www.jped.com.br
ORIGINAL
ARTICLE
TLR-2
and
TLR-4
expression
in
monocytes
of
newborns
with
late-onset
sepsis
夽
,
夽夽
Ana
C.C.
Redondo
a,
Maria
E.J.R.
Ceccon
a,∗,
Ana
L.
Silveira-Lessa
b,
Camila
Quinello
c,
Patrícia
Palmeira
c,
Werther
B.
Carvalho
d,
Magda
Carneiro-Sampaio
eaNeonatalIntensiveCareUnit,InstitutodaCrianc¸a,HospitaldasClínicas,FaculdadedeMedicina,UniversidadedeSãoPaulo
(USP),SãoPaulo,SP,Brazil
bDepartmentofParasitology,InstitutodeCiênciasBiomédicas,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil
cLaboratoryofMedicalInvestigation(LIM-36),InstitutodaCrianc¸a,HospitaldasClínicas,FaculdadedeMedicina,Universidade
deSãoPaulo(USP),SãoPaulo,SP,Brazil
dDepartmentofNeonatology,FaculdadedeMedicina,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil eDepartmentofPediatrics,FaculdadedeMedicina,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil
Received1October2013;accepted26December2013 Availableonline27May2014
KEYWORDS
Sepsis/immunology; Innateimmunity; Toll-likereceptor2; Toll-likereceptor4; Newborn;
Monocytes
Abstract
Objective: Toanalyzetoll-likereceptor(TLR)-2andTLR-4expressioninmonocytesofnewborns withlate-onsetsepsis.
Methods: Thisprospectivestudyincluded27full-termnewbornsaged8to29days,with clin-icalandlaboratorydiagnosisoflate-onsetsepsis.Tennewborns(37%)hadpositivecultures. Cytokinesweremeasured bycytometricbeadarrayinperipheralblood,whileTLR-2,TLR-4 expression,andmedian fluorescenceintensity(MFI)were determined by immunophenotyp-ingperipheralwholebloodmonocytes,andwereanalyzedwithaBDFACSDivaflowcytometer (Becton,DickinsonandCompany,USA).Acomparisonwasperformedwithhealthyadults. Results: Microorganismswereidentifiedin37%ofthesesepticnewborns,andallofthemhad highlevels ofpro-inflammatorycytokines (IL-8, IL-6,IL-1)and anti-inflammatorycytokine (IL-10)corroboratingtheinflammatory/septicprocess.Inmonocytes,thefrequencyofTLR-4 expressionwashigherininfectednewborns(p=0.01).
Conclusion: Thisstudy investigatedtheinnate immune responseinseptic newborns.Septic newborns thatreliedalmostexclusively ontheinnateimmune system showedlittle invivo responseatmonocyteactivation,suggestingimpairedimmuneresponseandincreased suscep-tibilitytoinfection.
©2014SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
夽 Pleasecitethisarticleas:RedondoAC,Ceccon ME,Silveira-LessaAL, QuinelloC,PalmeiraP,CarvalhoWB,etal.TLR-2andTLR-4
expressioninmonocytesofnewbornswithlate-onsetsepsis.JPediatr(RioJ).2014;90:472---8.
夽夽StudyconductedatInstitutodaCrianc¸a,HospitaldasClínicas,FaculdadedeMedicina,UniversidadedeSãoPaulo,SãoPaulo,Brazil.
∗Correspondingauthor.
E-mail:maria.esther@hc.fm.usp.br(M.E.J.R.Ceccon).
http://dx.doi.org/10.1016/j.jped.2013.12.012
PALAVRAS-CHAVE
Sepse/imunologia; Imunidadeinata; Receptor2toll-like; Receptor4toll-like; Recém-nascidos; Monócitos
ExpressãodoTLR-2edoTLR-4emmonócitosderecém-nascidosatermocomsepse tardia
Resumo
Objetivos: AnalisaraexpressãodosTLR-2eTLR-4emmonócitosderecém-nascidoscomsepse tardia.
Métodos: Trata-sedeumestudoprospectivocom27recém-nascidosatermoentre8e29dias de vidacomdiagnóstico clínico elaboratorialdesepse tardiadosquais dez (37%) apresen-taramculturapositiva.AscitocinasforamdeterminadasportestedeCBAemsangueperiférico enquantoqueaexpressãoeMFI(medianadeintensidadedefluorescência)dosTLR-2eTLR-4 foi determinadoporimunofenotipagemem monócitosde sangueperiféricototal atravésde análisepelocitômetrodefluxoBDFACSDiva. Ogrupousadopara comparac¸ãofoideadultos saudáveis.
Resultados: Microrganismosforamidentificadosem37%dospacienteseestesjuntamentecom ospacientescomsepseclínicativeramníveiselevadosdecitocinaspró-inflamatórias(IL-8,IL-6, IL-1)edecitocinaanti-inflamatória(IL-10)corroborandooprocessoinflamatório/infeccioso. Nomonócito,afrequênciadeexpressãodoTLR-4foimaiselevada(p=0,01).
Conclusões: Esteestudoanalisouarespostaimuneinatanorecém-nascidocomsepse. Recém-nascidossépticosquedependemquaseexclusivamentedosistemaimuneinatoapresentaram poucarespostainvivonaativac¸ãodemonócitosoquesugereumarespostaimunedeficientee maiorsusceptibilidadeàinfecc¸ão.
©2014SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Sepsisrepresentsamajorcauseofmorbidityandmortalityin thenewborn,1,2whoseseverityisproportionaltothe
inter-actionbetweenthehostandthecausativeagent,triggering a cascadeof events responsible for the immune response expression.
Late-onsetsepsisoccursafter72hoursoflife,with clin-icalsignsandsymptomsthatmaybesubtleandnonspecific early in the infection, which are often misinterpreted or mistakenfor other non-infectiousclinicalconditions. Nev-ertheless,itsevolutioncanbefulminant,leadingtoseptic shock, disseminated intravascular coagulation, and death withinhours.3---5
Differentlyfromtheadult,severaldegreesofdeficiency have been described inthe newbornregarding theinnate and adaptive immune responses.6 At birth, the adaptive
immuneresponseisimpaired,bothbytheminimalinutero antigenexposureandbyBandTeffectorcelldysfunction.1
Becauseofthat,thenewbornreliesontheeffectivenessof theinnateimmuneresponseandthepassive protectionof maternalantibodiesacquiredtransplacentally.7
A primordial part of the innate immune response triggercorrespondstotheactivationofthetoll-like recep-tors (TLRs), expressed on the surface of monocytes, macrophages,dendriticcells,lymphocytes,epithelialcells, orinthecytoplasmofdifferenttissuecells.Ofthetentypes ofreceptorsdescribedinhumans,TLR-2 andTLR-4 mainly recognizecomponentsofGram-positiveandGram-negative bacteria,respectively.
Thesignaltransductionactivationrecruitsseveral intra-cellularproteins(MyD88,IRAK,andTRAF-6),whichtrigger the activation of JNK (Jun amino-terminal kinases) and
ERK (extracellular signal-regulated kinases) pathways in thecascadeofmitogen-activatedproteinkinases(MAPKs). Thisinduces theactivationoftranscription factors activa-tor protein 1 (AP-1) and nuclear factor kappa B (NF-kB) thatexpressseveralgenesdirectlyinvolvedinthe produc-tion of inflammatory cytokines in response to infection, playing a key role in the amplification of cell immune response.8---11
Monocytesareantigen-presenting cells that actonthe inflammatoryprocessandasasourceofmacrophagesand dendritic cells. After activation through TLRs, there is an increase in the expression of costimulatory molecules (CD80andCD86),whichareimportantintheearlyadaptive immuneresponseandcytokineproduction.12
The regulation of the immune system at birth results ina biased TLR neonatalresponse by stimulating alower productionofpro-inflammatorycytokinesand demonstrat-inglowermulti-functionality.13,14 Onlyinthecourseoflife
docytokinelevelsbecomeequivalenttothoseoftheadult individual.15 However,intheneonatalperiod,quantitative
andqualitativechangesinTLRsandcellsparticipatinginthe innate immune responsehave been described when com-pared to the adult individual, which are proportional to gestationalage at birth.16 These differences could
eluci-date the increasedsusceptibility to infection observed in theneonatalagegroup.1,17
Therefore,despitethegrowingawarenessofthe impor-tance of the TLR system in protecting newborns against infections,18muchstillneedstobeclarifiedaboutthe
Methods
This was a prospective study, whose convenience sample included27full-termnewbornstransferredtotheneonatal intensivecareunit(NICU)oftheInstitutodaCrianc¸a- Hos-pitaldasClínicasdaFaculdadedeMedicinadaUniversidade deSãoPaulo(HCFMUSP),fromFebruaryof2011toJanuary of2013.Patientswhosegestationalagerangedfrom37to 42weeksandshowedclinicaland/orlaboratorysymptoms (completebloodcountandC-reactiveprotein)ofneonatal sepsis from72hoursup to 30 days of life at the time of admissionorduringhospitalization,whichledtothestart ofantibiotictherapy,wereincludedinthestudy.
Exclusioncriteriawerefactorsthatalonewouldalterthe immune response, such as: diagnosis of congenital infec-tions,inbornerrorsofmetabolism,useofanti-inflammatory drugs(indomethacin,ibuprofen,andsteroids),diagnosisof intracranial hemorrhage confirmed by skull ultrasound or computedtomography andsurgeryin theweek before,in additiontothoseinwhichthedateofsamplecollectiondid notpermitanalysis.
The criteria used to define and classify the diagnosis regardingclinicalseverity(sepsis,severesepsis,andseptic shock)werethosementionedin theSurvivingSepsis Cam-paign(2012),adjustedfor agerangebasedonthecriteria of Goldstein (2005) as determined by the consensus.19,20
Thecompletebloodcounts(CBC)analysiswasbasedonthe HematologicScoringSystem(HSS)ofRodwell,whereasCRP (C-reactive protein) level > 10mg/L was considered sug-gestiveofinfection.ThestudywasapprovedbytheEthics CommitteeforAnalysisofResearchProjectsoftheHCFMUSP (CAPPesq).
Sampling
After an informed consent was obtained from the legal guardians of the newborn, blood samples were collected byvenipuncture froma peripheral vein for assessment of infectiouspicture(CBC,CRP,andblood culture)and anal-ysis of cytokines and monocytes. The time of collection wasstandardizedtooccurwithinthefirst24hoursfromthe suspecteddiagnosisofinfectionandthestartofantibiotic therapy.An aliquot of 0.5mL of blood was distributed in aseparator tube withgel for cytokine measurement,and 1.5mL was placed in a tube with EDTA (Ethylenediamine
tetraaceticacid)forimmunophenotyping,whichwereboth performedattheLaboratoryofMedicalInvestigation36(LIM 36)ofInstitutodaCrianc¸aofHCFMUSP.
Cytokineanalysis
Cytokines TNF- ␣, IL-1 , IL-2, IL-4, IL-6, IL-8, and IL-10 weremeasuredinserumsamplesusingahumancytometric bead array (CBA) set (Cytometricbead array BDOptEIATM
SetHuman,Becton,DickinsonandCompany,USA)according tothemanufacturer’sinstructions.
AnalysisofTLRs
Immunophenotyping was performed with the sample red bloodcells,whichwerecollectedintubescontainingEDTA after beingprepared for additionof pre-established anti-bodies(Table1)andprocesseduntiltheyweretransferred tobereadintheflowcytometerFACSIILRSFortessa (Bec-ton,DickinsonandCompany,USA).Themonocytepopulation wasdefinedbygatingofCD14+HLA-DR+cellsusingthesize (forward scatter-FSC)andcellgranularityasparameters (sidescatter-SSC)asdescribedintheliterature.20
The analysis was performed in FlowJo software (Tree Star- Ashland,OR, UnitedStates),whereeach cell popu-lationwasanalyzedseparatelybygatingusingcellsizeand granularityasparameters.Thesamesoftwareprovideddata representingthemedianfluorescenceintensity(MFI)ofthe respectivemarkers.
Theabsolutenumbersofpopulationswerecalculatedby multiplying the percentage indicated for each population in flow cytometryand theabsolute number of leukocytes determinedbyautomaticcellcounter.
Bloodcontrolsamplesfromhealthyadults
Peripheral blood samples were obtained from 27 healthy adults andused for test standardization and control.The selectioncriteriawere:healthyadultvolunteers;aged 18-35 years;of both genders;withnegative serologicaltests forhumanimmunodeficiencyvirus (HIV),HTLVI/II(Human TlymphotropicvirustypeI/II),hepatitisBandC,syphilis, and Chagasdisease; andno symptomsof infection during thecollectionperiod.
