h tt p : / / w w w . b j m i c r o b i o l . c o m . b r /
Environmental
Microbiology
Evaluation
and
enhancement
of
heavy
metals
bioremediation
in
aqueous
solutions
by
Nocardiopsis
sp.
MORSY1948,
and
Nocardia
sp.
MORSY2014
Mervat
Morsy
Abbas
Ahmed
El-Gendy
a,b,
Ahmed
Mohamed
Ahmed
El-Bondkly
c,∗ aDepartmentofBiologicalSciences,FacultyofSciences,KingAbdulazizUniversity(KAU),Jeddah,SaudiArabiabChemistryofNaturalandMicrobialProductsDepartment,NationalResearchCentre,Dokki,Giza,Egypt cGeneticsandCytologyDepartment,NationalResearchCentre,Dokki,Giza,Egypt
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received4May2015 Accepted20February2016 Availableonline29April2016 AssociateEditor:CynthiaCanêdoda Silva
Keywords: Biosorption Heavymetals Deadandlivebiomass Nocardiopsis
Nocardia Wastewater
a
b
s
t
r
a
c
t
An analysis of wastewater samples collected from different industrial regions of Egypt demonstrated dangerously high levels of nickel (0.27–31.50mgL−1), chromium (1.50–7.41mgL−1)andzinc(1.91–9.74mgL−1)intheeffluents.Alarmingly,theseheavymetals areamongthemosttoxicknownonestohumansandwildlife.Sixty-nineActinomycete iso-lates derivedfromcontaminatedsiteswereevaluatedundersingle, binary,andternary systemsfortheirbiosorptioncapacityforNi2+,Cr6+andZn2+fromaqueoussolutions.The results ofthestudy identifiedisolatesMORSY1948and MORSY2014asthemostactive biosorbents.Phenotypicandchemotypiccharacterizationalongwithmolecular phyloge-neticevidenceconfirmedthatthetwostrainsaremembersoftheNocardiopsisandNocardia genera,respectively.Theresultsalsoprovedthatforboththestrains,heavymetalreduction wasmoreefficientwithdeadratherthanlivebiomass.Theaffinityofthedeadbiomassof MORSY1948strainforNi2+,Cr6+ andZn2+undertheoptimizedpHconditionsof7,8and 7,respectivelyat40◦Ctemperaturewith0.3%biosorbentdosagewasfoundtobeas fol-lows:Ni2+(87.90%)>Zn2+(84.15%)>Cr6+(63.75%).However,thedeadbiomassofMORSY2014 strainunderconditionsofpH8and50◦
Ctemperaturewith0.3%biosorbentdoseexhibited thehighestaffinitywhichwasasfollows:Cr6+(95.22%)>Ni2+(93.53%)>Zn2+(90.37%).All heavymetalsunderstudywerefoundtoberemovedfromaqueoussolutionsinentirety whenthesorbentdosagewasincreasedto0.4%.
©2016SociedadeBrasileiradeMicrobiologia.PublishedbyElsevierEditoraLtda.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:ahmedbondkly@yahoo.com(A.M.A.El-Bondkly).
http://dx.doi.org/10.1016/j.bjm.2016.04.029
Introduction
Themostsignificantsourcesofheavymetalcontamination are humanactivitiesand industries suchaselectroplating, electroforming, painting, petrochemicals, chemical manu-facturing,pigments, rechargeablebatteries, electronicsand computerequipment,metals(coatings,platingandfinishing operations),steel,manufacturingdetergents,andcoins.The dischargeofuntreatedmetal-containingeffluentintothe nat-uralenvironmentinquantitiesthatexceedprescribedlimitsis becominganissueofgreatconcernandiscausing consterna-tiontoenvironmentalistsaswellasgovernmentagencies.1,2 Theexamplesofsuchmetalsthatare knowntobe signifi-cantlytoxictohumansaswellastheecologicalenvironment include chromium (Cr6+), nickel (Ni2+), zinc (Zn2+), copper
(Cu2+),lead(Pb2+),cadmium(Cd2+),andmercury(Hg2+).
Tech-nologiesforremovingheavymetals,includingmethodologies suchaschemicalprecipitation,ion-exchange,reverse osmo-sis,electro-dialysis, and ultra-filtration,are routinely being usedfortreatingindustrialwastewaterbutbecausetheysuffer fromdisadvantagessuchasbeingextremelyexpensivewhile being inefficientatmetalremoval and becausethey result ingenerationoftoxiccompounds,theyare nowbeingseen asbothuneconomicalandunfavorable.3Thereis,therefore, apressingneedforthedevelopmentofhighlyselectiveyet cheapandefficientalternativesthatcanmitigateheavymetal concentrations in wastewaterto environmentally accepted levels.Onesuchpromisingmethodologybeingdevelopedis thebioremediationofheavymetalsfromaqueoussolutions usingcertainspecifictypesofmetabolicallyactive(livecells) or inactive (dead cells) microbial biomasses.4 Studies have indicatedthatActinomycetesasbiosorbentsarebothefficient aswellaseconomicalintreatingeffluentsandremovingtoxic metalsfrom wastewater;this propertyisattributedtothe presenceofalargenumberoffunctionalgroupsontheircell wallsandtheirfilamentousmorphology.4–7
Thepresent studyisaimedassessingtheabilityof Acti-nomycetes toremove toxicheavy metals suchas Ni2+, Cr6+
andZn2+,from aqueoussolutions. Itisalsoanobjectiveof
thisstudytoimprovetheadsorptioncapacityofselected bio-sorbentstrains byoptimizingthe removalparameters (pH, temperature,biomassnature,deadoralive,biomassdosage, heavy metalconcentrations versus different contacttime). Wealsoaimtodeterminethedesorptionandrecovery effi-ciencyofthesemetalsfromthebiosorbentbiomassesandto determinethemetaltoxicityandregenerationabilityof Acti-nomycetesfollowingexposuretoawiderangeofheavymetals thatmightbepresentinrealindustrialwastewater.
Materials
and
methods
Chemicalsandfactoryeffluents
Deionized waterwas used forthe preparation ofstandard heavymetalsolutions(concentration:2gL−1).Freshlydiluted
solutionsofvaryingconcentrations(mgL−1:50,100,200,300,
400,and500)thatwereusedinallexperimentswereprepared byseriallydilutingwithdeionizedwater.Binaryandternary
metalsolutionswerepreparedusing100mgL−1foreachone
ofthemetalsunderstudyandmixingthesameinequal pro-portions.ThepHofthemetalionsolutionswasadjustedto thedesiredvaluesusingeitherconcentratedHNO3(65%)or
1MNaOH.8
Effluents belonging to a variety of industries were col-lected from ten different industrial regions ofEgypt (10th ofRamadan,GesrElSuez,Badrcity,6thofOctober,Shubra El-Kheima,Sadatcity,BorgEl-Arab,Abu-Rawash,free zone-Nasr city and El-Amerya,Egypt). Thecollected wastewater samples were filteredand placedin sterile 250mLconical flaskscontaining2.5mLnitricacid(conc.).Theseflaskswere kept at 4◦C until analyzed for their Ni2+, Cr6+ and Zn2+
contents.Thisassessment,conductedwithin24hof collec-tion,wasexecutedbyAtomicAbsorptionSpectrophotometer (AAS,Model-MseriesThermo-Scientific,NIOSH)aspreviously reportedbyEl-Gendyetal.9
IsolationandidentificationofActinomycetesbiosorbents
Actinomycetesbiosorbents were isolated from environments that are naturally rich in heavy metals such as polluted soils(10thofRamadanand6thofOctober,Egypt)andwater drainageareassuchastheNileDeltainLowerEgypt.Forsoil samples,about 100gofairdriedsoilwascollectedin plas-ticbags;pollutedwatersampleswerecollectedinscrewcap testtubesandstoredat4◦Ctostopanybiologicalactivityuntil processing(24h).ForisolationofActinomycetes,pollutedwater drainage samples were filteredand inoculated into Actino-mycetesisolationmediumusingtheserialdilutiontechnique. Soil sampleswere prepared byheat treatment at60◦C for 45minfollowed byovernight extraction in600mL distilled water by shaking. The samples were then centrifuged for 10minat4000rpm;and100Laliquotsof10−3to10−5
dilu-tionsofthesupernatantwereseriallydilutedandplatedon soilextractagar.10 Theplates wereincubated for7daysat 28◦C and Actinomycetescolonieswere identifiedvisually by their toughleatheryappearance.Thepresenceofbranched vegetativemyceliawithaerialmyceliaandsporeformation weremicroscopicallyanalyzedandisolatesfulfillingall crite-riaweretransferredperiodicallytotrypticsoyagarmedium. The69Actinomyceteisolatesobtainedwereanalyzedfortheir biosorptionefficiencyandthemostactivebiosorbentisolates, MORSY1948andMORSY2014wereselectedandidentifiedas previouslydescribed.11–25
Molecularidentification
depositedintheGenBankdatabaseunderaccessionnumbers: KP979750andKP979751,respectively.
