REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
Official Publication of the Brazilian Society of Anesthesiologywww.sba.com.br
SCIENTIFIC
ARTICLE
Prophylactic
use
of
pregabalin
for
prevention
of
succinylcholine-induced
fasciculation
and
myalgia:
a
randomized,
double-blinded,
placebo-controlled
study
Vinit
K.
Srivastava
a,∗,
Sanjay
Agrawal
b,
Vikrant
K.
Nimbhorkar
a,
Abhishek
Mishra
a,
Sunil
Sharma
a,
Prasanta
K.
Panda
aaApolloHospitalsBilaspur,Chhattisgarh,India
bHimalayanInstituteofMedicalSciences,Dehradun,India
Received19May2014;accepted7August2014 Availableonline27November2014
KEYWORDS Pregabalin; Succinylcholine; Fasciculation; Myalgia
Abstract
Background: Succinylcholineiscommonlyusedtoachieveprofoundneuromuscularblockadeof rapidonsetandshortduration.
Objective: The present study compared the efficacy of pregabalin for prevention of succinylcholine-inducedfasciculationandmyalgia.
Design:Prospective,randomized,placebocontrolled,doubleblindedstudy.
Materialsandmethods: Patientsofbothgendersundergoingelectivespinesurgerywere ran-domly assigned to two groups. Patients in Group P (pregabalin group) received 150mg of pregabalinorally1hpriortoinductionofanesthesiawithsipsofwaterandpatientsinGroup C(controlgroup)receivedplacebo.Anesthesiawasinducedwithfentanyl1.5mcg/kg, propo-fol 1.5---2.0mg/kg followedbysuccinylcholine1.5mg/kg.The intensityoffasciculationswas assessedby anobserverblinded tothegroupallotment ofthepatientona4-pointscale. A blinded observer recordedpostoperative myalgiagrade after 24h ofsurgery.Patientswere providedpatient-controlledanalgesiawithfentanylforpostoperativepainrelief.
Results:Demographicdataofbothgroupswerecomparable(p>0.05).Theincidenceofmuscle fasciculation’swasnotsignificantbetweentwogroups(p=0.707),whilemorepatientsingroup Chadmoderatetoseverefasciculation’scomparedtogroupP(p=0.028).Theincidenceand severityofmyalgiaweresignificantlyloweringroupP(p<0.05).
Conclusion: Pregabalin150mgpreventssuccinylcholine-inducedfasciculationsandmyalgiaand alsodecreasesthefentanylconsumptioninelectivesinesurgery.
© 2014SociedadeBrasileirade Anestesiologia.Publishedby ElsevierEditoraLtda.Allrights reserved.
∗Correspondingauthor.
E-mail:drvinit75@gmail.com(V.K.Srivastava).
http://dx.doi.org/10.1016/j.bjane.2014.08.004
PALAVRAS-CHAVE Pregabalina; Succinilcolina; Fasciculac¸ão; Mialgia
Usoprofiláticodepregabalinaparaprevenc¸ãodemialgiaefasciculac¸ãoinduzidas porsuccinilcolina:estudorandômico,duplo-cegoecontroladoporplacebo
Resumo
Justificativa:A succinilcolina écomumente usada para atingir um bloqueio neuromuscular profundo,deiníciorápidoedecurtadurac¸ão.
Objetivo:Opresenteestudocomparouaeficáciadepregabalinanaprevenc¸ãodemialgiae fasciculac¸ãoinduzidasporsuccinilcolina.
Desenho:Estudoprospectivo,randômico,duplo-cegoecontroladoporplaceboo.
Materiaisemétodos:Pacientes de ambos os sexos submetidos à cirurgia eletiva de coluna foramaleatoriamentedivididosemdoisgrupos.OspacientesdoGrupoP(pregabalina) rece-beram150mgdepregabalinaoral1horaantesdainduc¸ãodaanestesiaeospacientesdoGrupo C(controle)receberamplacebo.Aanestesiafoiinduzidacomfentanil(1,5mcg/kg)epropofol (1,5-2,0mg/kg),seguidosdesuccinilcolina1,5mg/kg.Aintensidadedafasciculac¸ãofoi avali-adaporumobservador,cegoparaaalocac¸ãodosgrupos,usandoumaescalade4pontos.Um observadorcegoregistrouograupós-operatóriodemialgiaapós24horasdecirurgia.Parao alíviodadornopós-operatório,fentanilfoiusadoem sistemadeanalgesiacontroladapelo paciente.
