SOCIEDADE BRASILEIRA DE ORTOPEDIA E TRAUMATOLOGIA
w w w . r b o . o r g . b r
Update
Article
Regenerative
potential
of
the
cartilaginous
tissue
in
mesenchymal
stem
cells:
update,
limitations,
and
challenges
夽
Ivana
Beatrice
Mânica
da
Cruz
a,b,
Antônio
Lourenc¸o
Severo
c,
Verônica
Farina
Azzolin
b,
Luiz
Filipe
Machado
Garcia
b,
André
Kuhn
c,
Osvandré
Lech
c,∗aUniversidadeFederaldeSantaMaria(UFSM),CentrodeCiênciasdaSaúde,SantaMaria,RS,Brazil
bUniversidadeFederaldeSantaMaria(UFSM),LaboratóriodeBiogenômica,SantaMaria,RS,Brazil
cUniversidadeFederaldaFronteiraSul(UFFS),HospitalSãoVicentedePaulo,InstitutodeOrtopediaeTraumatologia,PassoFundo,
RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12February2016
Accepted15February2016
Availableonline6December2016
Keywords:
Stemcells
Cartilaginoustissue
Regenerativepotential
a
b
s
t
r
a
c
t
Advancesinthe studieswithadultmesenchymalstemcells(MSCs)haveturnedtissue
regenerativetherapyintoapromisingtoolinmanyareasofmedicine.Inorthopedics,oneof
themainchallengeshasbeentheregenerationofcartilagetissue,mainlyindiarthroses.In
theinductionoftheMSCs,inadditiontocytodifferentiation,themicroenvironmental
con-textofthetissuetoberegeneratedandanappropriatespatialarrangementareextremely
importantfactors.Furthermore, itis knownthatMSC differentiationis fundamentally
determined bymechanisms such as cell proliferation (mitosis),biochemical-molecular
interactions,movement,celladhesion,andapoptosis.AlthoughtheuseofMSCsfor
car-tilageregenerationremainsataresearchlevel,thereareimportantquestionstoberesolved
inordertomakethistherapyefficientandsafe.Itisknown,forinstance,thattheexpansion
ofchondrocytesincultivation,neededtoincreasethenumberofcells,couldendup
produc-ingfibrocartilageinsteadofhyalinecartilage.However,thelatestresultsarepromising.In
2014,thefirststageI/IIclinicaltrialtoevaluatetheefficacyandsafetyoftheintra-articular
injectionofMSCsinfemorotibialcartilageregenerationwaspublished,indicatingadecrease
ininjuredareas.Oneissuetobeexploredishowmanymodificationsinthearticulate
inflam-matoryenvironmentcouldinducedifferentiationofMSCsalreadyallocatedinthatregion.
Suchissuearosefromstudiesthatsuggestedthatthesuppressionoftheinflammation
mayincreasetheefficiencyoftissueregeneration.Consideringthecomplexityoftheevents
relatedtothechondrogenesisandcartilagerepair,itcanbeconcludedthattheroadahead
isstilllong,andthatfurtherstudiesareneeded.
©2016PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradeOrtopedia
eTraumatologia.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
creativecommons.org/licenses/by-nc-nd/4.0/).
夽
StudyconductedattheInstituteofOrthopedicsandTraumatologyofPassoFundo,PassoFundo,andattheHealthSciencesCenter,
UniversidadeFederaldeSantaMaria(UFSM),SantaMaria,RS,Brazil.
∗ Correspondingauthor.
E-mails:lech@lech.med.br,ensino@iotrs.com.br(O.Lech).
http://dx.doi.org/10.1016/j.rboe.2016.11.005
2255-4971/©2016PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradeOrtopediaeTraumatologia.Thisisanopen
Potencial
regenerativo
do
tecido
cartilaginoso
por
células-tronco
mesenquimais:
atualizac¸ão,
limitac¸ões
e
desafios
Palavras-chave: Células-tronco
Tecidocartilaginoso
Potencialregenerativo
r
e
s
u
m
o
Osavanc¸osnosestudoscomcélulas-troncomesenquimais(CTMs)adultastornouaterapia
regenerativatecidualumaferramentapromissoraemdiversasáreasdamedicina.Na
orto-pedia,umdosprincipaisdesafiostemsidoaregenerac¸ãodotecidocartilaginoso,sobretudo
emdiartroses.Nainduc¸ãodeCTMs,alémdacitodiferenciac¸ão,ocontexto
microambien-taldotecidoaserregenerado,bemcomoumadisposic¸ãoespacialadequada,sãofatores
deextremaimportância.Alémdisso,sabe-sequeadiferenciac¸ãodasCTMsébasicamente
determinadapormecanismoscomoproliferac¸ãocelular(mitose),interac¸ões
bioquímico-moleculares, movimento, adesão celulare apoptose.Apesar de ousode CTMsparaa
regenerac¸ãodacartilagemestaraindaemâmbitodepesquisa,existemquestões
impor-tantesaseremresolvidasparatornaressaterapêuticaeficazesegura.Sabe-se,porexemplo,
queaexpansãodecondrócitosemcultura,necessáriaparaaumentaronúmerodecélulas,
podeproduzirfibrocartilagem,enãocartilagemhialina.Noentanto,osúltimos
resulta-dossãopromissores.Em2014,foipublicadooprimeiroensaioclínicofaseI/IIparaavaliar
aeficáciaeaseguranc¸adainjec¸ãointra-articulardeCTMsnaregenerac¸ãodecartilagem
femorotibialehouveumadiminuic¸ão dasáreas lesadas.Umaquestãoa serexplorada
éoquantomodificac¸õesnopróprioambienteinflamatórioarticularpoderiaminduzira
diferenciac¸ãodeCTMsjáalocadasnaquelaregião.Talincógnitapartedoprincípiode
estu-dosquesugeremqueasupressãodainflamac¸ãoarticularaumentaria,potencialmente,a
eficiênciadaregenerac¸ãotecidual.Considerandoacomplexidadedoseventosrelacionados
àcondrogêneseeaoreparodacartilagem,conclui-sequeocaminhoaindaélongo,são
necessáriaspesquisascomplementares.
