www.jped.com.br
ORIGINAL
ARTICLE
Erythrocyte
oxidative
stress
markers
in
children
with
sickle
cell
disease
夽
Priscila
Bacarin
Hermann
a,∗,
Mara
Albonei
Dudeque
Pianovski
b,
Railson
Henneberg
a,
Aguinaldo
José
Nascimento
a,
Maria
Suely
Soares
Leonart
aaDepartmentofClinicalAnalysis,ClinicalLaboratory,UniversidadeFederaldoParaná(UFPR),Curitiba,PR,Brazil
bDepartmentofPediatricHematologyandOncology,HospitaldeClínicas,UniversidadeFederaldoParaná(UFPR),Curitiba,PR,
Brazil
Received6July2015;accepted16October2015 Availableonline24April2016
KEYWORDS
Oxidativestress; Sicklecelldisease; Children
Abstract
Objective: Todetermine eightparameters ofoxidative stress markersinerythrocytesfrom
childrenwithsicklecelldiseaseandcomparewiththesameparametersinerythrocytesfrom healthychildren,sinceoxidativestressplaysanimportantroleinthepathophysiologyofsickle celldiseaseandbecausethisdiseaseisaseriouspublichealthprobleminmanycountries.
Methods: Bloodsamples wereobtained from 45 children withsickle celldisease (21 males
and24femaleswithameanageof9years;range:3---13years)and280bloodsampleswere obtainedfromchildrenwithouthemoglobinopathies(137malesand143femaleswithamean ageof10years;range:8---11years),asacontrolgroup.Allbloodsampleswereanalyzedfor methemoglobin,reducedglutathione,thiobarbituricacidreactivesubstances,percentageof hemolysis,reactiveoxygenspecies,andactivityoftheenzymesglucose6-phosphate dehydro-genase,superoxidedismutase,andcatalase.DatawereanalyzedusingStudent’st-testandwere expressedasthemean±standarddeviation.Ap-valueof<0.05wasconsideredsignificant.
Results: Significantdifferenceswereobservedbetweenchildren withsicklecelldiseaseand
thecontrolgroupfortheparametersmethemoglobin,thiobarbituricacidreactivesubstances, hemolysis, glucose 6-phosphate dehydrogenase activity, and reactive oxygen species, with higherlevelsinthepatientsthaninthecontrols.
夽
Pleasecitethisarticleas:HermannPB,PianovskiMA,HennebergR,NascimentoAJ,LeonartMS.Erythrocyteoxidativestressmarkersin childrenwithsicklecelldisease.JPediatr(RioJ).2016;92:394---9.
∗Correspondingauthor.
E-mail:prihermann@hotmail.com(P.B.Hermann). http://dx.doi.org/10.1016/j.jped.2015.10.004
Conclusions: Oxidativestressparametersinchildren’serythrocytesweredeterminedusing sim-plelaboratorymethodswithsmallvolumesofblood;thesebiomarkerscanbeusefultoevaluate diseaseprogressionandoutcomesinpatients.
©2016SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/ 4.0/).
PALAVRAS-CHAVE
Estresseoxidativo; Doenc¸afalciforme; Crianc¸as
Marcadoresdeestresseoxidativoemeritrócitosdecrianc¸ascomdoenc¸afalciforme
Resumo
Objetivo: Determinarparâmetrosdeestresseoxidativoemeritrócitosdecrianc¸ascomdoenc¸a
falciformeecompará-loscomosmesmosparâmetrosemeritrócitosdecrianc¸assaudáveis,pois oestresseoxidativodesempenhaumimportantepapelnafisiopatologiadadoenc¸afalciforme, consideradaumsérioproblemadesaúdepúblicaemmuitospaíses.
Métodos: Foramobtidasamostrasdesanguede45crianc¸ascomdoenc¸afalciforme(21meninos
e24meninascommédiade9anos,variac¸ãode3a13anos)e280amostrasdesanguedecrianc¸as semhemoglobinopatias(137meninose143meninascommédiade10anos,variac¸ãode8a 11anos),comogrupocontrole.Emtodasasamostrasforamdeterminadosmeta-hemoglobina, glutationareduzida,substânciasreativasao ácidotiobarbitúrico,porcentagemdehemólise, espéciesreativasdeoxigênioeatividadedasenzimasglucose6-fosfatodesidrogenase, superóx-idodismutaseecatalase.OsdadosforamanalisadoscomotestetdeStudenteforamexpressos comomédia±desviopadrão.Umvalordep<0,05foiconsideradosignificativo.
