromCH.TheSwedishversion
oftheBathAnkylosingSpondylitisDiseaseActivityIndex. Reliabilityandvalidity.ScandJRheumatol.1999;111 Suppl:10–6.
9. BrandtJ,WesthoffG,RudwaleitM,ListingJ,ZinkA,BraunJ, etal.AdaptionandvalidationoftheBathAnkylosing SpondylitisDiseaseActivityIndex(Basdai)forusein Germany.ZRheumatol.2003;62:264–73.
10.CardielMH,Londo ˜noJD,GutierrezE,Pacheco-TenaC, Vazquez-MelladoJ,Burgos-VargasR.Translation, cross-culturaladaptation,andvalidationoftheBath AnkylosingSpondylitisFunctionalIndex(Basfi),theBath AnkylosingSpondylitisDiseaseActivityIndex(Basdai),and theDougadosFunctionalIndex(DFI)inaSpanishspeaking populationwithspondyloarthropathies.ClinExpRheumatol. 2003;21:451–8.
11.AkkocY,KaratepeAG,AkarS,KirazliY,AkkocN.ATurkish versionoftheBathAnkylosingSpondylitisDiseaseActivity Index:reliabilityandvalidity.RheumatolInt.2005;25: 280–4.
12.ElMiedanyY,YoussefS,MehannaA,ShebryaN,AbuGamraS, ElGaafaryM.Definingdiseasestatusinankylosing
spondylitis:validationandcross-culturaladaptationofthe ArabicBathAnkylosingSpondylitisFunctionalIndex(Basfi), theBathAnkylosingSpondylitisDiseaseActivityIndex (Basdai),andtheBathAnkylosingSpondylitisGlobalscore (Basg).ClinRheumatol.2008;27:605–12.
13.CusmanichKG(Dissertac¸ãodeMestrado)Validac¸ãoparaa línguaportuguesadosinstrumentosdeavaliac¸ãodeíndice funcionaleíndicedeatividadededoenc¸aempacientescom espondiliteanquilosante.FaculdadedeMedicinada UniversidadedeSãoPaulo;2006.
14.VanderHeijdeD,LieE,KvienTK,SieperJ,VandenBoschF, ListingJ,etal.ASDAS,ahighlydiscriminatoryAsas-endorsed diseaseactivityscoreinpatientswithankylosingspondylitis. AnnRheumDis.2009;68:1811–8.
15.MachadoP,LandewéR,LieE,KvienTK,BraunJ,BakerD,etal. AnkylosingSpondylitisDiseaseActivityScore(Asdas): definingcut-offvaluesfordiseaseactivitystatesand improvementscores.AnnRheumDis.2011;70:47–53. 16.DougadosM,vanderLindenS,JulinR,HuitfeldB,AmorB,
CalinA,etal.TheEuropeanSpondyloarthropathyStudy Grouppreliminarycriteriafortheclassificationof spondyloarthropathy.ArthritisRheum.1991;34: 1218–27.
17.VanderLindenS,ValkenburgHA,CatsA.Evaluationof diagnosticcriteriaforankylosingspondylitis.Aproposalfor modificationoftheNewYorkcriteria.ArthritisRheum. 1984;27:361–8.
18.MollJMH,WrightV.Psoriaticarthritis.SeminArthritis Rheum.1973;3:55–78.
19.KingsleyG,SieperJ.ThirdInternationalWorkshopon ReactiveArthritis,23–26September1995,Berlin,Germany. AnnRheumDis.1996;55:564–84.
20.GallinaroAL,VenturaC,Sampaio-BarrosPD,Gonc¸alvesCR. Espondiloartrites:análisedeumasériebrasileiracomparada aumagrandecasuísticaibero-americana(estudoRespondia). RevBrasReumatol.2010;50:581–9.
21.Heuft-DorenboschL,VanTubergenA,SpoorenbergA, LandewéR,DougadosM,MielantsH,etal.Theinfluenceof peripheralarthritisondiseaseactivityinankylosing spondylitispatientsasmeasuredwithBathAnkylosing SpondylitisDiseaseActivityIndex.ArthritisRheum. 2004;51:154–9.
22.TaylorWJ,HarrisonAA.CouldtheBathAnkylosing
SpondylitisDiseaseActivityIndex(Basdai)beavalidmeasure ofdiseaseactivityinpatientswithpsoriaticarthritis? ArthritisRheum.2004;51:311–5.
23.Fernandez-SueiroJL,WillischA,Pertega-DiazS,TasendeJA, Fernández-LópezJC,VillarNO,etal.ValidityoftheBath AnkylosingSpondylitisDiseaseActivityIndexforthe evaluationofdiseaseactivityinaxialpsoriaticarthritis. ArthritisCareRes.2010;62:78–85.
24.EderL,ChandranV,ShenH,CookRJ,GladmanDD.IsAsdas betterthanBasdaiasameasureofdiseaseactivityinaxial psoriaticarthritis?AnnRheumDis.2010;69:2160–4.
25.GlintborgB,OstergaardM,KroghNS,DreyerL,KristensenHL, HetlandML.Predictorsoftreatmentresponseanddrug continuationin842patientswithankylosingspondylitis treatedwithanti-tumournecrosisfactor:resultsfrom8 years’surveillanceintheDanishnationwideDanbioregistry. AnnRheumDis.2010;69:2002–8.
therapyinankylosingspondylitis:aprospectivelongitudinal observationalcohortstudy.ArthritisResTher.2011;13:R94. 27.CalinA,GarrettS,WhitelockH,KennedyLG,O’HeaJ,Malorie
P,etal.Anewapproachtodefiningfunctionalabilityin ankylosingspondylitis:thedevelopmentoftheBath AnkylosingFunctionalIndex.JRheumatol.1994;21:2281–5. 28.ValimV,MarianelliBF,BortoluzzoAB,Gonc¸alvesCR,Bragada
SilvaJA,XimenesAC,etal.Aplicac¸ãodoBasfi(Bath AnkylosingSpondylitisFunctionalIndex)numacoortede pacientesdoRegistroBrasileirodeEspondiloartrites(RBE). RevBrasReumatol.1492(submetido).
29.RoussouE,SultanaS.Spondyloarthritisinwomen: differencesindiseaseonset,clinicalpresentation,andBath AnkylosingSpondylitisDiseaseActivityandFunctional indices(BasdaiandBasfi)betweenmenandwomenwith spondyloarthritides.ClinRheumatol.2011;30:
121–7.
30.RoussouE,SultanaS.Earlyspondyloarthritisinmultiracial society:differencesbetweengender,race,anddisease subgroupswithregardtofirstsymptomatpresentation,main problemthatthediseaseiscausingtopatients,and