©2016PublicadoporElsevierEditoraLtda.emnomedeSociedadeBrasileirade
OrtopediaeTraumatologia.Este ´eumartigoOpenAccesssobumalicenc¸aCCBY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Thehumanbodyfundamentallyoriginatesfromembryonic
stemcells:ectoderm,mesoderm, andendoderm.Itisfrom
thesethreeleafletsthatthe230celltypesfoundinthebody
aredifferentiated.Inadifferentiatedorganism,manytissues
retainadultstemcelllinesthatworkintissuereplacement
andregeneration;themostabundantonesareofmesodermal
origin,themesenchymalstemcells(MSCs).MSCsarefound
invariousplacesinthebody,suchasintheredbonemarrow,
hairfollicles,muscle,umbilicalcord,dentalpulp,adipose
tis-sue,bone,andcartilage,amongothers.1Withtheincreased
knowledgeonadultMSCs,theirclinicalusefortissue
regen-erationhasbecomequiteattractive.However,understanding
andeffectivelyandsafelyhandlingMSCsisstilla
consider-ablechallenge,especiallyintissueswithdifficultregeneration,
suchascartilage.
Inthiscontext,thisreviewaimedtoprovideanupdateon
themainprocessesrelatedtomorphodifferentiationandits
potentialrole intheregenerationofcartilage tissue.
There-fore,theinformationcontainedhereinisbasedonscientific
journalarticlesindexedinthedatabasesofPubMed-MEDLINE
andSciELO.
Cartilaginous
tissue
and
challenges
for
regeneration
Instructuralterms,thearticularcartilageisrichin
extracel-lularmatrix,inwhichchondrocytesaredistributed,whether
isolatedorarrangedinclonalgroupsinsmallcellcolonies.2
Chondrocytes areresponsible forsecreting cartilage matrix
components such as collagen, proteoglycans, and
glyco-proteins. Cartilage tissue receives nutrition via capillaries
contained in the perichondrium, a connective tissue that
surroundsthecartilageandhasadultMSCstermed
chondrob-lasts.
Nonetheless, as the cartilages lining the bonesof
mov-ablejointsdonothaveperichondrium,theyreceivenutrition
throughthesynovialfluidpresentinthejointcavities.
Syno-vial fluidis a plasma ultrafiltrate that passes through the
synovialmembrane,whereitreceivesmucopolysaccharides
that contain hyaluronic acid and a small amount of high
molecular weight proteins. Therefore, even with a large
amountofcollagenprotein,thesmallamountofcellular
com-ponentsincartilagetissuehindersitsregenerationcapability
leadingrepetitivejointinjuriestowardatendencytobecome
Inorder tounderstand the roleof MSCsin adulttissue
regeneration,it is importanttorememberthat the human
organismismadeofcellsthat havedistinctdifferentiation
andfunctions.4 Itshould benoted thattissueregeneration
is not only focused on the induction of undifferentiated
MSCs in a differentiated cell. Each type of tissue has an
extracellular matrix, witha key role in body homeostasis.
Thus,themicroenvironmentalcontext(extracellularmatrix)
ofthetissuebeingregeneratedmustbetakeninto
considera-tion.
Five causal mechanisms are crucial forcell
differentia-tionintissuesandorgans,aswellasthetissueregeneration
processitself,namely:cellproliferation(mitosis),
biochemi-calandmolecularinteractions,movement,celladhesion,and
apoptosis.Asthesemechanismsareofgreatimportancefor
handlingMSCswiththeperspectiveofdeveloping
cartilagi-noustissueregenerationtechniques,theywillbethefocusof
thisreview.
Proliferation
and
cellular
senescence
TounderstandingreaterdepththebiologyofMSCs,itis
neces-sarytounderstandsomeofthemechanismsassociatedwith
thecellcycle.Eukaryoticcellsdividebymitosis,considered
thefinalstageofthecycle,inwhichthetwonewlyborncells
willperformtheirrespectivemetabolicfunctions.
Nonetheless, the mitotic division is not an unlimited
process.Astheydivide,mostspecializedcellslosetheir
proli-ferativecapacity.Somecellsthatwillnolongerdivideremain
constantlyin the gap1phase (G1)of mitosis, which isthe
caseofthevastmajorityofchondrocytes.Newchondrocytes
areformedfromchondroblastsfromtheperichondrium;itis
throughthismechanismthatthecartilagetissueisrenewed,
albeit slowly when compared, for example, withbone
tis-sue.Conversely,somematurespecializedcells undergothe
entire cell cycle until they lose their ability to proliferate,
byaprocesstermedreplicativesenescenceorcellularaging
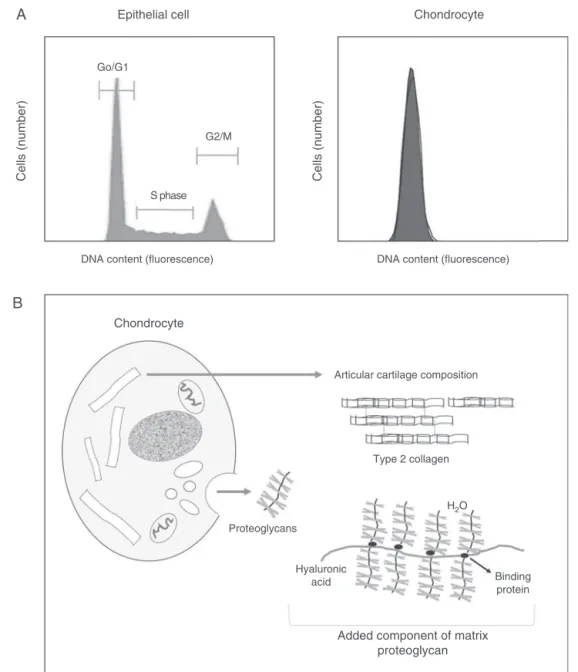
(Fig.1).
Cellularagingistriggeredbychangesoccurringinthe
ter-minalregionofthechromosome,knownastelomere,which
comprises a single-stranded deoxyribonucleic acid (DNA)
molecule, in contrast with the double-stranded structure
presentintheremainderofthegeneticmaterial.Telomeric
DNA consists of a sequence of six nucleotides – thymine,
thymine, adenine, guanine, guanine, guanine (TTAGGG) –
whichrepeatsthousandsoftimes.Thischromosomalregionis
synthesizedbythereversetranscriptaseenzymetelomerase,
which synthesizes DNA and uses a ribonucleic acid (RNA)
moleculeasascaffold.
In cell division, a small telomere shortening is always
observed.In embryonic cells,the telomere isreconstituted
bytheactionoftelomerase.Inspecializedcells,telomerase
geneissilenced;therefore,whentelomericshorteningoccurs,
telomere reconstitution is not possible. Overthe divisions
(approximately 50–80 mitoses), the telomere becomes too
shortandstartstoinhibitmitosis,thusconstitutingcellular
senescenceorHayflicklimit.
Unlikespecializedcells,inMSCs,thetelomerasegeneis
active;therefore,withinthebody,suchcellsdonotpresent
a sharp cellularaging.However, theMSC proliferation rate
isextremelylowandthusthenumberofthesecellsinbody
tissuesisquitelimited.Thisisanimportantchallengetobe
overcomeinregenerativetherapy.
Somestudieshavesuggestedthattheinductionofinvitro
MSC proliferationcanbedonebyexposuretoreactive
oxy-genspecies(ROS)molecules,suchashydrogenperoxide.The
study byBornes etal.5 showedthatinvitrochondrogenesis
ofMSCsinducedinsheepbonemarrowincreased
prolifera-tionandcelldifferentiation.However,itappearsthat,despite
theincreaseincellgrowth,cellsstarttopresentsignificant
DNAdamage,indicatingchromosomalinstability.6Thestudy
byMachadoetal.6supportstheresultsoftheresearch
con-ducted by Brand et al.,7 suggesting that in vitro exposure
tooxidative stressinducescellularsenescencein
chondro-cytes.
Inturn,the study byMachado etal.6 showed areversal
ofreplicativesenescencemarkersinhumanMSCscollected
throughliposuctionbysupplementingculturemediumwitha
hydroalcoholicguarana(Paulliniacupana)extract.Theguarana
seed,usedfortheproductionoftheextract,isrichincaffeine,
theophylline,theobromine,andcatechins.
AnotherverysurprisingresultwasdescribedbySadeghiet
et al.,8 who investigatedtheeffect ofestrogen
supplemen-tation on chondrogenesis induced in MSCs derived from
adiposetissue.Itwasobservedthatthepresenceofestrogen
hadnegativeeffectsonthechondrogenesisprocessby
inhib-itingtheexpressionofthecollagen2geneandreducingthe
expressionoftheaggrecanproteingene.
Cell
differentiation
All bodycells andtissuesareformedfrom thezygote,ina
highlycontrolledtranscriptionalregulationprocess.In
gen-eral,theDNAoftheeukaryoticgenehasaninitialsequence
of nucleotides known asthe promoter region. Itis in this
regionthat thesignalingmoleculesbind,allow(ornot) the
transcription, and determining the amount of RNA to be
transcribed.Thismodulationisknownasgeneregulation,a
mechanism throughwhicheach cell typeisformedbythe
production of proteins in different shapes and quantities.
Endogenousmolecules,suchastranscriptionfactorsand
hor-mones,candifferentiallyregulategenes.Likewise,molecules
derivedfromthediet,suchasresveratrol(presentingrapes),
inducetheproductionofsirtuins,proteinsthatincreasecell
life.
Underinvitroconditions,theinductionofMSC
differentia-tioninthepresenceofcertainmoleculesisverywellknown.
Notwithstanding,whenMSCsareplacedinaninjuredorgan,
itisnotalwayspossibletoknowwhetherthe
microenviron-mentalconditionswillfavortheinductionofdifferentiation
(even when the inducing agents are co-inserted with the
cells).
Other molecules that regulate the maintenance of the
undifferentiatedstateofMSCs,ensuringtheir
pluripotential-ityandself-renewal,havebeenidentified.Thisisthecaseof
Oct-4,Nanog,andSox-2,whicharefoundbothinhumansand
inmice.9Whenthisproteinisnolongerexpressed,thecellhas
Epithelial cell
A
B
Chondrocyte
Chondrocyte
Go/G1
G2/M
S phase
Cells (number) Cells (number)
DNA content (fluorescence)
Type 2 collagen
Proteoglycans
Hyaluronic acid
Added component of matrix proteoglycan
Binding protein H2O
Articular cartilage composition DNA content (fluorescence)
Fig.1–Comparisonofthecellcycleofanepithelialcellandofachondrocyte,assessedbyflowcytometry.(A)Inan epithelialtissue,cellsarefoundinphaseG0/G1,S,andG2/M,whileinthecartilagetissuemostchondrocytesareintheG1 phase.Onlychondroblastsfromperichondriumwillpresentacompletecellcycle.(B)Chondrocytes,onceformed,are usuallyclusteredinabouteightcellsthatcontinuouslysecreteanextracellularmatrixcomposedmainlyoftype2collagen, proteoglycans,andhyaluronicacid.
Toinducechondrogenicdifferentiation,MSCsarecultured
without the presence of fetal or adult serum (animal or
human), which is typically used to nourishthe cells, and
uponexposuretogrowthfactorb3.11Thus,thecellsdevelop
a multilayer with a proteoglycan-rich extracellular matrix.
In 10–14-day cultures, cells begin to producetype 2
colla-gen,characteristicofthe articularcartilage.Moreover,they
presentpositivesurfacemarkersforchondrocytesand
typi-calcellgapsvisibleatopticalmicroscopy.Thechondrocytes
remainviableuntilapproximately90daysafterinitiationof
differentiation.12
Chondrogenesis is induced through various inducing
molecules, especially through supplementation of culture
mediumwithTFN-3,IGF-1,BMP-2, andBMP-6.
Chondrod-ifferentiationinductionisconfirmedbytheidentificationof
markerssuchastype2collagen,Sox-9,andaggrecan,through
analysisofgeneexpressionusingreal-timequantitative
poly-merasechainreaction(PCR).
Inaddition todifferentialregulationofgeneexpression,
methylationisanepigeneticmodificationthatusuallyoccurs
inthepromoterregionofthegenetobesilenced.This
Genescanalsobesilencedviatheacetylationprocess,which
preventshistonesfrom becomingrelaxedwhenthe DNAis
exposedtotranscriptionalregulation.13
Adhesion
and
cell
movement,
and
production
of
scaffolds
Duringembryogenesis,inadditiontodifferentiationprocess,
cellsneedtomigrateorgrowtowardaspecificlocationand
remaintheretoperformtheirroles.Celladhesionand
move-ment,which occur by chemical and spatial signals, are of
vitalimportance forcombining individual cells ina
three-dimensionalformat,suchasinbodytissuesandorgans.
Celladhesionmechanismsarehighlyregulatedduring
tis-sue morphogenesis. Reversible phosphorylation by protein
kinaseC(PKC)isakeyeventincelladhesionandmigration
duringchondrogenesis.14
Adhesionandcellmovementarealsorelatedtothe
archi-tectural formation of tissues and organs. In vitro studies
haveshownthatMSCsrespondtotheirenvironmental
for-mat and thatin vivocells are alsoinduced to differentiate
bythe topographical characteristics ofthe tissue inwhich
they are arranged. Such evidence boosted the field of
tis-sueengineering, whichcombinescellulartherapywithuse
ofbiomaterialscaffolds.Thisareainvolvestheuseof
compat-ibleandbiodegradablematerialsthatactasamatrixforcell
growth.Scaffoldsaresimplemediainwhichcellsare
culti-vatedtocreateatissueinvitro.
Apart from providing mechanical support and spatial
orientation for cell growth and differentiation, the
struc-ture ofthe scaffold must allow the transportof nutrients,
metabolites,growthfactors,andotherimportantregulatory
moleculestothecellsandextracellularmatrix.Scaffoldscan
beproducedfromnaturalorsyntheticmolecules.Among
nat-uralbiomaterials,collagen,hyaluronicacid,hydroxyapatite,
andglycosaminoglycansarenoteworthy.15
Electrospinningproducesscaffolds formedbyfibersthat
canphysicallymimicanaturalextracellularmatrix.This
con-ditioncreatesasuitablemicroenvironmentforcellandtissue
differentiation.Thecreationoffibersofdifferentdiametersby
electrospinningisperformedusingpolymersolutionsapplied
toamagneticfield.Thepolylactic-co-glycolicacid(PLGA)
poly-merhasbeenwidelyusedintheproductionofscaffoldsby
electrospinning, because it isbiodegradable, bioabsorbable,
andbiocompatible.TheuseofPLGA-basedscaffoldshasbeen
approvedinhumans bytheUS FoodandDrug
Administra-tion (FDA).Investigationshaveshown thatthis biomaterial
caninducegrowthindifferentcelltypes,suchasfibroblasts,
osteoblasts,andchondrocytes.16
Anothertechnologyderivedfrom electrospinningis
bio-electrospinning, which uses the processing of cellular
suspensionsthataresubjectedtoahighintensityelectricfield
andinducedtopassthroughasharpneedle,generatingfine
dropletsthatcontaincells.Thus,thescaffoldisbuiltwithcells
alreadyintegrated.Thistechniqueallowsforahomogeneous
distributionofMSCsinthescaffoldandtherefore,agreater
regenerativepotential.15Consideringthelimitedregenerative
capacityofcartilaginoustissue,themixtureofbiomaterials
and stem cells appears to be the most promising option,
althoughfurtherstudiesareneededtoconfirmthe efficacy
andsafetyofthismethod.
Apoptosis
and
inflammation
in
cartilage
degeneration
and
regeneration
Cells have the ability to self-regulate not only the rate
of proliferation and differentiation, but also their deathin
manysituations,fromaneventknownasapoptosisor
pro-grammedcelldeath.Unlikenecrosisandautophagy,apoptosis
is a highly coordinated mechanism and does not cause a
specific inflammatory process.17 However, evidence shows
that chronic inflammation induces disorganization of the
extracellular matrix and apoptosis of chondrocytes, which
consequently leads tocartilage destruction. Thisoccurs in
manydegenerativediseases,suchasrheumatoidarthritisand
osteoarthritis.18
It is known that monocytes/macrophages are essential
componentsoftheinnateimmunesystemandhaveavariety
offunctions.Theycontroltheonsetandresolutionof
inflam-mation byphagocytosis,releaseofinflammatorycytokines,
reactiveoxygenspecies(ROS),andactivationoftheacquired
immune system. Under normal circumstances, monocytes
circulateinthebloodstreamforashortperiodbefore
spon-taneouslyenteringinapoptosis.Thepresenceofstimulatory
factors inhibits the apoptosis of monocytes that
differen-tiate into macrophages, which can live for a long time in
tissues.19,20
Macrophages produce many substances that are
rele-vanttoimmuneresponseandcoordinatetheinflammatory
process (inflammatory cytokines L-1, IL-6, TNF␣, and
the anti-inflammatory cytokine IL-10). Furthermore, they
produce factors that are critical in combating
microor-ganisms (such as oxygen metabolites and nitric oxide)
and factors that promote tissue repair (such as fibroblast
growth factor), among others.21 Nowadays, two types of
macrophageactivationintheinflammatoryresponseare
rec-ognized:the“classicalactivation”and“alternativeactivation”
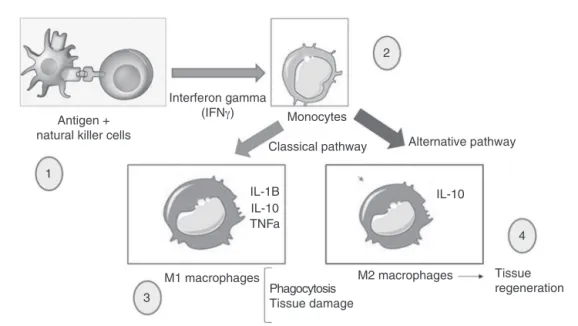
(Fig.2).
Inthisprocess,whenanincreaseintheactivationofM1
macrophagescomparedtoM2macrophagesisobserved,there
willbepoortissuerepairwithcontinueddestructionintissues
withlowregenerativecapacity,suchascartilage.
Inosteoarthritis,theoccurrenceofintra-articular
inflam-mationwithsynovitisindicatesthatsynovialfluidcanbethe
sourceofinflammatorycytokines andproteolyticenzymes.
In synovitis, there isrelease ofproinflammatory cytokines
suchasIL-1andTNF;thesemoleculeshaveaninhibitory
effectonthetype2collagenandaggrecanproductionby
chon-drocytes.Furthermore,thesecytokinescausethereleaseof
metalloproteinasesandaggrecanasesthatdegradethematrix,
whichresultsinthedestructionofcartilage.Othermolecules,
suchasMIL-1andTNF,mayalsobeinvolvedinapoptosis
ofchondrocytesbyincreasingthereleaseofnitricoxideand
prostaglandinE2(PGE2).18
Althoughthere are MSCsinjoint tissue,synovial
Antigen + natural killer cells
Interferon gamma
(IFNγ) Monocytes
Classical pathway Alternative pathway
2
1
3
M1 macrophages M2 macrophages Tissue
regeneration Phagocytosis
Tissue damage
4 IL-10
IL-1B IL-10 TNFa
Fig.2–MacrophagesactivatedbytheclassicalpathwayactasinflammationinducersandarecalledM1.Thesemacrophages producehighlevelsofIL-2andlowlevelsofIL-10.Studieshavealsoshownthattheactivationofmacrophagesis
dependentonthestimulationofinflammatorycytokinesproducedbyhelperlymphocytesorNKcells,inparticulargamma interferon(INF␥).Theactivatedmacrophagesthathavemicrobialandtumoricidalactivityarecharacterizedbysecreting
largeamountsofcytokinesandproinflammatorymediators.Inthisinflammatoryresponse,thesemacrophagesrelease proinflammatorycytokines,suchasIL-1,IL-6,TNF␣,andalsoproducereactiveoxygenspecies(ROS)suchassuperoxide
anionandhydrogenperoxide,aswellasreactiveintermediates,suchasnitricoxide.Shortlyafterphagocytosis,the macrophagesdiebyprogrammedcelldeath,knownasapoptosis.The“alternativeactivation”involvesthestimulationof macrophagesbymoleculessuchastheinterleukinsIL-4andIL-13,whichleadstoanincreaseinIL-10anti-inflammatory cytokinelevelsandinducestissuerepair(anti-inflammatoryresponse).Inthebody,theproinflammatoryimmuneresponse isusuallyfollowedbyananti-inflammatoryimmuneresponse.Thisisimportantfortissuerepairafteramicrobialinfection orphysicalinjury.Theimbalancebetweenthetworesponsescanleadtochronicdiseasessuchasosteoarthritis.
to regenerate cartilage tissue has not been fully
eluci-dated. It is known that the chondrogenesis process is
triggered by factors such as bone morphogenetic proteins
(BMPS) and growth factors such as TGF-. These factors
actongenes, suchastranscription factorSRY-box9(Sox9),
whichisessentialforchondrocytedifferentiation.Sox9
con-trolsthetranscriptionofgenesthatsynthesizeextracellular
matrix molecules, such as type 2 collagen and
aggre-can, while alsosuppressing the formation ofhypertrophic
chondrocytes.
Chronicinflammatoryprocessesappeartonegatively
influ-ence thedifferentiation ofMSCsinto chondrocytes. Inthis
case,thecytokineIL1andTNF-ahaveasuppressiveeffect
onchondrogenesis.Thisisbecausethesecytokinesinhibitthe
expressionofthe Sox9genebysuppressingthe expression
oftheTFG-molecule(animportantinitiationfactorinthe
differentiationof chondrocytes) and increasingthe
expres-sion of the Smad7 molecule (a chondrogenesis inhibitor).
Theinflammatory cytokineIL-17, which is a key molecule
in chronic inflammation processes, also has the ability to
suppresschondrogenesis.Thismoleculesuppressesthe
phos-phorylation of Sox9 protein and prevents its regenerative
action.22
Therefore,consideringalltheevidenceontheimportant
roleofchronicinflammationinthe regenerativeprocess of
cartilage,itisclearthat,inaninflammatoryenvironmentwith
highlevelsofIL-1,TNF,andIL-17,MSCsmaynotrespond
adequatelytoregenerativetherapy.Thisisbecausethesecells
can be induced to apoptosis before they differentiate into
chondrocytes.
Clinical
applications
of
stem
cells
in
cartilage
tissue
regeneration
Manypreclinicalandclinicalstudiesinvolvingpotential
car-tilage regeneration with MSCs are conducted for various
diseases,includingosteoarthritis.AlthoughtheuseofMSCs
forcartilageregenerationisstillattheresearchlevel,thereare
importantissuestoberesolvedtomakethisaneffectiveand
safetherapy.
Theimplantationofchondrocytesfromoneregionofthe
bodytotheinjuredregionhasthedisadvantageofrequiring
twosurgicalprocedures.Theneedforgrowingchondrocytes
inculturetoincreasethenumberofcellstobeimplantedis
alsoanothermajorproblem,asthesecellsmaydedifferentiate
andproducefibrocartilageinsteadofhyalinecartilage.23–26
Inanattempttominimizetheseproblems,someauthors
begantoresearchtheeffectofintra-articularMSCinjection
Eligible patients=22
18 patients allocated to MSCs (IIA- MSC) intra-articular injection
09 allocated to phase 1
03 IIA- MSC low dose
03 IIA- MSC moderate dose
03 IIA- MSC high dose
2 IIA- MSC high dose
03 IIA-CTM high dose
Follow-up: 6 months Follow-up:
6 months Follow-up:
6 months Follow-up:
6 months
09 allocated to phase 1
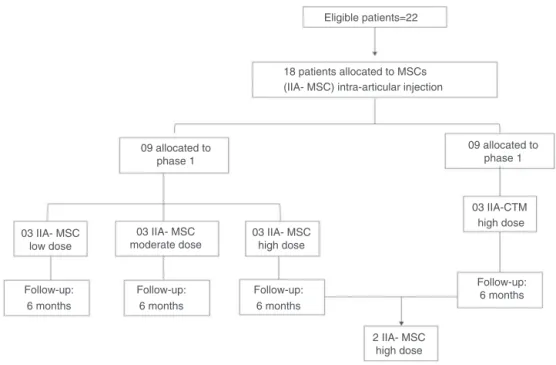
Fig.3–OverallexperimentaldesignofthestudybyJohnetal.33(2014),whoassessed,inaphaseI/IIclinicaltrialtheeffect
ofintra-articularinjectionontheregenerationofthekneecartilageinpatientswithosteoarthritis.MSCswereobtained fromabdominallipoaspirate,culturedinthelaboratory,andinjectedinthejointafterthreeweeks.Lowdose=1×107;
moderatedose=5×107;highdose=1×108cellsinsaline.Pharmacologicaltherapywasdiscontinued,withtheexceptionof
ketoprofenadministration.
hasmanyadvantages,sinceitwouldavoidsurgeryinmany
cases.27–32Nonetheless,thefirstphaseI/IIclinicaltrialto
eval-uatetheefficacyandsafetyofintra-articularinjectioninknee
articular cartilage regenerationthrough clinical,laboratory,
radiological,arthroscopic,andhistologicalanalyseswasonly
publishedin2014,byJoetal.(Fig.3).33
TheinjectedMSCs wereobtainedbyliposuctionof
sub-cutaneous abdominal fat; the obtained MSCs were tested
for their viability, purity (with evaluation of CD31
mark-ers,CD34,CD45),identity(withevaluationofCD73markers,
CD90),sterility,andlackofcontaminationwithendotoxinsor
mycoplasma.
The procedures for intra-articular injection were
per-formedinthe supinepositionwithspinalanesthesia three
weeksafter liposuction.A standard arthroscopic
examina-tionwasperformedandthekneearticularcartilagelesions
were measured with a calibrated and graduated
arthro-scopicprobe,inaccordancewiththeInternationalCartilage
Repair Society (ICRS)classificationofcartilage lesions.The
MSCsdilutedinsalinewereinjected, withoutdebridement,
synovectomy, or meniscectomy during the procedure. No
serious adverse effects were reported and the quality of
knee condition was assessed by the Western Ontario and
McMasterUniversitiesArthritisIndex(WOMAC),andshowed
significant improvements in patients receiving high
con-centrationofintra-articularMSCs.Thecartilagedefect size
decreased in the medial femoral and tibial condyles, and
alsointhegroupsreceiving highdosesofMSCs.Thus,the
authors concluded that intra-articular injection of 1×108
cellsimprovedthefunctionofosteoarthritickneeandpain,
without causing adverse effects, through the reduction of
cartilagedefectsbyregeneratingtissuesimilartohyaline
car-tilage.
Final
considerations
AlthoughtheresultsofJoetetal.33are encouraging,Kondo
et al.,18 in their review on the subject, pointed out that
the results of additional studies involving multiple
pro-tocols are needed in order to truly prove the efficacy
and safety of this procedure. Furthermore, these latest
authors commented that further investigations are being
conducted, aimingto improve efficiencyofMSC
differenti-ation into chondrocytes byanalyzing the supplementation
of culture media with various regulatory molecules, such
as TGF-1–3; BMP-2, -4, -6, -7; FGF-2, and IGF-1, among
others. In addition to these, some compounds such as
dexamethasone and ATP have shown a positive effect on
chondrogenesis.
Another important question concernsthe inflammatory
microenvironmental conditions of the MSC injection site.
Evidence shows that MSCs have immunosuppressive and
anti-inflammatoryeffects.Nonetheless,suppressionofjoint
inflammationcouldpotentiallyincreasetheefficiencyof
tis-sueregeneration.
Inthiscase,whatremainsunclear,requiringfurther
jointenvironment itself couldinduce thedifferentiation of
MSCsalreadyallocatedintheregion.Theanswertothis
ques-tioncouldleadtothedevelopmentofregenerativetechniques
associatedwith the already well-establishedsurgical
tech-niques, without transplantation of MSCs to other parts of
thebody.However,consideringthecomplexityoftheevents
relatedtochondrogenesisandcartilagerepair,theroadahead
isstilllongandaconsiderablevolumeofadditionalresearch
isneeded.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. BakshD,SongL,TuanRS.Adultmesenchymalstemcells: characterization,differentiation,andapplicationincelland genetherapy.JCellMolMed.2004;8(3):301–16.
2. AmorinB,ValimVS,LemosNE,MoraesJúniorL,SilvaAMP, SilvaMAL,etal.Mesenchymalstemcells–characterization, cultivation,immunologicalproperties,andclinical
applications.RevHCPAFacMedUnivFedRioGddoSul. 2012;32(1):71–81.
3. AlbertsB,BrayD,LewisJ,RaffM,RobertsK,WatsonJD. Molecularbiologyofthecell.3rded.NewYork:Garland Publishing;1994.p.971–84.Theextracellularmatrixof animals.
4. BobisS,JarochaD,MajkaM.Mesenchymalstemcells: characteristicsandclinicalapplications.FoliaHistochem Cytobiol.2006;44(4):215–30.
5. BornesTD,JomhaNM,SierraAM,AdesidaAB.Hypoxic cultureofbonemarrow-derivedmesenchymalstromalstem cellsdifferentiallyenhancesinvitrochondrogenesiswithin cell-seededcollagenandhyaluronicacidporousscaffolds. StemCellResTher.2015;6(84):1–17.
6. MachadoAK,CadonáFC,AzzolinVF,DornellesEB,BarbisanF, RibeiroEE,etal.Guaraná(Paulliniacupana)improvesthe proliferationandoxidativemetabolismofsenescent adipocytestemcellsderivedfromhumanlipoaspirates.Food ResInt.2015;67:426–33.
7. BrandlA,HartmannA,BechmannV,GrafB,NerlichM,Angele P.Oxidativestressinducessenescenceinchondrocytes.J OrthopRes.2011;29(7):1114–20.
8. SadeghiF,EsfandiariE,HashemibeniB,AtefF,SalehiH, ShabaniF.Theeffectofestrogenontheexpressionof cartilage-specificgenesinthechondrogenesisprocessof adipose-derivedstemcells.AdvBiomedRes.2015; 4:43.
9. RoddaDJ,ChewJL,LimLH,LohYH,WangB,NgHH,etal. TranscriptionalregulationofnanogbyOCT4andSOX2.JBiol Chem.2005;280(26):24731–7.
10.LeeJ,KimHK,RhoJY,HanYM,KimJ.ThehumanOCT-4 isoformsdifferintheirabilitytoconferself-renewal.JBiol Chem.2006;281(44):33554–65.
11.MackayAM,BeckSC,MurphyJM,BarryFP,ChichesterCO, PittengerMF.Chondrogenicdifferentiationofcultured humanmesenchymalstemcellsfrommarrow.TissueEng. 1998;4(4):415–28.
12.PittengerMF,MackayAM,BeckSC,JaiswalRK,DouglasR, MoscaJD,etal.Multilineagepotentialofadulthuman mesenchymalstemcells.Science.1999;284(5411): 143–7.
13.TamburiniBA,TylerJK.Localizedhistoneacetylationand deacetylationtriggeredbythehomologousrecombination pathwayofdouble-strandDNArepair.MolCellBiol. 2005;25(12):4903–13.
14.MattaC,MobasheriA.Regulationofchondrogenesisby proteinkinaseC:emergingnewrolesincalciumsignalling. CellSignal.2014;26(5):979–1000.
15.GargT,GoyalAK.Biomaterial-basedscaffolds–currentstatus andfuturedirections.ExpertOpinDrugDeliv.
2014;11(5):767–89.
16.SachlosE,ReisN,AinsleyC,DerbyB,CzernuszkaJT.Novel collagenscaffoldswithpredefinedinternalmorphologymade bysolidfreeformfabrication.Biomaterials.2003;24(8): 1487–97.
17.SorrentinoG,ComelA,MantovaniF,DelSalG.Regulationof mitochondrialapoptosisbyPin1incancerand
neurodegeneration.Mitochondrion.2014;19PtA:88–96. 18.KondoM,YamaokaK,TanakaY.Acquiringchondrocyte
phenotypefromhumanmesenchymalstemcellsunder inflammatoryconditions.IntJMolSci.2014;15(11):21270–85. 19.Wiktor-JedrzejczakW,GordonS.Cytokineregulationofthe
macrophage(Mphi)systemstudiedusingthecolony stimulatingfactor-1-deficientop/opmouse.PhysiolRev. 1996;76(4):927–47.
20.SavillJ,FadokV.Corpseclearancedefinesthemeaningofcell death.Nature.2000;407(6805):784–8.
21.CruvinelWM,MesquitaJúniorD,AraújoJAP,CatelanTTT, SouzaAWS,SilvaNP,etal.Sistemaimunitário–ParteI: Fundamentosdaimunidadeinatacomênfasenos mecanismosmolecularesecelularesdaresposta inflamatória.RevBrasReumatol.2010;50(4):434–61. 22.MorissetS,FrisbieDD,RobbinsPD,NixonAJ,McIlwraithCW.
IL-1ra/IGF-1genetherapymodulatesrepairofmicrofractured chondraldefects.ClinOrthopRelatRes.2007;462:
221–8.
23.vonderMarkK,GaussV,vonderMarkH,MüllerP. Relationshipbetweencellshapeandtypeofcollagen synthesisedaschondrocyteslosetheircartilagephenotypein culture.Nature.1977;267(5611):531–2.
24.BrittbergM,LindahlA,NilssonA,OhlssonC,IsakssonO, PetersonL.Treatmentofdeepcartilagedefectsintheknee withautologouschondrocytetransplantation.NEnglJMed. 1994;331(14):889–95.
25.KnutsenG,DrogsetJO,EngebretsenL,GrøntvedtT,IsaksenV, LudvigsenTC,etal.Arandomizedtrialcomparingautologous chondrocyteimplantationwithmicrofracture.Findingsatfive years.JBoneJtSurgAm.2007;89(10):2105–12.
26.VanlauweJ,SarisDB,VictorJ,AlmqvistKF,BellemansJ, LuytenFP.Five-yearoutcomeofcharacterizedchondrocyte implantationversusmicrofractureforsymptomaticcartilage defectsoftheknee:earlytreatmentmatters.AmJSports Med.2011;39(12):2566–74.
27.MurphyJM,FinkDJ,HunzikerEB,BarryFP.Stemcelltherapy inacaprinemodelofosteoarthritis.ArthritisRheum. 2003;48(12):3464–74.
28.LeeKB,HuiJH,SongIC,ArdanyL,LeeEH.Injectable
mesenchymalstemcelltherapyforlargecartilagedefects–a porcinemodel.StemCells.2007;25(11):
2964–71.
29.CentenoCJ,BusseD,KisidayJ,KeohanC,FreemanM,KarliD. Increasedkneecartilagevolumeindegenerativejointdisease usingpercutaneouslyimplanted,autologousmesenchymal stemcells.PainPhysician.2008;11(3):343–53.
31.DavatchiF,AbdollahiBS,MohyeddinM,ShahramF,NikbinB. Mesenchymalstemcelltherapyforkneeosteoarthritis. Preliminaryreportoffourpatients.IntJRheumDis. 2011;14(2):211–5.
32.EmadedinM,AghdamiN,TaghiyarL,FazeliR,MoghadasaliR, JahangirS,etal.Intra-articularinjectionofautologous
mesenchymalstemcellsinsixpatientswithknee osteoarthritis.ArchIranMed.2012;15(7):422–8. 33.JoCH,LeeYG,ShinWH,KimH,ChaiJW,JeongEC,etal.