Resultados: Foramobservadasdiferenc¸assignificativasentreascrianc¸ascomdoenc¸afalciforme
eogrupocontroleparaosparâmetrosmeta-hemoglobina,substânciasreativasaoácido tio-barbitúrico,porcentagemdehemólise, espéciesreativasde oxigênioe atividadedaenzima glucose6-fosfatodesidrogenase,comníveisaumentadosnospacientes.
Conclusões: Foi possível determinar parâmetros de estresse oxidativo em eritrócitos de
crianc¸as,com técnicas laboratoriaissimples e pequenos volumes de sangue.Esses biomar-cadorespodemserúteisnaavaliac¸ãodaprogressãoedosresultadosdetratamentosdadoenc¸a. ©2016SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Este ´eumartigo OpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4. 0/).
Introduction
Sickle cell disease is one of the most common hemato-logic disordersin theworld andis a seriouspublichealth problem in many countries, including Brazil.1 There are
over 2 million Brazilian carriers of the sickle gene, and this disease is estimated to have an incidence of one in every 1000 live births. In 2001, a decree of the Ministry ofHealthincludedscreeningforhemoglobinopathiesinthe pre-existingscreeningprograms.2
Sickle cell disease has been characterized as a multi-system disease, associated with episodes of acute illness andprogressiveorgandamage,whichbeginsininfancyand is primarilyresponsible fora shortenedlife expectancyin affectedpatients.3Ratesofmorbidityandmortalityarestill
highforpatientswithsicklecelldisease.InBrazil,upto25% ofthechildrenaffecteddiedduringtheirfirst5yearsoflife, butearlydiagnosisandtreatmentmightreducetheserates andimprovetheirqualityoflife.4
Sickle hemoglobin results from a substitution of glu-tamic acid to valine at the sixth amino acid position of the -globin chain.5 This ostensibly minor change is the
origin of hemoglobin S, and is responsible for significant changes in the stability and solubility of the molecule.6
The tendency of deoxygenated hemoglobin S to undergo polymerization underlies the innumerable expressions of thesicklingsyndromeswithintravascularhemolysis.7 Free
plasmahemoglobinisabletoinitiatelipidperoxidation,and theheme,which readilydissociates frommethemoglobin, may contribute significantly to oxidative stress,8 which
might play a significant role in the pathophysiology of sicklecelldisease-relatedmicrovasculardysfunction, vaso-occlusion,anddevelopmentoforgandamage.9Biomarkers
ofoxidativestresscanthereforebepotentiallyuseful,both toidentifypatientswhoareathighriskofoxidativedamage andtoevaluatetheeffectsofanti-oxidativetherapies.10
Thepurposeofthisworkwastoevaluatetheparameters ofoxidativestressinerythrocytesfromchildrenwithsickle cell disease, including percentages of hemolysis, methe-moglobin,reducedglutathione,thiobarbituricacid-reactive substances, glucose 6-phosphate dehydrogenase activity, reactiveoxygenspecies,andtheanti-oxidantenzymes cata-laseandsuperoxidedismutase.
Methods
Chemicals
saponin, trichloroacetic acid, and thiobarbituric acid weresupplied by VetecLtda (Riode Janeiro, RJ, Brazil). Sodium citrate, tris(hydroxymethyl)aminomethane, and methanol were obtained from Merck (Darmstadt, Ger-many). G6-PD activity was determined using a PD410 kit by Randox Laboratories (Antrim, United Kingdom). All organic solvents were of high quality and were double-distilled, and all the other chemicals were of analytical grade.
Bloodsamples
Bloodsamples were obtained from 45 children diagnosed with sickle cell disease (21 males and 24 females with a mean age of 9 years; range: 3---13) at the hematope-diatric department of Hospital de Clínicas, Universidade FederaldoParaná(UFPR).Acontrolgroupconsistedof280 children without hemoglobinopathies (137 males and 143 femaleswithameanageof10yearsold;range:8---11years) whowereparticipants of theuniversity extensionproject entitled ‘‘Incidenceof anemia and parasitic infections in school-aged children inmunicipal schools of metropolitan region of Curitiba-Parana --- Brazil,’’ from UFPR. The use ofhumansubjectswasapprovedbytheEthicalCommittee forResearchInvolvingHumans,HospitaldeClínicas,UFPR. Informedconsentwasobtainedfromtheguardiansforallthe children.Childrenwithanyhematologicalalterationwere excludedfromthestudy.
