w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
High
resolution
peripheral
quantitative
computed
tomography
for
the
assessment
of
morphological
and
mechanical
bone
parameters
Henrique
Fuller
a,
Ricardo
Fuller
b,
Rosa
Maria
R.
Pereira
a,∗aBoneLaboratoryMetabolism,RheumatologyDivision,MedicineSchool,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
bRheumatologyDivision,HospitaldasClÍnicas,MedicineSchool,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received17January2014 Accepted6July2014
Availableonline5January2015
Keywords:
Highresolutionperipheral
quantitativecomputedtomography (HR-pQCT)
Structuralparameters Radius
Tibia
a
b
s
t
r
a
c
t
Highresolutionperipheralquantitativecomputedtomographyisanewtechnology com-mercially available for <10 years that allows performing in vivo assessment of bone parameters.Highresolutionperipheralquantitativecomputedtomographyassessesthe trabecularthickness,trabecularseparation,trabecularnumberandconnectivitydensity and,inaddition,corticalbonedensityandthicknessandtotalbonevolumeanddensity inhigh-definitionmode,whichadditionallyallowsobtaining digitalconstructsofbone microarchitecture.Theapplicationofmathematicstocaptureddata,amethodcalledfinite elementanalysis,allowstheestimationofthephysicalpropertiesofthetissue, simulat-ingsupportedloadsinanon-invasiveway.Thus,highresolutionperipheralquantitative computedtomographysimultaneously acquiresdata previouslyprovided separatelyby dualenergyX-rayabsorptiometry,magneticresonanceimagingandhistomorphometry, aggregatingbiomechanicalestimatespreviouslyonlypossibleinextractedtissues. This methodhasasatisfactoryreproducibility,withcoefficientsofvariationrarelyexceeding 3%.Regardingaccuracy,themethodshowsafairtogoodagreement(r2=0.37–0.97).
Themainclinicalapplicationofthismethodisinthequantificationandmonitoringof metabolicbonedisorders,morefullyevaluatingbonestrengthandfracturerisk.In rheuma-toidarthritispatients,thisallowsgaugingthenumberandsizeoferosionsandcysts,in additiontojointspace.Inosteoarthritis,itispossibletocharacterizethebonemarrow edema-likeareasthatshowacorrelationwithcartilagebreakdown.
Givenitshighcost,highresolutionperipheralquantitativecomputedtomographyisstill aresearchtool,butthehighresolutionandefficiencyofthismethodrevealadvantagesover themethodscurrentlyusedforboneassessment,withapotentialtobecomeanimportant toolinclinicalpractice.
©2014ElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthor.
E-mail:rosamariarp@yahoo.com(R.M.R.Pereira).
http://dx.doi.org/10.1016/j.rbre.2014.07.010
Tomografia
computadorizada
quantitativa
periférica
de
alta
resoluc¸ão
para
avaliac¸ão
de
parâmetros
morfológicos
e
funcionais
ósseos
Palavras-chave:
Tomografiacomputadorizada quantitativaperiféricadealta resoluc¸ão(HR-pQCT) Parâmetrosestruturais Rádio
Tíbia
r
e
s
u
m
o
Atomografiacomputadorizadaquantitativaperiféricadealtaresoluc¸ão(HR-pQCT)éuma novatecnologiadisponívelcomercialmentehámenosde10anosquepermiteafeiturade examesinvivoparaaavaliac¸ãodeparâmetrosósseos.AHR-pQCTavaliaaforma,onúmero,o volume,adensidade,aconectividadeeaseparac¸ãodastrabéculas;adensidadeeaespessura doossocorticaleovolumeeadensidadetotal,emaltadefinic¸ão,oquepermiteaconstruc¸ão digitaldamicroarquiteturaósseaadicionalmente.Aaplicac¸ãodecálculosmatemáticosaos dadoscapturados,métododenominadoelementofinito(FE),permiteaestimativadas pro-priedadesfísicasdotecidoesimulacargassuportadasdeformanãoinvasiva.Dessemodo, aHR-pQCTadquiresimultaneamentedadosantesfornecidosseparadamentepela densit-ometriaóssea,pelaressonânciamagnéticaepelahistomorfometriaeagregaestimativas biomecânicasantessópossíveisemtecidosextraídos.Areprodutibilidadedométodoé sat-isfatória,comcoeficientesdevariac¸ãoqueraramenteultrapassamos3%.Quantoàacurácia, osparâmetrosapresentamderegularaboaconcordância(r2=0,37–0,97).
A principal aplicac¸ão clínica é na quantificac¸ão e no monitoramento das doenc¸as osteometabólicas,porqueavaliademodomaiscompletoaresistênciaósseaeoriscode fratura.Naartritereumatoidepermite-seaaferic¸ãodonúmeroedotamanhodaserosõese doscistos,alémdoespac¸oarticular.Naosteoartriteépossívelcaracterizarasáreas edema-símilequeguardamcorrelac¸ãocomadegradac¸ãodacartilagem.
Restritasaindaauminstrumentodepesquisa,dadooseuelevadocusto,aaltaresoluc¸ão eaeficiênciamostram-secomovantagensemrelac¸ãoaosmétodosatualmenteusadospara aavaliac¸ãoóssea,comumpotencialparatornar-seumaimportanteferramentanaprática clínica.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Inthe1990s,theincorporationofdualenergyX-ray absorp-tiometry(DXA)inclinical practiceconsiderablyboostedthe knowledgeofmetabolicbonediseasesandtheestablishment offracturerisk.However,bonestrengthalsodependson tis-suemicroarchitecture.Thus,thehistomorphometricanalysis
hasbecomenecessary tosupplement the boneevaluation,
inferring its spatial properties. But this isan invasive and
expensive method that can only be performed from bone
samples.
Inthisscenario,anewinvivomethodforassessingbone microarchitectureandvolumetricbonemineraldensity(BMD) inhigh-quality3Demerges:highresolutionperipheral quanti-tativecomputedtomography(HR-pQCT).Thistechnologywas originallydesignedfortheanalysisofmaterialssuchassnow, concrete,gems,andsoon.Subsequently,thetechnologycame tobeusedforthestudyofbiologicalmaterialssuchasteeth, implants,boneand,morerecently,cartilage.Inaddition,the methodalsoallowedtheanalysisofbiomechanicalproperties oftheanalyzedmaterial,withtheuseofacomplex mathe-maticalprocess.
Itsuseformedicalpurposeshasgrownrapidlyinrecent years,becausethemethodrevealsindetailtheinternal struc-ture ofin vivoand ex vivo biological materials.The use of HR-pQCT is still largely confined to the field of scientific research,giventhatthereislessthanhalfahundreddevices worldwideinoperationtoperformtheexamination1andonly
two inBrazil.Due toits greatpotential, wepresent herea reviewofmethodologicalaspectsofHR-pQCTanditspotential clinicalapplication.
