ANTIDEPRESSANT-LIKE EFFECT OF NITRIC OXIDE SYNTHASE
INHIBITORS AND SILDENAFIL AGAINST
LIPOPOLYSACCHARIDE-INDUCED DEPRESSIVE-LIKE BEHAVIOR IN MICE
V. S. TOMAZ,aR. C. CORDEIRO,aA. M. N. COSTA,a D. F. DE LUCENA,aH. V. NOBRE JU´NIOR,b
F. C. F. DE SOUSA,aS. M. M. VASCONCELOS,a M. L. VALE,cJ. QUEVEDOd,eAND D. MACEˆDOa* aNeuropharmacology Laboratory, Department of Physiology and Pharmacology, Universidade Federal do Ceara´, Fortaleza, CE, Brazil
bDepartment of Clinical and Toxicological Analysis, School of Pharmacy, Laboratory of Bioprospection and Experiments in Yeast, LABEL, Federal University of Ceara´, Fortaleza, CE, Brazil cLaboratory of Inflammation and Cancer Pharmacology,
Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceara´, Fortaleza, CE, Brazil dLaboratory of Neurosciences, Graduate Program in Health Sciences, Health Sciences Unit, University of Southern Santa Catarina, Criciuma, SC, Brazil
eCenter for Experimental Models in Psychiatry, Department of Psychiatry and Behavioral Sciences, The University of Texas Medical School at Houston, Houston, TX, USA
Abstract—Inflammation, oxidative and nitrosative stress underlie depression being assessed in rodents by the systemic administration of lipopolysacharide (LPS). There is an increasing body of evidence of an involvement of nitric oxide (NO) pathway in depression, but this issue was not investigated in LPS-induced model. Thus, herein we evaluated the effects of NO-pathway-modulating drugs, named aminoguanidine,L-NAME, sildenafil and L-arginine, on the behavioral (forced swimming test [FST], sucrose preference [SPT] and prepulse inhibition [PPI] of the startle)
and neurochemical (glutathione [GSH], lipid peroxidation, IL-1b) alterations in the prefrontal cortex, hippocampus and striatum as well as in BDNF levels in the hippocampus 24 h after LPS (0.5 mg/kg, i.p.) administration, a time-point related to depressive-like behavior. Twenty-four hours post LPS there was an increase in immobility time in the FST, decrease in sucrose preference and PPI levels accompanied by a decrease in GSH levels and an increase in lipid perox-idation, IL-1b and hippocampal BDNF levels suggestive of a depressive-like state. The pretreatment with the NOS inhibitors,L-NAME and aminoguanidine as well as sildenafil prevented the behavioral and neurochemical alterations induced by LPS, although sildenafil andL-NAME were not able to prevent the increase in hippocampal BDNF levels induced by LPS. The iNOS inhibitor, aminoguanidine, and imipramine prevented all behavioral and neurochemical alterations induced by LPS.L-arginine did not prevent the alterations in immobility time, sucrose preference and GSH induced by LPS. Taken together our results show that the NO-cGMP pathway is important in the modulation of the depressive-like alterations induced by LPS. Ó2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Key words: depressive-like behavior, LPS, nitric oxide, oxidative stress, neuroinflammation, BDNF.
INTRODUCTION
Major depressive disorder (MDD) is a stress-related illness that affects 4.4–20% of general population
(Bakish, 2001). Stress regulates proinflammatory
cytokines (e.g. IL-1b, IL-6, and TNF-
a
), cyclooxygenase-2 and lipid peroxidation. Indeed, MDD patients present increased levels of proinflammatory cytokines, for instance, IL-6, IL-1b and TNF-a
in the blood (Maes, 1999) and cerebrospinal fluid (Levine et al., 1999). In addition, these inflammatory cytokines can practically interact with every pathophysiologic target relevant to depression, including neurotransmitter metabolism, neuroendocrine function and synaptic plasticity (Maes,1999; Tsigos and Chrousos, 2002) being correlated
in humans, for instance IL-1b, with anhedonia
(DellaGioia et al., 2013). Within this context,
brain-derived neurotrophic factor (BDNF), a key factor in neuroplasticity, is decreased in MDD (McNally et al., 2008).
The poor control of the immune/inflammatory response is related to the absence of clinical therapeutic
http://dx.doi.org/10.1016/j.neuroscience.2014.03.025
0306-4522/Ó2014 IBRO. Published by Elsevier Ltd. All rights reserved. *Corresponding author. Address: Department of Physiology and Pharmacology, Universidade Federal do Ceara´, Rua Cel. Nunes de Melo 1127, 60431-270 Fortaleza, CE, Brazil. Tel: +55-85-3366-8337; fax: +55-85-3366-8333.
E-mail addresses:daniellesilmacedo@gmail.com,daniellesm2000@ yahoo.com(D. Maceˆdo).
Abbreviations:AMINO, aminoguanidine; ANOVA, analysis of variance; BDNF, brain-derived neurotrophic factor; cGMP, cyclic guanosine monophosphate; DTNB, 5,5-dithiobis-(2-nitrobenzoic acid); eNOS, endothelial NOS; FST, forced swimming test; HC, hippocampus; IDO, indoleamine 2,3-dioxygenase; IL-1b, interleukin 1b; IMI, imipramine; iNOS, inducible NOS; L-Arg, L-arginine; L-NAME, (Nx-nitro-L-arginine methyl ester hydrochloride); LPS, lipopolysacharide; MDA, malondialdehyde; MDD, major depressive disorder; NF-jB, nuclear factor-jB; NMDAR, N-methyl-D-aspartate receptor; nNOS, neuronal NOS; NO, nitric oxide; NOS, NO synthases; O&NS, oxidative and nitrosative stress; OFT, open field test; PDE5, phosphodiesterase 5; PFC, prefrontal cortex; PPI, prepulse inhibition; Sal, saline; SEM, standard errors of the mean; sGC, soluble guanylate cyclase; SIL, sildenafil citrate; SPT, sucrose preference test; ST, striatum; TBA, thiobarbituric acid; TBARS, thiobarbituric-acid reacting substances; TCA, trichloroacetic acid; TLR4, toll-like receptor 4; TNF-a, tumor necrosis factora.
benefit of antidepressants in patients with treatment-resistant depression (Maes et al., 1997; Carvalho et al., 2013). Currently, treatment resistant depression occurs in up to 40% of the patients diagnosed with MDD (Rush et al., 2012).