Avenousbloodsampleof5mLwascollectedfromeach patientinK3-EDTAcoatedtubes.Aliquots(200L)ofwhole bloodwereseparatedfordeterminationofG6-PDactivity. Then,sampleswerecentrifugedat3000×gfor10min.The plasma and the buffy coat were removed by aspiration, andtheerythrocyteswerewashedwithphosphatebuffered saline(PBS)(NaCl,150mmol/L;NaH2PO4,1.9mmol/L;and
Na2HPO4,8.1mmol/L)threetimes.Finally,redbloodcells
weresuspendedinPBSsolutionandwatertoobtain suspen-sions with hematocrits of approximately10% and 40% for PBSsolutionandof approximately40% forwater solution. Hemoglobinconcentrationwasmeasuredinallsuspensions. Notallanalyses wereperformed in eachspecimendue to thelimitedvolumesavailable.
Hematologicparameters
ThecompletebloodcountwasdeterminedusingthePentra 80electroniccellcounter(HoribaMedical,Japan).
Methemoglobinconcentration
Methemoglobinconcentrationwasdeterminedaccordingto a method basedon Naoum et al.11 adapted to small
vol-umes.Aliquots(100L)of10%erythrocytesuspensionswere hemolyzedwith100Lof 1% saponinand werestabilized in1000Lof60mmol/Lphosphatebuffer;theabsorbance wasthendeterminedat630nm(formethemoglobin)andat 540nm(foroxyhemoglobin).Methemoglobinconcentration wasexpressed as a percentagein relation to hemoglobin concentration.
Reducedglutathionedetermination
Reduced glutathione(GSH) concentration wasdetermined byamethodpreviouslydescribedbyBeutler,12byevaluating
thereductionof5,5′-dithiobis(2-nitrobenzoicacid)(DTNB) bysulfhydrylcompoundsfromtheformationofayellow col-ored anionic product whose absorbance was measured at 412nm.Aliquotsof50Lof40%suspensionofredbloodcell inPBSwereused.TheGSHconcentrationwasexpressedin
mol/gHb.
Lipidperoxidation
Lipidperoxidationofredbloodcellmembraneswasassessed based onCesquiniet al.13 Aliquots(600L) of a 10%
sus-pension of red blood cell were added to 250L of 25% trichloroacetic acid and 600L of 1% thiobarbituric acid, boiled for 15min at 100◦C, and cooled for 5min at 0◦C. The absorbance of the thiobarbituric acid reactive sub-stances (TBARS) formed was then read at 532nm using
ε=156/(mmolecm)andtheconcentrationsareexpressedin
nmol/gHb.
Measurementofhemolysis
Hemolysis of red blood cell was carried out as described byBanerjeeetal.,14adaptedtomicroplatesbymixing10%
suspensionofredbloodcellinPBSwithvaryingamountsof AAPHsolution(providingfinalconcentrationsof50,100,and 150mmol/L).Thisreactionmixturewasincubatedfor3hat 37◦Cwithshaking.Theextentofhemolysiswasdetermined spectrophotometricallybymeasuringtheabsorbanceofthe hemolysateat540nminamicroplatereader(Thermo Sci-entific, Thermo Plate, USA).Red blood cells in a solution of 200mmol/L of AAPH were used asthe 100% hemolysis control.
Activityofglucose6-phosphatedehydrogenase (G6-PD)
Aliquots(200L)of wholeblood beforeerythrocyte isola-tionwerewashedwith2mLPBSthreetimes.G6-PDactivity wasdeterminedusing theCobas Miraautomated analyzer (Roche, Mannheim,Germany) withthe PD410 commercial kit (Randox,Antrim,United Kingdom) asdescribed in the manufacturer’smanual.