Preparationofliveanddeadbiomasses
TheliveanddeadbiomassesoftheActinomyceteisolateswere used individually as natural biosorbents to test the biore-movalofNi2+,Cr6+andZn2+fromaqueoussolutions.Ten-day
oldculturespores(106CFUmL−1)ofeachActinomyceteisolate
weretransferredindividuallyinto500mLErlenmeyerflasks containing100mLbroth medium[(gL−1):peptone, 4; yeast
extract,2;glucose,10]andincubatedat30◦C,150rpmona rotaryshakerfor7days.Thereafter,theresultantbiomassof eachActinomyceteisolatewaspelletizedbyfiltrationthrough filterpapers(WhatmanNo.1),washedfivetimeswith0.1M NaClfollowedbysteriledistilledwaterinordertoremovenon biomassparticles.Deadbiomassexperimentswereperformed as described previously by Vijayaraghavan and Yun.30 The inactivecellswerewashedwith0.1MNaClfollowingwhich theyweretransferredtopreweightedaluminumfoilcapsand driedinanovenat60◦Cuntilconstantweightwasobtained. Toassesscompletedeathofthedriedcells,thesampleswere inoculatedintoPetridishescontainingthemediumdescribed previously;absenceofanygrowthwaspresumedtobe indica-tiveofpositiveresults.Livebiomasswasobtainedbyairdrying thecellsfollowedbypulverizingthemtoafinepowderusing aporcelainmortar.Theconcentrationsofbothliveanddead biomasses were calculatedsubsequent towhich theywere storedat4◦Ctillrequired.31,32
EvaluationofmetalbiosorptioncapacityofActinomycete isolates
Unless stated otherwise, the biosorption tests were con-ductedusingquick-fitflaskscontaining3gL−1deadbiomass
oftheindividualActinomycetesorbentunderstudy (biosorb-entdosage)in100mLaliquotsofmetalsolutioncontaining 100mgL−1 ofoneofthemetalionsofinterest.Flaskswere
keptonrotaryshakers(150rpm)at30◦CandpH6.0for3h. Subsequently,thesampleswerecentrifugedat10,000rpmfor 15min.Thesupernatantswere analyzedforresidual heavy metalsusingAtomicAbsorptionSpectrophotometer.Presence of Ni2+, Cr6+ and Zn2+ was determined by using different
lampsspecificforeachmetalatspecificwavelengths.Metal solutionswithoutbiomassadditionservedascontrol. Exper-imentswereconductedinduplicateandaveragevalueswere computed.Thefollowingequationwasusedtocomputethe in-solutionmetalbiosorptionefficiency(R)foreachmetalion byeach isolate;and theresultswereexpressedin percent-age terms:PercentBiosorption (R)=(Ci−Cf)/Ci×100, where
Cicorrespondstotheinitialmetalionconcentrationofthe aqueoussolutionandCfcorrespondstotheresidual concen-tration.Inaddition,metalbiosorptioncalculationsforeach metalunderbinaryandternaryconditionsand indifferent combinations orin realwastewaterconditions were calcu-latedbythefollowingequations:R1(%)=(C1i−C1f)/C1i×100,
R2(%)=(C2i−C2f)/C2i×100andR3(%)=(C3i−C3f)/C3i×100,
whereR1,R2 and R3are thebiosorptionefficienciesofthe first,second and third metal respectively(%); C1i, C2iand C3iare initial concentration offirst, second and the third
metal, respectively (mgL−1) and C1f,C2f and C3fare final,
post-biosorption,concentrationsofthefirst,secondandthird metalrespectively(mgL−1).33
FactorsaffectingtheefficiencyoftheNi2+,Cr6+andZn2+
bioremovalprocessbyMORSY1948andMORSY2014 isolates
For analyzingtheimpactofpH,biosorptionbyMORSY1948 andMORSY2014wascarriedoutwithvaryingpHvalues(2.0, 4.0,6.0,7.0,8.0,9.0,10and11)underconditionsinwhich3gL−1
biomass was dispersed in 100mLof a solution containing 100mgL−1ofindividualmetalofinterest.Theexperimentwas keptatcontinuousshaking(150rpm)for3hat30◦C follow-ingwhichtheaqueoussolutionswerecentrifugedandeach supernatantwasanalyzedforresidualmetalconcentration.
Foranalyzingtheeffectoftemperature,experimentswere conductedatdifferenttemperaturepoints(25,30,35,40,45, 50,55,60and65◦C)underoptimumpHfollowingwhichthe sampleswere analyzedforresidual metalconcentrationas describedabove.
Differentweightsofbiomass,rangingfrom0.05%to0.5%, were dispersed in each metal solution under optimized parameterstodetermineconditionsformaximummetalion biosorption. Flasks were leftforequilibration and then the solutionswerecentrifuged(10,000rpm;15min)andthefinal concentrationofeachmetalanditsbiosorptionefficiency(%) weredeterminedusingtheproceduresdescribedearlier.
Theeffectofinitialmetalconcentration(50,100,200,300, 400and500mgL−1ofNi2+,Cr6+andZn2+,separately)was
stud-iedbyanalyzingbiosorptionunderconditionswhereinallthe parameters (pH,temperature, and biosorbent dosage)were optimumforeachstrain.Flaskswereallowedtoattain equi-libriumontherotaryshakerand sampleswerecollectedat regulartimeintervals(10,30,60,120,180,240,300,360,420 and1440min)inordertodeterminebioremovalefficiency(%).
Desorptionexperiments
Toevaluatethedesorption efficiencyforeach heavymetal, Ni2+,Cr6+ andZn2+loadedbiomassesweredriedat60◦Cfor 24hafterattainingequilibriumofsorptionatoptimum condi-tions.Theexposureofthedriedbiomassto1MH2SO4for4hin
eachcycleallowedtheheavymetaltobereleased.Thereafter, thedesorbedmetalwasanalyzedand desorptionefficiency wascalculatedasdescribedbyChuetal.34
Determinationofmetaltoxicity
Inordertodeterminethetoxiceffectsofthevariousmetal ions(Ni2+,Cr6+,andZn2+)onthegrowthofbiosorbentstrains
MORSY1948andMORSY2014,andalsotodeducetheir toler-ancetowardotherheavymetalsthatmightbefoundpresent in contaminated sites and industrial wastewaters, spores suspension (107CFUmL−1) ofeach isolate was individually inoculated onto variety of cultivation media. The culture mediausedwereasfollows:(a)starchcaseinmedium(gL−1:
starch10,caseinpowder1),(b)St1medium(gL−1:peptone
Table1–AnalysisofindustrialwastewatercollectedfromdifferentindustrialregionsofEgypt.