What
is
HR-pQCT?
HR-pQCT isan imaging techniquethat uses computerized
processing of X-ray attenuation (measured in Hounsfield
Units,HU)fortheacquisitionofsectionalimages,inthesame waythataconventionalCTscandoes.Fromtheslices,itis pos-sibletoproduceathree-dimensional(3D)high-qualitymodel.
AlthoughHR-pQCTisalsooftenconfusedwithComputed
Microtomography(MicroCTorCT),thesetermsarenot
syn-onymous. While CT has a very high resolution of up to
fractionsofm(micron)andevaluatesingreatdetailthe mor-phologyofthesamples,itsuseisrestrictedtoexvivoanalysis.2
On the other hand,HR-pQCT,specifically, isanequipment whoseresolutionreachesonlytensofmicrometers;thissize isslightlylargerthanthatrepresentedbythetrabecular struc-ture,butalsoallowsadetailedanalysisoftissuemorphology. Inaddition,thismethoddifferentiatesitselffromCTasto the possibility to perform rapidtests in vivo.3,4 Thestudy
ofbone structureswith CTwas introduced in1989,5 and soonbecame thegoldstandardfortheevaluationof three-dimensionalbonestructure.
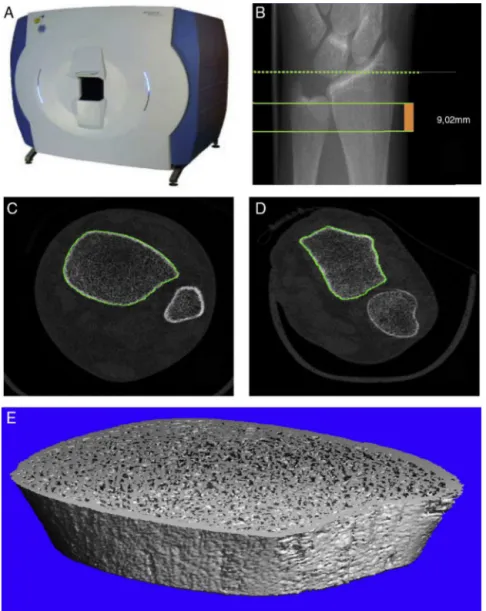
Fig.1–(A)ScancoXtremeCTHR-pQCTdevice.(B)ReferencestandardizedplanesforHR-pQCT.Thedottedlineindicatesthe referenceplane,whilethesolidlinesindicateinitialandfinalplanesofthetest,comprisingathicknessof9.02mm.(C)The sectionalimageoftheleg,highlightingthetibialperiosteumcontour(ingreen).(D)Thesectionalimageoftheforearm, highlightingtheradialperiosteumcontour(ingreen).(E)Constructionofa3Dmodeloftibia,afterinitialanalysis.
measurethree-dimensional humanbone microarchitecture
in vivo, the XtremeCT (SCANCO Medical AG, Brüttisellen,
Switzerland).(Fig.1A).Nevertheless,theabilitytomeasurethe averagetrabecularthicknessisstilllimitedbythemaximum resolutionofthemachine.6–8
Despitetheabilitytocarryonmorphologicalscanningof thetissue microstructure,therewas stillnoproper wayto
estimate the mechanical properties of the material under
analysisinvivo.Theimprovedresolutionof3Dimages pro-videdbythisnewdevicecoupledwithcomputer-basedfinite elementanalysis(FEA)modeling9providesestimatesof
func-tionalpropertiesofthematerial.
Image
acquisition
and
results
ThestandardtestwithXtremeCTevaluatesdistalradiusand tibiain vivo.10 Thelimbbeing scannedisimmobilized ina
carbon fiber shell to avoidartifacts resulting from motion, which could lead tothe needfor rescanning.11–13 Initially,
a scout view, essentially a two-dimensional X-ray scan,
is obtained to determine a precise region for the
three-dimensional measurement (Fig. 1B). Eachsite includes110
computerized tomography slices, totaling an extension of
9.02mm along the axial axis ofthe bone. The acquisition
of these images takes about 3min. The standard
proto-colistypicallyconductedwiththefollowingsettings:X-ray tube current=95mA, X-ray tube potential=60kVp, voxel size=82m,anda1536×1536matrix.
TheHR-pQCTsingle-scaneffectiveradiationdoseisless than 5Sv.14 Somestudiesestimatethat itisaround3Sv permeasure.15Theinternationalrecommendationisthatthe
averageannualdoseforplannedradiationexposuremustnot exceed 20Sv/year, measuredover a definedperiodoffive years.16,17Forcomparison,achestX-rayexposestoaradiation
Table1–HR-pQCTmainboneparameters.
Abbreviation Parameter Description Unit
Structuralparameters
BV/TV Bonevolumeratio Ratiobetweenbonevolume
andtotalvolumeoftissue
–
Tb.N Trabecularnumber Meannumberoftrabeculae permm
Tb.Th Trabecularthickness Meanthicknessoftrabeculae mm
Tb.Sp Trabecularseparation Meanspacebetween
trabeculae
mm
Tb.1/N.SD Inhomogeneityofnetwork Standarddeviationofthe
inverseofnumberof trabeculae
mm
Ct.Th Corticalthickness Averagecorticalthickness mm
Co.Po Corticalporosity Ratiobetweenporevolume
andtotalcorticalvolume
–
Tt.Ar Totalbonearea Cross-sectionalarea mm2
Ct.Ar Corticalbonearea Meanoftheareaoccupiedby
corticalbone
mm2
Tt.Ar Trabecularbonearea Meanoftheareaoccupiedby
trabecularbone
mm2
Densityparameters
BMD(D100) Bonemineraldensity Totalvolumetricdensity mgHA/cm3
Tb.BMD(Dtrab) Trabecularbonemineraldensity Trabecularvolumetricdensity mgHA/cm3
Dmeta Metatrabecularbonemineraldensity(40%) Externaltrabecularvolumetric
density(40%oftrabecular volume)
mgHA/cm3
Dinn Innertrabecularbonemineraldensity(60%) Innertrabecularvolumetric
density(60%oftrabecular volume)
mgHA/cm3
Meta/Inn Ratiometatoinnerbonemineraldensity Ratiobetweenouterandinner
trabecularbonedensity
–
Ct.BMD(Dcomp) Corticalbonemineraldensity Corticalvolumetricdensity mgHA/cm3
Aftertheacquisitionofimages,thesystemautomatically performsaninitialevaluationthatconsistsoftwoprocesses: (1)processingofdigitaldatainsectionalimages(Fig.1CandD) and(2)constructionofa3Dmodel(Fig.1E).Subsequently,itis necessarytodeterminethecompartments.Thefirstcontour ischaracterizedbytheouterenvelopeoftheradius,which isthenusedtodefinethefullcompartment.Thesoftwareis providedwithasemiautomaticcontouringalgorithm(Fig.1C andD).