Superoxidedismutaseactivity
Theenzymeactivitywasbasedonamethodadaptedfrom Beutler12 of the auto-oxidation of pyrogallol. Aliquots of
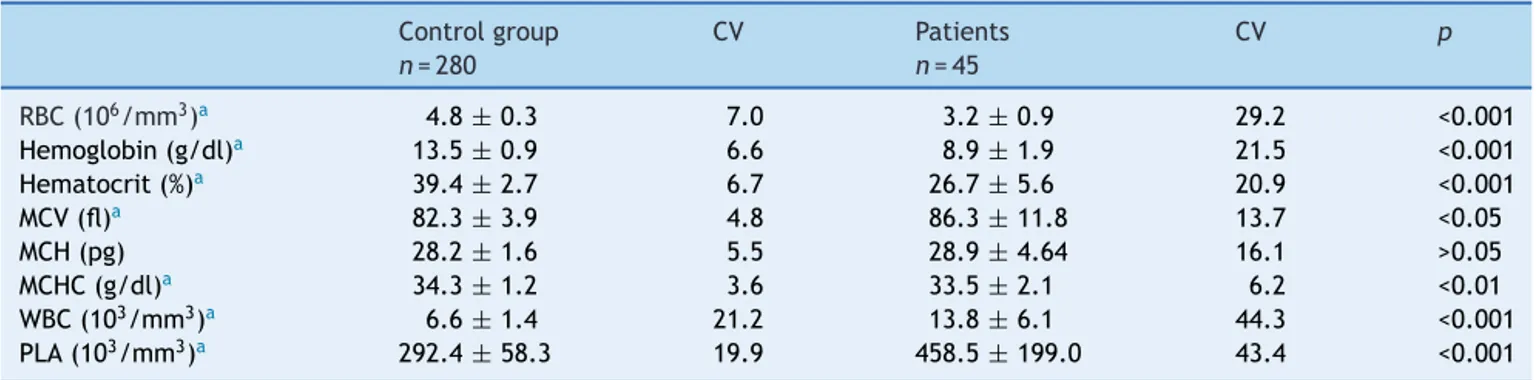
Table1 Hematologicalvaluesinhealthychildren(controlgroup)andpatientswithsicklecelldisease.
Controlgroup CV Patients CV p
n=280 n=45
RBC(106/mm3)a 4.8±0.3 7.0 3.2±0.9 29.2 <0.001
Hemoglobin(g/dl)a 13.5±0.9 6.6 8.9±1.9 21.5 <0.001
Hematocrit(%)a 39.4±2.7 6.7 26.7±5.6 20.9 <0.001
MCV(fl)a 82.3±3.9 4.8 86.3±11.8 13.7 <0.05
MCH(pg) 28.2±1.6 5.5 28.9±4.64 16.1 >0.05
MCHC(g/dl)a 34.3±1.2 3.6 33.5±2.1 6.2 <0.01
WBC(103/mm3)a 6.6
±1.4 21.2 13.8±6.1 44.3 <0.001
PLA(103/mm3)a 292.4±58.3 19.9 458.5±199.0 43.4 <0.001
RBC,redbloodcells;MCV,mediumcorpuscularvolume;MCH,mediumcorpuscularhemoglobin;MCHC,mediumcorpuscularhemoglobin concentration;WBC,whitebloodcells;PLA,platelets;CV,Pearson’scoefficientofvariation(%).Dataarepresentedasmean±standard deviation.
a Statisticallysignificancedifference(Student’st-test).
extractrequiredtoinhibitpyrogallolauto-oxidationby50% wasusedtodeterminethelevelofenzymeactivity.
Catalaseactivity
Theenzymeactivitywasdeterminedbyamethodadapted fromBeutler12 thatmeasurestherateofdecompositionof
hydrogen peroxide by catalase spectrophotometrically at 240nm. Aliquotsof 50L of 40% suspension of red blood cell wereadded to450Lof a hemolyzing solutionof  -mercaptoethanol(0.7mmol/L)andEDTA(0.27mol/L).This solutionwasdiluted1:100inPBSand10Lofthefinal solu-tion was added to 990L of hydrogen peroxide solution. The decrease in absorbance of the system wasmeasured for10min.
Intracellularreactiveoxygenspecies
Reactive oxygen species were determined according to a methodbasedonLópez-Revueltaetal.15adapted tosmall
volumes of blood samples in a microplate. Erythrocytes (995L of 10%, v/v suspension in PBS) were incubated with5L of dichlorodihydrofluorescein-diacetate (DCFDA, 10mol/L) at 37◦C for 30min. This suspension wasdiluted in 9.0mL of PBS and 37.5L of this was then added to 112.5L of PBSin 96-well plates.Determinationof reac-tive oxygen specieswasperformed using a GloMax®-Multi
MicroplateMultimodeReaderfluorimeter(Promega Corpo-ration,USA).Undertheseconditions,DCFDAwashydrolyzed to 2′,7′-dichlorodihydrofluorescein (DCFH
2), which then
becameavailableforoxidationbyreactiveoxygenspeciesto producefluorescent2,7-dichlorofluorescein(DCF). Fluores-cencewasdeterminedat530nmafterexcitationat495nm. Reactiveoxygenspeciesformationwasexpressedas fluores-cenceunits(UF)/gHb.