Resultados: Osdadosdemográficosdeambososgruposeramcomparáveis(p>0,05).A incidên-ciadefasciculac¸ãomuscularnãofoisignificativaentreosdoisgrupos(p=0,707),enquantomais pacientesdoGrupoCapresentaramfasciculac¸ãodemoderadaagraveemrelac¸ãoaoGrupoP (p=0,028).AincidênciaeagravidadedemialgiaforamsignificativamentemenoresnogrupoP (p<0,05).
Conclusão:Pregabalina(150mg)previnemialgia efasciculac¸ãoinduzidas porsuccinilcolina, alémdediminuioconsumodefentanilemcirurgiaeletivadecoluna.
©2014SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Todosos direitosreservados.
Introduction
Succinylcholineisashortactingdepolarizingmuscle relax-antwithrapidonsetandshortdurationofaction.Itsuseis associatedwithanumberofsideeffectslikefasciculation, postoperativemyalgia, increasedserumlevelsof creatine kinaseandpotassium,malignanthyperthermia, myoglobin-uria, raised intraocular pressureand intracranialpressure precluding its routine use.1,2 Fasciculations are relatively
benignside effects ofits use;most anesthesiologists
pre-fertopreventthemduetoapossibleassociationbetween
fasciculationsandpostoperativemyalgia.
Differentpre-treatmentmodalitieshavebeenattempted
toreduce theincidenceandseverityoffasciculationsand
myalgia. This includes precurarization with a small
dose of non-depolarizing muscle relaxant,3 pre
succinyl-cholineuse of lidocaine,4 calcium gluconate,5 magnesium
sulphate,6nonsteroidalanti-inflammatorydrugs (NSAIDs),7
dexmedetomidine,8 benzodiazepines,4 remifentanil,9
phenytoin10 orketorolac.11Theefficacyofeachisvariable.
Pregabalinanditspredecessor,gabapentin,areanalogs
oftheinhibitoryneurotransmittergammaaminobutyricacid
(GABA).Asgabapentin12hasbeenfoundtoprevent
succinyl-cholineinducedfasciculationandmyalgia,pregabalinmay
beanalternativeofthiswithbetterresults.
Withthisaim,thisrandomized,double-blinded,
placebo-controlledstudy wasinstitutedtoinvestigatewhetheruse
of preoperative pregabalinadministration hasany effects
on succinylcholine-induced fasciculation’s and myalgia in
subjects undergoingmicrodiscectomy undergeneral
anes-thesia.
Materials
and
methods
Thisprospective,randomized,placebo-controlledstudywas
conductedafterapprovalfromtheInstitutionalEthics
Com-mittee and written informed consent from the patients
undergoing elective spine surgery under general
anesthe-sia. The study was registered at Clinical Trials.gov (Ref.:
CTRI/2013/08/003925).
Sixty-fourpatients, aged20---60 years,either sex, ASA
physicalstatusIorII, scheduledforelective spinesurgery
were included in the study. Patients with a history of
seizure disorders, preoperative ingestion of pregabalinor
gabapentin, hyperkalemia, systemic illness like
hyperten-sion,diabetes,impairedkidneyorliverfunctions,increased
intracranial and intraocular pressure, pregnant or
breast-feeding females and patients with known sensitivity to
pregabalinwereexcludedfromthestudy.Thepatientswere
randomlyallocatedtotwoequalgroupswiththehelpofa
computer generated table of randomnumbers to receive
followingdrugs.
GroupP(pregabalingroup)
Patients received pregabalin 150mg orally with sips of
Table1 Demographicdata.
GroupC(n=32) GroupP(n=32) p-Value
Meanage(yrs) 47.03±10.12 48.87±7.97 0.4212
Male/female 21/11 19/13 0.796
Weight(kg) 61.19±8.30 63.28±10.06 0.367
Spinesurgery
Cervical/thoracic/lumbar 12/1/19 10/0/22 0.496
Durationofsurgery(min) 138.69±33.43 133.44±39.42 0.5676
Dataarepresentedaseithermeanvalues±SDorbyabsolutenumbers.
GroupC(controlgroup)
Patientsreceivedsimilarlookingplacebotabletorallywith sipsofwater,1hbeforetheinductionofanesthesia.