Table1 Monocytecellsurfacemarkers.
Monoclonalantibody Clone Fluorochrome Manufacturer
HLA-DR L243 APCCy7 BDBiosciences
CD14 M5E2 Pacificblue BDBiosciences
CD80 BB1 FITC BDBiosciences
CD86 2331(FUN-1) PECy5 BDBiosciences
TLR-2(CD282) 11G7 Alexa647 BDBiosciences
TLR-4(CD284) HTA125 PE BioLegenda
HLA-DR,humanleukocyteantigenclassII-DRlocus;CD,clusterofdifferentiation;TLR,toll-likereceptor;APCCy7,allophycocyanin com-binedwithcyaninedye;Pacificblue,6,8-difluoro-7-hydroxycoumarinfluorophore;FITC,fluoresceinisothiocyanate;PECy5,conjugate ofphycoerythrinandcyaninedye;Alexa647,fluorochromeforreadingbetween594and633nm;PE,R-phycoerythrin.Clone,refersto thespecifiedmarkersofmonoclonalantibodies.
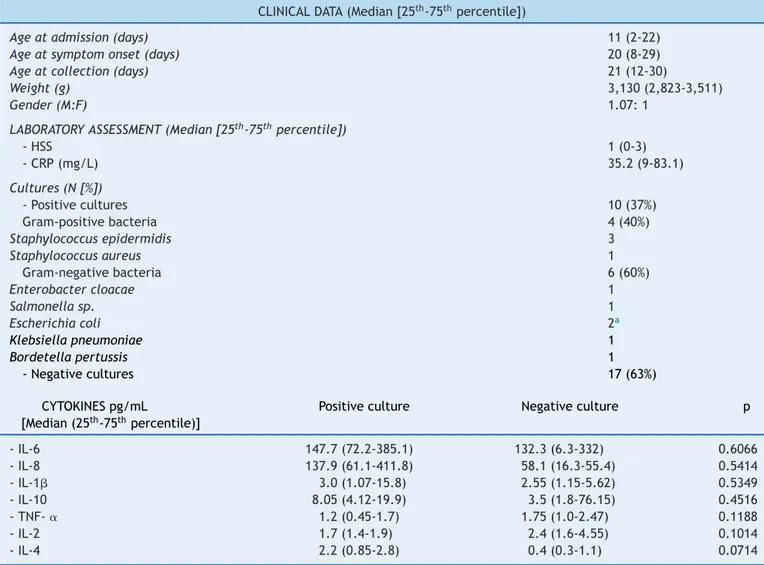
Table2 Distributionof27newbornsaccordingtotheclinicalandlaboratorydata.
CLINICALDATA(Median[25th-75thpercentile])
Ageatadmission(days) 11(2-22)
Ageatsymptomonset(days) 20(8-29)
Ageatcollection(days) 21(12-30)
Weight(g) 3,130(2,823-3,511)
Gender(M:F) 1.07:1
LABORATORYASSESSMENT(Median[25th-75thpercentile])
-HSS 1(0-3)
-CRP(mg/L) 35.2(9-83.1)
Cultures(N[%])
-Positivecultures 10(37%)
Gram-positivebacteria 4(40%)
Staphylococcusepidermidis 3
Staphylococcusaureus 1
Gram-negativebacteria 6(60%)
Enterobactercloacae 1
Salmonellasp. 1
Escherichiacoli 2a
Klebsiellapneumoniae 1
Bordetellapertussis 1
-Negativecultures 17(63%)
CYTOKINESpg/mL
[Median(25th-75thpercentile)]
Positiveculture Negativeculture p
-IL-6 147.7(72.2-385.1) 132.3(6.3-332) 0.6066
-IL-8 137.9(61.1-411.8) 58.1(16.3-55.4) 0.5414
-IL-1 3.0(1.07-15.8) 2.55(1.15-5.62) 0.5349
-IL-10 8.05(4.12-19.9) 3.5(1.8-76.15) 0.4516
-TNF-␣ 1.2(0.45-1.7) 1.75(1.0-2.47) 0.1188
-IL-2 1.7(1.4-1.9) 2.4(1.6-4.55) 0.1014
-IL-4 2.2(0.85-2.8) 0.4(0.3-1.1) 0.0714
HSS,hematologicscoringsystemofRodwell;CRP,C-reactiveprotein;IL,interleukin;TNF-␣,tumornecrosisfactoralpha.
a OnepatientwithpositiveurinecultureforEscherichiacoli.
Statisticalanalysis
Thenewborndatawererecordedonthecollectionformsand storedinspreadsheetsfromthestatisticalpackage Graph-PadPrism®,release6.0cforMacOSX(GraphPadSoftware, LaJollaCalifornia,UnitedStates).Forqualitativevariables, absoluteandrelativefrequencies(percentageandnumber ofcases)werecalculated,whereasfor quantitative varia-bles,themedian,theminimumandmaximumvalues,and interquartilerange (P25-P75)were calculatedfor each of thereceptorsevaluatedineachanalysis.TheMann-Whitney testwasusedtocomparethemedianofmarkersbetween the groups, while the comparative analysis of the medi-ans of morethan twogroups usedthe Kruskal-Wallis test withDunn’spost-test.The testswereperformed with95% confidence intervals and a p-value< 0.05 wasconsidered significant.
Results
Clinical and laboratory characteristics of the 27 infants included in the study are shown in Table 2. It can be observed that most patients (62.9%) had symptoms
compatible with severe sepsis (44.4%) and septic shock (18.5%),whichoccurredatamedianageof20daysoflife; deathwasobservedinonlytwopatients(7.4%).Amongthe 17 patients who had negative cultures, all showed clear clinicalsignsandsymptomsofinfectionandcompatible lab-oratoryalterationsin theCBCandCRP levelsat diagnosis andsubsequentclinicalanalysis,andthuswereconsidered patientswithclinicalsepsis.
Of these patients withclinical sepsis, eight (47%) had aclinical picturecompatiblewithsepsis,five(29.5%)with severesepsis,andfour(23.5%)withsepticshock.Moreover, themeasurementofpro-inflammatory cytokineswas simi-larin infected patients regardless of positivecultures, as shown in Table 2. Culture positivityin material collected fromsterile fluids (blood,urine,and CSF)occurred inten patients(37%),withdistributionaccording totheisolated agentasshowninthesametable.
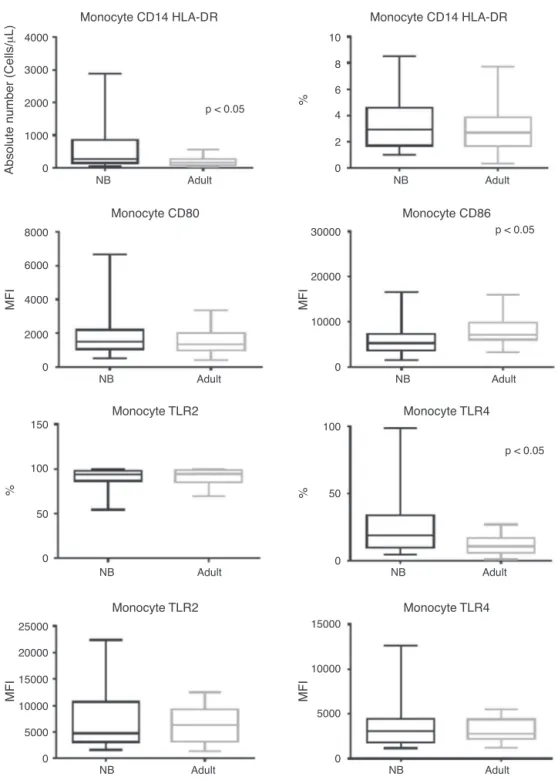
Regardingmonocytes,itcanbeobservedthatdespitethe factthattheirtotalabsolutenumberwashigherinnewborns whencomparedtoadults(p<0.0001),therewasasimilar frequencyofmonocytesbetweengroups(Fig.1).
Monocyte CD14 HLA-DR
Monocyte CD80
Monocyte TLR2
Monocyte CD86
Monocyte TLR4
Monocyte TLR2 Monocyte TLR4
Absolute number (Cells/
μ
L)
MFI MFI
%
4000
3000
2000
1000
0
2000 4000 6000 8000
0 0
MFI MFI
0 5000 10000 20000
15000 25000
0 5000 10000 15000 0 50
%
0 50 100
150 100
10000 20000 30000
NB Adult
NB Adult NB Adult
NB Adult
NB Adult
NB Adult
NB Adult
p < 0.05
Monocyte CD14 HLA-DR
%
0 2 4 6 8 10
NB Adult
p < 0.05
p < 0.05
Figure1 Boxplotofabsolutenumbers(cells/L),frequency(%),andmedianfluorescenceintensity(MFI)ofCD80,CD86,TLR-2,
andTLR-4activationmolecules.Valuesexpressedasmedian±interquartilerange(P25-P75);preferstostatisticalsignificance. Samplenumberofthe‘‘newborn’’and‘‘adult’’groups=27.
NB,newborn;HLA-DR,humanleukocyteantigenclassII-DRlocus;CD,clusterofdifferentiation;TLR,toll-likereceptor.
patients showed maintenance of CD80 and TLR-2 expres-sion(p = 0.822 and p = 0.825, respectively), while there wasahigherfrequencyforTLR-4insepticnewbornswhen comparedtoadults(p=0.0043).
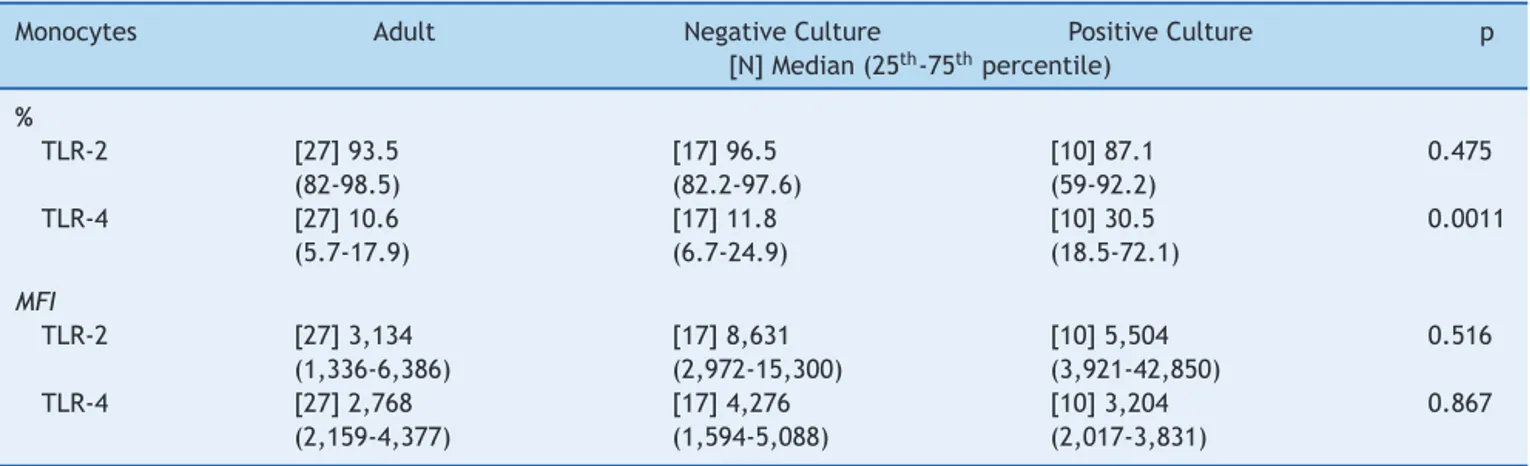
Culturepositivitywasassociated toa higherfrequency ofTLR-4whencomparedtoadultsandpatientswith nega-tivecultures(Table3),whiletherewasasimilarfrequency betweengroupsintheevaluationofTLR-2.
Despitetheidentificationofsomebacteria,theanalysis betweenculturepositivityaccordingtothetypeofbacteria
identifiedandthetypes ofTLR wasnotperformed dueto thesamplenumber.
Discussion
Table3 Median frequency(%)and MFIoftoll-likereceptors 2and4inperipheralbloodmonocytesofinfected newborns (positiveornegativeculture),andadultperipheralblood.
Monocytes Adult NegativeCulture PositiveCulture p
[N]Median(25th-75thpercentile)
%
TLR-2 [27]93.5
(82-98.5)
[17]96.5 (82.2-97.6)
[10]87.1 (59-92.2)
0.475
TLR-4 [27]10.6
(5.7-17.9)
[17]11.8 (6.7-24.9)
[10]30.5 (18.5-72.1)
0.0011
MFI
TLR-2 [27]3,134
(1,336-6,386)
[17]8,631 (2,972-15,300)
[10]5,504 (3,921-42,850)
0.516
TLR-4 [27]2,768
(2,159-4,377)
[17]4,276 (1,594-5,088)
[10]3,204 (2,017-3,831)
0.867
preferstostatisticalsignificanceusingtheKruskal-Wallistest. MFI,medianfluorescenceintensity;TLR,toll-likereceptor.
in newborns with infection caused by Gram-positive and Gram-negativebacteria,andincreasedexpressionofTLR-2 inpatientswithclinicalsepsis.
Monocytes arephagocyticcells, and play an important roleininnateandacquiredimmunitythroughtheireffects ontheinflammatoryprocessorasasourceofmacrophages and dendritic cells. The behavior of TLRs in the new-born,eitherinhealthyorinfectedinfants,iscontroversial. AccordingtoLevy etal.,althoughthe basalexpressionof TLRsinmonocytesoffull-termnewbornsissimilartothatof adultindividuals,thefunctionalconsequencesofactivation arequitedifferent,asalowerproductionofcytokinesand lowerexpressionofco-stimulatorymoleculesareobserved innewborns.18
Itwasobservedthatinthepresenceofaninfectious stim-ulus,thepopulationofassessednewbornsshowedalower numberofactivatedcells (lowerMFIfor CD86thanin the adultindividualandMFIforCD80similartoadultindividuals without infection)in peripheral blood,suggesting a lower activitypotentialofthiscelltypeinnewborns,whichisin accordancewiththeliterature.18---21
Regarding TLR-2, it was observed that it was widely expressed in neonatal monocytes (93.9%) and monocytes of adult individuals without infection (94.6%). The same similarity was observed regarding MFI for this receptor (MFI=4,748and6,386,respectively)regardlessofculture positivity, contradicting findings in the literature that demonstrate an up-regulation in the expression of TLR-2 in monocytes isolated from patients with sepsis, as well assignificantchangesinTLR-2expression.22,23 Thus,inthe
presentstudy,inwhichallnewbornshadclinicaland labora-torysignsofinfection,itwasobservedthatdespitepresence of the necessary tools to recognize the invading antigen (TLR andco-stimulatory molecules),theydid notundergo theup-regulationexpectedintheoverallanalysis.
Meanwhile,contrarytowhatwasreportedby Viemann etal.,1 the presence ofa positive culture wasassociated
withahigherfrequencyinTLR-4expressioninrelationtothe adultindividualwithanegativeculture,probablysecondary to the fact that most patients with positive cultures had Gram-negativebacteria(60%)isolatedfromtheculture.As
forthesimilarexpressionofthesamereceptorinnewborns withnegative cultures and in adult patients, it may sug-gestatendencytoimmunoparalysisthatwouldhaveshifted themedian tolowerlevels,similartothe levelsfound in healthyadultindividuals,asfournewbornsfromthisgroup progressedtosepticshock.
The present study has limitations that should be emphasized,suchasthesamplesizeandthepointwise char-acteristicoftheinfectiouspictureassessment.However,the resultsstillsuggestthatatthetimeofdiagnostic hypothe-sisofinfectioninthenewborns,TLR-2andTLR-4receptors hadahigherexpressionininfants withinfection,butwith maintenanceofexpressionofcostimulatorymolecules, indi-catinga possible deficiency in the cell activation process invivo.
Thus,althoughlargerextrapolationscannotbemade,the authorsbelieveitwouldbeintriguingtoassesstheresponse ofTLRaswellasthatofcellactivationinnewbornsduring theevolutionofaclinicalinfection.
Funding
Fundac¸ão de Amparo à Pesquisa do Estado São Paulo -FAPESP.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthorswould liketothankFAPESPfor theirfinancial support.
References
2.DellingerRP,LevyMM,CarletJM,BionJ,ParkerMM,Jaeschke R,etal.SurvivingSepsisCampaign:internationalguidelinesfor managementofseveresepsisandsepticshock:2008.CritCare Med.2008;36:296---327.
3.Camacho-GonzalezA,SpearmanPW,StollBJ.Neonatal infec-tiousdiseases:evaluationofneonatalsepsis.PediatrClinNorth Am.2013;60:367---89.
4.Bedford Russell AR. Neonatal sepsis. Paediatr Child Health. 2011;21:265---9.
5.CianciarulloMA,CostaIC,CecconME,KrebsVL.Newandold markersoninfectioninneonatalsepsisdiagnostic:criticvision. Pediatria(SãoPaulo).2008;30:107---17.
6.KollmannTR,CrabtreeJ,Rein-WestonA,BlimkieD,Thommai F,WangXY,etal.NeonatalinnateTLR-mediatedresponsesare distinctfromthoseofadults.JImmunol.2009;183:7150---60. 7.Yoon HS. Neonatal innate immunity and toll-like receptor.
KoreanJPediatr.2010;53:985---8.
8.MuzioM,BosisioD,PolentaruttiN,D’amicoG,StoppacciaroA, MancinelliR,etal.Differentialexpressionandregulationof toll-likereceptors(TLR)inhumanleukocytes:selectiveexpression ofTLR3indendriticcells.JImmunol.2000;164:5998---6004. 9.TippingPG.Toll-likereceptors:theinterfacebetweeninnate
andadaptiveimmunity.AmSocNephrol.2006;17:1769---71. 10.BochudPY,Calandra T.Pathogenesisofsepsis: newconcepts
andimplicationsforfuturetreatment.BMJ.2003;326:262---6. 11.HigginsSC,LavelleEC,McCannC,KeoghB,McNeelaE,Byrne
P,et al. Toll-likereceptor 4-mediatedinnate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J Immunol.2003;171:3119---27.
12.KadowakiN,HoS,AntonenkoS,MalefytRW,KasteleinRA,Bazan F,etal.Subsetsofhumandendriticcellprecursorsexpress dif-ferent toll-like receptors and respond to differentmicrobial antigens.JExpMed.2001;194:863---9.
13.NguyenM,LeuridanE,ZhangT,DeWitD,WillemsF,VanDamme P,etal.Acquisitionofadult-likeTLR4andTLR9responsesduring thefirstyearoflife.PLoSOne.2010;5:e10407.
14.PrabhuDasM,AdkinsB,GansH,KingC,LevyO,RamiloO,etal. Challenges ininfant immunity:implications for responses to infectionandvaccines.NatImmunol.2011;12:189---94. 15.KollmannTR,LevyO,MontgomeryRR,GorielyS.Innateimmune
functionbyToll-likereceptors:distinctresponsesinnewborns andtheelderly.Immunity.2012;37:771---83.
16.Förster-WaldlE,SadeghiK,TamandlD,GerholdB,HallwirthU, RohrmeisterK,etal.Monocytetoll-likereceptor4expression and LPS-induced cytokine production increase during gesta-tionalaging.PediatrRes.2005;58:121---4.
17.Wynn JL, Levy O. Role of innate hostdefenses in suscepti-bilitytoearly-onsetneonatalsepsis. ClinPerinatol.2010;37: 307---37.
18.Levy O. Innate immunity of the newborn: basic mecha-nisms and clinical correlates. Nat Rev Immunol. 2007;7: 379---90.
19.DellingerRP,LevyMM, RhodesA, AnnaneD,GerlachH,Opal SM,et al.Survivingsepsiscampaign:internationalguidelines formanagementofseveresepsisandsepticshock:2012.Crit CareMed.2013;41:580---637.
20.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sep-sis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6: 2---8.
21.Sánchez-TorresC,García-RomoGS,Cornejo-CortésMA, Rivas-CarvalhoA,Sánchez-SchmitzG.CD16+andCD16-humanblood monocytesubsetsdifferentiateinvitrotodendriticcellswith different abilities to stimulate CD4+ T cells. Int Immunol. 2001;13:1571---81.
22.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge:repurificationoflipopolysaccharideeliminatessignaling throughbothhumanandmurinetoll-likereceptor2.JImmunol. 2000;165:618---22.