Industry Region Metalanalysis(mgL−1)
Ni2+ Cr6+ Zn2+
SteelWool 10thofRamadan 25.16 3.1 5.84
Highvoltage 10thofRamadan 31.50 2.0 6.01
Electric 10thofRamadan 18.22 3.15 3.15
Painting 10thofRamadan 2.09 7.25 9.74
Food 10thofRamadan 0.85 1.80 1.91
Steel GesrElSuez 20.25 4.2 6.05
Preciseindustries GesrElSuez 11.38 3.0 4.00
Printing GesrElSuez 6.82 2.90 4.02
Chemicalandmedical BadrCity 4.59 4.13 6.51
Electromechanicalengineering 6thofOctober 29.16 5.06 3.00
Electric ShubraEl-Kheima 18.41 7.41 4.83
Electric SadatCity 24.36 3.98 2.77
Electric BorgEl-Arab 17.91 4.25 5.47
Chloride AbuRawash 8.15 4.99 5.00
Medicalsupplies freezone,Nasrcity 2.90 2.80 6.10
Food El-Amerya 0.27 1.50 2.00
Thresholdlimitvaluesofdischargeheavymetalsinto:
Undergroundreservoir,Nileandcanals(Law48/82) 0.1 0.05 1
Sewagesystem(Law44/2000) 1.0 0.05 2
Coastalenvironment(Law4/94) 0.1 0.05 2
5,K2HPO42),and(d)themodifiedKuster’smedium(gL−1:
glyc-erol 10,casein 0.3,KNO3 2, K2HPO4 2, NaCl2, MgSO4 0.05,
CaCO3 0.02).For the purposeofthe experimentthe
differ-entmediaweresupplementedwithindividualsterilefiltered metalions,concentrationsrangingfrom50to1500gmL−1;
someoftheheavymetalsincludedNi2+,Zn2+,Cr6+,Fe3+,Cu2+,
Cd2+,Pb2+,Co2+,Hg2+,Mn2+ andAr2+.Heavymetal
concen-trationsthat are required for50% inhibition(IC50)and the
minimum inhibitoryconcentration (MIC)were determined. Controlswerepreparedbyinoculatingthe samemediabut withoutanymetalsupplementation.Cultureswereincubated at28◦Ctillgrowthyieldincontrolflaskswasmaximaland nofurtherincreaseintheirlevelswasobserved;atthispoint biomasswasquantifiedforallcultures.Theregeneration abil-ityofbothMORSY1948andMORSY2014strainsweretestedby subculturingthemfromtheheavymetalstreatedculturesinto non-heavymetalsmedia.Foranalysispurposes,thevaluefor biomassproductioninmetaltreatedcultureswasexpressed asapercentageofthatobtainedinuntreatedcontrolcultures; thelaterwereconsideredas100%asperthemethoddescribed byDeepikaandKannabiran35andEl-Gendyetal.9
Results
and
discussion
Analysisofindustrialwastewatersamplesfromdifferent industrialregionsofEgypt
Theanalysisofeffluentstakenfromdifferentfactories repre-sentingdifferentindustrialregionsofEgypt(10thofRamadan, GesrElSuez,Badrcity,6thofOctober,ShubraEl-Kheima,Sadat city,BorgEl-Arab,Abu-Rawash,freezone–Nasrcityand El-Amerya)showedthat Ni2+ concentrationinthese effluents
rangedbetween0.27–31.50mgL−1 andthatthehighestNi2+
concentrationvalues(18.41–31.50mgL−1)weredetectedinthe
effluentsinregionswhereinelectromechanical,electrical,and steelindustrieswerelocated;thelowestconcentrationswere
detectedinregionswherefood industrieswere based(0.27 and 0.85mgL−1,Table1).ItwasobservedthatCr6+
concen-trationintheseeffluentsrangedfrom1.50to7.41mgL−1 but thatofZn2+ rangedbetween1.91and 9.74mgL−1.Whereas
thehighestamountsofCr6+ weredetectedintheindustrial
wastewater of electrical and painting industries (7.41 and 7.25mgL−1,respectively),thehighestamountsofZn2+ were
detectedintheeffluentsofpaintingandchemical&medical industries(9.74and6.51mgL−1,respectively;Table1).Asper
thethresholdlimit(TLV),governing thedischargeof indus-trialeffluentsintoundergroundreservoirs(Nilebranchesor canals),sewageandcoastalenvironment,thecontentofNi2+,
Cr6+ andZn2+ mustnotexceed(0.1,0.05and 1mgL−1,Law
48/82), (0.1,0.05 and 2mgL−1, Law4/94) and(1.0, 0.05 and 2mgL−1,Law44/2000).Asaresultofthistreatmentof
indus-trialwastewatersinordertodecreasetheenvironmentalload isimperativeforprotectingboththeenvironmentaswellas publichealth.Previousstudieshavealsoreporteddangerously high concentrations oftoxic metalsions such as mercury, chromium,cadmium,zinc,copper,lead,andnickelin indus-trialwastewatersandeffluents.4,9,36,37
Evaluation
of
metal
removing
potential
of
Actinomycete
isolates
and
their
function
as
biosorbents
Insinglemetalsystem
AllActinomyceteisolates(69)obtainedfrompollutedsiteswere found toremoveappreciableamountsofthe heavymetals understudy(Ni2+;Cr6+andZn2+)asisclearlyevidentfromthe
biosorptionvalues(%)ofthesemetals(Table2).Amongallthe isolatesthatweretested,itwasfoundthatthebiomassof iso-lateMORSY1948supportedthehighestremovalofZn2+(67.4%)
followedbyNi2+(60.1%)alongwithsignificantremovalofCr6+
Table2–Comparativerepresentationofbiosorptionefficiency(%)ofvariousNi2+,Cr6+,Zn2+heavymetalsunderdifferent
metalsystemsbyActinomycetebiosorbentisolates(biosorptionconditionsare100mgL−1ofsuchheavymetal,0.3%of
biosorbent,pH6.0at30◦Cand150rpmfor3h).
Biosorbents Metalsystem/biosorption(%)
Singlemetalsystem Binarymetalsystem Ternarymetalsystem
Ni2+ Cr6+ Zn2+ Ni2++Cr6+ Ni2++Zn2+ Cr6++Zn2+ Ni2++Cr6++Zn2+
Ni2+ Cr6+ Ni2+ Zn2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+
Morsy-1948 60.1 47.4 67.4 79.5 51.0 72.48 71.28 54.94 75.62 74.0 58.4 81.3
Morsy-1949 35.4 20.0 38.0 32.0 16.4 30.2 27.3 19.0 35.1 28.1 16.0 42.3
Morsy-1950 19.0 18.7 55.0 17.0 15.0 19.0 42.7 16.5 41.0 16.4 13.9 58.4
Morsy-1951 56.2 20.0 41.2 54.5 14.5 30.1 59.0 17.0 57.6 47.5 12.7 42.0
Morsy-1952 31.9 24.6 60.0 38.2 19.4 40.0 59.5 21.8 56.9 43.0 15.8 50.9
Morsy-1953 60.0 30.0 49.4 59.0 22.1 55.3 48.0 26.5 45.0 54.1 20.2 43.7
Morsy-1954 29.5 20.8 55.0 29.0 15.9 26.7 53.6 17.5 51.5 21.7 10.2 50.5
Morsy-1955 58.1 9.1 49.7 55.1 2.0 52.8 46.0 6.1 45.4 50.0 1.9 39.9
Morsy-1956 50.0 13.5 67.0 44.2 9.3 45.1 67.0 12.2 46.0 41.0 7.0 66.0
Morsy-1957 22.9 46.0 61.5 19.7 43.2 18.0 59.9 48.8 59.2 13.5 42.5 56.5
Morsy-1958 20.8 18.5 49.6 17.0 14.9 15.4 48.0 16.0 47.0 13.0 11.6 34.3
Morsy-1959 55.2 10.0 50.8 51.9 31.0 51.6 46.9 37.3 46.4 50.6 30.0 45.1
Morsy-1960 47.0 40.7 30.2 45.0 34.8 40.9 30.0 37.2 26.8 37.4 31.7 25.0
Morsy-1961 21.6 21.0 19.0 18.0 35.7 16.2 17.5 40.0 15.0 13.8 34.1 19.7
Morsy-1962 47.3 39.0 60.0 44.5 30.8 40.9 66.8 35.4 54.3 38.6 30.0 54.1
Morsy-1963 50.8 40.2 58.5 47.9 35.6 43.5 55.0 39.0 56.4 40.0 33.5 52.8
Morsy-1964 41.2 50.0 60.0 46.3 44.5 37.3 45.9 48.5 60.0 36.1 43.0 60.0
Morsy-1965 25.0 20.1 61.5 23.4 15.0 20.1 59.4 19.0 54.1 17.5 11.7 51.9
Morsy-1966 42.7 40.0 56.0 41.0 36.2 37.0 55.0 37.1 652.5 35.3 35.8 50.0
Morsy-1967 50.3 40.6 57.4 48.5 34.9 45.7 54.0 40.0 54.0 40.8 30.3 51.7
Morsy-1968 58.0 41.8 30.0 56.0 37.0 51.0 37.1 38.5 33.2 47.4 36.0 32.5
Morsy-1969 50.2 50.0 49.0 49.1 46.1 43.6 45.7 48.0 41.6 40.6 42.8 35.1
Morsy-1970 38.5 39.4 62.8 36.2 33.5 30.3 66.5 35.8 61.0 30.0 32.0 58.6
Morsy-1971 47.4 40.0 53.4 45.0 36.4 44.0 49.9 36.9 47.5 43.2 35.4 47.0