Allthepatientswerepremedicatedwithorallorazepam 2mg and ranitidine 150mg night before,and 2h prior to surgery. The study drugs were given to the nurse atten-dantinidenticalenvelopesmarkedPandC.Thenatureof themedicationswasnotknowntothenurseattendantwho administeredthedrugsasperinstructions.
Intheoperatingroom,afterestablishingthebasic mon-itoringanesthesiawasinduced byinjection(inj.) fentanyl 1.5mcg/kg, propofol 1.5---2.0mg/kg and succinylcholine 1.5mg/kgbodyweight.Theintensityoffasciculation’swas assessedbyanobserverblindedtothegroupallotmentof thepatientona4-pointscaleasAbsent(0);Mild---fine fas-ciculation’sattheeyes,neck,faceorfingerswithoutlimb movement (1);Moderate ---fasciculation’s occurring bilat-erallyorobviouslimbmovement(2);Severe---widespread, sustainedfasciculation’s(3).
The patients wereintubatedwithan appropriatesized cuffedendotrachealtubeafterassessingcompletemuscular relaxationbysingletwitchneuromuscularmonitoring. Anes-thesia wasmaintainedwithoxygen:nitrousoxide(O2:N2O;
33:66)andsevoflurane.Vecuroniumbromide0.1mg/kgwas givenafterendotrachealintubation. Intermittentdosesof fentanylandvecuroniumbromidewereadministeredduring surgeryasindicated.Aftercompletionofsurgery, neuromus-cular blockade was reversed and patients shiftedto post anesthesiacareunit(PACU).
In PACUpatientsreceived postoperativeanalgesiawith fentanyl (5mcg/mL)through patient controlled analgesia (PCA) pump (Smith Medical ASD, Inc., USA). The total fentanyl requirement in the first 24h was recorded. Any complications like postoperative nausea, vomiting, dizzi-ness,somnolence,vertigo,confusion,blurredvisionanddry mouthwerealsobeingrecordedandmanagedaccordingly.
Theincidenceandseverityofmyalgiawereassessedby ablindedobserver24haftersurgicalintervention,utilizing afour-pointratingscaleandgradedas:absenceofmuscle pain(0);musclestiffness,limitedtooneareaonly(1); mus-clepainor stiffnessnoticedspontaneouslybythepatient, whichmayrequireanalgesic therapy(2);andgeneralized, severeorincapacitatingdiscomfort(3).
The postoperative sedation level was assessed by the Ramsay sedationscore which consistsof the following six grades: anxious (1),cooperative and tranquil(2), respon-dingtocommandsonly(3),briskresponsetolightglabellar
tap(4),sluggishresponsetolightglabellartap(5),andno responsetolightglabellartap(6).
Sample size calculation was based on the pilot study, wheretheincidenceoffasciculationwasfoundtobe96%. Weaimedtodecreasetheincidenceby50%withpregabalin pre-treatment.Withapowerof80%andtypeIerrorof5%, wecalculatedthat30subjectswererequiredpergroup.To takecareofanydropouts,weenrolled32patientsineach group.
Statistical analysiswasperformed usingtheGraph pad prism6.0statisticalsoftware.Thedemographicdatawere analyzedbyStudentt-test.Maleandfemaledatawere ana-lyzedwiththeChisquaretest.Theconsumptionoffentanyl andsedation ingroups were analyzed byusingStudent t -test.Theincidenceandseverityoffasciculationandmyalgia wereanalyzedusingFisher’sexacttest.Ap-valueof<0.05 wasconsideredstatisticallysignificant.
Results
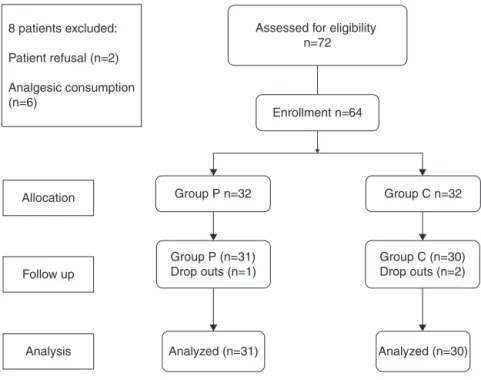
Seventy-twopatientswereassessedforeligibilitybetween September2013andFebruary2014.Sixty-fourpatientswere includedin thestudyafter randomizationand 61patients (95.3%)completed thestudy (Fig.1). Eight patientswere
notincluded in this study onaccount of patient’s refusal
(2 patients), preoperative history of analgesic
consump-tion(6 patients). Three patients were excluded fromthe
study following initial randomization on account of need
of postoperative ventilator support (2 patients) and PCA
pump failure(1 patient);their data has been included in
thecomparisonofdemographicprofile,however,theywere
notsubjectedtofurtherstatisticalanalysis(Fig.1).
Therewerenosignificant differencesbetweenthe two
groupswithrespecttoage,gender,bodyweight,typeand
durationofsurgery(p>0.05)(Table1).
Theoverallincidenceofmusclefasciculation’swas83.9%
ingroupPand90%ingroupC(p=0.707)(Table2).Thegrades
ofmusclefasciculation’sobservedweremild(57.7%),
mod-erate (34.6%) and severe (7.7%) in Group P, while 22.2%,
59.2%,and18.5%respectivelyinGroupC.Morepatientsin
GroupChadmoderate toseverefasciculation’s compared
toGroupP(p=0.028).
Six(19.3%)patients ofgroup Pand14 (46.7%)patients
ofgroup Chad postoperativemyalgia after 24h (p=0.03)
(Table 3).The severityofmyalgia waslessin thegroup P
comparedtogroupC(5and1vs.10and4ofGrade1and
8 patients excluded:
Patient refusal (n=2)
Analgesic consumption (n=6)
Enrollment n=64
Group P n=32
Group P (n=31) Drop outs (n=1)
Analyzed (n=31) Analysis
Follow up Allocation
Analyzed (n=30) Group C (n=30) Drop outs (n=2) Group C n=32 Assessed for eligibility
n=72
Figure1 Studydesign.
Table2 Theincidenceandseverityoffasciculations.
GroupC(n=30) GroupP(n=31) p-Value
Fasciculations
No 3(10%) 5(16.1%)
Yes 27(90%) 26(83.9%) 0.707
Severityoffasciculations
Mild 6(22.2%) 15(57.7%)
Moderate 16(59.2%) 9(34.6%) 0.028 Severe 5(18.5%) 2(7.7%)
Dataarepresentedasnumberswithpercentage.
ofmyalgia of grade 3in any studygroups. No association betweentheincidence/gradeoffasciculation’sandmyalgia wasdemonstrated.
The consumption of fentanyl in the first 24h was sig-nificantly less in the group P compared to the group C (674.03±137.84mcgvs.1002.67±214.43mcg)(p<0.001). SedationscorewassignificantlyhigheringroupP(p=0.004) (Table4).
Table3 Theincidenceandseverityofmyalgia.
GroupC(n=30) GroupP(n=31) p-Value
Myalgia
No 16(53.3%) 25(80.7%)
Yes 14(46.7%) 6(19.3%) 0.030
Severityofmyalgia
Mild 10(71.4%) 5(83.3%)
Moderate 4(28.6%) 1(16.7%) 0.020
Severe 0 0
Dataarepresentedasnumberswithpercentage.
Discussion
Succinylcholineisthebestdrugfor rapidlyprovidingideal conditionsforshortproceduresrequiringendotracheal intu-bation. Unfortunately, its useis associatedwith muscular fasciculation’sandpostoperativemyalgia.
Fasciculation produced by succinylcholine have been attributed to a prejunctional depolarizing action of suc-cinylcholine, resulting in repetitive firing of the motor nerve terminals and antidromic discharges that mani-fests as an uncoordinated muscle contractions.13 Various
drugs have been found to influence the fasciculation’s
and the mechanisms proposed ranges from impairing
release of acetylcholine by morphine and narolphine,9
impairneurohumoraltransmissionatperipheralmuscarinic
receptorsbymorphine,9 blockingtheprejunctional
recep-tors by non depolarizing muscle relaxants,3 motor nerve
membrane stabilization by reduction of calcium ions by
diphenylhydantoin,10 inhibition of calcium releaseleading
todecreaseinacetylcholinereleasebymagnesium.6
The mechanism of the inhibitory action of pregabalin
onsuccinylcholineinducedmusclefasciculationisunclear.
Since intracellular calcium accumulation is important for
enhancingthespeedandstrengthofthefasciculation’sand
the contractionof theintrafusalmusclefibers, theeffect
ofpregabalinonvoltage-gatedcalciumchannelsmaybea
possiblemechanism ofdecreasingthemusclecontractions
leadingtofasciculation’s.14
Postoperative myalgia following the use of
succinyl-choline is a common, troublesome clinical problem.