Afterobtainingthiscontour,thenextnecessarystepisto determinetheinnercontourdelineatingcorticalfrom trabe-cularbone,withthegoalofobtainingisolateddatarelatingto eachofthecompartments.Thisisacomplexprocess,because their boundary isnot always well defined. Where the cor-texisrather thickor highlyporous,theboundary between thecompartmentsmaybeinaccurate.18Thisprocedure
auto-maticallycreatesthedifferentcompartmentsbasedonimage processing.19,20
Anotheraspectthatmustbeestablishedwithrespectto trabecularboneistodescribeandquantifytheplateand rod-like structure ofbone. While rod-like trabeculae havetwo connections(calleddisjunctive)attachedtotheadjacentbone andonlyonecontactsurfacewiththebonemarrow,plate-like trabeculaehaveonlyonecontactsurfacewiththeadjoining bone(inallitsperimeter)andtwowiththebonemarrow(one oneachsideofthedisc).20Thisprocessofrod-likeand
plate-liketrabeculaeseparationisperformedautomaticallybythe
software,havinganinfluenceontheresultsofsomeofthe parameters.
Aseriesofteststodeterminethemainboneparameters usedintheliteraturefollows.1Thus,mathematicalalgorithms
arerequiredthatallowsuchcalculations.Themanufacturer’s software already includescomputer scripts containing the equations.
Thesescriptsincludethedefinitionofbonevolume,bone volumedensity,structuremodelindex,trabecularthickness, trabecular separation, trabecular number and connectivity density,andcorticalthickness.Thedegreeofcorticalporosity isthemostrelevantcorticaldataobtained.Table1 discrimi-natesthemainparametersandtheirterminology,asusedin themedicalliterature.
Finite
element
analysis
modeling
FEA isa numericaltechniqueofengineering, which, when
applied to medicine, allows a quantitative and
qualita-tive estimation of biomechanical propertiesresulting from
bone microarchitecture, by means of complex differential
equations.21–23
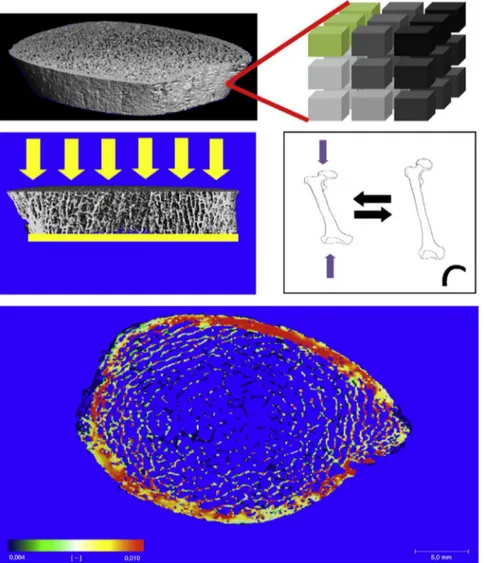
Fig.2–(A)Voxelconversiontechnique.Inthescheme,eachofthecubestotherightisavoxelwithaspecificelasticity,here representedbydifferentshadesofgray.(B)Virtualcompressiontest,performedbythefiniteelementsoftware.Application ofcompressiveforces(inyellow).(C)IllustrationofYoung’smodulus.Theremovalofthecompressionforces(inyellow) causesthematerialtoreturntoitsoriginalshape.(D)Exampleoftheresultofananalysiswiththefiniteelementmethod; axialbonesection.Inred,areassubjectedtogreaterstress;ingreen,areasunderlessstress.
softwareemploystheso-called“voxelconversiontechnique” (Fig.2A)tocreatefiniteelementmodels(FiniteElement Soft-ware,V.1.13,Scanco MedicalAG, Switzerland,January2009 ManufacturerHandbook).Inthistechnique,thevector infor-mationobtainedinthemodelisconvertedintoblockscalled voxels,whichhaveidenticalshapeandsize.Thevoxelsare shapedlikecubes,beingthesmallestunitthatmakesupthe imageoftheanalyzedmaterial.Eachvoxelisregisteredwith oneamong255gradationsofelasticityrecognizedbythe sys-temtoperformthemathematicalcalculations.Thestandard analysisofFEAcomprisesavirtualresistancetest,namely,the computerestimatesandanalyzesthebehaviorofthebone tis-suewhenitissubmittedtoacompressiveforcealongitsmajor axis(Fig.2B).
For analysis of FE, two mechanical properties of the
bone under study must be estimated, considering that
no histofunctional study of bone tissue is being carried
out:
• ThefirstofthemistheYoung’smodulus,ameasureofthe abilityof amaterial toreturn to its original shapeafter removalofastressforce,thusindicatingthetissue’s elastic-ity.Thismeasureisvalidonlyintherangeofforcesinwhich theelasticdeformationoccurs,namely,whenthereis nei-thermicrorupturenorchangeinbonestructure,enablingit toreturntoitsoriginalform.
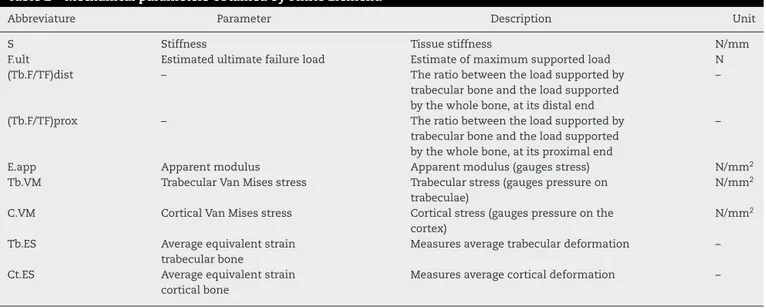
Table2–MechanicalparametersobtainedbyFiniteElement.
Abbreviature Parameter Description Unit
S Stiffness Tissuestiffness N/mm
F.ult Estimatedultimatefailureload Estimateofmaximumsupportedload N
(Tb.F/TF)dist – Theratiobetweentheloadsupportedby
trabecularboneandtheloadsupported bythewholebone,atitsdistalend
–
(Tb.F/TF)prox – Theratiobetweentheloadsupportedby
trabecularboneandtheloadsupported bythewholebone,atitsproximalend
–
E.app Apparentmodulus Apparentmodulus(gaugesstress) N/mm2
Tb.VM TrabecularVanMisesstress Trabecularstress(gaugespressureon
trabeculae)
N/mm2
C.VM CorticalVanMisesstress Corticalstress(gaugespressureonthe
cortex)
N/mm2
Tb.ES Averageequivalentstrain
trabecularbone
Measuresaveragetrabeculardeformation –
Ct.ES Averageequivalentstrain
corticalbone
Measuresaveragecorticaldeformation –
Thevaluesofthesevariablesarenotyetfullyestablished, andhencetheirusevariesaccordingtotheliteratureused. ThenormalrangeofYoung’smodulususedisbetween10GPa and22.5GPa(GigaPascalisamultipleofthestandardunitof pressureintheinternationalsystem,definedasNewton/m2).