Statisticalanalyses
StatisticalanalysiswasperformedusingStatistica8.0 soft-ware (StatSoft, USA). No outliers were identified. The Kolmogorov---Smirnov test wasused to assess the normal-ity and all parameters were distributed normally. Data
wereexpressedasmean±standarddeviationandcompared betweengroupsusingStudent’st-test;ap-value<0.05was consideredsignificant.
Results
Datafrombloodcountsofhealthychildrenandpatientswith sickle cell disease are illustrated in Table 1. Statistically significant differences were observed for all parameters, exceptformediumcorpuscularhemoglobin(MCH;p<0.05). Datafromoxidative stressparametersareillustratedin
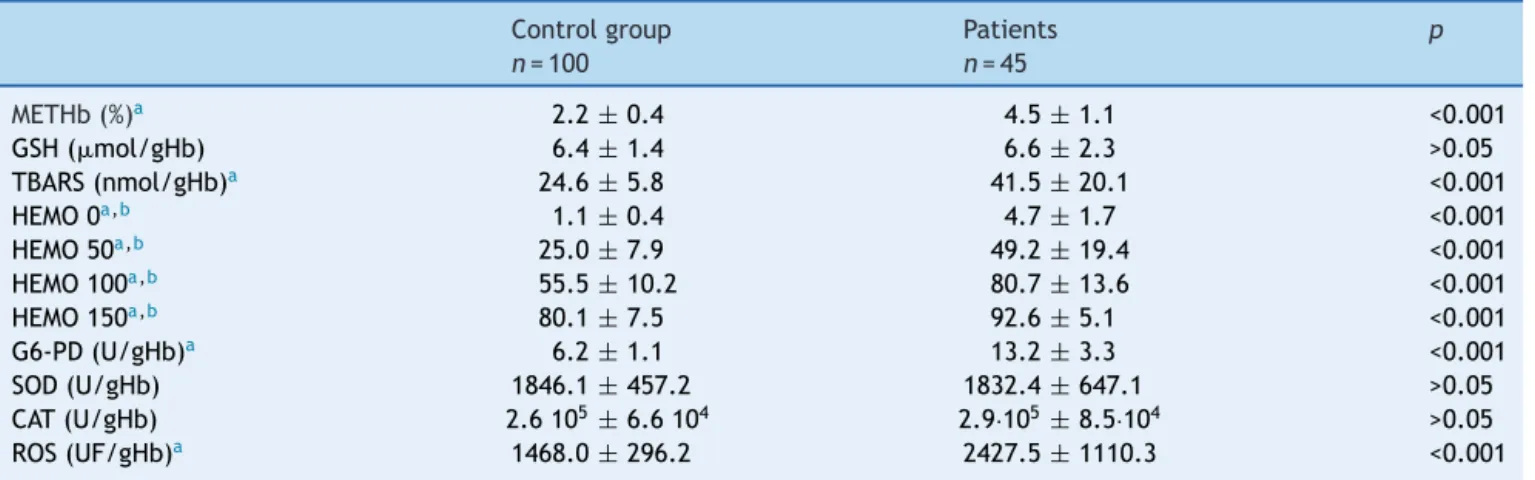
Table 2, comparing patients with sickle cell disease with healthychildren. Statistically significant differences were observedformethemoglobin,TBARS,percentageof hemol-ysis,G6-PDactivity,andreactiveoxygenspecies(p<0.05).
Discussion
Normalerythrocytessufferoxidativestressduetothe pro-ductionofreactiveoxygenspeciesthatresultsfromoxygen metabolism. However, this is efficiently repaired by the highlypowerfulantioxidantsystemsofthecellwithoutany problematiceffect.Oxidativestressoccursasaresultofan imbalancebetweenreactiveoxygenspeciesproductionand antioxidantdefenses.16
In sickle celldisease, oxidative stress may result from high levels of meta hemoglobin S, which is less sta-ble than meta hemoglobin A, leading to intravascular hemolysis,17ischemia-reperfusioninjury,chronic
inflamma-tion, and higher auto-oxidation of sickle hemoglobin.18,19
Manypotential antioxidants are of interest in relation to sicklecelldisease,20andseveralstudieshavedemonstrated
significantincreasesinstressmarkersanddiffering behav-iorinantioxidantdefensesystemsinpatientswithsicklecell diseasewhencomparedtothoseinhealthysubjects.21
Thepresentresultsforbloodcountsconfirmseveral fea-turesof sickle cell disease that are already known,such as the hemolytic anemia,21 evidenced by low levels of
hemoglobin7 andincreasedlevelsofwhiteblood cellsand
platelets.6
Aspreviouslydemonstrated,8methemoglobinlevelsare
Table2 Oxidativestressparametersinnormalchildren(controlgroup)andpatientswithsicklecelldisease.