Succinylcholine-induced postoperative myalgia is most
frequent onthe first postoperativeday. The exact
patho-physiologyforsuccinylcholineinducedmyalgiaisnotclear.
Various proposed mechanisms of its causation include
increased myoplasmic calcium concentrations, membrane
Table4 Postoperativesedationscore(Ramsaysedationscore)andfentanylconsumptionwithin24haftersurgery.
GroupC(n=30) GroupP(n=31) p-Value
Fentanylrequirements(mcg) 1002.67±214.43 674.03±137.84 <0.0001
Sedationscore 2.27±0.66 2.71±0.69 0.0048
Dataarepresentedasmeanvalues±SD.
freeradicalsresponsibleformuscledamageleadingto post-operativemyalgia.15---18Variousdrugshavebeen utilizedto
lookintoblockingthesespecifictargetstodecreasemyalgia.
Pregabalin inhibits Ca2+ currents via
high-voltage-activatedchannels containingthe ␣2␦ subunit,19 reducing
neurotransmitter release (e.g. glutamate, substance P,
calcitonin,noradrenaline,gene-relatedpeptide)and
atten-uating the postsynaptic excitability,14 providing the basis
for itsantinociceptive efficacy in post-operativepain.20,21
Theabovefactsmayalsobeaplausibleexplanationforits
efficacyinreducingsuccinylcholineinducedmyalgia.
We utilized pregabalin over gabapentin to assess its
effectonfasciculationandmyalgiaaspregabalinhashigher
bioavailability(90%vs.33---66%),rapidabsorption(withpeak
plasma levels at: 1h vs. 3---4h) and a linear increase in
plasmaconcentrationasitsdoseisincreased.Lowerdoses
ofpregabalinthanthatofgabapentin(2---4-foldlowerdoses)
haveasimilaranalgesiceffectonneuropathicpain,which
makespregabalinmoreadvantageousin terms ofthe side
effectsofdosage.22
Useofinductionagentslikethiopentoneorpropofolhave
been demonstratedtohave nobearing onsuccinylcholine
induced fasciculation though less myalgia is seen when
thiopentoneisusedincomparisontopropofol.15Maddineni
etal.23observedthatthereisnodifferenceinpostoperative
myalgiawhenpropofolwassubstitutedforthiopentonebut
accordingtoMcClymont,24 propofolisbetterthan
thiopen-tonetocontrolmyalgia.
Our study is in agreement with various studies of
reductionofpostoperativepainaswellasopioids
require-mentswiththeuseofpreoperativepregabalin.Reubenand
colleagues25observedthatuseofpreoperativepregabalinin
patients undergoinglumbar laminectomywasaseffective
as celecoxib in reducing postoperative pain and
patient-controlledmorphineconsumption. Agarwaletal.reported
effectivenessofthesinglepreoperativeoraldoseof150mg
pregabalininreducingpostoperativepainandfentanyl
con-sumptioninlaparoscopiccholecystectomy.26
Therearesomelimitationswiththisstudy:(a)thestudy
designwasobservational,andwemeasureasubjective
vari-able(fasciculation)ratherthanobjectivevariables(increase
inpotassium, myoglobin,andCPK) and(b) thisis asingle
institutionalstudyandourresultsmaynotbegeneralized.
Furtherstudies indifferentsettingsand groupofpatients
maygiveabetterunderstandingofuseofpregabalin.
Conclusion
Our study demonstrated that the preoperative
adminis-tration of 150mg pregabalin significantly decreased the
severityofmusclefasciculation’s,without aneffectonits
incidence.Italsodecreased theincidenceandseverityof
succinylcholine-induced myalgia as well as postoperative
fentanylconsumption.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Perry JJ,Lee JS, Sillberg VA, et al. Rocuroniumversus suc-cinylcholineforrapidsequenceinductionintubation.Cochrane DatabaseSystRev.2008;16:CD002788.
2.FarhatK,PashaAK,JafferyN.Biochemicalchangesfollowing succinylcholineadministrationafterpretreatmentwith rocuro-niumatdifferentintervals.JPakMedAssoc.2014;64:146---50.
3.True CA, Carter PJ. A comparison of tubocurarine, rocuro-nium, and cisatracurium in the prevention and reduction of succinylcholine-induced muscle fasciculations. AANA J. 2003;71:23---8.