TheYoung’smoduluscanbesetseparatelyforbothtrabecular andcorticalbone.Ontheotherhand,thePoissonratiois0.3 formoststudies.25–30
Theapplicationofthistechniquehasproduced,inasimple andfastway,ahugeamountofdata,previouslyonlyobtained frominvasive,costlyandtime-consumingprocedures.These aredatathatestimatethesupportedloadandthe deforma-tionsoftheboneasawhole,andineachofitsregions.Table2
liststhemainparametersobtainedfromthismethod.
Accuracy
The analysesof HR-pQCT accuracy are based on the gold
standard for measurement of bone microarchitecture, the
CT.Comparisonsareperformedoncadaverdata,mostlydue totheinabilitytoperforminvivotestswithCTdevices.
Overall,theparametersexhibitgood tomoderate agree-ment(r2=0.37–0.97).Itisnoted,however,thatsome param-eterssuchas BV/TV (r2=0.91–0.97)3,31 and Tb.Sp(r2=0.91)3
exhibitedanexcellentcorrelation,whileparameterssuchas Tb.1/N.SD(r2=0.62–0.71)4andTb.Th(r2=0.42–0.64)3,31 hada
lowercorrelation.
Tjongetal.3alsoinvestigatedtheimpactofdifferentvoxel
sizes on HR-pQCT and its correlation with gold standard
parameters. Alternating between standardized values by
XTremeCT(41m;82m; and123m),it ispossibleto
sig-nificantly change the accuracy. With a voxel of 123m,
Tb.Thpresents r2=0.37;increasing the resolutionto41m onereachesr2=0.82.Similarly,Tb.Spcanvaryfromr2=0.78 to r2=0.95. But some parameters, especially BMD, are lit-tleinfluencedbytheincreasedresolution.Tb.BMDremains atr2=0.84–0.85inthevariousresolutionscompared.While improvingtheaccuracy,decreasingthevoxelsizeimpliesina
greaterexaminationtimeand,therefore,thechancesof occur-renceofartifactsresultingfrompatientmotionaremultiplied. Totalbonemineraldensity(D100),inadditiontotheother
BMDparametersobtainedwithHR-pQCT,mayalsobe
com-paredwiththoseobtainedbyDualenergyX-raydensitometry (DXA).Itisimportanttonote,however,thatwhileHR-pQCT and CTcalculatevolumetricdensities(vBMD), DXA calcu-latesdensityperarea(aBMD).Thecorrelationshowninthe
comparisonbetweenHR-pQCTandDXA,dependingon the
parameteranalyzed,canvaryfromr2=0.37tor2=0.73,being
maximumwhenthecomparisonismadebetweentotalvBMD
andaBMD.32Itcanbenoted,however,thattherearestillfew
studies focused on showingthe correlation between these
parameters.
Reproducibility
Todate,few studiesreporting thereproducibilityofresults obtainedbyHR-pQCTwerepublished.Mostofthesestudies showthattheequipment,whenusedinaccordancewith stan-dardizedandwell-definedprotocols,reacheslowcoefficients ofvariation.Severalaspectsinfluencethe reproducibilityof results,amongwhichstandouttheparameterbeinganalyzed, theprotocolsused,thebonebeingevaluatedandthecorrect accomplishmentofcalibrationprotocols.
TheparametersderivedfromHR-pQCTcanbedividedinto those concerningstructuralmeasures and those relatedto
BMD(Table1).Comparingthereproducibility,itcanbenoticed
thatthisfactorisgreaterinthesecondversusfirstgroup.While thestructuralmeasurescanachievecoefficientsofvariation ofupto3.2–4.4%,thoserelativetobonemineraldensityhardly exceed1%.33,34 Theexplanationforthisfact isthat, inthe
Asforthe protocols employed,these mustbevery well defined,toimprovereproducibility.Theyinclude:patient posi-tioning,fixingthelimbintothesupportshell,andthechoiceof thereferenceplane,amongothers.Basically,allthesefactors cangiverisetothreetypesoferrors:artifactsofmovement, reducingtheoverlapindifferentmeasurements,andchanges inangulation.Thelackofacomfortablepositionandthe non-fixation into the supportshell can leadto patient motion, whichincreasesthevariationbetweentests.Thechoiceofthe referenceplane(boundariesofboneareaanalyzed)cancause significantdiscrepanciesintheresultsobtained33;inthis
mea-surement,thechangeofonly1mmcanleadtoavarietyof
11% in the tissue sampleanalyzed. Asto the bone
evalu-ated,accordingtoBoutroyetal.34 inmostcasestheresults
forthetibiahavehighercoefficientsofvariation,when com-paredtosimilarparametersfortheradius.ButMacNeiletal.33
observedthatradiusmeasurementsaremoreproneto move-mentartifacts.
Regarding XtremeCT, it is important to note that the
properconductofdailyandweeklycalibrationprotocolswas extremelyimportantformaintaininghighstandardsof repro-ducibility and low variability in the short and long term, besidesmulticenterreproducibility.35
Applications
TheuseofHR-pQCTinbiologicaltissuesgeneratedan enor-mousrangeofpossibilitiesforscientificresearch,andthiscan beobservedbytheexponentialincreaseinthenumberof pub-licationsthatmakeuseofthistechnologyinrecentyears.The functionalanalysiswiththefiniteelementmethodhasfurther expandedthenumberofapplicationsofthetechnique.
Since its first use for bone assessment, this has been the main application of HR-pQCT. Studies indicate that it ispossibletoevaluatetheprofileofbonemicroarchitecture throughoutlife,theriskoffractures,mineralizationandthe developmentofbonediseases(e.g.,osteoporosis).Theeffectof drugsanddietsinboneformation,resorptionandmorphology alsocanbeverified.Currently,theuseofHR-pQCThasbeen
extendedtothediagnosis and monitoringofinflammatory
arthropathies,suchasrheumatoidarthritisand osteoarthri-tis.However,thepracticaluseofthemethodstillseemsmuch morepromisingfor,andrespondstogapsin,osteoporosis;in osteoarthritisandrheumatoidarthritis,itsusefulnessisstill morerelatedtoresearch.