Controlgroup Patients p
n=100 n=45
METHb(%)a 2.2±0.4 4.5±1.1 <0.001
GSH(mol/gHb) 6.4±1.4 6.6±2.3 >0.05
TBARS(nmol/gHb)a 24.6±5.8 41.5±20.1 <0.001
HEMO0a,b 1.1±0.4 4.7±1.7 <0.001
HEMO50a,b 25.0±7.9 49.2±19.4 <0.001
HEMO100a,b 55.5±10.2 80.7±13.6 <0.001
HEMO150a,b 80.1
±7.5 92.6±5.1 <0.001
G6-PD(U/gHb)a 6.2±1.1 13.2±3.3 <0.001
SOD(U/gHb) 1846.1±457.2 1832.4±647.1 >0.05
CAT(U/gHb) 2.6105±6.6104 2.9·105±8.5·104 >0.05
ROS(UF/gHb)a 1468.0±296.2 2427.5±1110.3 <0.001
METHb,methemoglobin;GSH,reducedglutathione;TBARS,thiobarbituricacidreactivesubstances;HEMO,hemolysis;G6-PD,glucose 6-phosphatedehydrogenase;SOD,superoxidedismutase;CAT,catalase;ROS,reactiveoxygenspecies.Datapresentedasmean±standard deviation.
aStatisticallysignificantdifference(Student’st-test).
b Percentagesofhemolysiswithadditionof0---150mmol/LofAAPHsolutions.
an electron transfer in the bonding interaction between thehemeandthe oxygen(O2) inoxygenated hemoglobin.
When hemoglobin deoxygenates, the heme iron normally remainsintheferrousstate.20 Inthisexchange,alterations
wherein hemoglobinautoxidizes resultin methemoglobin, withthehemeironinferricstate.8Alterationsin
erythro-cytefunctionorstructurecanleadtoanenhancedflowof methemoglobinthatcanleadtooxidativestress.15
The increased intra- and extra-erythrocytic oxidative stressinduceslipidperoxidationandmembraneinstability.14
TBARSisoneoftheexistingbiomarkers,andthisevaluation isanindirectquantificationoflipidperoxidationprocesses, which makes it a good indicator of pro-oxidant stimuli. In accordance with results reported previously,19,20,22 the
presentstudyobservedsignificantlyhigherlevelsofTBARS inpatientswithsicklecelldiseasethaninthecontrols.
Rigidanddeformedsickleerythrocyteshaveashortened lifespanand undergobothintravascular andextravascular hemolysis.23Higherpercentagesofhemolysisinerythrocyte
fromchildren with sickle celldisease than in the control groupwereobserved,bothinbasalsuspensionsof erythro-cytesandinsuspensionsincubatedwithanoxidizingagent. G6-PDisanimportantenzymerelatedtotheantioxidant defense in erythrocytes.20 Higher activity of this enzyme
inpatients withsickle cell diseasewas found thanin the controlgroup.Itwaspreviouslyreportedthaterythrocytes from patients with sickle cell disease have an increased percentageofreticulocytes,whiletheactivityofG6-PDin reticulocytes is normal,but declines exponentiallyasthe redcellsage.24
Sickle cells spontaneously generate approximately two timesmorereactiveoxygenspeciesthannormalredblood cells.25 Inaccordancewiththefindingsof Georgeetal.,26
elevatedlevelsofreactiveoxygenspeciesinsickle erythro-cyteswerealsodemonstrated.
Reducedglutathione(GSH)ispresentathigh concentra-tions in erythrocytesand acts by itself or viaglutathione peroxidase as a major reducing source to maintain cell integrity.17 ThemeasurementsofGSHanditsoxidizedform
glutathione disulfide (GSSG) have been considered useful indicatorsofinvivooxidativestress.27Themajorityof
stud-iesofadultswithsicklecelldiseasereportedsomedeficitsof endogenoussynthesisofGSH,probablyduetoits consump-tionbyincreasedoxidantproduction.26,28AlthoughRusanova
etal.22showedhighlevelsofGSHinpediatricpatientswith
sicklecelldisease,thepresentstudyfoundnodifferencein GSHlevelsbetweenchildrenwithsicklecelldiseaseandthe controlgroup.
Superoxidedismutasecanconvertsuperoxideto hydro-gen peroxide, and catalase can remove excess hydrogen peroxide.16 AccordingtoSilva etal.,20 the increased
pro-oxidant generation in sickle cell disease results in an antioxidantdeficiency.However,therearesome discrepan-ciesbetweenstudiesonsuperoxidedismutaseandcatalase levelsinthisdisease,withsomestudiesobservingincreased activityandothersobservingdecreasedlevels.29Anincrease
intheseenzymesactivitypotentiallyconstitutesadefense mechanism in response toincreasedoxidative stress,19 or
might beaconsequenceof increasedreticulocytecontent in blood samples from patients with sickle cell disease. However,a decreasein enzyme levelswasrelatedto dis-easeseverityinpatients.20,22Theseseeminglycontradictory
findingscouldbeduetodifferencesintheextentof oxida-tive stress, disease severity, enzyme polymorphism, and the enzyme co-factor.29 The present results showed no
differencebetweentheactivitiesoftheseenzymesin chil-drenwithsicklecelldiseaseandthoseinhealthychildren, accordingwithChoetal.30 withregardtocatalase.These
results may be due to large individual variability found amongpatients.
Inlightofevidencesuggestingthatanexcessofoxidative stress has implications in sickle cell disease pathophysio-logy,theassessmentofoxidativestressparametersinthese patientsmayprovideuseful informationregardingtheuse of current medications andmay leadto thedevelopment of newtherapeutic strategies.10,19,20 Monitoringthe
However, the use of an isolated biomarker and the mea-surement of individual antioxidants are not likely to be usefulindexesofoxidativestatus.Theoxidant---antioxidant balance involves biochemical reactions that require the evaluationofmanyendpoints.26
Thepresentstudyevaluatedeightoxidativestress mark-ers,includingpro-oxidantandantioxidantparameters.The resultsindicatethepresenceofahyperoxidative statusin childrenwithsicklecelldisease,whichcanbeobservedby theirhighlevelsofmethemoglobin,TBARS,hemolysis, reac-tiveoxygenspecies,andG6-PDactivity.Simpletechniques wereusedtodeterminetheseparametersusingsmall vol-umesofblood.Theseparametersthatappearedalteredin childrenwithsicklecelldiseasecanbeusefulinthe evalu-ationofdiseaseprogressionandtreatment.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Felix AA, Souza HM, Ribeiro SB. Epidemiologic and social aspects of sickle cell disease. Rev Bras Hematol Hemoter. 2010;32:203---8.
2.RamalhoAS,MagnaLA,dePaiva-e-SilvaRB.Government Direc-tiveMS# 822/01: uniqueaspects ofhemoglobinopathies for publichealthinBrazil.CadSaudePublica.2003;19:1195---9.
3.ThompsonBW,MillerST,RogersZR,ReesRC,WareRE,Waclawiw MA, et al. The pediatric hydroxyurea phase III clinical trial (BABYHUG):challengesofstudydesign.PediatrBloodCancer. 2010;54:250---5.
4.Sabarense AP, Lima GO, Silva LM, Viana MB. Characteriza-tionofmortalityinchildrenwithsicklecelldiseasediagnosed through the Newborn Screening Program. J Pediatr (Rio J). 2015;91:242---7.
5.Aslan M, Freeman BA. Redox-dependent impairment of vas-cular function in sickle cell disease. Free Radic Biol Med. 2007;43:1469---83.
6.Wang WC. Sickle cell anemia and other sickling syndromes. In:Greer JP, FoersterJ,Lukens JN, RodgersGM,Paraskevas F,GladerB,editors.Wintrobes’sclinicalhematology.11thed. Baltimore:LippincottWilliams&WilkinsPublishers;1999. p. 1293---311.
7.SteinbergMH.Managementofsicklecelldisease.NEnglJMed. 1999;340:1021---30.
8.HansonMS,PiknovaB, KeszlerA,DiersAR, WangX,Gladwin MT,et al. Methaemalbumin formationin sickle cell disease: effectonoxidative proteinmodificationandHO-1induction. BrJHaematol.2011;154:502---11.
9.MorrisCR,SuhJH,HagarW,LarkinS,BlandDA,SteinbergMH, etal.Erythrocyteglutaminedepletion,alteredredox environ-ment,andpulmonaryhypertensioninsicklecelldisease.Blood. 2008;111:402---10.
10.Rees DC, Gibson JS. Biomarkers in sickle cell disease. Br J Haematol.2012;156:433---45.
11.NaoumPC,RadispielJ,MoraesMS.Spectrometricmeasurement ofmethemoglobinwithoutinterferenceofchemicalor enzy-maticreagents.RevBrasHematolHemoter.2004;26:19---22.
12.BeutlerE.Redcellmetabolism.Amanualofbiochemical meth-ods.3rded.NewYork:Grune&Stratton;1984.
13.CesquiniM,TorsoniMA,Stoppa GR, OgoSH.t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids.BiomedPharmacother.2003;57:124---9.
14.BanerjeeA,KunwarA,MishraB,PriyadarsiniKI.Concentration dependentantioxidant/pro-oxidantactivityofcurcumin stud-iesfromAAPHinducedhemolysisofRBCs.ChemBiolInteract. 2008;174:134---9.
15.López-RevueltaA,Sánchez-GallegoJI,Hernández-HernándezA, Sánchez-YagüeJ, Llanillo M. Membrane cholesterolcontents influence the protective effects of quercetin and rutin in erythrocytesdamagedbyoxidativestress.ChemBiolInteract. 2006;161:79---91.
16.Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species:oxidativedamageandpathogenesis.CurrSci.1999;77: 658---66.
17.vanZwieten R,Verhoeven AJ,Roos D.Inborndefectsinthe antioxidantsystemsofhumanredbloodcells.FreeRadicBiol Med.2014;67:377---86.
18.DasguptaT,FabryME,KaulDK.Antisickling propertyoffetal hemoglobinenhancesnitricoxide bioavailabilityand amelio-ratesorganoxidativestressintransgenic-knockoutsicklemice. AmJPhysiolRegulIntegrCompPhysiol.2010;298:R394---402.
19.GiziA,PapassotiriouI,ApostolakouF,LazaropoulouC, Papas-tamatakiM,KanavakiI,etal.Assessmentofoxidative stress inpatientswithsicklecelldisease:theglutathionesystemand theoxidant---antioxidantstatus.BloodCellsMol Dis.2011;46: 220---5.
20.SilvaDG,BeliniJuniorE,deAlmeidaEA,Bonini-DomingosCR. Oxidativestressinsicklecelldisease:anoverviewof erythro-cyte redox metabolism and current antioxidant therapeutic strategies.FreeRadicBiolMed.2013;65:1101---9.
21.BeliniJuniorE,daSilvaDG,TorresLDS,deAlmeidaEA,Cancado RD,ChiattoneC,etal.Oxidativestressandantioxidantcapacity insicklecellanaemiapatientsreceivingdifferenttreatments and medicationsfor differentperiodsoftime.Ann Hematol. 2012;91:479---89.
22.Rusanova I, EscamesG, CossioG, de BoraceRG, Moreno B, ChahbouneM,etal.Oxidativestressstatus,clinicaloutcome, and-globingeneclusterhaplotypesinpediatricpatientswith sicklecelldisease.EurJHaematol.2010;85:529---37.
23.Banerjee T, Kuypers FA. Reactive oxygen species and phos-phatidylserineexternalizationinmurinesickleredcells.BrJ Haematol.2004;124:391---402.
24.McGannPT,WareRE.Hydroxyureaforsicklecellanemia:what have we learnedand what questions still remain?CurrOpin Hematol.2011;18:158---65.
25.GladerB. Hereditary hemolytic anemiasdueto enzyme dis-orders. In: Greer JP, Foerster J, Lukens JN, Rodgers GM, ParaskevasF,GladerB,editors.Wintrobes’sclinicalhematology. 11thed.Baltimore:LippincottWilliams& WilkinsPublishers; 1999.p.1115---40.
26.George A,Pushkaran S, KonstantinidisDG,KoochakiS,Malik P, Mohandas N, et al. Erythrocyte NADPH oxidase activity modulated byRac GTPases,PKC, and plasmacytokines con-tributes to oxidative stress in sickle cell disease. Blood. 2013;121:2099---107.
27.MagalhãesSM.Oxidativestatusinsicklecellanemia.RevBras HematolHemoter.2011;33:177---8.
28.FatimaM,KesharwaniRK,MisraK,Rizvi SI.Protectiveeffect oftheaflavinonerythrocytes subjectedtoinvitrooxidative stress.BiochemResInt.2013;2013:649759.
29.Daak AA, Ghebremeskel K, Mariniello K, Attallah B, Clough P, Elbashir MI. Docosahexaenoic and eicosapentaenoic acid supplementation does not exacerbate oxidative stress or intravascular haemolysis in homozygous sickle cell patients. ProstaglandinsLeukotEssentFattyAcids.2013;89:305---11.