4.HassaniM,SahraianMA.Lidocaineordiazepamcandecrease fasciculation induced by succinylcholine during induction of anesthesia.MiddleEastJAnesthesiol.2006;18:929---31.
5.Shrivastava OP,Chatterji S, KachhawaS,et al. Calcium glu-conatepretreatmentforpreventionofsuccinylcholine-induced myalgia.AnesthAnalg.1983;62:59---62.
6.KumarM,TalwarN,GoyalR,etal.Effectofmagnesiumsulfate with propofol induction of anesthesia on succinylcholine-induced fasciculations and myalgia. J Anaesthesiol Clin Pharmacol.2012;28:81---5.
7.Rahimi M,Makarem J,GoharriziAG.Succinylcholine-induced myalgiainobstetricpatientsscheduledforcaesareansection --- diclofenac vsplacebo patches. Middle East J Anesthesiol. 2009;20:417---22.
8.CelebiN,CanbayO,CilH,etal.Effectsofdexmedetomidine onsuccinylcholine-inducedmyalgiaintheearlypostoperative period.SaudiMedJ.2013;34:369---73.
9.YunMJ,KimYH,GoYK,etal.Remifentanilattenuatesmuscle fasciculationsbysuccinylcholine.YonseiMedJ.2010;51:585---9.
10.HattaV,SaxenaA,KaulHL.Phenytoinreduces suxamethonium-inducedmyalgia.Anaesthesia.1992;47:664---7.
11.Leeson-Payne CG, Nicoll JM, Hobbs GJ. Use of ketorolac in the preventionof suxamethonium myalgia.Br JAnaesth. 1994;73:788---90.
12.Pandey CK, Tripathi M, Joshi G, et al. Prophylactic use of gabapentin for prevention of succinylcholine-induced fasciculation and myalgia: a randomized, double-blinded, placebo-controlledstudy.JPostgradMed.2012;58:19---22.
13.Hartman GS, Fiamengo SA, Riker WF Jr. Mechanism of fasciculations and their prevention by d-tubocurarine or diphenylhydantoin.Anesthesiology.1986;65:405---13.
14.FinkK,DooleyDJ,MederWP,etal.InhibitionofneuronalCa(2+) influxbygabapentinandpregabalininthehumanneocortex. Neuropharmacology.2002;42:229---36.
16.McLoughlin C,ElliottP,McCarthyG, et al.Muscle painsand biochemicalchangesfollowingsuxamethoniumadministration aftersixpretreatmentregimens.Anaesthesia.1992;47:202---6.
17.Wong SF, Chung F. Succinylcholine-associated postoperative myalgia.Anaesthesia.2000;55:144---52.
18.Allen DG. Skeletal muscle function: role of ionic changes in fatigue,damageand disease. ClinExp PharmacolPhysiol. 2004;31:485---93.
19.BauerCS,RahmanW,Tran-van-MinhA,etal.Theanti-allodynic alpha(2)deltaligand pregabalininhibitsthetraffickingofthe calciumchannelalpha(2)delta-1subunittopresynaptic termi-nalsinvivo.BiochemSocTrans.2010;38:525---8.
20.KavoussiR.Pregabalin: frommoleculetomedicine.Eur Neu-ropsychopharmacol.2006;16:S128---33.
21.ClarkeH,BoninRP,OrserBA,etal.Thepreventionofchronic postsurgical pain using gabapentin and pregabalin: a com-bined systematic review and meta-analysis. Anesth Analg. 2012;115:428---42.
22.Dauri M, Faria S, Gatti A, et al. Gabapentin and pre-gabalin for the acute post-operative pain management. A systematic---narrativereviewoftherecentclinicalevidences. CurrDrugTargets.2009;10:716---33.
23.MaddineniVR,MirakhurRK,CooperAR.Myalgiaandbiochemical changes following suxamethonium after induction of anaes-thesia with thiopentone or propofol. Anaesthesia. 1993;48: 626---8.
24.McClymontC.Acomparisonoftheeffectofpropofolor thiopen-toneontheincidenceandseverityofsuxamethonium-induced myalgia.AnaesthIntensiveCare.1994;22:147---9.
25.ReubenSS,BuvanendranA,KroinJS,etal.Theanalgesic effi-cacyofcelecoxib,pregabalin,andtheircombinationforspinal fusionsurgery.AnesthAnalg.2006;103:1271---7.