The
use
of
HR-pQCT
in
osteoporosis
and
fracture
risk
assessment
Osteoporosis(OP) ischaracterizedby acompromised bone
strength,predisposingtheindividualtotheriskoffractures.36
Dual emission X-ray densitometry (DXA) is still the gold
standardfordiagnosis,monitoringandclinicalinvestigation ofthe patient with osteoporosis.37 However, bone mineral
density(BMD)onlycorrespondstoapartofbonestrength.38
Thus, for the assessment of fracture risk, BMD
measure-mentsshouldbeassociatedwithotherfactorsthatinfluence bone strength: cortical thickness and porosity, trabecular microstructureandbonegeometry.39Thesecombinedfactors
contributetodefinethebiomechanicalpropertiesofbone tis-sue,suchasstiffnessandsupportedload.31
TheabilityofHR-pQCTtodefinetheseparametersofbone architecture,inconjunctionwiththeabilityoftheFEAfor
esti-mating biomechanicalproperties, makesthis techniquean
excellenttoolforosteoporosisevaluation.Ithasbeenshown byseveralstudiesthatthisdatasetiscloselylinkedtotherisk offracturesinOP,10,22,40andalsothatindividualswithsimilar
resultsobtainedbyDXAmayhavelargedifferencesinfracture risk,duetotheabovefactors.41,42
The
use
of
HR-pQCT
in
monitoring
therapy
Some,43–46butnotall,47studiessuggestthatchangesinBMD
duringthetherapyofosteoporosiscorrelatetothereduction infracturerisk.Ameta-analysisof12clinicaltrialsconcluded thataBMDimprovementinthespinecomprisesonlyasmall partofthereductioninfracturerisk.48Thus,toevaluatethe
therapeuticaspects,theuseofparametersthatmeasurenot onlytheBMDisinorder,butalsothebonemicroarchitecture. Severalstudiesalreadypublishedhavedemonstratedthat therapeutictreatmentsforosteoporosiscanbring improve-mentinmanyboneparameters.Thus,HR-pQCTisatoolthat
allowsamuchmoredetailedassessmentoftreatment
com-paredwithDXA.
Cheungetal.1reportedseveralstudiesontheuseof
HR-pQCT in monitoring osteoporosis treatment. Research
conducted with alendronate,49–52 zoledronic acid,53
ibandronate,54,55 denosumab,51 strontium ranelate,49,50
odanacatib56,57andteriparatide53,58candemonstratechanges
mainlyintheparametersofvBMDforthevariousbone com-partments,corticalthickness,maximumsupportedloadand trabecularnumber.
The
use
of
HR-pQCT
in
rheumatoid
arthritis
Boneerosionsarecloselylinkedtotheprogressionof rheuma-toidarthritis(RA).Therefore,monitoringoftheselesionsisan earlyprognosticparameterandanimportantinputto mon-itortheeffectivenessoftreatment.59,60Currently,amongthe
imagingmethods,conventionalradiographyisthemostused tool in clinical practice to aid in the diagnosis and mon-itoring ofRA for evaluating erosions and bone loss,61 but
besidesbeingasemiquantitativeprocedure,theidentification oflesionsinthistypeoftesttakes6–12monthstobecome apparent.62
Stachetal.63 showedthatitispossibletoadaptthe
HR-pQCT device for evaluating the volume of bone erosions.
Thisinformationisobtainedbymeasuringdistancesin var-iousdirectionsintheslicesmade.64,65 Thecurrentanalysis
methodisstillnotautomatednorstandardized,but prelimi-naryresultsofsomestudiesshowthatthehigh-resolutionof HR-pQCTallowsaprecisecharacterizationofboneerosions onamuchgreaterdegreeofdetailthantraditionalmethods.61
Thetechniquealsoallowstheevaluationofthejointspaceof metacarpophalangealandproximalinterphalangealjoints,66
Recent studies show that the data obtained with
HR-pQCT inpatients with RA havea higher sensitivity, when
comparedtodata obtainedbyconventional radiographs in
correlationwithmarkersofbonecatabolismandanabolism (r=0.393–0.474).67ThisshowsthatHR-pQCTcannotonly
eval-uatemomentarilyRA,butalsoshowdiseaseprogression.
The
use
of
HR-pQCT
in
osteoarthritis
Inosteoarthritis (OA), acartilage lesion is accompaniedby alterationsinsubchondralboneandmarrowspace.Magnetic resonance imaging can connect cartilage injury toregions wheretherearetheso-calledbonemarrowedema-like(BMEL)
injuries, which are areas of high signal on T2-weighted
images.68 Inthese placesit ispossibletoobserve, in
addi-tiontoedema,necrosisofadipocytes,increaseoffibroustissue andanacceleratedbonemetabolism.However,themagnetic
resonance is unable to determine which changes in bone
microarchitecturearepresent,andhowtheyrelatetodisease. There are still few studies demonstrating the efficacy
ofassessmentwithHR-pQCTinthe diagnosis and
progno-sisofOA,but Kazakia et al.69 demonstrated that, inBMEL
injuries,importantchangesoccurinsomeboneparameters. Theseauthors69evaluatedfragmentsofsubchondralboneof
the tibiain patientstreated withknee arthroplasty due to osteoarthritis.Theyfoundthattherewasasignificantincrease
in volumetricbone mineral density (vBMD) and bone
vol-ume/totalvolume(BV/TV),alongwithtrabecularthickening (Tb.Th).Alsointhisstudy,whendataobtainedwithHR-pQCT werecoupledtothebonespectroscopicanalysis,areduction inthe mineral/matrixratio was noted. In fact, histological evaluationsreveal that,intheseBMELareas,aninfiltration ofmarrowspacesbyafibrouscollagennetworkandintense boneremodelingoccur.Therelationshipbetweenthesebone changeswithcartilagedamageisnotyetfullyknown.Itis
possiblethatthe measurementofthesedata mayhave an
importantclinicalroleinOA.
Perspectives
AlthoughHR-pQCThasbeenlaunchedonthemarketlessthan 10yearsago,thistechnologyhasalreadyamyriadof medi-calapplications.Groupsofresearchersworldwidehavebeen workingtofindnewwaystoexploititsfullpotential.Fornow, thedeviceisstillatoolrestrictedtoresearch;butconsidering thethingsithasbeenabletoassess,theunderlyingviewis that,inashorttime,HR-pQCTwillbecomeanimportant clin-icaltool.However,thecurrentcostsarestillanobstacletoits fullclinicaluse.
Itshigh resolutionand non-invasive characteristics,the evaluationin vivo, and its speed and efficiencyare advan-tageouspointsovertraditionalmethodsofmeasuringbone
mineral density and histomorphometry for bone studies.
Thus, HR-pQCT can be used foran efficient and accurate
assessmentofthe developmentofdiseases such as
osteo-porosis,osteoarthritisandrheumatoidarthritis.Thus,inthe future,theincorporationofthemeasurementsofthis tech-nologyonclassificationcriteriaandinthestagingofvarious
clinicalconditionsmayoccur.Itwillbeabsolutelyessentialto carryoutfurtherstudiesonsafety,accuracyand reproducibil-ityoftheanalysespromotedbyHR-pQCTinvariousdiseases andintheagingprocess,whencomparedtowhatisalready establishedbyCT,DXAandhistomorphometry.