Based on the outlined role of cytokines in depression, the systemic administration of the endotoxin lipopolysaccharide (LPS) has been used to trigger depressive-like alterations in rodents (De La Garza Ii,
2005; Dantzer et al., 2008; Custodio et al., 2013) and
depressive mood in humans (Grigoleit et al., 2011). Lipopolysacharide activates toll-like receptor 4 (TLR4)
(Hoshino et al., 1999). The TLR4 signaling pathway
activates nuclear factor-
j
B (NF-j
B), which leads to the production of proinflammatory cytokines (Akira andTakeda, 2004). Recently, preclinical evidences pointed
toward an activation of TLR4 signaling pathway in mice prefrontal cortex (PFC) after repeated restraint/acoustic stress exposure being responsible for triggering neuroinflammation at PFC level and regulating gut barrier function/permeability (Garate et al., 2011).
The behavioral alterations induced by LPS are time-related. In this regard, the depressive-like behavior induced by this endotoxin occurs 24 h after administration in rodents (Custodio et al., 2013; Ohgi
et al., 2013) and was prevented by antidepressants
such as fluoxetine, paroxetine (Ohgi et al., 2013) and imipramine (Ferreira Mello et al., 2013).
Aside from inflammation, increased oxidative and nitrosative stress (O&NS) (Maes et al., 2011; Leonard
and Maes, 2012) are related to MDD. The occurrence of
nitrosative insult in vivo is observed in inflammatory processes, neurotoxicity and ischemia (Sayre et al., 2007; Sßenesß et al., 2007; Leonard and Maes, 2012) as well as during neurotransmission through
N-methyl-D-aspartate receptor (NMDAR) activation (Calabrese
et al., 2007). Inflammation together with excessive
excitatory neurotransmission and alterations in NMDAR subunits are important features of MDD (Zarate et al.,
2006; Feyissa et al., 2009; Leonard and Maes, 2012).
Nitric oxide production in mammalian cells is a result of the enzymatic oxidation of L-arginine by NO
synthases (NOS). This gas is regarded as a ubiquitous, janus-faced signaling molecule in the regulation of key functions in the immune, cardiovascular and nervous system (Calabrese et al., 2007).
Nitric oxide synthases comprise a family of three related proteins that modulate diverse biological processes such as neurotransmission, vascular homeostasis and immunological surveillance (Alderton
et al., 2001). These enzymes namely endothelial
(eNOS), neuronal (nNOS) and inducible NOS (iNOS) present different functions and subcellular distribution
(Alderton et al., 2001). In this regard, nNOS and eNOS
are constitutive enzymes being responsible for the production of low quantities of NO. In contrast, iNOS mediates neurotoxic events due to the overproduction of NO (Ainscough and Brodie, 1995; Calabrese et al., 2007). Recently, NO-pathway-modulating drugs are gaining increasing relevance in the study of depression because NOS inhibitors, e.g. L-NAME (a nonspecific NOS
inhibitor), aminoguanidine (a specific iNOS inhibitor) and sildenafil (a phosphodiesterase 5 – PDE5 inhibitor) are presenting antidepressant-like activity in rodents (Bettio et al., 2012; Montezuma et al., 2012; Zhang et al., 2013) that was reversed by L-arginine, a NO precursor
(Joca and Guimara˜es, 2006). By contrast, a recent
study showed that NOS inhibitors potentiated LPS-induced sickness behavior (Ribeiro et al., 2013). Thus, the issue regarding the role of NO in LPS-induced behavioral alterations needs to be clarified.
To our best knowledge there are no reports exploring the role of NO on LPS-induced depressive-like symptoms, with the exception of two recent studies of our research group that demonstrated, 24 h after the LPS challenge, a decrease in nitrite levels in the PFC, hippocampus (HC) and striatum (ST) (Custodio et al., 2013) that was restored by imipramine (Ferreira Mello et al., 2013).
Thus, based on the putative role of NO in depression and the absence of studies exploring the effects of this gas on the model of depressive-like behavior induced by immune challenge we decided to test the hypothesis that NO-pathway-modulating drugs, i.e. the NOS inhibitors L-NAME and aminoguanidine as well as
sildenafil could present antidepressant-like effect against LPS-induced depressive-like behavior in mice. Furthermore, we assessed whether the effects were associated with alterations in oxidative stress parameters (i.e. reduced glutathione – GSH and lipid peroxidation), IL-1b and BDNF levels in discrete areas named prefrontal cortex, hippocampus and striatum of the mice brain following the immune challenge with LPS.
EXPERIMENTAL PROCEDURES
Animals
Adult male Swiss mice (8 weeks) weighing 20–30 g were housed eight per cage in standard polycarbonate cages (4220.520 cm) with standard environmental conditions (22 ± 1°C, humidity 60 ± 5% and 12-h light–dark cycle) and food and water ad libitum. The animals were obtained from the Central Animal Facility of the Federal University of Ceara. The experimental procedures were performed in the period from 8:00 to 14:00 h. All experiments were performed according to the Guide for the Care and Use of Laboratory Animals, from the US Department of Health and Human Services
(Resources, 1996) and adhered to the Brazilian
legislation on animal experimentation (law n° 11.794 of 10/08/2008). The experimental protocol was approved by the Federal University of Ceara Animal Care and Use Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs
Lipopolysaccharide (LPS) from Escherichia coli, strain 055:B5, aminoguanidine hydrochloride, L-NAME
Corp., St. Louis, USA were used. All drugs were freshly prepared and diluted in saline solution.
Experimental design
The LPS dosage (0.5 mg/kg, i.p.) was chosen based on previous studies evaluating depressive-like behavioral changes and neurochemical alterations in mice (Lejuez
et al., 2011; Custodio et al., 2013; Ohgi et al., 2013).
For behavioral and neurochemical determinations the animals were randomly divided into groups of eight animals each. The administration of the drugs was performed through single intraperitoneal injections of the drugs alone (saline – Sal, imipramine 10 mg/kg – IMI, aminoguanidine 75 mg/kg – AMINO, L-NAME 30 mg/kg,
sildenafil citrate 5 mg/kg – SIL orL-arginine 150 mg/kg –
L-Arg) or followed by LPS administration separated by a 30-min interval (Sal + LPS, IMI + LPS, AMINO + LPS,
L-NAME + LPS, SIL + LPS or L-Arg + LPS). The
doses of NO-pathway-modulating drugs were chosen based on previous studies showing neuroprotective effects (Kaviani et al., 2004; Montezuma et al., 2012;
Custodio et al., 2013; Heydarpour et al., 2013;
Vasconcelos Rios et al., 2013). Imipramine was used as
a standard antidepressant. Twenty-four hours after the administration of LPS or saline (control group) the animals were subjected to the behavioral tests. For this purpose, different animals from each group were submitted to the behavioral determinations of open field, forced swimming or prepulse inhibition (PPI) tests only once. Since our goal was to evaluate depressive-like behavior and the NO-pathway-modulating drugs when administered alone did not display any alterations in the forced swimming test nor in the open field test we decided to conduct the neurochemical determinations only in controls and in LPS-treated animals. For neurochemical determinations mice were sacrificed by cervical dislocation. The brain areas prefrontal cortex (PFC), hippocampus (HC) and striatum (ST) were dissected and immediately stored at 80° C until assayed.
Behavioral tests
Forced swimming test (FST). In this test, animals
were subjected individually to the analysis of the depressive-like behavior, based on a model adapted
from Porsolt et al. at 1977. In this task the immobility
period of the animal (during 6 min) is registered and, the greater this time, the lower the animal’s motivation to escape, representing, thus, a depressive-like behavior (Russell et al., 2008). Animals were placed individually in acrylic cylinder (25 cm high, 10 cm in diameter and 8 cm in depth) containing water at 25°C. Mice were unable to escape or touch the bottom of the cylinder. Any mouse appearing to have difficulty keeping its head above the water was removed from the cylinder and excluded from the study.
Open field test (OFT). This test was adapted from the
model initially proposed byArcher (1973) and was used
here to evaluate the locomotor activity of the animals. The experimental trial was conducted in a dark room with red light where the mice were individually placed in a transparent acrylic box (808030 cm) divided equally into nine quadrants. Each animal was placed in the center of the arena immediately before the test and allowed to explore it for 5 min. The number of squares crossed by the animals were registered and used as a parameter of locomotor activity. The arena was cleaned with a 5% ethanol solution between each test animal.
Sucrose preference test. The test was performed 24 h
after LPS administration to address LPS-induced anhedonia. The procedure (Mao et al., 2014) consisted of an adaptation period 72 h before the test in which rats were trained to adapt to sucrose solution with two bottles of 1% (w/v) sucrose solution placed in each cage. Twenty-four hours later, sucrose solution in one bottle was replaced with tap water during 24 h. After this adaptation period rats were deprived of water and food for a further 24 h. For the SPT the rats were housed in individual cages with free access to two bottles containing 100 ml of sucrose solution (1% w/v) and 100 ml of water. After 1 h, the volumes of consumed sucrose solution and water were recorded and the sucrose preference was calculated as follows: % sucrose consumption = sucrose consumption/ (water + sucrose consumption)100.
Evaluation of prepulse inhibition (PPI) of the startle
response. Recently, decreases in PPI levels are being
associated with depressive-like behavior in rodents
(Tejeda et al., 2010; Custodio et al., 2013). Thus, we
decided to determine the effect of NO-pathway-modulating drugs on PPI levels of animals submitted to an immune challenge with LPS. To do this, the animals were subjected to a stabilimeter, which consisted of a wire-mesh cage (844.5 cm) suspended within a PVC frame (2599 cm) attached to the response platform with four thumbnail-screws. The stabilimeter and platform were located inside a ventilated plywood sound-attenuating chamber (646040 cm) (Insight, Sa˜o Paulo, Brazil). The floor of the stabilimeter consisted of six stainless steel bars. The startle reaction of the animals generated a pressure on the response platform and analog signals were amplified, digitized and analyzed by a software of the startle measure system (Insight, Sa˜o Paulo, Brazil), which also controlled other parameters of the session (intensity of the acoustic stimulus, inter-stimulus interval, etc.). Two loudspeakers located 10 cm above the floor, on each lateral side of the acoustic isolation chamber, were used to deliver the prepulse stimulus, the acoustic startle stimulus and continuous background noise (65 dB). Calibration procedures were conducted before the experiments to ensure equivalent sensitivities of the response platforms over the protocol.
(pulse alone—120 dB, 50 ms duration), with an inter-trial interval of 15 s. The purpose of this phase was to allow within-session habituation to the startle stimulus. Thereafter, the PPI modulation of the acoustic startle was tested in 74 trials pseudo-randomly divided into seven different categories presented with an inter-trial interval of 15 s: 20 presentations of pulse alone (120 dB, 50 ms duration), eight presentations of each prepulse intensity alone (70, 75 and 80 dB, 3000 Hz frequency, and 20 ms duration) and 10 presentations of each prepulse intensity + pulse (with 50 ms interval)
(Blaszczyk et al., 2000; Gururajan et al., 2010). Mean
amplitude of startle response to pulse-alone (P) and prepulse-pulse (PP + P) trials were calculated for each subject. The PPI level of each mouse was determined by expressing the prepulse + pulse startle amplitude as a percentage decrease from pulse-alone startle amplitude, according to the following formula:% PPI = 100 [100(PP/P)]. Using this formula, a 0% value denotes no difference between amplitude of startle response to pulse alone and to the prepulse + pulse and, consequently, no PPI.
Neurochemical determinations
Evaluation of GSH levels. Reduced glutathione (GSH)
levels were evaluated to estimate endogenous defenses against oxidative stress. The method was based on Ellman’s reagent (DTNB) reaction with free thiol groups
(Ellman, 1959). The brain areas were diluted in EDTA
0.02 M buffer (10% w/v) and added to a 50% trichloroacetic acid solution. After centrifugation (3000 rpm/15 min), the supernatant of the homogenate was collected and mixed with 0.4 M tris–HCl buffer, pH 8.9 and 0.01 M 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB). The resultant yellow color was immediately read at 412 nm using a spectrophotometer (Beckman coulter UV/Visible). Results were calculated based on a standard glutathione curve and expressed as ng of GSH/g wet tissue.
Evaluation of Lipid peroxidation. Lipid peroxide
formation was analyzed by measuring the thiobarbituric-acid reacting substances (TBARS) in the homogenates
(Draper et al., 1993) as an index of ROS production.
The samples were mixed with 1 mL of trichloroacetic acid 10% (TCA) and 1 mL of thiobarbituric acid 0.67% (TBA), then heated in a boiling water bath for 15 min and immediately kept cold in a bath of ice. Lipid peroxidation was assessed by the absorbance at 532 nm and expressed as
l
mol of malonaldehyde (MDA)/g tissue.Immunoassays for IL1-band BDNF. The brain areas,
PFC, HC and ST were homogenized in eight volumes of phosphate-buffered saline (PBS) buffer with protease (EMD Biosciences) and phosphatase (Sigma–Aldrich) inhibitors and centrifuged (10,000 rpm, 5 min). BDNF was estimated only in the HC. For IL-1b and BDNF determinations 50
l
L samples were used. The immunoenzymatic assay (ELISA) was performedaccording to the manufacturer’s protocol (R&D systems, Minneapolis, MN, USA) and expressed in pg/g tissue.
Statistical analyses
Data from behavioral and neurochemical determinations are presented as mean ± SEM (standard errors of the mean) and were compared using the Tukey’s test. For the analyses of the PPI produced by the three different prepulse intensities, repeated measures two-way analysis of variance (ANOVA) with ‘‘experimental groups’’ and ‘‘prepulse intensities’’ as factors was used, with the Bonferroni’s test for post hoc comparisons. The significance level was set at p60.05. The statistical program used was GraphPad Prism 5.0 Version for Windows, GraphPad Software (San Diego, CA, USA).
RESULTS
As a first step we assessed the alterations in the immobility time in the FST induced by the administration of the NO-pathway-modulating drugs alone and associated with LPS (Fig. 1A). The results showed that the administration of the drugs alone caused no alteration in the immobility time with the exception of imipramine that decreased this parameter [F(5, 36) = 4.594, P< 0.05] as compared to control animals. As expected LPS significantly increased immobility time
FST
Control IMI L-Arg L-NAME Sil Amino Sal IMI L-Arg L-NAME Sil Amino
0 50 100 150 200
LPS *
*
#
# #
#
A
*
Im
m
o
b
ili
ty
ti
m
e
(
s)
in
FS
T
OFT
Control IMI L-Arg L-NAME Sil Amino Sal IMI L-Arg L-NAME Sil Amino 0
50 100 150
LPS *
*
B
Num
b
e
r
of
C
ros
s
ing
s
in
OF
T
(5
mi
n
)
[F(6, 48) = 33.45,P< 0.0001] when compared to control animals. The LPS-induced increase in immobility time was maintained by the pretreatment with the NO donor,
L-arginine (P< 0.001). On the other hand, a significant
reduction in the immobility time was observed in the animals pretreated with the selective inducible nitric oxide inhibitor (iNOS), aminoguanidine, and the tricyclic antidepressant imipramine (P< 0.05) when compared to the control and LPS-treated groups. The prior administration of sildenafil and L-NAME, significantly
decreased the immobility time when compared to the LPS group (P< 0.0001). We next determined the alterations in the locomotor activity since LPS administration is related to sickness behavior that presents a complex behavioral phenotype with the occurrence of hypolocomotion. In our results locomotor activity was unchanged by NO-pathway-modulating drugs, either alone [F(5, 42) = 4.526, P< 0.001], associated with LPS and by LPS alone when compared to control animals. Only the treatment with imipramine alone and associated with LPS augmented this behavioral parameter [F(6, 48) = 22.90, P< 0.0001]
(Fig. 1B). This absence of hypolocomotion gives an idea
that the animals were not in sickness behavior.
Based on the absence of behavioral alterations in the FST and OFT induced the NO-pathway-modulating drugs when administered alone, we decided to conduct the other determinations only in LPS-treated animals and controls.
To better evaluate LPS-induced anhedonia the SPT was performed (Fig. 2). The results showed that 24 h after the endotoxin administration the animals displayed a significant decrease in sucrose preference when compared to control animals [F(6, 38) = 14.99, P< 0.001]. The administration of imipramine (P< 0.001), sildenafil (P< 0.05), L-NAME (P< 0.05)
and aminoguanidine (P< 0.001) prevented the decrease in sucrose preference when compared to LPS-treated animals. Mice that underwent L-arginine
administration prior to LPS decreased sucrose preference when compared with control animals (P< 0.05).
Regarding PPI data (Fig. 3) two-way ANOVA revealed significant main effects of ‘‘prepulse intensities’’
[F(2, 90) = 7.18, P< 0.0013] and ‘‘experimental groups’’ [F(6, 90) = 12.59, P< 0.0001] with a significant interaction [F(12, 90) = 3.94, P< 0.0001] between these parameters. Bonferroni post hoc test showed a significant decrease in PPI levels 24 h after LPS administration in the prepulse intensities of 70 (P< 0.05), 75 (P< 0.05) and 80 dB (P< 0.05) when compared to control animals. The administration of imipramine, L-arginine, sildenafil, L-NAME and
aminoguanidine was able to prevent the reduction in PPI levels caused by the systemic administration of LPS. In addition, the pretreatment with imipramine,
L-arginine, L-NAME and aminoguanidine in the prepulse
intensity of 70 andL-arginine in the prepulse intensity of
75, increased PPI levels when compared to control animals (P< 0.05).
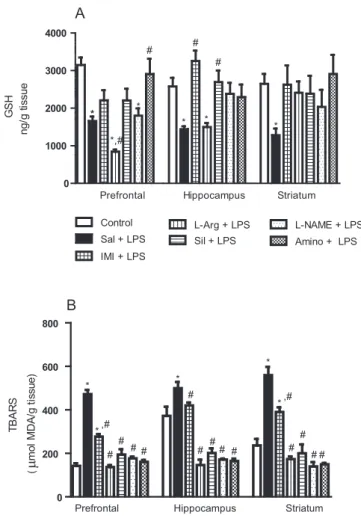
One key aspect of depression is a defect in antioxidant defenses (Kubera et al., 2013). Based on this evidence we observed that the levels of the endogenous antioxidant GSH (Fig. 4A) decreased in the PFC, HC and ST of animals treated with LPS when compared to controls [F(6, 120) = 4.238, P< 0.0001]. In the PFC, the pretreatment with L-arginine caused a
significant reduction in GSH levels as compared to the control and LPS-treated animals (P< 0.01), while
L-NAME pretreatment caused a significant decrease in
GSH levels when compared to control animals (P< 0.05). Only aminoguanidine was able to prevent the decrease in GSH levels induced by LPS in the PFC (P< 0.05). In the HC the pretreatment with L-arginine
also significantly decreased GSH levels as compared to control animals (P< 0.05). In this brain area imipramine and sildenafil significantly prevented the decreases in GSH levels induced by LPS (P< 0.01). In the ST no significant alterations were observed in the groups pretreated with NO-pathway-modulating drugs or with imipramine.
A significant increment in lipid peroxidation, measured by TBARS levels, was observed in all brain areas of animals treated with LPS as compared to controls [F(20, 147) = 34.41, P< 0.0001] (Fig. 4B). The administration of imipramine, L-arginine, sildenafil, L-NAME and aminoguanidine significantly prevented the
increases in lipid peroxidation induced by LPS (P< 0.05). Since depressive-like behavioral changes induced by LPS are related to the induction of proinflammatory cytokines in the brain, the levels of IL-1b were measured (Fig. 5). LPS administration caused a significant increase in the content of IL-1b when compared to control animals in the PFC [F(6, 50) = 6.150, P< 0.0001], HC [F(6, 48) = 10.27, P< 0.0001] and ST [F(6, 49) = 8.69, P< 0.0001]. In the PFC only imipramine and L-arginine were able to significantly
prevent the increase in IL-1binduced by LPS, while in the HC and ST the pretreatment with L-NAME,
aminoguanidine, sildenafil, L-arginine and imipramine
significantly prevented the increase in IL-1b levels induced by LPS.
Because oxidative stress and inflammation alters the brain levels of neurotrophic factors, the content of BDNF was evaluated in the HC, the main site of neurogenesis
Control Sal IMI L-Arg L-NAME Sil Amino
0 20 40 60 80 100
LPS *
#
# #
#
Suc
ro
s
e
co
nsu
m
ptio
n
(
%)
*
in the adult brain. Twenty-four hours after the systemic administration of LPS a significant increase in BDNF levels was demonstrated as compared to controls. Only
imipramine and aminoguanidine prevented the increase in BDNF levels induced by LPS [F(6, 54) = 15.52, P< 0.0001] (Fig. 6).
0 20 40 60 80 100
L-Arg + LPS
Sil + LPS
L-NAME + LPS
Amino + LPS Control
Sal + LPS
IMI + LPS
* * *
#
#
#
# #
*,# #
#
# # #
*,# *,#
*,#*,#
70 dB 75 dB 80 dB
%
P
repul
s
e
i
nhi
bi
ti
on
Fig. 3.Percent of pre-pulse inhibition of startle (PPI), using three prepulse intensities (70, 75 and 80 dB) of animals treated with LPS (Sal + LPS) or pretreated with the NO-pathway-modulating drugs [L-arginine (L-arg), sildenafil (sil),L-NAME or aminoguanidine (amino)] or the antidepressant imipramine (IMI) before LPS. Bars represent means ± standard error of the means (SEM) of the percent of PPI of eight animals/group.⁄P< 0.05 vs. control,#P< 0.05 vs. LPS according to a two-way ANOVA followed by the Bonferroni’s test.
0 1000 2000 3000 4000
GS
H
ng/
g
t
issue
Control
Sal + LPS
IMI + LPS
L-Arg + LPS Sil + LPS
L-NAME + LPS Amino + LPS *
#
*,#
* #
* #
* *
* A
Prefrontal Hippocampus Striatum
0 200 400 600 800
*
*,#
# #
# # *
#
# # # #
*
*,#
# # # #
Prefrontal Hippocampus Striatum
TB
A
R
S
(
mo
l
MDA/
g
t
iss
ue
)
B
DISCUSSION
The present study is the first to demonstrate that drugs that modulate the NO-pathway such as the NOS inhibitors, L-NAME and aminoguanidine as well as
sildenafil prevent the depressive-like behavior induced by the systemic administration of LPS. The antidepressant-like effect of these drugs was accompanied by restoration of GSH levels, decrease in lipid peroxidation and IL-1b levels in the PFC, HC and ST. Aminoguanidine also reestablished BDNF levels altered by LPS in the HC showing overall a similar profile to the tricyclic antidepressant imipramine used here as a standard antidepressant drug. L-arginine, in
turn, was not able to prevent LPS-induced alterations in the tests used here to evaluate antidepressant-like effect, i.e. FST and sucrose preference test.
It is widely demonstrated that inflammation underlies MDD. The proinflammatory state associated to immune alterations involved in this disorder triggers several other mechanisms, such as: activation of indoleamine 2,3-dioxygenase (IDO), the enzyme that metabolizes
L-tryptophan by cytokines and cortisol (Maes et al., 1993, 1994); increase in LPS translocation from gram-negative enterobacteria, giving rise to the leaky gut syndrome in depression (Maes et al., 2008); decrease in antioxidant levels and increase in oxidative and nitrosative stress (O&NS) pathways (Bilici et al., 2001; Maes et al., 2011); reduction in the levels of zinc, related to the altered immune/inflammatory response (Maes et al., 1997); and damage to mitochondria (Gardner et al., 2003).
This proinflammatory state observed in MDD can be triggered by the systemic administration of LPS. Indeed, the activation of TL4R by LPS increases the synthesis of cytokines, O&NS (Akira and Takeda, 2004) eliciting alterations similar to those of depressive- and anxiety-like behaviors in rodents (Dantzer et al., 2008; Ohgi
et al., 2013) and humans (Grigoleit et al., 2011;
DellaGioia et al., 2013). On the other hand, chronic
unpredictable stress, an acknowledged animal model of depression, aggravates LPS-induced NFkB activation in the frontal cortex and hippocampus (Munhoz et al., 2006). These findings were confirmed by the lower inflammatory response and lipid peroxidation in the brain, accompanied by better behavioral outcome in response to immobilization stress in TLR4-deficient mice (Hoshino et al., 1999). In addition, it was recently shown that LPS from bacterial translocation is responsible, at least in part, for the TLR4 upregulation seen after chronic mild stress leading to the release of inflammatory mediators in the central nervous system (Garate et al., 2011).
Herein we used the time-period of 24 h post LPS to assess depressive-like symptoms, not sickness behavior. Sickness behavior peaks 2 h after the endotoxin administration and is characterized by malaise, pyrexia, lethargy, behavioral inhibition, hypolocomotion, exploration, anhedonia, among others (De La Garza Ii, 2005). In our study, the confirmation of the absence of sickness behavior 24 h after LPS came from the OFT in which no animal displayed decreased locomotor activity as compared to the control group.
Prefrontal cortex
Control Sal IMI L-ARG SIL L-NAME Amino 0
5 10 15 20 25
*
# #
A
IL
-1
pg
/
g
ti
s
s
u
e
LPS
Hippocampus
Control Sal IMI L-ARG Sil L-Name Amino
0 5 10 15 20 25
*
#
# # #
#
B
IL
-1
pg
/
g
ti
s
s
u
e
LPS
Striatum
Control Sal IMI L-ARG Sil L-Name Amino
0 10 20 30 40 50
*
#
# # # #
C
IL
-1
pg
/
g
ti
s
s
u
e
LPS
Fig. 5.Levels of IL-1b in the prefrontal cortex, hippocampus and striatum of animals treated with LPS (Sal + LPS) or pretreated with the NO-pathway-modulating drugs [L-arginine (L-arg), sildenafil (sil), L-NAME or aminoguanidine (amino)] or the antidepressant imipra-mine (IMI) before LPS. Bars represent means ± standard error of the means (SEM) of eight animals/group. ⁄P< 0.05 vs. control, #P< 0.05 vs. LPS according to ANOVA followed by the Tukey’s multiple comparison test.
Control Sal IMI L-ARG Sil L-Name Amino
0 200 400 600 800
*
* *
*
# #
BDN
F
(pg/
g
ti
s
s
u
e
)
LPS
Imipramine, on the contrary, increased this activity, but this was expected because previous studies of our group showed the same pattern of locomotor activity (Ferreira Mello et al., 2013).
Therefore, based on the putative role of NO on brain functioning (Calabrese et al., 2007) and also on the importance of nitrosative stress in MDD (Maes et al., 2011) we attempted to use NO-pathway -modulating drugs to better elucidate the role of this system in the depressive-like symptoms elicited by LPS. In this regard, in our results, NOS inhibitors and sildenafil presented antidepressant-like effects in the FST, sucrose preference test and increased PPI levels. These drugs when administered alone caused no behavioral alterations in the FST and OFT.
Behavioral determinations such as FST and sucrose preference test are used to address depressive phenotype in rodents. Especially sucrose preference paradigm is a method to determine motivational deficits, a core feature of depression (Rygula et al., 2005). In our study the results obtained in the FST were confirmed by the sucrose preference test. In both paradigms the deficits induced by LPS were prevented by L-NAME,
aminoguanidine and sildenafil, but not byL-arginine.
Another behavioral determination performed in our study was the PPI. Evidences point to lower PPI levels in patients with MDD when compared to non-depressed patients, although the levels of PPI are higher in depressed patients when compared to schizophrenic patients (Perry et al., 2004). Hence, during severe depressive states MDD patients are also characterized by clinical gating deficits as they fail to inhibit intrusive negative thoughts (Teasdale, 1983). Deficits in central inhibitory mechanisms, i.e. the organism’s ability to inhibit or gate responses to sensory, motor, or cognitive information are manifested in several neuropsychiatric disorders and are evaluated using the PPI test
(Swerdlow and Geyer, 1993).
We recently showed a decrease in PPI levels in mice 24 h after LPS administration correlating to the occurrence of depressive-like behavior (Custodio et al., 2013). Herein the decrease in PPI levels induced by LPS was prevented by all drugs tested, including
L-arginine that was not able to prevent the
depressive-like behavior induced by LPS in the FST and sucrose preference test. A possible explanation to this controversy regardingL-arginine is that the levels of PPI
are decreased only in patients with high levels of depression and/or anhedonia not in low depressed ones (Kaviani et al., 2004). Following this line of reasoning we can infer that PPI is not a good behavioral parameter to study depressive-like behavior and antidepressant drugs induced by LPS as we previously suggested (Custodio et al., 2013).
From the NO-pathway-modulating drugs evaluated in this study, as mentioned above, sildenafil, a PDE5 inhibitor causing intracellular accumulation of cGMP, presented antidepressant-like effects. Indeed, activation of the soluble guanylate cyclase (sGC), increase of cGMP formation and action of cGMP-dependent protein kinases have been suggested as the main signal
transduction pathway of NO related to its neuroprotective effects (Bettio et al., 2012). These effects are: regulation of hippocampal neurogenesis and synaptic plasticity (Moreno-Lopez and Gonzalez-Forero, 2006); regulation of the release of classical neurotransmitters, for instance, increase of dopamine and serotonin release (Prast and Philippu, 2001) and reduction of ROS formation (Teasdale, 1983).
In this study sildenafil prevented GSH decrease, lipid peroxide formation and IL-1bincrease induced by LPS. Only the alterations in BDNF levels induced by LPS were not prevented by sildenafil. To date, the effects of sildenafil on depression are still controversial. Previous report showed that this drug enhanced the activity of the atypical antidepressant drugs, mianserin and tianeptine, in the FST (Socala et al., 2012), by contrast several other studies exhibited inhibition of antidepressants action by sildenafil (Wang and Robinson, 1997; Dhir and
Kulkarni, 2007; Ludka et al., 2013). In humans sildenafil
is used for managing sexual dysfunction induced by antidepressants (Taylor et al., 2013). To our knowledge this is the first report to show that sildenafil alone presents antidepressant-like effect in the model of LPS-induced depressive-like behavior.
Numerous studies indicate that NOS inhibitors (Dhir
and Kulkarni, 2007; Zhang et al., 2013) exert
antidepressant effects in animal studies. Indeed nNOS-derived NO was demonstrated to exert a negative control on the hippocampal neurogenesis. This was confirmed by the antidepressant-like effects presented by nNOS / mice (Zhou et al., 2007). In addition
L-NAME at moderate doses increased the release of
serotonin in the hypothalamus (Prast and Philippu, 2001). Further evidences came from the recent preclinical findings showing that 10 mg/kg, i.p. ketamine (an antidepressant dosage) decreased nNOS activity
(Zhang et al., 2013). Thus, the antidepressant-like
effects of L-NAME against LPS-induced depressive-like
behaviors observed here were partially predictable. From the NOS inhibitors used here aminoguanidine presented the best results. This conclusion was based on the prevention by this drug of all behavioral and neurochemical alterations induced by LPS. Similar effects to those of aminoguanidine were observed in the animals treated with imipramine. Aminoguanidine is a selective inhibitor of iNOS. This enzyme is responsible for an overproduction of O&NS, activation of NF-
j
B resulting in inflammatory and oxidative processes leading to neurotoxicity (Sayre et al., 2007). Chronic social isolation, an animal model of depression caused upregulation of iNOS in the PFC of rats (Zlatkovic andFilipovic, 2013). Likewise, LPS administration also
induced iNOS expression in cortical neurons (Kim et al., 2002). Thus, iNOS seems to be an important target for the development of antidepressant drugs, although the evidences point toward a possible participation of this mechanism in imipramine antidepressant effects as well (Montezuma et al., 2012).
contribute to nitrosative stress, mitochondrial damage and cell death in neurons, important features of depression. Recently, the administration of GSH by i.c.v. route to mice presented antidepressant effect in the FST and tail suspension test whereas the inhibition of extracellular GSH catabolism prevented this antidepressant-like effect. No behavioral alterations were observed after GSH depletion (Rosa et al., 2013). Despite the antidepressant-like effect shown by GSH, the precise mechanisms involved in this action needs to be further addressed. In this study onlyL-arginine, that was devoid
of the antidepressant effect, was not able to prevent the deficit in GSH content induced by LPS.
Another important point to be considered is that, in our study, only imipramine and aminoguanidine prevented BDNF alterations induced by LPS. In line with our findings, it was demonstrated in animals submitted to social defeat, an ecologically and ethologically relevant animal model of psychosocial stress, that 2 h after stress, BDNF protein and mRNA expressions were increased in the medial PFC, while 28 days after stress this elevation was observed in the medial amygdala and ventral tegmental area (Fanous et al., 2010).
The exposure of microglial cells to LPS in vitro increased the production of BDNF, and this increase was accompanied by microglia proliferation (Gomes
et al., 2013). Meanwhile, patients with major depression
present decreased serum levels of BDNF (Brunoni
et al., 2008). Thus, this increase in BDNF levels may be
a possible limitation of some animal models of depression. To further understand the participation of BDNF in depression induced by LPS, the newly proposed model based on a chronic administration of LPS during 4 months must be performed (Kubera et al., 2013).
CONCLUSION
Our results showed using the model of depressive-like symptoms induced by LPS that: (i) sildenafil alone presented antidepressant-like effect; (ii) the effect of aminoguanidine was similar to the tricyclic antidepressant imipramine, giving further evidences for a possible iNOS inhibitor effect of imipramine
(Montezuma et al., 2012). Further studies are needed,
using the model of depression (Kubera et al., 2013) induced by the chronic administration of LPS, to better determine the influence of NO pathway in depression.
REFERENCES
Ainscough EW, Brodie AM (1995) Nitric oxide—some old and new perspectives. J Chem Educ 72.
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511.
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615. Archer J (1973) Tests for emotionality in rats and mice: a review.
Anim Behav 21:205–235.
Bakish D (2001) New standard of depression treatment: remission and full recovery. J Clin Psychiatry 26:5–9.
Bettio LE, Cunha MP, Budni J, Pazini FL, Oliveira A, Colla AR, Rodrigues AL (2012) Guanosine produces an antidepressant-like
effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res 234:137–148.
Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O (2001) Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 64:43–51.
Blaszczyk JW, Tajchert K, Lapo I, Sadowski B (2000) Acoustic startle and open-field behavior in mice bred for magnitude of swim analgesia. Physiol Behav 70:471–476.
Brunoni AR, Lopes M, Fregni F (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11:1169–1180.
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8:766–775.
Carvalho LA, Torre JP, Papadopoulos AS, Poon L, Juruena MF, Markopoulou K, Cleare AJ, Pariante CM (2013) Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord 148:136–140.
Custodio CS, Mello BS, Cordeiro RC, de Araujo FY, Chaves JH, Vasconcelos SM, Nobre Junior HV, de Sousa FC, Vale ML, Carvalho AF, Macedo DS (2013) Time course of the effects of lipopolysaccharide on prepulse inhibition and brain nitrite content in mice. Eur J Pharmacol 713:31–38.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. De La Garza Ii R (2005) Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev 29:761–770. DellaGioia N, Devine L, Pittman B, Hannestad J (2013) Bupropion
pre-treatment of endotoxin-induced depressive symptoms. Brain Behav Immun 31:197–204.
Dhir A, Kulkarni SK (2007) Involvement ofL-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of venlafaxine in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 31:921–925.
Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M (1993) A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med 15:353–363.
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77.
Fanous S, Hammer Jr RP, Nikulina EM (2010) Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Nat Rev Neurosci 167:598–607.
Ferreira Mello BS, Monte AS, McIntyre RS, Soczynska JK, Custodio CS, Cordeiro RC, Chaves JH, Mendes Vasconcelos SM, Nobre Junior HV, Florenco de Sousa FC, Hyphantis TN, Carvalho AF, Macedo DS (2013) Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res 47:1521–1529.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B (2009) Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 33:70–75.
Garate I, Garcia-Bueno B, Madrigal JL, Bravo L, Berrocoso E, Caso JR, Mico JA, Leza JC (2011) Origin and consequences of brain Toll-like receptor 4 pathway stimulation in an experimental model of depression. J Neuroinflamm 8:1742–2094.
Gardner A, Johansson A, Wibom R, Nennesmo I, von Dobeln U, Hagenfeldt L, Hallstrom T (2003) Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord 76:55–68. Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Goncalves
of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflamm 10:1742–2094.
Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M (2011) Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6:2.
Gururajan A, Taylor DA, Malone DT (2010) Effect of testing conditions on the propsychotic action of MK-801 on prepulse inhibition, social behaviour and locomotor activity. Physiol Behav 99:131–138.
Heydarpour P, Salehi-Sadaghiani M, Javadi-Paydar M, Rahimian R, Fakhfouri G, Khosravi M, Khoshkish S, Gharedaghi MH, Ghasemi M, Dehpour AR (2013) Estradiol reduces depressive-like behavior through inhibiting nitric oxide/cyclic GMP pathway in ovariectomized mice. Horm Behav 63:361–369.
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752.
Joca S, Guimara˜es F (2006) Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Eur Neuropsychopharmacol 185:298–305.
Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V (2004) Affective modulation of the startle response in depression: influence of the severity of depression, anhedonia, and anxiety. J Affect Disord 83:21–31.
Kim EJ, Kwon KJ, Park J-Y, Lee SH, Moon C-H, Baik EJ (2002) Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Behav Brain Res 941:1–10. Kubera M, Curzytek K, Duda W, Leskiewicz M, Basta-Kaim A,
Budziszewska B, Roman A, Zajicova A, Holan V, Szczesny E, Lason W, Maes M (2013) A new animal model of (chronic) depression induced by repeated and intermittent lipopolysaccharide administration for 4 months. Brain Behav Immun 31:96–104.
Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL (2011) Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav Modif 35:111–161. Leonard B, Maes M (2012) Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36:764–785.
Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V (1999) Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 40:171–176.
Ludka FK, Zomkowski AD, Cunha MP, Dal-Cim T, Zeni AL, Rodrigues AL, Tasca CI (2013) Acute atorvastatin treatment exerts antidepressant-like effect in mice via theL-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur Neuropsychopharmacol 23:400–412.
Maes M (1999) Major depression and activation of the inflammatory response system. Adv Exp Med Biol 461:25–46.
Maes M, Meltzer HY, Scharpe S, Bosmans E, Suy E, De Meester I, Calabrese J, Cosyns P (1993) Relationships between lower plasmaL-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res 49:151–165.
Maes M, Scharpe S, Meltzer HY, Okayli G, Bosmans E, D’Hondt P, Vanden Bossche BV, Cosyns P (1994) Increased neopterin and interferon-gamma secretion and lower availability ofL-tryptophan in major depression: further evidence for an immune response. Psychiatry Res 54:143–160.
Maes M, Vandoolaeghe E, Neels H, Demedts P, Wauters A, Meltzer HY, Altamura C, Desnyder R (1997) Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Prog Neuro-Psychopharmacol Biol Psychiatry 42:349–358.
Maes M, Kubera M, Leunis JC (2008) The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29:117–124.
Maes M, Galecki P, Chang YS, Berk M (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuro-Psychopharmacol Biol Psychiatry 35:676–692.
Mao QQ, Huang Z, Zhong XM, Xian YF, Ip SP (2014) Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav Brain Res 261:140–145.
McNally L, Bhagwagar Z, Hannestad J (2008) Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr 13:501–510.
Montezuma K, Biojone C, Lisboa SF, Cunha FQ, Guimaraes FS, Joca SR (2012) Inhibition of iNOS induces antidepressant-like effects in mice: pharmacological and genetic evidence. Neuropharmacology 62:485–491.
Moreno-Lopez B, Gonzalez-Forero D (2006) Nitric oxide and synaptic dynamics in the adult brain: physiopathological aspects. Rev Neurosci 17:309–357.
Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C (2006) Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 26:3813–3820.
Ohgi K, Kajiya H, Okamoto F, Nagaoka Y, Onitsuka T, Nagai A, Sakagami R, Okabe K (2013) A novel inhibitory mechanism of nitrogen-containing bisphosphonate on the activity of Cl( ) extrusion in osteoclasts. Naunyn Schmiedebergs Arch Pharmacol 386:589–598.
Perry W, Minassian A, Feifel D (2004) Prepulse inhibition in patients with non-psychotic major depressive disorder. J Affect Disord 81:179–184.
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nat Rev Neurosci 266:730–732.
Prast H, Philippu A (2001) Nitric oxide as modulator of neuronal function. Prog Neurobiol 64:51–68.
Resources IoLA (1996) Guide for the care and used of laboratory animals. National Academies Press.
Ribeiro DE, Maiolini VM, Soncini R, Antunes-Rodrigues J, Elias LL, Vilela FC, Giusti-Paiva A (2013) Inhibition of nitric oxide synthase accentuates endotoxin-induced sickness behavior in mice. Pharmacol Biochem Behav 103:535–540.
Rosa JM, Dafre AL, Rodrigues AL (2013) Antidepressant-like responses in the forced swimming test elicited by glutathione and redox modulation. Behav Brain Res 253:165–172.
Rush AJ, Wisniewski SR, Zisook S, Fava M, Sung SC, Haley CL, Chan HN, Gilmer WS, Warden D, Nierenberg AA, Balasubramani GK, Gaynes BN, Trivedi MH, Hollon SD (2012) Is prior course of illness relevant to acute or longer-term outcomes in depressed out-patients? A STAR⁄D report. Psychol Med 42:1131–1149.
Russell JA, Douglas AJ, Brunton PJ (2008) Reduced hypothalamo-pituitary-adrenal axis stress responses in late pregnancy: central opioid inhibition and noradrenergic mechanisms. Ann N Y Acad Sci 1148:428–438.
Rygula R, Abumaria N, Flu¨gge G, Fuchs E, Ru¨ther E, Havemann-Reinecke U (2005) Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162:127–134. Sayre LM, Perry G, Smith MA (2007) Oxidative stress and
neurotoxicity. Chem Res Toxicol 21:172–188.
Sßenesß M, Kazan N, Cosßkun O¨, Zengi O, Inan L, Yu¨cel D (2007) Oxidative and nitrosative stress in acute ischaemic stroke. Ann Clin Biochem 44:43–47.
activity of two atypical antidepressant drugs, mianserin and tianeptine, in the forced swim test in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 38:121–126.
Swerdlow NR, Geyer MA (1993) Prepulse inhibition of acoustic startle in rats after lesions of the pedunculopontine tegmental nucleus. Behav Neurosci 107:104–117.
Taylor MJ, Rudkin L, Bullemor-Day P, Lubin J, Chukwujekwu C, Hawton K (2013) Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev 31.
Teasdale JD (1983) Negative thinking in depression: cause, effect, or reciprocal relationship? Adv Behav Res Ther 5:3–25.
Tejeda H, Chefer V, Zapata A, Shippenberg T (2010) The effects of kappa-opioid receptor ligands on prepulse inhibition and CRF-induced prepulse inhibition deficits in the rat. Eur Neuropsychopharmacol 210:231–240.
Tsigos C, Chrousos GP (2002) Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53:865–871.
Vasconcelos Rios ER, Moura Rocha NF, Rodrigues Carvalho AM, Freire Vasconcelos L, Leite Dias M, de Carvalho Lima CN, Soares
Lopes K, Cavalcante Melo FH, de Franca Fonteles MM (2013) Involvement of the nitric oxide/cyclic guanylate monophosphate pathway in the pilocarpine-induced seizure model in mice. Eur J Pharmacol 91:131–134.
Wang X, Robinson PJ (1997) Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. J Neurochem 68:443–456.
Zarate Jr CA, Singh JB, Carlson PJ, et al (2006) A randomized trial of an n-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864.
Zhang GF, Wang N, Shi JY, Xu SX, Li XM, Ji MH, Zuo ZY, Zhou ZQ, Yang JJ (2013) Inhibition of theL-arginine-nitric oxide pathway mediates the antidepressant effects of ketamine in rats in the forced swimming test. Pharmacol Biochem Behav 24:8–12. Zhou Q-G, Hu Y, Hua Y, Hu M, Luo C-X, Han X, Zhu X-J, Wang B, Xu
J-S, Zhu D-Y (2007) Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem 103:1843–1854.
Zlatkovic J, Filipovic D (2013) Chronic social isolation induces NF-kappaB activation and upregulation of iNOS protein expression in rat prefrontal cortex. Neurochem Int 63:172–179.
![Fig. 1. (A) Immobility time (s) in the forced swimming test (FST) and (B) number of crossings in the open field test (OFT) of animals treated with [ L -arginine (L-arg), sildenafil (sil), L -NAME, aminoguani-dine (amino)] or the antidepressant imipramine (I](https://thumb-eu.123doks.com/thumbv2/123dok_br/15361425.564949/4.892.467.813.627.1008/immobility-swimming-crossings-arginine-sildenafil-aminoguani-antidepressant-imipramine.webp)
![Fig. 2. Sucrose preference measured as% of sucrose consumption of animals treated with [ L -arginine (L-arg), sildenafil (sil), L -NAME, aminoguanidine (amino)] or the antidepressant imipramine (IMI) alone or prior to LPS administration](https://thumb-eu.123doks.com/thumbv2/123dok_br/15361425.564949/5.892.72.428.608.1011/sucrose-preference-consumption-sildenafil-aminoguanidine-antidepressant-imipramine-administration.webp)