Morsy-1972 30.0 45.8 28.1 33.0 37.8 25.0 87.2 41.9 84.0 22.0 35.9 20.0
Morsy-1973 22.8 44.0 60.2 18.0 40.0 16.6 66.0 41.7 65.3 13.9 38.6 60.3
Morsy-1974 53.5 38.2 20.0 46.7 31.9 48.5 14.9 35.2 12.7 44.0 28.7 10.9
Morsy-1975 49.8 53.0 65.0 50.0 50.0 43.9 65.3 52.0 50.4 42.0 49.4 55.8
Morsy-1976 38.4 42.0 58.3 33.5 36.8 30.4 54.1 39.5 51.6 26.3 35.0 47.0
Morsy-1977 51.5 53.0 25.0 47.4 48.0 46.6 22.5 50.6 15.9 43.4 44.5 12.2
Morsy-1978 20.0 30.0 60.4 18.9 24.2 14.8 66.0 28.0 52.1 14.0 21.2 45.5
Morsy-1979 54.5 20.8 49.0 54.1 15.3 47.1 47.3 17.2 44.0 42.5 15.0 38.4
Morsy-1980 20.3 19.0 44.0 17.1 15.0 16.5 41.6 18.0 38.7 13.8 13.7 36.0
Morsy-1981 33.9 22.7 60.5 30.0 18.4 30.0 56.0 19.9 52.0 27.5 16.6 50.3
Morsy-1982 54.2 40.4 34.0 50.7 34.9 46.1 31.2 37.5 30.3 45.2 34.0 30.0
Morsy-1983 19.1 14.0 67.1 16.8 10.2 14.3 64.0 11.8 64.5 11.2 9.1 60.6
Morsy-1984 47.6 39.1 14.0 46.4 32.8 40.5 11.1 35.4 10.3 38.1 29.5 9.5
Morsy-1985 50.8 40.5 50.2 50.0 32.9 64.9 48.0 37.5 44.0 45.4 29.0 41.9
Morsy-1986 50.5 10.7 61.3 47.9 5.1 44.2 50.0 10.0 37.2 20.7 1.8 25.4
Morsy-1987 37.0 19.0 50.0 35.5 16.5 34.0 41.9 19.0 40.5 33.0 17.2 32.6
Morsy-1988 53.5 30.0 56.3 51.0 23.0 49.6 52.4 26.0 42.7 45.3 21.6 39.3
Morsy-1989 49.6 9.9 60.0 45.9 5.0 40.4 59.1 7.3 65.9 40.0 2.5 65.1
Morsy-1990 32.4 35.0 60.6 30.0 29.0 28.0 66.7 33.5 66.3 25.8 27.4 60.0
Morsy-1991 36.0 20.2 60.5 32.7 12.0 31.5 57.5 17.8 53.0 29.0 10.5 50.2
Morsy-1992 51.9 50.0 49.0 50.0 40.9 44.7 45.0 45.0 41.7 54.0 39.7 39.2
Morsy-1993 54.5 44.5 30.0 52.7 39.5 52.0 26.2 43.1 23.1 50.1 38.0 20.0
Morsy-1994 13.0 40.4 57.7 11.6 40.0 8.9 52.3 40.2 50.4 7.9 39.1 50.0
Morsy-1995 55.0 38.9 60.0 41.8 32.7 40.8 57.0 36.2 57.0 39.3 31.0 55.5
Morsy-1996 44.6 22.0 61.3 44.0 20.0 42.1 68.4 21.3 65.6 39.5 19.3 58.1
Morsy-1997 60.0 50.3 30.0 55.9 44.6 50.2 30.0 47.1 41.0 47.8 42.8 35.2
Morsy-1998 19.1 20.7 65.3 16.3 13.5 15.0 71.5 16.5 59.5 14.6 12.8 59.0
Morsy-1999 44.9 10.0 19.4 43.0 7.8 46.9 25.8 18.0 15.2 46.5 16.5 22.9
Morsy-2000 47.1 40.6 50.0 47.0 35.7 46.3 44.5 37.4 42.8 45.0 33.2 41.6
Morsy-2001 59.5 47.0 50.0 55.0 40.3 53.0 39.0 45.0 41.0 52.2 39.5 37.5
Morsy-2002 45.0 30.3 41.0 44.0 22.0 40.8 33.0 25.5 35.6 40.0 20.9 32.8
Table2–(Continued)
Biosorbents Metalsystem/biosorption(%)
Singlemetalsystem Binarymetalsystem Ternarymetalsystem
Ni2+ Cr6+ Zn2+ Ni2++Cr6+ Ni2++Zn2+ Cr6++Zn2+ Ni2++Cr6++Zn2+
Ni2+ Cr6+ Ni2+ Zn2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+
Morsy-2004 90.0 20.0 28.5 88.1 15.0 80.2 25.5 27.2 25.4 80.0 12.6 80.0
Morsy-2005 25.4 45.3 40.6 24.0 40.1 27.9 37.9 43.0 32.0 25.9 39.4 27.1
Morsy-2006 60.0 48.0 35.2 58.0 44.5 55.5 33.0 46.1 32.5 50.6 43.1 30.5
Morsy-2007 15.3 39.6 57.0 11.9 36.0 11.0 53.2 39.0 62.4 10.3 37.8 55.0
Morsy-2008 18.0 14.2 64.2 18.0 9.2 16.5 59.0 11.5 69.2 16.0 8.0 66.7
Morsy-2009 35.0 50.0 23.5 32.7 35.0 29.9 25.1 37.9 29.6 30.5 32.9 24.7
Morsy-2010 9.9 20.5 20.5 7.9 16.0 4.7 17.2 17.6 17.0 10.9 14.5 15.8
Morsy-2011 58.0 20.0 41.3 56.0 15.2 55.4 37.4 17.3 26.4 52.6 15.0 29.0
Morsy-2012 39.6 40.7 64.0 37.1 35.8 33.2 60.0 37.0 60.0 33.0 33.8 60.0
Morsy-2013 52.1 50.1 30.5 40.0 42.4 44.0 45.0 45.9 38.0 39.3 41.7 41.6
Morsy-2014 50.5 59.4 62.8 60.65 68.19 69.19 51.81 86.2 41.6 62.92 77.64 32.0
Morsy-2015 50.4 20.0 50.5 50.0 17.0 48.5 42.9 18.2 46.8 75.0 15.6 40.0
Morsy-2016 55.0 49.3 47.0 49.4 42.5 48.0 45.1 47.5 40.5 47.2 41.5 40.0
MORSY2014exhibitedthehighestremovalefficiencyforCr6+
(59.4%)followedbyZn2+(62.8%)andNi2+(50.5%).This
behav-ioroftheMORSY2014straincanbeexplainedbyitssuperior abilitytosequestersubstantialamountsoftheheavymetals fromaqueoussolutionwhencomparedtotheotherisolates. Boththestrains,MORSY1948andMORSY2014,wereselected forfurtherstudies.Itiswellestablishedthatadaptedmicrobial populationsarepronetoexhibithigherresistancetoheavy metals as compared to populations of non-contaminated sites.9,36 Reports published by previous studies have sup-portedtheroleofActinomycetesintheremovaloftoxicmetals fromthe contaminatedsoil;examples include:Streptomyces coelicolor(Cu2+),S.pimprina(Cd2+),S.rimosus(Cd2+,Pb2+,Cu2+,
Zn2+,Fe3+ andCr6+),Streptoverticilliumcinnamoneum(Pb2+),S.
rimosus(Fe3+),S.rimosus(Ni2+),NocardiaerythropolisIAM1399
(Th4+andU6+),S.rimosusandStreptoverticilliumcinnamoneum
(Zn2+),Actinomycetestrains(Cr6+),Streptomycessp.19H(Au2+),
Arthrobacterspecies(Cr3+andCr6+).1,6,36,38,39Itishypothesized thatthe superiormetaladsorbing capacityofActinomycetes mightbeduetotherelativelyhighphosphoruscontentoftheir cellwall,asitisknownthatthemajormetalbindingsiteisthe teichoicacidmoiety.5,31
Inbinaryandternarymetalsystems
Althoughrealwastewatertreatmentsystems oftenhaveto dealwithamixtureofheavymetals,mostresearchworkstill focusesonthesorptioncapacityunderasinglemetalsystem. AsisevidentfromTable2,themetalbiosorptioncapacityof Actinomyceteisolatesunderbinary andternarysystemswas observedtoexhibitnosignificantinterferenceconsequentto competitionbetweenmetalsforbioremoval.Incaseofthe iso-lateMORSY1948,whenin thepresenceofabinary system composedofNi2++Zn2+ orCr6++Zn2+,itwasobservedthat
thepresenceofZn2+ledtoanenhancementofthe
biosorp-tioncapacityofNi2+andCr6+by20.6%and15.9%,respectively.
Thisobservationwasinparallelwithresultsthatsuggestedan increaseintheremovalefficiencyofZn2+,inthesebimetallic
systems,by5.8%and12.2%,respectively.Boththeseresults
aresuggestiveoflesserobservableinterferencebetweenthese three metalsforbinding tothe metalbinding siteson the biomassofMORSY1948(Table2). Similarly,itwasobserved thatinabinarysystemcomposedofCr6+andNi2+,theremoval
capacityofMORSY1948wasestimatedtobe51.0%and79.5% as comparedto 47.4% and 60.1% in singlesystem, respec-tively.IncaseofthesecondstrainMORSY2014,Zn2+removal
wasobservedtodeclineby17.5%overitsmaximumremoval efficiency of62.8% whenpresent in abinary system along withNi2+.Incontrast,asignificant37%enhancementinNi2+
removalwasobservedinthepresenceofZn2+ assecondary
metal.When thedeadbiomassofMORSY2014wasusedas abiosorbent,itwasalsoobservedthatthepresenceofZn2+
enhancedtheremovalofCr6+inbimetallicsystemcomposed
ofCr6++Zn2+(59.4%–86.2%)butCr6+suppressedZn2+removal
efficiencyto41.6%.Interestingly,inthebimetallicsystemof Cr2++Ni2+therewasnoobservablecompetitionbetweenthe
biosorptioncapacitiesofbothmetalionsastheremoval capa-bilityforbothmetalswereseentoincreaseby14.8%and20.1%, respectively(Table2).
In a multi-metallic ternary mixture composed of Ni2++Cr6++Zn2+inaqueoussolution,itwasobservedthatthe
removalefficiency theMORSY1948 biomassincreasedfrom 60.1%,47.4%and67.4%to74%,58.4%and81.3%,respectively (Table2).Similarly,incaseofMORSY2014the removal effi-ciencyforNi2+andCr6+wasobservedtosignificantlyincrease
by24.6%and30.7%,respectivelywhenobservedinaternary system.HoweverthesamecannotbesaidforZn2+which
wit-nessedasharpdecreasefrom62.8%inthesinglemetalsystem to32.0%intheternarymetalsystem.Thisobservationcanbe hypotheticallyexplainedasaconsequenceresultof compet-itive interactions formetalbindingsites andaccumulation ofZn2+aswellastheothertwometals,Ni2+andCr6+,inside
the MORSY2014 biomass (Table 2). Very limited literature is availableregardingsorption ofmetalsfrom heavymetal mixtures.ApublishedstudybyKaewsam40reportedthatin mixedmetalsystems,Cu2+uptakewassignificantlyaffected
bypresenceofotherheavymetalssuchasAg+,Mn2+,Co2+,
theorderofadsorptionpotentialofdifferentheavymetals, incaseofS.viridochromogeneswasZn2+>Cu2+>Pb2+>Cd2+in
singleandmixedmetalreactions, whereas thesame forS. chromofuscusK101wasZn2+>Pb2+>Fe2+≥Cu2+≥Cd2+.7
Identification
the
high
biosorption
potential
MORSY1948
and
MORSY2014
isolates
CharacteristicsoftheMORSY1948strain
The phenotypic and chemotaxonomic characteristics of MORSY1948strain,assummarizedinTable3,werefoundto beconsistentwiththosedescribedforthegenusNocardiopsis. Thematurevegetativehyphaewerelong,well-developedand fragmentedintorod-shapedelements.Thestraindisplayed awhiteaerialmyceliumwhichwasobservedtoturninto a yellowish-whiteshadeinoldercultures.Also,thesubstrate myceliumwasyellowishbrownincolorwhichmatchedwith that observedfor most ofthe Nocardiopsis species. Soluble pigments were produced but melanin formation was not observed. Growth patterns observed can be classified as good in case of yeast extract-malt extract agar, Bennett’s potato starch agar, yeast extract asparagine glucose agar, glycerolasparagineagarandpotatodextroseagar;moderate growthwasseenonoatmealagar,riceagar,CzapekDoxagar and Hickey and Tresner’s agar and poor growth was seen on inorganic salts-starch agar and nutrient agar (Table 3). ChemotaxonomiccharacteristicsofstrainMORSY1948were consistentwithits classificationasamember ofthegenus Nocardiopsis.Testssuchastheliquefactionofgelatin,catalase activity,nitrate reduction,milkcoagulationand peptoniza-tion were positive while the acid fast test was negative. MORSY1948strainwascapableofutilizingribose,arabinose, fructose,mannose,glucose,mannitol,xylose,glycerol, galac-tose,trehalose,sucrose,maltose,adonitol,andcellobioseas asolecarbonsourcebutnotfucose,melibiose,lactose, rham-nose,sorbitol,inositol,cellulose,andraffinose.Besidesthis,it wasunabletodegradeTween80(Table3).StrainMORSY1948 exhibitedrobust growth underthe followingrange of con-ditions:temperature–30,37and45◦C,pH–between5and 11,andNaClconcentrationsashighas20%(w/v).Thestrain wassusceptibletoantimicrobialagentssuchasmitomycinC, cefotaxime,amikacin,andlysozyme(Table3).Thewhole-cell hydrolysates were found to contain meso-diaminopimelic acid as the only peptidoglycan diamino acid without a characteristic sugar (type III). Polar lipid pattern revealed the presence ofthe diagnostic phosphatidyl ethanolamine (PE),diphosphatidylglycerol(DPG),phosphatidylcholine(PC), phosphatidylglycerol(PG),phosphatidylmethylethanolamine (PME),andphosphatidylinositol(PI)(patternIII12).This phos-pholipidpatternisknowntobefoundinthespeciesofthe genera Nocardiopsis, Actinopolyspora, Saccharopolyspora, and Pseudonocardia.Nocardiopsisstrains,however,caneasilybe dif-ferentiatedfromthesetaxabytheoccurrenceofPME,presence ofhighamountsofPG,andthelackofhydroxy-phosphatidyl ethanolamine.Thepredominantmenaquinonesfoundwere: MK-9(H2)(8%),MK-9(H4)(10%),MK-10(22%),MK-10(H2)(17%),
MK-10(H4)(7%), MK-10(H6)(20%), MK-10(H8) (13%)and
MK-11(H8)(3%).Thecombinationoffatty acidsinthisstrain is
unique among Nocardiopsis species16,20 as it is composed
of C 16:0 (2.1%), C 18:1(10.3%), iso-C16:0 (29.3%), iso-C17:0 (3.8%),iso-C18:0(2.9%),anteiso-C15:0(16.2%),anteiso-C17:0 (10.7%),C17:18c(5.9%),C18:19c(7.1%),10-methyl-C17:0 (8.5%) and 10-methyl-C18:0 (3.2%) (fatty acid type3d). The highamountofanteiso-fattyacidsincombinationwith 10-methyl-branchedfattyacids(fattyacidtype3d)isdiagnostic ofspeciesbelongingtothegenusNocardiopsis.Moreover,the DNA G+C content was equivalent to 71.2mol% (Table 3). The members ofthe genus Nocardiopsis are known to pro-ducebioactivemetabolitessuchasgriseusinD,apoptolidin, methylpendolmycin,thiopeptideandnaphthospirononeA.41
CharacteristicsofMORSY2014strain
The MORSY2014 strain was subjected to a broad range of phenotypic and chemotaxonomic analysis. The strain was characterizedasstrictly aerobic,gram positiveand slightly acid-fast. Pale white aerial mycelium with well-developed yellowish orange substrate mycelium that had extensively irregularbranchedhyphaewithatendencytofragmentation intorodsandcoccoidelementswasobserved.Other charac-teristics notedwere: growth on agar, filamentousmargins, beadedappearance,andsolublepigmentsformation(Table4). Thestrainexhibitedpoorgrowthonoatmealagar,moderate growth oninorganicsalts-starch agar,riceagarandCzapek Doxagarandgoodgrowthonyeastextractasparagineglucose agar,Bennett’spotatostarchagar,HickeyandTresner’sagar and potatodextroseagar.Althoughthe strain waspositive for catalase, urease, and -galactosidase activity, but was negative for arylsulfatase activity. The strain was able to utilize d-ribose,melibiose, d-fructose, mannose,d-glucose, d-mannitol,glycerol,d-galactose,trehalose,lactose,sorbitol,
inositol, adonitoland salicin but notl-arabinose,d-fucose, d-xylose,l-rhamnose,sucrose,maltoseandraffinose.Casein, gluconicacid,propionicacid,uricacid,hypoxanthine,Tween 80,Tween20,l-valine,tyrosine,andurea wereobservedto
beutilizedbytheMORSY2014 strainbutthe samewasnot observed for ornithine, adenine, and xanthine. Growth on Bennett agarwas observedat25◦C aswell asat37◦C and also at pH 10 but no growth was observed at 45◦C or at pH 12 (Table 4). MORSY2014 strain exhibited susceptibility to antimicrobial agents such as mitomycin C, rifampicin, cefotaxime,amikacin,ciprofloxacin,andimipenem.TheG+C content ofthe genomicDNA was63.9mol%.Thefatty acid patterndetectedinMORSY2014strainwasasfollows(%):C13: 0(3.22),C14:0(10.71),C15:0(1.15),C16:0(24.25),C16:1(16.11), C17:0(1.22), C18:1(6.34),10-methylC18:0(6.35),C16:1
Table3–PhenotypicandchemotaxonomiccharacteristicsofMORSY1948isolate.
Properties CharacteristicsofMORSY1948isolate Properties Characteristicsof
MORSY1948isolate
Phenotypiccharacters Decompositionof
Aerialmycelium White Xanthine +
Substratemycelium Yellowishbrown Tween-80 −
Fragmentation Dividedintorodshapedspores Tween-20 +
Melaninproduction − Casein +
Solublepigment + Alanine +
Acidfast − l-Serine +
Growthoccurson l-Valine +
Inorganicsalts-starchagar Poor L-Threonine +
Nutrientagar Poor Tyrosine +
Riceagar Moderate Adenine +
Yeastextractasparagineglucoseagar Good Growthat
Yeastextract-maltextractagar Good 30◦C +
Glycerolasparagineagar Good 37◦C +
CzapekDoxagar Moderate 45◦C +
Bennett’spotatostarchagar Good pHrange 5–11
HickeyandTresner’sagar Moderate NaCltolerance(%) 0–20
Oatmealagar Moderate Resistantto
Potatodextroseagar Good 5-Fluorouracil +
Chemotypiccharacters MitomycinC −
Liquefactionofgelatin + Rifampicin +
Nitratereduction + Sulfamethoxazole +
Milkcoagulation + Amoxicillin +
Milkpeptonization + Cefotaxime −
Catalaseactivity + Gentamicin +
Utilizationof(1.0%,w/v) Amikacin −
d-Ribose + Ciprofloxacin +
l-Arabinose + Imipenem +
d-Fructose + Lysozyme −
Mannose + Cell-walltype Meso-diaminopimelicwithno
characteristicsugar(III)
d-Glucose +
D-Manitol + Majorcellularfatty
acids(%)
d-Xylose + C16:0 2.1
Glycerol + C18:1 10.3
d-Galactose + Iso-C16:0 29.3
Trehalose + Iso-C17:0 3.8
Sucrose + Iso-C18:0 2.9
Maltose + Anteiso-C15:0 16.2
Adonitol + Anteiso-C17:0 10.7
Cellobiose + C17:18c 5.9
d-Fucose − C18:19c 7.1
Melibiose − 10-methyl-C17:0 8.5
Lactose − 10-methyl-C18:0 3.2
l-Rhamnose − DNAG+Ccontent
(mol%)
71.2
Sorbitol − Predominant
menaquinones
MK-9(H2)(8%),MK-9(H4)(10%), MK-10(22%),MK-10(H2)(17%), MK-10(H4)(7%),MK-10(H6)(20), MK-10(H8)(13%),MK-11(H8)(3%)
Inositol −
Cellulose −
Raffinose −
Decompositionof Majorpolarlipids Phosphatidylethanolamine,
diphosphatidylglycerol, phosphatidylcholine, phosphatidylglycerol,
phosphatidylmethylethanolamine, phosphatidylinositol(PIII)
Glutamicacid +
Propionicacid +
Urea +
Table4–phenotypicandchemotypiccharacteristicsofMORSY2014isolate.
Properties Characteristicsof
MORSY2014isolate
Properties Characteristicsof
MORSY2014isolate
Phenotypiccharacters Decompositionof
(0.5%,w/v)
Aerialmycelium Palewhite Hypoxanthine +
Substratemycelium Yellowish
orange
Xanthine −
Filamentousmargins + Tween-80 +
Growthintoagar + Tween-20 +
Roughandwaxycolony + l-Valine +
Solublepigment + Ornithine −
Fragmentationintorodsandcoccoidelements + Tyrosine +
Beaded + Urea +
Acidfast + Adenine −
Growthoccurson Growthat(Bennett’s
agar)
Inorganicsalts-starchagar Moderate 30◦C +
Riceagar Moderate 37◦C +
Yeastextractasparagineglucoseagar Good 45◦C −
CzapekDoxagar Moderate pH10 +
Bennett’spotatostarchagar Good pH12 −
HickeyandTresner’sagar Good NaCltolerance(%) Upto18
Oatmealagar Poor Resistantto
Potatodextroseagar Good 5-Fluorouracil +
Chemotypiccharacters MitomycinC −
Liquefactionofgelatin + Rifampicin −
Nitratereduction + Sulfamethoxazole +
Catalase + Amoxicillin +
Urease + Cefotaxime −
-Galactosidase + Gentamicin +
Arylsulfatase − Amikacin −
Utilizationof(1.0%,w/v) Ciprofloxacin −
d-Ribose + Imipenem −
l-Arabinose − Lysozyme +
d-Fucose − Majorfattyacids(%)
Melibiose + C13:0 3.22
d-Fructose + C14:0 10.71
Mannose + C15:0 1.15
d-Glucose + C16:0 24.25
D-Manitol + C16:1 16.11
d-Xylose − C17:0 1.22
Glycerol + C18:1 6.34
d-Galactose + 10-methylC18:0 6.35
Trehalose + C16:19c 1.25
Lactose + C17:18c 1.19
l-Rhamnose − C18:19c 6.20
Sucrose − C16:17c 1.73
Maltose − C16:1cis 20.28
Sorbitol + Cellwalltype Meso-diaminopimelicwith
arabinoseandgalactoseas characteristicsugars(IV)
Inositol +
Adonitol + G+C(mol%) 63.9
Salicin + Majorquinones CycloMK-8(H4)(78.26),MK-8(H2)
(15.47%),MK-8(H4)(5.12%)and MK-8(H6)(1.15%)
Raffinose −
Decompositionof(0.5%,w/v) MajorPolarlipids Phosphatidylethanolamine,
Phosphatidylinositol, diphosphatidylglycerol, phosphatidylinositolmannoside
Casein +
Gluconicacid +
Propionicacid +
16SrDNAsequenceandphylogeneticanalysesofhyper activestrains
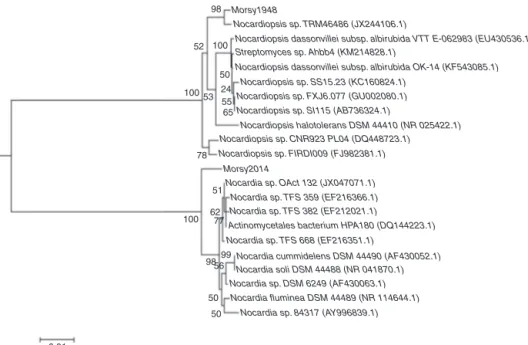
The almost-complete 16S rDNA sequences of strains MORSY1948 and MORSY2014(accession numbers KP979750 andKP979751,respectively)werecomparedtothesequences of members of the order Actinomycetales. It was observed that the members of the genus Nocardiopsis and Nocardia, respectively, were the closest phylogenetic neighbors. The values were seen to range between 96% (Nocardiopsis das-sonvilleiSubsp.albirubidaVTTE-062983)and98%(Nocardiopsis sp. TRM46486) forisolate MORSY1948 to 98% (Nocardia sp. OAct 132) and 99% (N. cummidelens DSM 44490) for isolate MORSY2014 (Fig. 1). Based on the 16S rDNA analyses and phylogenetic data,it was concludedthatboth theisolates, MORSY1948 and MORSY2014, merit species status within the genus Nocardiopsis and Nocardia, respectively. Isolates MORSY1948andMORSY2014canbedifferentiatedfromthe Nocardiopsisand Nocardiaspeciesbya combinationof mor-phological, physiological, chemotaxonomic and 16S rDNA analysesdata.Basedontheseresults,itwasconcludedthat strainsMORSY1948andMORSY2014arespeciesofthegenus NocardiopsisandNocardia;andhenceweregiventhenamesas Nocardiopsissp.MORSY1948andNocardiasp.MORSY2014.
Factors
that
affect
the
biosorption
process
of
heavy
metals
by
live
and
dead
biomasses
of
Nocardiopsis
sp.
MORSY1948,
and
Nocardia
sp.
MORSY2014
strains
Effectofdifferenttemperatures
Ananalysis ofthe strains Nocardiopsis sp.MORSY1948 and Nocardiasp.MORSY2014forreductionefficiencyatdifferent
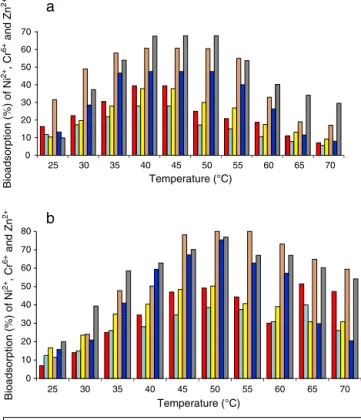
temperatures revealedthatirrespective ofgrowth tempera-ture,reductionefficiencyforallheavymetalsunderstudywas higherfordeadbiomassratherthanitwasforlive(Fig.2aand b).BioremovalcapacityofNi2+,Cr6+andZn2+wasincreased
by 60.5%,47.5% and 67.8% whennon-active cells of Nocar-diopsissp.MORSY1948wereincubatedat40◦Cascompared totheirlivecounterparts(Fig.2a).Theremovalefficiencyat 40◦CforthedeadbiomassofNocardiopsissp.MORSY2014for Ni2+, Cr6+ and Zn2+ was 1.45 fold, 2.11 fold, and 1.55 fold
higherthanthatofthelivebiomass(Fig.2b).Althoughliving biomasshasanadditionalcapacityforheavymetal biosorp-tionasaresultofmetabolicentrapment,nonlivingbiomasses haveseveraladvantagestooffer.Theseincludetheireaseof treatment,theirstrongaffinityformetalionsbecauseofthe lackofaprotonproductionmechanism duringmetabolism andnometaltoxicityissuesofthetypethatcanresultincell deathinlivecells.Additionally,thecultivationoflivebiomass requiressupplementationwithnutrients,whichcanincrease thebiologicalandchemicaloxygendemandsonthetreated water.7OurresultsarelinewiththatreportedbySimeonova et al.,31 AlTurk and Kiki4 and Daboor et al.7 inwhich the authorsclaimthatmaximumbiosorptionofheavymetalswas obtainedwhendeadbiomassesofS.fradiae,halophilic Actino-mycetesandS.chromofuscusK101wereusedforthepurpose. Interestingly,itwasobservedthatwhen100mgL−1solutions
ofmetalions,Ni2+,Cr6+andZn2+,wereindividuallyapplied
onto0.3%biomassofMORSY1948orMORSY2014,temperature wasdiscoveredtofunctionasasignificantparameter influenc-ingbiosorption.Thisindicatesthatthebiosorptionprocessis endothermicinnature.
By increasing the temperature from 30◦C to 40◦C, the removalofNi2+,Cr6+andZn2+byNocardiopsissp.MORSY1948
strain was elevatedfrom 22.5%, 17.3%and 19.7% to39.4%, 28.0%, 37.9%, respectively (live biomass) and from 49.0%, 28.5%and37.2%to60.5%,47.5%and67.8%,respectively(dead
Morsy1948
98
52 100
50
53
100 24
55 65
78
51
62 77 100
985699
50
50
0.01
Nocardiopsis sp. TRM46486 (JX244106.1)
Nocardiopsis dassonvillei subsp. albirubida VTT E-062983 (EU430536.1) Streptomyces sp. Ahbb4 (KM214828.1)
Nocardiopsis dassonvillei subsp. albirubida OK-14 (KF543085.1) Nocardiopsis sp. SS15.23 (KC160824.1)
Nocardiopsis sp. FXJ6.077 (GU002080.1) Nocardiopsis sp. SI115 (AB736324.1)
Nocardiopsis halotolerans DSM 44410 (NR 025422.1) Nocardiopsis sp. CNR923 PL04 (DQ448723.1) Nocardiopsis sp. FIRDI009 (FJ982381.1)
Morsy2014
Nocardia sp. OAct 132 (JX047071.1) Nocardia sp. TFS 359 (EF216366.1) Nocardia sp. TFS 382 (EF212021.1)
Actinomycetales bacterium HPA180 (DQ144223.1) Nocardia sp. TFS 668 (EF216351.1)
Nocardia cummidelens DSM 44490 (AF430052.1) Nocardia soli DSM 44488 (NR 041870.1) Nocardia sp. DSM 6249 (AF430063.1) Nocardia fluminea DSM 44489 (NR 114644.1)
Nocardia sp. 84317 (AY996839.1)
0 10 20 30 40 50 60 70 80
Bioadsorption (%) of Ni
2+
, Cr
6+
and Zn
2+
Bioadsorption (%) of Ni
2+
, Cr
6+
and Zn
2+
70 65 60 55 50 45 40 35 30 25
Temperature (°C) 0
10 20 30 40 50 60 70
a
b
70 65 60 55 50 45 40 35 30 25
Ni (II) removal by the live biomass Ni (II) removal by the dead biomass
Cr (VI) removal by the dead biomass
Zn (II) removal by the dead biomass Cr (VI) removal by the live biomass
Zn (II) removal by the live biomass
Temperature (°C)
Fig.2–(a)Effectofdifferenttemperaturesonthe
biosorptioncapacityofNi2+,Cr6+andZn2+(%)fromaquous solutionbytheliveanddeadcellsofNocardiopsissp. MORSY1948.(b)Effectofdifferenttemperaturesonthe biosorptioncapacityofNi2+,Cr6+andZn2+(%)fromaquous solutionbytheliveanddeadcellsofNocardiasp.
MORSY2014.
biomass)(Fig.2a).ForNocardiasp.MORSY2014(livebiomass), thepotentsorptionpercentageforNi2+,Cr6+andZn2+at50◦C was determined as49.2%, 38.5% and 50.2% respectively as comparedto14%,15%and23.6%at30◦C.Thesameforthe deadbiomasswasestimatedto be80.0%,75.4%and 76.9% respectivelyat50◦Cascomparedto24.1%,20.9%and39.4% at30◦C(Fig.2b).Theincreaseinadsorptionpercentagewith elevationintemperaturecanbeattributedtotheseveral fac-torssuchasachangeintheporesizeoftheadsorbentleading toagreaterinter-particlediffusionwithinthepores,the cre-ationofnewactivesitesonthesorbent,atemperature-based accelerationofsomeslowadsorptionsteps,anenhancement inthemobilityofmetalionsfromthebulksolutiontowardthe adsorbentsurface,and/oranenhancementinthechemical affinityofthemetalcationsforthesurfaceofadsorbent.42
EffectofdifferentpHvalues
ByincreasingpHfrom2to7,absorptioncapabilityofthelive biomassofNocardiopsissp.MORSY1948towardNi2+andCr6+
wasincreased2.21foldand4.39foldrespectively,whileatpH 8,Zn2+bioremovalefficiencyincreased3.89fold.Itwasnoticed
thatthedeadbiomassremoved87.9%and63.75%ofNi2+and
Cr6+ atpH7,respectively, and84.15%ofZn2+ wasremoved
atpH 8(Table 5). In caseofNocardia sp.MORSY2014,only 61.46%(Ni2+),62.52%(Cr6+)and67.91%(Zn2+)removal
poten-tialwasachievedbythelivebiomassofthestrainwhichisin sharpcontrasttothemuchsuperiorremovalefficiencyofthe deadbiomassatpH8[93.53%(Ni2+),95.22%(Cr2+)and90.37%
(Zn2+)](Table5).Thebiosorptioncapacityofbothstrainswas
observedtosignificantlydecreaseatacidicpHvalues.Thisis hypothesizedtobeduetothefactthatH+ionscompetewith
metalionsforthenegatively chargedbindingsites thereby hinderingthemfromreachingthebindingsitesofthe adsor-bent (repulsiveforces).However,atpH4andabove,anion exchangemechanism occursvia H+ ionandthe negatively
chargedgroupsofbiomassresultinadrasticincreaseinthe heavymetalremovalefficiency.30
Effectofbiosorbentdosage
Theeffectofbiosorbentdosage(0.05%–0.5%)onsorption effi-ciency inaqueoussolutionsunderoptimizedparametersis presentedinTable6. Theresultsindicate thatwhena bio-sorbentdosage(asinthedeadbiomass)wasincreasedfrom 0.05% to 0.3%,the removalofNi2+, Cr6+ and Zn2+ byboth
Nocardiopsissp.MORSY1948aswellasNocardiasp.MORSY2014 increasedrapidlyfrom87.90%,63.75%and84.15%to93.53%, 89.22%and90.37%,respectively.Moreover,athigher concen-trationsofsorbentdosage(0.4%and0.5%),itwasobservedthat theheavymetalsunderstudywereremovedfromtheaqueous solutionsinentirety.Thisisbecausewhiletheconcentration ofmetalionsremainsthesameinsolution,therearemore bio-sorbentbindingsitesavailableathigherdosagesthanthere are ata lower dosage,which leads tobinding ofall avail-ablemetalions.Asimilartrendwithrespecttothebiomass effect was reported for the biosorption of Cd2+, Cu2+ and
Zn2+byS.lunalinharesiiandCr3+byStreptomycessp.(MB2)and
S.noursei.43
Effectofinitialheavymetalconcentrationsversusvarying contacttime
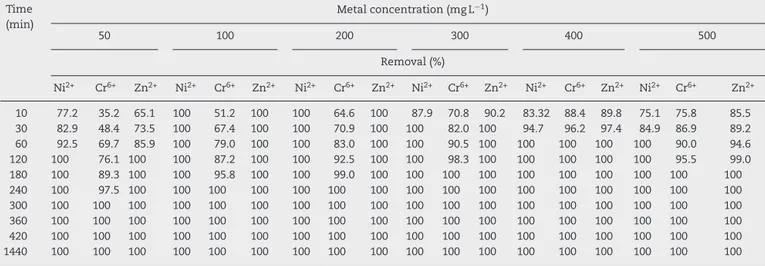
Initial concentration of heavy metal ions in the solution playsakeyrole asadrivingforceforovercomingthemass transferresistance betweenthe aqueousand solid phases. EquilibriumstageforbothNi2+ and Zn2+ bythebiomassof
strainNocardiopsissp.MORSY1948wasreachedfasterwhen their concentrations were increasedto 100 and 200mgL−1
within10min,respectively. For Cr6+ it took240minforthe
biomass to reach equilibrium at the same concentrations (Table7a).Incaseofhighermetalionconcentrations(300,400 and500mgL−1),equilibriumpointoftheadsorptionprocess wasreacheduponvaryingthecontacttimesasfollows:Ni2+–
0,60and60min;Cr6+–180,60and180minandZn2+–30,60
and180min,respectively.Ontheotherhand,thetimeprofile of the metal biosorption at different metal concentrations bythebiomassofNocardiasp.MORSY2014strainwassingle, smoothandcontinuousleadingtosaturationasillustratedin
Table7b.Theheavymetalionremovalefficiencyreachedin 10minatspecificmetalconcentrationsisasfollows:43.51%, 74.3%and 49.6%atconcentrationof50mgL−1; 66.7%,100%
Table5–EffectofpHonbiosorptionefficiency(%)oftheheavymetalsNi2+,Cr6+andZn2+byliveanddeadbiomassof
Nocardiopsissp.MORSY1948andNocardiasp.MORSY2014.
Biosorbent Heavymetal Biosorptionefficiency(%)atdifferentpHvalues
2 4 6 7 8 9 10 11
Nocardiopsissp.MORSY1948 (livecells)
Ni2+ 19.23 27.08 39.4 42.54 39.59 17.50 11.65 7.15
Cr6+ 8.14 20.51 28.0 35.72 31.00 26.23 11.47 9.07
Zn2+ 12.06 18.93 37.9 41.40 46.91 34.72 20.51 12.32
Nocardiopsissp.MORSY1948 (deadcells)
Ni2+ 25.39 42.17 60.5 87.90 87.90 70.04 53.58 50.05
Cr6+ 18.50 20.36 47.5 63.75 63.75 52.57 38.35 19.49
Zn2+ 14.92 29.08 67.8 75.15 84.15 68.31 40.01 32.28
Nocardiasp.MORSY2014(live cells)
Ni2+ 5.08 18.40 49.32 59.65 61.46 42.70 38.24 22.70
Cr6+ 11.54 22.00 38.47 60.85 62.52 60.38 31.90 12.04
Zn2+ 18.72 35.09 50.32 64.30 67.91 54.02 40.85 26.16
Nocardiasp.MORSY2014 (deadcells)
Ni2+ 14.85 47.95 80.30 88.71 93.53 80.81 51.93 30.78
Cr6+ 20.04 34.78 75.52 84.57 95.22 77.10 70.07 42.05
Zn2+ 25.37 50.03 76.87 87.95 90.37 85.20 61.44 50.00
Table6–Effectofbiosorbetdosage(%)onbiosorptionefficiency(%)oftheheavymetalsNi2+,Cr6+andZn2+bythedead
biomassofNocardiopsissp.MORSY1948andNocardiasp.MORSY2014.
Biosorbent Heavymetal Biosorptionefficiency(%)atdifferentbiosorbentdosage(%)
0.05 0.1 0.2 0.3 0.4 0.5
Nocardiopsissp.MORSY1948 (deadcells)
Ni2+ 13.77 41.90 60.19 87.90 100 100
Cr6+ 9.52 23.37 54.48 63.75 100 100
Zn2+ 16.05 41.52 72.57 84.15 100 100
Nocardiasp.MORSY2014 (deadcells)
Ni2+ 15.80 53.20 67.50 93.53 100 100
Cr6+ 19.27 34.81 70.29 89.22 100 100
Zn2+ 11.91 40.32 66.12 90.37 100 100
Table7a–EffectofdifferentinitialconcentrationsofmetalionsonitsremovalbythedeadbiomassofNocardiopsissp. MORSY1948atdifferentcontacttimes.
Time (min)
Metalconcentration(mgL−1)
50 100 200 300 400 500
Removal(%)
Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+
10 77.2 35.2 65.1 100 51.2 100 100 64.6 100 87.9 70.8 90.2 83.32 88.4 89.8 75.1 75.8 85.5
30 82.9 48.4 73.5 100 67.4 100 100 70.9 100 100 82.0 100 94.7 96.2 97.4 84.9 86.9 89.2
60 92.5 69.7 85.9 100 79.0 100 100 83.0 100 100 90.5 100 100 100 100 100 90.0 94.6
120 100 76.1 100 100 87.2 100 100 92.5 100 100 98.3 100 100 100 100 100 95.5 99.0
180 100 89.3 100 100 95.8 100 100 99.0 100 100 100 100 100 100 100 100 100 100
240 100 97.5 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
300 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
360 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
420 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
1440 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
63.75% at a concentration of 200mgL−1; 83.6%, 100% and
71.4% at a concentration of 300mgL−1; 80.7%, 85.0% and 55.3%ataconcentrationof400mgL−1and71.0%,81.3%and
52.0% at a concentration of 500mgL−1 for Ni2+, Cr6+, and
Zn2+,respectively(Table7b).ForNocardiasp.MORSY2014,the
equilibriumstagewasreachedin(300,60and120min),(240, 10and120min),(120,10and120min)and(120,10and60min) forNi2+,Cr6+andZn2+atconcentrationsequivalentto50,100,
200and300mgL−1respectively;butathigherconcentrations,
i.e.,400and500mgL−1, itwasobservedthattimetaken to
reach equilibriumwas300,120and180min(Ni2+,Cr6+ and
Zn2+,respectively).Theincreaseinthebioremovalefficiency
(%)observedasaresultofincreaseintheinitial concentra-tion ofNi2+,Cr6+ and Zn2+ (mgL−1)canbeattributedtoan
Table7b–EffectofdifferentinitialconcentrationsofmetalionsonitsremovalbythedeadbiomassofNocardiasp. MORSY2014atdifferentcontacttimes.
Time (min)
Metalconcentration(mgL−1)
50 100 200 300 400 500
Removal(%)
Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+
10 43.51 74.3 49.6 66.7 100 51.9 75.8 100 63.75 83.6 100 71.4 80.7 85.0 55.3 71.0 81.3 52.0
30 60.47 89.5 68.3 74.4 100 55.0 83.1 100 76.3 93.5 100 74.0 87.3 92.8 70.1 79.5 88.5 66.8
60 76.8 100 83.5 88.2 100 70.3 92.4 100 91.27 96.5 100 100 90.2 99.0 84.0 83.0 97.4 80.5
120 82.1 100 100 93.1 100 100 100 100 100 100 100 100 94.1 100 97.3 89.2 100 94.3
180 95.5 100 100 98.9 100 100 100 100 100 100 100 100 96.8 100 100 92.6 100 100
240 99.3 100 100 100 100 100 100 100 100 100 100 100 97.9 100 100 94.9 100 100
300 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
360 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
420 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
1440 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100 100
interactionsandcoverageofactivesitesresultsinadecrease intheremovalefficiencyofheavymetalions.
Application
of
biosorption
for
real
wastewater
treatment
Inordertoassesstheapplicabilityoftheoptimizedprocedure, we attempted to apply our pilot biosorption experimental resultstoreal wastewatersamplessoastomove fromthe experimentalsetuptothereal-worldapplicationstage.The experiment was conducted on 100mL of pre-filtered real wastewatersamples,insteadofthepre-mademetalsolutions atdifferentpHvalues(pH2–11)underthesameoptimized con-ditionsandthebioremovaldataforbothsetsofexperiments wasfoundtobesimilar(Fig.3).Themaximumionremoval capacityofthedeadbiomassofNocardiopsissp.MORSY1948 forreal industrial wastewaterwas 100%for Ni2+ and Cr6+
atpH 7.0 and the same for Zn2+ at pH 8.0. Along similar
lines,thedeadbiomassofNocardiasp.MORSY2014wasalso observedtoremove100%ofallheavymetalsunderstudyfrom realindustrialwastewaterunderoptimizedconditions(Fig.3). AstudybyAlTurkandKiki4 recommendedthat halophilic
Actinomycetesbiomasshasthepotentialforremovalofheavy metalsfromrawindustrialwastewater.Moreover,inastudyby ChatterjeeandChandra,44theefficiencyofGeobacillus thermod-enitrificansbiomassinremovingheavymetalsfromreal-world effluentwasdetermined.
0 20 40 60 80 100
Bioadsorption (%) of Ni
2+
,
Cr
6+
and Zn
2+
120
2 3 4 5 6
pH 7 8 9 10 11
Ni (II) removal by Nocardia sp. MORSY2014 Cr (VI) removal by Nocardia sp. MORSY2014 Zn (II) removal by Nocardia sp. MORSY2014 Cr (VI) removal by Nocardiopsis sp. MORSY1948 Ni (II) removal by Nocardiopsis sp. MORSY1948 Zn (II) removal by Nocardiopsis sp. MORSY1948
Fig.3–AdsorptionofNi2+,Cr6+andZn2+(%)fromreal industrialwastewaterbythedeadbiomassofNocardiopsis sp.MORSY1948andNocardiasp.MORSY2014.
Desorption
Ni
2+,
Cr
6+and
Zn
2+heavy
metals
Torecoverheavymetalsforreuseofthebiosorbent,desorption efficiencyshouldalsobeconsidered.Thedatapresentedin
Table8illustratesthatmetalionssorbedormetaliondesorbed decreasedfromcycle1tocycle5.Biosorptionefficiencywas observedtoreducefrom100%forNi2+,Cr6+andZn2+afterthe firstcycleto94.32%,97.14%and96.81%,respectively,afterthe
Table8–DesorptionofNi2+,Cr6+andZn2+fromthebiomassofNocardiopsissp.MORSY1948andNocardiasp.MORSY2014.
No.ofcycles Sorptionefficiency(%) Desorptionefficiency(%)
MORSY1948 MORSY2014 MORSY1948 MORSY2014
Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+ Ni2+ Cr6+ Zn2+
1 100 100 100 100 100 100 99.12 91.18 99.29 98.53 99.70 98.1
2 98.63 99.06 100 94.57 100 97.75 98.28 88.27 99.04 92.18 99.01 96.3
3 96.51 98.20 99.45 90.85 99.4 96.22 97.53 87.50 98.02 89.26 98.65 94.18
4 95.96 98.20 97.09 88.71 99.0 95.08 95.70 85.74 95.90 85.95 98.00 92.16