Itwillbeimportantalsotodetermineandconsolidatethe standardsofnormalityfordifferentpopulations.Some stud-iesusingcontrolgroupsforcomparisonhavebeenpublished, butthereisstillnotonecomprehensivestudyinthisdirection fortheBrazilianpopulation.Presently,thestudygroupatthe LaboratoryforBoneMetabolism,MedicineSchool, Universi-dadedeSãoPaulo(LIM-17)isconductingastudytodetermine
normality curvesforHR-pQCTand FiniteElement Analysis
parameterswithasampleofover400healthywomenaged
over20years.
Atthispoint,itisworthmentioningsomelimitationsof
theassessmentsperformedwithHR-pQCTandinthe
appli-cationoffiniteelement analysismodeling.One ofthemis thattheachievementofparametersofstrengthandstiffness dependson functionalestimates andon theapplicationof mathematical modelsthat, inmany cases,are notentirely reliablerepresentationsofreality.Moreover,itisnotyetreally clearhowthemorphofunctionalchangesobservedin periph-eral bones(radiusand tibia)maycorrelate withtherestof theskeleton.Anotherlimitationtobeemphasizedisrelated totheresolutionofthedevicethat,despitebeingthe high-estavailabletodayfortestinginvivo,isnotstillsufficientto individuallyassesstrabeculae.
Finally,itisnecessarytoconsolidatethestandardization ofmethodsofacquisitionandanalysisofimageswith HR-pQCTtechnology.Todoso,onemustkeepinmindthepatient positioning,system settings,the initialand final planesof
imagingforthesitemeasurementsandthemostimportant
parametersinthevariousassessments.Regardingfinite ele-mentanalysismodeling(whichonlyrecentlyisbeingusedin bonestudies),itisreallyimportanttodefinepatternsofbone functionalproperties(Young’smodulusandPoisson’sratio).
Funding
Conselho Nacional de Desenvolvimento Científico e
Tec-nológico (CNPQ nr. 300559/2009-7 for RMRP), Federico
Foundation(RMRP).
Conflict
of
interest
Theauthorsdeclarenoconflictofinterests.
r
e
f
e
r
e
n
c
e
s
1.CheungAM,AdachiJD,HanleyDA,KendlerDL,DavisonKS,
JosseR,etal.High-resolution-peripheralquantitative
computedtomographyfortheassessmentofbonestrength
andstructure:areviewbytheCanadianBoneStrength
WorkingGroup.CurrOsteoporosRep.2013;11:136–46.
2.BouxseinML,BoydSK,ChristiansenBA,GuldbergRE,Jepsen
microstructureinrodentsusingmicro-computed
tomography.JBoneMinerRes.2010;25:1468–86.
3. TjongW,KazakiaGJ,BurghardtAJ,MajumdarS.Theeffectof
voxelsizeonhigh-resolutionperipheralcomputed
tomographymeasurementsoftrabecularandcorticalbone
microstructure.MedPhys.2012;39:1893–903.
4. BurghardtAJ,KazakiaGJ,MajumdarS.Alocaladaptive
thresholdstrategyforhighresolutionperipheralquantitative
computedtomographyoftrabecularbone.AnnBiomedEng.
2007;35:1678–86.
5. FeldkampLA,GoldsteinSA,ParfittAM,JesionG,Kleerekoper
M.Thedirectexaminationofthree-dimensionalbone
architectureinvitrobycomputedtomography.JBoneMiner
Res.1989;4:3–11.
6. NieburGL,YuenJC,HsiaAC,KeavenyTM.Convergence
behaviorofhigh-resolutionfiniteelementmodelsof
trabecularbone.JBiomechEng.1999;121:629–35.
7. GuldbergRE,HollisterSJ,CharrasGT.Theaccuracyofdigital
image-basedfiniteelementmodels.JBiomechEng.
1998;120(2):289–95.
8. NagarajaS,CouseTL,GoldbergRE.Trabecularbone
microdamageandmicrostructuralstressesunderuniaxial
compression.JBiomech.2005;38:707–16.
9. VanRietbergenB,HuiskesR,EcksteinF,RüegseggerP.
Trabecularbonetissuestrainsinthehealthyandosteoporotic
humanfemur.JBoneMinerRes.2003;18:1781–8.
10.VicoL,ZouchM,AmiroucheA,FrereD,LarocheN,KollerB,
etal.High-resolutionperipheralquantitativecomputed
tomographyanalysisatthedistalradiusandtibia
discriminatespatientswithrecentwristandfemoralneck
fractures.JBoneMinerRes.2008;23:1741–50.
11.PialatJB,BurghardtAJ,SodeM,LinkTM,MajumdarS.Visual
gradingofmotioninducedimagedegradationin
high-resolutionperipheralcomputedtomography:impactof
imagequalityonmeasuresofbonedensityand
micro-architecture.Bone.2012;50:111–8.
12.SodeM,BurghardtAJ,PialatJB,LinkTM,MajumdarS.
QuantitativecharacterizationofsubjectmotioninHR-pQCT
imagesofthedistalradiusandtibia.Bone.2011;48:
1291–7.
13.PauchardY,LiphardtAM,MacdonaldHM,HanleyDA,Boyd
SK.Qualitycontrolforbonequalityparametersaffectedby
subjectmotioninhigh-resolutionperipheralquantitative
computedtomography.Bone.2012;50:1304–10.
14.XtremeRevision5.05.ScancoMedicalAg.18July2005. BassersdorfSwitzerland.
15.KrugR,BurghardtAJ,MajumdarS,LinkTM.High-resolution
imagingtechniquesfortheassessmentofosteoporosis.
RadiolClinNorthAm.2010;48:601–21.
16.ICRP.RecommendationsoftheInternationalCommissionon
RadiologicalProtection,vol.103.ICRP;2007.p.2–4.
17.WrixonAD.NewICRPrecommendations.JRadiolProt.
2008;28:161–8.
18.ZebazeRM,Ghasem-ZadehA,BohteA,Iuliano-BurnsS,
MiramsM,PriceRI,etal.Intracorticalremodellingand
porosityinthedistalradiusandpost-mortemfemursof
women:across-sectionalstudy.Lancet.2010;375:1729–36.
19.LaibA,HäuselmannHJ,RüegseggerP.Invivohighresolution
3D-QCTofthehumanforearm.TechnolHealthCare.
1998;6:329–37.
20.VanRuijvenLJ,GiesenEB,MulderL,FarellaM,VanEijdenTM.
Theeffectofbonelossonrod-likeandplate-liketrabeculae
inthecancellousboneofthemandibularcondyle.Bone.
2005;36:1078–85.
21.VanRietbergenB,HeinansH,HuiskesR,OdgaardA.Anew
methodtodeterminetrabecularboneelasticpropertiesand
loadingusingmicromechanicalfinite-elementmodels.J
BiomechEng.1995;28:69–81.
22.BoutroyS,VanRietbergenB,Sornay-RenduE,MunozF,
BouxseinML,DelmasPD.Finiteelementanalysisbasedon
invivoHR-pQCTimagesofthedistalradiusisassociated
withwristfractureinpostmenopausalwomen.JBoneMiner
Res.2008;23:392–9.
23.MacNeilJA,BoydSK.Improvedreproducibilityof
high-resolutionperipheralquantitativecomputed
tomographyformeasurementofbonequality.MedEngPhys.
2007;29:1096–105.
24.SundarSS,NandlalB,SaikrishnaD,MalleshG.Finiteelement
analysis:amaxillofacialsurgeon’sperspective.JMaxillofac
OralSurg.2012;11:206–11.
25.PistoiaW,VanRietbergenB,LochmullerEM,LillCA,Eckstein
F,RuegseggerP.Image-basedmicro-finite-elementmodeling
forimproveddistalradiusstrengthdiagnosis:movingfrom
benchtobedside.JClinDensitom.2004;7:153–60.
26.TurnerCH,RhoJ,TakanoY,TsuiTY,PharrGM.Theelastic
propertiesoftrabecularandcorticalbonetissuearesimilar:
resultsfromtwomicroscopicmeasurementtechniques.J
Biomech.1999;32:437–41.
27.RhoJY,AshmanRB,TurnerCH.Young’smodulusoftrabecular
andcorticalbonematerial:ultrasonicandmicrotensile
measurements.JBiomech.1993;26:111–9.
28.VanRietbergenB,HuiskesR,WeinansH,OdgaardA,KabelJ.
Theroleoftrabeculararchitectureintheanisotropic
mechanicalpropertiesofbone.Bonestructureand
remodeling.WorldScientific.1995:137–45.
29.RhoJY,TsuiTY,PharrGM.Elasticpropertiesofhuman
corticalandtrabecularlamellarbonemeasuredby
nanoindentation.Biomaterials.1997;18:1325–30.
30.VanRietbergenB,OdgaardA,KabelJ,HuiskesR.
Relationshipsbetweenbonemorphologyandboneelastic
propertiescanaccuratelyquantifiedusinghigh-resolution
computerreconstructions.JOrthopRes.1998;16:23–8.
31.LiuXS,ZhangXH,SekhonKK,AdamsMF,McMahonDJ,
BilezikianJP,etal.High-resolutionperipheralquantitative
computedtomographycanassessmicrostructuraland
mechanicalpropertiesofhumandistaltibialbone.JBone
MinerRes.2010;25:746–56.
32.KazakiaGJ,BurghardtAJ,LinkTM,MajumdarS.Variationsin
morphologicalandbiomechanicalindicesatthedistalradius
insubjectswithidenticalBMD.JBiomech.2011;44:257–66.
33.MacNeilJA,BoydSK.Loaddistributionandthepredictive
powerofmorphologicalindicesinthedistalradiusandtibia
byhighresolutionperipheralquantitativecomputed
tomography.Bone.2007;41:129–37.
34.BoutroyS,BouxseinML,MunozF,DelmasPD.Invivo
assessmentoftrabecularbonemicroarchitectureby
high-resolutionperipheralquantitativecomputed
tomography.JClinEndocrinolMetab.2005;90:6508–15.
35.BurghardtAJ,PialatJB,KazakiaGJ,BoutroyS,EngelkeK,
PatschJM,etal.Multicenterprecisionofcorticaland
trabecularbonequalitymeasuresassessedbyhighresolution
peripheralquantitativecomputedtomography.JBoneMiner
Res.2013;28:524–36.
36.MillerPD.Clinicaluseofbonemassmeasurementsinadults
fortheassessmentandmanagementofosteoporosis.In:
FavusMJ,editor.Primeronthemetabolicbonediseaseand
disordersofmineralmetabolism.6thed.Washington,DC:
AmericanSocietyforBoneandMineralResearch;2006.p.
150–61.
37.MartinRM,CorreaPH.Bonequalityandosteoporosistherapy.
ArqBrasEndocrinolMetabol.2010;54:186–99.
38.WattsNB.Bonequality:gettingclosertoadefinition.JBone
MinerRes.2002;17:1148–50.
39.Sornay-RenduE,BoutroyS,MunozF,DelmasPD.Alterations
ofcorticalandtrabeculararchitectureareassociatedwith
ofdecreasedBMDmeasuredbyDXA:theOfelyStudy.JBone
MinerRes.2007;22:425–33.
40.KhoslaS,RiggsBL,AtkinsonEJ,ObergAL,McDanielLJ,Holets
M,etal.Effectsofsexandageonbonemicrostructureatthe
ultradistalradius:apopulation-basednoninvasiveinvivo
assessment.JBoneMinerRes.2006;21:124–31.
41.SteinEM,LiuXS,NickolasTL,CohenA,ThomasV,McMahon
DJ,etal.Abnormalmicroarchitectureandreducedstiffnessat
theradiusandtibiainpostmenopausalwomenwith
fractures.JBoneMinerRes.2010;25:2572–81.
42.LiuXS,SteinEM,ZhouB,ZhangCA,NickolasTL,CohenA,
etal.Individualtrabeculasegmentation(ITS)-based
morphologicalanalysesandmicrofiniteelementanalysisof
HR-pQCTimagesdiscriminatepostmenopausalfragility
fracturesindependentofDXAmeasurements.JBoneMiner
Res.2012;27:263–72.
43.HochbergMC,RossPD,BlackD,CummingsSR,GenantHK,
NevittMC,etal.Largerincreasesinbonemineraldensity
duringalendronatetherapyareassociatedwithalowerrisk
ofnewvertebralfracturesinwomenwithpostmenopausal
osteoporosis.FractureInterventionTrialResearchGroup.
ArthritisRheum.1999;42:1246.
44.WasnichRD,MillerPD.Antifractureefficacyofantiresorptive
agentsarerelatedtochangesinbonedensity.JClin
EndocrinolMetab.2000;85:231.
45.HochbergMC,GreenspanS,WasnichRD,MillerP,Thompson
DE,RossPD.Changesinbonedensityandturnoverexplain
thereductionsinincidenceofnonvertebralfracturesthat
occurduringtreatmentwithantiresorptiveagents.JClin
EndocrinolMetab.2002;87:1586.
46.EastellR,VrijensB,CahallDL,RingeJD,GarneroP,WattsNB.
Boneturnovermarkersandbonemineraldensityresponse
withrisedronatetherapy:relationshipwithfractureriskand
patientadherence.JBoneMinerRes.2011;26:1662.
47.SarkarS,MitlakBH,WongM,StockJL,BlackDM,HarperKD.
Relationshipsbetweenbonemineraldensityandincident
vertebralfractureriskwithraloxifenetherapy.JBoneMiner
Res.2002;17:1.
48.CummingsSR,KarpfDB,HarrisF,GenantHK,EnsrudK,
LaCroixAZ,etal.Improvementinspinebonedensityand
reductioninriskofvertebralfracturesduringtreatmentwith
antiresorptivedrugs.AmJMed.2002;112:281.
49.RizzoliR,ChapurlatRD,LarocheJM,KriegMA,ThomasT,
FrielingI,etal.Effectsofstrontiumranelateandalendronate
onbonemicrostructureinwomenwithosteoporosis:results
ofa2-yearstudy.OsteoporosInt.2012;23:305–15.
50.RizzoliR,LarocheM,KriegMA,FrielingI,ThomasT,DelmasP,
etal.Strontiumranelateandalendronatehavediffering
effectsondistaltibiabonemicrostructureinwomenwith
osteoporosis.RheumatolInt.2010;30:1341–8.
51.SeemanE,DelmasPD,HanleyDA,SellmeyerD,CheungAM,
ShaneE,etal.Microarchitecturaldeteriorationofcorticaland
trabecularbone:differingeffectsofdenosumaband
alendronate.JBoneMinerRes.2010;25:1886–94.
52.BurghardtAJ,KazakiaGJ,SodeM,dePappAE,LinkTM,
MajumdarS.AlongitudinalHRpQCTstudyofAlendronate
treatmentinpostmenopausalwomenwithlowbonedensity:
relationsamongdensity,corticalandtrabecular
microarchitecture,biomechanics,andboneturnover.JBone
MinerRes.2010;25:2558–71.
53.HansenS,HaugeEM,JensenJE,BrixenK.Differingeffectsof
PTH1-34,PTH1-84,andzoledronicacidonbone
microarchitectureandestimatedstrengthinpostmenopausal
womenwithosteoporosis.An18monthopen-labeled
observationalstudyusingHR-pQCT.JBoneMinerRes.
2013;28:736–45.
54.ChapurlatRD,LarocheM,ThomasT,RouanetS,DelmasPD,
DeVernejoulMC.Effectoforalmonthlyibandronateonbone
microarchitectureinwomenwithosteopenia-arandomized
placebo-controlledtrial.OsteoporosInt.2013;24:
311–20.
55.SchaferAL,BurghardtAJ,SellmeyerDE,PalermoL,Shoback
DM,MajumdarS,etal.Postmenopausalwomentreatedwith
combinationparathyroidhormone(1-84)andibandronate
demonstratedifferentmicrostructuralchangesattheradius
vs.tibia:thePTHandIbandronateCombinationStudy(PICS).
OsteoporosInt.2013;24:2591–601.
56.JayakarRY,CabalA,SzumiloskiJ,SardesaiS,PhillipsEA,Laib
A,etal.Evaluationofhigh-resolutionperipheralquantitative
computedtomography,finiteelementanalysisand
biomechanicaltestinginapre-clinicalmodelofosteoporosis:
astudywithodanacatibtreatmentintheovariectomized
adultrhesusmonkey.Bone.2012;50:1379–88.
57.CabalA,JayakarRY,SardesaiS,PhillipsEA,SzumiloskiJ,
PosavecDJ,etal.High-resolutionperipheralquantitative
computedtomographyandfiniteelementanalysisofbone
strengthatthedistalradiusinovariectomizedadultrhesus
monkeydemonstrateefficacyofodanacatiband
differentiationfromalendronate.Bone.2013;56:
497–505.
58.MacdonaldHM,NishiyamaKK,HanleyDA,BoydSK.Changes
intrabecularandcorticalbonemicroarchitectureat
peripheralsitesassociatedwith18monthsofteriparatide
therapyinpostmenopausalwomenwithosteoporosis.
OsteoporosInt.2011;22:357–62.
59.SchettG,RedlichK,SmolenJS.Inflammation-inducedbone
lossintherheumaticdiseases.In:FavusMJ,editor.Primeron
themetabolicbonediseasesanddisordersofmineral
metabolism.6thed.Philadelphia:LippincottWilliams&
Wilkins;2006.p.310.
60.FarrantJM,GraingerAJ,O’ConnorPJ.Advancedimagingin
rheumatoidarthritis:part2:erosions.SkeletalRadiol.
2007;36:381–9.
61.TöpferD,FinzelS,MuseykoO,SchettG,EngelkeK.
Segmentationandquantificationofboneerosionsin
high-resolutionperipheralquantitativecomputed
tomographydatasetsofthemetacarpophalangealjointsof
patientswithrheumatoidarthritis.Rheumatology(Oxford).
2014;53:65–71.
62.SokkaT.Radiographicscoringinrheumatoidarthritis:ashort
introductiontothemethods.BullNYUHospJtDis.
2008;66:166–8.
63.StachCM,BäuerleM,EnglbrechtM,KronkeG,EngelkeK,
MangerB,etal.Periarticularbonestructureinrheumatoid
arthritispatientsandhealthyindividualsassessedby
high-resolutioncomputedtomography.ArthritisRheum.
2010;62:330–9.
64.FinzelS,RechJ,SchmidtS,EngelkeK,EnglbrechtM,StachC,
etal.Repairofboneerosionsinrheumatoidarthritistreated
withtumournecrosisfactorinhibitorsisbasedonbone
appositionatthebaseoftheerosion.AnnRheumDis.
2011;70:1587–93.
65.AlbrechtA,FinzelS,EnglbrechtM,RechJ,HueberA,
SchlechtwegP,etal.ThestructuralbasisofMRIbone
erosions:anassessmentbymicroCT.AnnRheumDis.
2012;72:1351–7.
66.BarnabeC,SzaboE,MartinL,BoydSK,BarrSG.Quantification
ofsmalljointspacewidth,periarticularbonemicrostructure
anderosionsusinghigh-resolutionperipheralquantitative
computedtomographyinrheumatoidarthritis.ClinExp
Rheumatol.2013;31:243–50.
67.AschenbergS,FinzelS,SchmidtS,KrausS,EngelkeK,
EnglbrechtM,etal.Catabolicandanabolicperiarticularbone
changesinpatientswithrheumatoidarthritis:acomputed
tomographystudyontheroleofage,diseasedurationand
68.ZhaoJ,LiX,BolbosRI,LinkTM,MajumdarS.Longitudinal
assessmentofbonemarrowedema-likelesionsandcartilage
degenerationinosteoarthritisusing3TMRT1rho
quantification.SkeletalRadiol.2010;39:
523–31.
69.KazakiaGJ,KuoD,SchoolerJ,SiddiquiS,ShanbhagS,
BernsteinG,etal.Boneandcartilagedemonstratechanges
localizedtobonemarrowedema-likelesionswithin
osteoarthriticknees.OsteoarthritisCartilage.2013;21: