Contents lists available atScienceDirect
Toxicology in Vitro
journal homepage:www.elsevier.com/locate/toxinvit
Anticancer potential of benzothiazolic derivative (
E
)-2-((2-(benzo[
d
]thiazol-2-yl)hydrazono)methyl)-4-nitrophenol against melanoma cells
Zanair Soares Vasconcelos
a, Ana Carolina Lima Ralph
a, Danielle Queiroz Calcagno
b,
Gleyce dos Santos Barbosa
a, Tatiana do Nascimento Pedrosa
a, Lucas Pio Antony
a,
Marília de Arruda Cardoso Smith
c, Eliza de Lucas Chazin
d, Thatyana Rocha Alves Vasconcelos
d,
Raquel Carvalho Montenegro
e, Marne Carvalho de Vasconcellos
a,⁎aFaculty of Pharmaceutical Sciences, Federal University of Amazon, Amazon, Brazil bCenter on Oncology Research, Federal University of Pará, Pará, Brazil
cDepartment of Morphology and Genetics, Paulista School of Medicine, Federal University of São Paulo, São Paulo, Brazil dChemistry Institute, Fluminense Federal University, Rio de Janeiro, Brazil
eDrug Research and Development Center, Faculty of Medicine, Federal University of Ceará, Ceará, Brazil
A R T I C L E I N F O
Keywords:
AFN01
B-RAF
Cutaneous melanoma Cytotoxicity
A B S T R A C T
Malignant melanoma is an important type of cancer worldwide due to its aggressiveness and poor survival rate. Significant efforts to understand the biology of melanoma and approaches to treat the advanced disease are focused on targeted gene inhibitors. Frequently mutated genes, such as NRAS, B-RAF and TP53, significantly exceed the frequency of mutations of other genes, emphasizing their importance for future targeted therapies. Considering the antitumor activity of benzothiazolic derivatives, this study aimed to demonstrate the action of benzothiazolic (E)-2-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-4-nitrophenol (AFN01) against three estab-lished human melanoma cell lines that recapitulate the molecular landscape of the disease in terms of its genetic alterations and mutations, such as theTP53,NRASandB-RAFgenes. The results presented here indicate that AFN01, as a significant cytostatic and cytotoxic drug due to its induction of DNA fragmentation, causes single and double DNA strand breaks, consequently inhibiting cell proliferation, migration and invasion by promoting apoptosis. Our data suggest that AFN01 might be considered as a future therapeutic option for managing melanoma.
1. Introduction
Cutaneous melanoma is the most aggressive and treatment-resistant form of skin cancer (Ratnikov et al., 2017). Mutations in theB-RAF proto-oncogene, located on chromosome 7q, are elevated in human cancers (Wajapeyee et al., 2008) and are particularly frequent in human melanomas (40–70%) (Serratì et al., 2016; Sullivan and Flaherty, 2013). These alterations are more frequent than mutations in other genes, such asNRAS,TP16andTP53(Shinozaki et al., 2004), which culminate in the development of specific inhibitors for advanced mel-anoma treatment (Maldonado et al., 2003). Currently, systemic che-motherapy is unsatisfactory, since a small minority of patients respond
in an acceptable manner, thus indicating the importance of exploring other ways to prevent and treat this type of cancer (Brohem et al., 2011; Ferreres et al., 2009;Liu et al., 2015;Zhang and Zhang, 2016).
Benzothiazole compounds have been shown to be an important therapy alternative, since studies have shown that they have several pharmacological activities of interest (Facchinetti et al., 2012), such as antimalarial (Burger and Sawhney, 1968), antitubercular (Palmer et al., 1971), analgesic (Siddiqui et al., 2004), antidiabetic (Pujar et al., 2005), anticonvulsants (Siddiqui et al., 2007), antiviral (Akhtar et al., 2008), antihelmintic (Suresh et al., 2011), antimicrobial (Singh et al., 2014) and antitumor (Lindgren et al., 2014).
The substance (E)-2-((2-(benzo[d
]thiazol-2-yl)hydrazono)methyl)-https://doi.org/10.1016/j.tiv.2018.03.001
Received 27 September 2017; Received in revised form 22 February 2018; Accepted 1 March 2018
⁎Corresponding author at: Faculdade de Ciências Farmacêuticas, Universidade Federal do Amazonas, Av. General Rodrigo Octávio 6200, Coroado I, CEP: 69077-000 Manaus-Amazonas, Brazil.
E-mail address:marne@ufam.edu.br(M.C. de Vasconcellos).
Abbreviations:AFN01, (E)-2-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-4-nitrophenol; AO/EB, acridine orange/ethidium bromide; DCB, dacarbazine; DI, damage index; DMEM, Dulbecco's Modified Eagle's Medium; DMSO, dimethylsulfoxide; DNA, deoxyribonucleic acid; DX, doxorubicin; FBS, fetal bovine serum; HCT-8, ileocecal colorectal adenocarcinoma cell line; HL-60, promyelocytic leukemia cell line; IC50, Median Inhibition Concentration (concentration that reduces the effect by 50%); LMP, low melting point agarose; MDA-MB-435, melanoma cell line; NaCl, sodium chloride; NaOH, sodium hydroxide; NMP, normal melting point agarose
Available online 22 March 2018
0887-2333/ © 2018 Published by Elsevier Ltd.
4-nitrophenol (AFN01), in addition to being from the benzothiazole class, has a hydroxyl group in position R1 and a nitro group in position R4 (Francisco Nogueira et al., 2010). Nitro compounds have aroused interest in antitumor therapy acting as radiosensitizers, considering the bioreduction capacity of the nitro group that releases intermediates in the redox process (Francisco Nogueira et al., 2010;Paulai et al., 2009 andKrause et al., 2005). This ability to produce reactive oxygen species is partly responsible for the antitumor activity of benzothiazoles (Mohamed et al., 2017). However, the mechanism of this action is still unknown.
The present study evaluated the activity of the benzothiazole deri-vative AFN01 on cell viability, clonogenic activity, the mechanism of cell death, DNA damage, and the migration and invasion abilities of melanoma cell lines with different genetic landscapes and established its role as an important inducer of apoptosis. This ability is critical for drugs aimed at regulating cancer cell proliferation via processes that are either dependent or independent of the melanoma main mutated genes, includingTP53,NRASandB-RAF.
2. Materials and methods
2.1. AFN01 synthesis
The (E)-2-((2-(benzo[d ]thiazol-2-yl)hydrazono)methyl)-4-ni-trophenol (AFN01) (Fig. 1a) was prepared by reacting 2-hy-drazinobenzothiazole (1 equiv) with 2-hydroxy-5-nitrobenzaldehyde (1 equiv) in ethanol (10 mL). After stirring for 1 h at room temperature, the solid product obtained was collected and purified with cold alcohol and ethyl ether. Compound AFN01 was identified and characterized by spectral data, as reported previously (Francisco Nogueira et al., 2010).
2.2. Cell lines and culture conditions
The human malignant melanoma cells Sk-Mel-19, 28 and 103 were kindly provided by Silvya Stuchi Maria-Engler (School of Pharmaceutical Sciences, University of Sao Paulo). The cells were grown in 75-cm2 culture flasks as adherent monolayer cultures in Dulbecco's Modified Eagle's Medium (DMEM, Life Technologies, Foster City, CA) supplemented with 10% fetal bovine serum (FBS, Vitrocell), 100μg/mL streptomycin and 100 U/mL penicillin and were incubated at 37 °C with a 5% CO2atmosphere. The genetic differences in each cell line are presented inTable 1.
2.3. Viability of human melanoma cell lines
Cell viability was assessed by Alamar Blue (Ahmed et al., 1994) and trypan blue exclusion assays (Freshney, 2011).
2.3.1. Alamar blue assay
The Alamar Blue assay was performed as previously described (Ahmed et al., 1994). In a 96-well plate, Sk-Mel-19, 28 and 103 were cultured at concentrations of 104cells per well. After 24 h of incuba-tion, the cells were treated with different concentrations of AFN01 (0–10μM). Doxorubicin (DX) and dacarbazine (DCB) were used as a positive control (0–10μM). The negative control received the same amount of dimethylsulfoxide (DMSO) from the highest concentration of test solutions (< 0.1%). After the treatment time (24/48/72 h), 10μL of the Alamar Blue solution (0.4% stock solution, diluted 1:20 in DMEM without FBS) was added to each well of the plate and was incubated at 37 °C for 3 h. Fluorescence was measured using a microplate reader (DTX800 Beckman and Coulter). Viability was expressed as the IC50and was obtained using nonlinear regressions based on three replicates per concentration level.
2.3.2. Trypan blue exclusion assay
The trypan blue assay was performed as previously described
(Freshney, 2011). The cells were seeded at a density of 1.5 × 104cells/ well in 24-well plates. After 24 h, the cells were treated with different concentrations of AFN01 (1.0, 2.5 and 5.0μM). Doxorubicin (DX) and dacarbazine (DCB) were used as positive controls (5μM), and DMSO (< 0.1%) was the negative control. After the treatment period (24/48/ 72 h), the cells were harvested and centrifuged at 2400gfor 3 min. The supernatant was discarded, and the cell pellets suspended in 90μL of DMEM and 10μL of Trypan Blue. After 1 min, the cells were quantified as viable and non-viable in a Neubauer chamber. The results were compared by a two-way analysis of variance with Tukey's post-test.
2.4. Differential staining by hematoxylin/eosin (HE)
To assess the morphological changes, HE was performed and the Sk-Mel cell lines 19, 28 and 103 were seeded into 24-well plates (3.0 × 104cells/mL) and were treated with 1.0, 2.5 and 5.0
μM of AFN01. Doxorubicin (DX) and dacarbazine (DCB) were used as the positive controls (5μM), and DMSO (< 0.1%) was the negative control. After 24, 48 and 72 h of treatment, the cells were harvested and sus-pended in 100μL of PBS and were then cytocentrifuged on slides where they werefixed and stained with a LaborClin®rapid panoptic dye kit. The slides were analyzed under an optical microscope for the evalua-tion of morphological changes comparing the treated and untreated cells. The registry of the cellular alterations was performed using the LAS EZ Software for the acquisition of images from Leica®.
2.5. Differential staining by acridine orange and ethidium bromide (AO/ EB)
To determine the type of cell death caused by AFN01, differential staining using AO/EB was performed as previously described (Kasibhatla et al., 2006). The cells were grown in 12-well plates and were treated with three concentrations of AFN01 (1.0, 2.5 and 5.0μM) for 24, 48 and 72 h. Doxorubicin (DX) and dacarbazine (DCB) were used as positive controls (5μM), and DMSO (< 0.1%) was the negative control. After the treatment time, the cell aliquots were suspended in 20μL of phosphate buffered saline (PBS) and were stained with 1μL of an aqueous solution of AO/EB (100μg/mL). The cell suspension was immediately examined using fluorescence microscopy (Leica Micro-systems) at a 40× magnification. Three hundred cells were counted per sample and were classified as viable (uniform bright green nuclei with an organized structure), apoptotic (orange to red nuclei with condensed to fragmented chromatin and green cytoplasm) or necrotic (uniformly orange to red nuclei with an organized structure and red cytoplasm). The results were analyzed by a two-way analysis of variance followed by Tukey's post-test.
2.6. Clonogenic assay
To measure the ability of a single cell to grow into a colony, a clonogenic assay was performed. The viable cells (400 cells/well) were plated, and after 24 h of seeding, the treatment was performed with the following concentrations of AFN01: 1.0; 2.5 and 5.0μM. Doxorubicin (DX) and dacarbazine (DCB) were used as positive controls (5μM), and DMSO (< 0.1%) was the negative control. Colony growth was observed for 7–10 days, maintaining ideal growing conditions (Plumb, 1999). After this period, the cells werefixed with methanol and stained with a 0.1% crystal violet solution. The number of colonies was quantified, and the results of the triplicates were compared by a one-way variance analysis with Tukey's post-test. The cytotoxic effect was observed by the reduction in the number of colonies, and the cytostatic effect was as-sessed by the reduction in the number of cells in each colony.
2.7. Comet assay
Fig. 1.a. AFN01 molecular configuration; b., c. and d. Viability of the human melanoma cell lines Sk-Mel-19, 28 and 103 after treatment with AFN01 for 24, 48 and 72 h. The cell viabilities of the Mel-19, Mel-28 and Sk-Mel-103 cells were assessed by trypan blue staining. The vehicle, 0.1% dimethylsulfoxide (DMSO), was used as the negative control, and 5μM Dacarbazine (DCB) and 5μM
alkaline and neutral comet assays were performed. The alkaline assay was conducted according toSingh et al. (1988), with small modifi ca-tions in the number of cells and lysis solution. Sk-Mel cells (5 × 105 cells/well) were treated with 1.0, 2.5 and 5.0μM of AFN01 for 3 h. Doxorubicin (DX) and dacarbazine (DCB) were used as positive controls (5μM), and DMSO (< 0.1%) was the negative control. The slides were prepared by mixing 50μL of the treated cell suspension in 100μL of 1% low melting point (LMP) agarose and layering this mixture on micro-scope slides that were previously covered with normal melting point (NMP) agarose. Then, the microscope slides were placed in a lysis so-lution (2.5 M sodium chloride, 100 mM ethylene diamine tetra acetic acid, 10 mM Tris, 10% dimethylsulfoxide and 1% Triton X-100) at 4 °C overnight to remove cellular proteins. After this period, the slides were immersed in electrophoresis buffer [300 mM NaOH, 1 mM EDTA pH 13] for 20 min to unwind the DNA, and then, they were subjected to electrophoresis (20 V; 300 mA) for 20 min at 4 °C in the dark (Singh et al., 1988). After electrophoresis, the slides were neutralized with 0.4 M Tris-HCl pH 7.5 and were dried overnight at room temperature. The neutralized and dehydrated slides were stained with ethidium bromide (2 ng/mL) before scoring. One hundred cells were scored for each sample (50 per slide), and the DNA damage was classified using a previously described visual scoring method (Collins et al., 2001).
The neutral comet assay was performed as previously described (Wojewódzka et al., 2002). The procedures for the cell treatments, slide preparations, lysis, neutralization and staining were identical to those used to perform the alkaline assay. Nevertheless, the electrophoresis buffer was 300 mM sodium acetate and 100 mM Tris-HCl pH 8.5, and the electrophoresis was run at 25 V and 300 mA for 20 min in the dark (Collins et al., 2001;Singh et al., 1988;Wojewódzka et al., 2002). The data analysis was based on the mean and standard deviation of three independent experiments. We used the analysis of variance (One-way
ANOVA) followed by Tukey's test, with a significance level of 5% (p< 0.05) in the GraphPad Prism version 6.0 program to verify the occurrences of significant differences among the different groups.
2.8. Wound healing assay
A wound healing assay was performed according toLiang et al., 2007. Sk-Mel cell lines were plated into 6-well plates (106cells/mL) and were incubated up to complete confluence. After this period, the “scratch” was made along the diameter of the well. The cells were treated with three concentrations of AFN01 (1.0, 2.5 and 5.0μM). Doxorubicin (DX) and dacarbazine (DCB) were used as positive controls (5μM), and DMSO (< 0.1%) was the negative control. The cells were photographed after 48 h, and the measurement of the space scratch were done using the Leica LAS EZ imaging software. The results were analyzed by a one-way variance analysis followed by Tukey's post-test.
2.9. Cell invasion assay
To provide an in vitro system to study cell invasion, inserts with 8μM pores from the manufacturer Bencton and Dickinson (BD) were packed in 24 well plates. Within the inserts 20μL of a laminin-rich multiprotein compound (Matrigel BD) diluted 1: 6 in serum-free DMEM (approximately 2–3 mg/mL protein) were added, and these inserts were incubated for 30 min in a CO2 oven at 37 °C. Then, 5 × 104Sk-Mel cells/200μL serum-free DMEM was plated into the inserts. After cul-turing, AFN01 (1.0, 2.5 and 5.0μM) or 0.1% DMSO (negative control) was added into the inserts. In the wells, outside the inserts, 300μL of DMEM with 10% FBS was added. After 24 h, the lower chamber culture medium was discarded, and the remaining cells were fixed in 3.7% formaldehyde in PBS for 10 min at room temperature. Then, the bottom chamber was washed three times with distilled water, and the cells were stained with a 0.5% toluidine blue solution in 2% Na2CO3for 20 min at room temperature. The excess of dye was removed by three washes with distilled water. The Matrigel®and the cells that remained on top of the insert were removed with the aid of a cotton swab. Cells that traversed the pores and attached to the lower surface of the insert and the bottom of the lower chamber were visualized and counted under an inverted microscope (Lochter et al., 1997).
3. Results
3.1. Evaluation of AFN01 cytotoxicity in human melanoma cells
The cytotoxic activity of AFN01 was assessed by the Alamar Blue and trypan blue assays. The in vitro cytotoxicity assays of AFN01 against the Sk-Mel cell lines revealed that AFN01 exhibited more ef-fective cytotoxic activities than the reference drugs (DCB IC50= 100μM and DX IC50≥10μM) for melanoma on Sk-Mel-19 (IC50= 9.91μM) and Sk-Mel-28 (IC50= 6.02μM) cell lines in 24 h. The same was not observed in Sk-Mel-103 (IC50≥10μM) (Table 2).
AFN01 exerted a concentration and time-dependent cytotoxic effect Table 1
Some frequently mutated genes of the human metastatic melanoma lines: Sk-Mel-19, Sk-Mel-28 and Sk-Mel-103.
Adapted fromBrohem et al., 2012.
Cell type/line p53 p14 B-RAF NRAS
(RNAm) (V600E) (éxon 3)
Sk-Mel-19 wt + Mutant wt
Sk-Mel-28 R273HR NE Mutant wt
Sk-Mel-103 wtR + wt Q61R
+: expresses. wt: wild-type. mRNA: messenger RNA. NE: Nt expressed.
p53 (R273H): hot spot mutation of the protein, resulting in the substitution of arginine for histidine at codon 273. This mutation leads to gain of function, loss of tumor suppressor functions and acquisition of new oncogenic activities. B-RAF (V600E): mutation caused by the substitution of thymine for adenine in codon 1799 (c.1799T), resulting in the substitution of valine for glutamic acid (p.V600E).
NRAS (Q61R): amino acid substitution at position 61 of glutamine to arginine.
Table 2
Cytotoxic evaluation using Alamar Blue assay. 50% inhibitory concentration (IC50) of AFN01, dacabarzine (reference drug) and doxorubicin (positive control) inμM.
Cell line AFN01 IC50(μM)
Doxorubicin IC50(μM)
Dacarbazine IC50(μM)
24 h 48 h 72 h 24 h 48 h 72 h 24 h 48 h 72 h
Sk-Mel-19 9.91 (6.92–14.20)
5.07 (4.23–6.75)
4.87 (4.12–5.76)
> 10 4.61 (3.61–5.87)
2.93 (2.57–3.35)
> 100 > 100 > 100
Sk-Mel-28 6.02 (4.96–7.29)
5.47 (4.53–6.61)
4.72 (4.09–5.44)
> 10 > 10 4.52 (3.73–5.47)
> 100 > 100 > 100
Sk-Mel-103 > 10 > 10 > 10 > 10 1.87 (1.36–2.57)
1.12 (0.99–1.26)
according to the results of the trypan blue assay (Fig. 1b–d). AFN01 caused significant cell death in all the cell lines tested, and at a con-centration of 5μM, AFN01 promoted a more effective decrease in via-bility than those of the positive controls, except at 24 h and 72 h for the Sk-Mel 103 cell (Fig. d). Interestingly, the Sk-Mel-103 cells were the less sensitive to AFN01. In contrast, the Sk-Mel-19 cells were the most sensitive and were followed by the Sk-Mel-28 cells at all the times tested (Fig. 1b).
3.2. Morphological alterations in the Sk-Mel cell lines
The HE differential staining assay was performed to verify the cell morphologies after the AFN01 treatment. As shown in Fig. 2a, the AFN01 caused important morphological changes that were absent in the negative control cells. All the concentrations of AFN01 tested (1.0, 2.5 and 5.0μM) presented cell alterations, such as cell lysis, in-tracellular vacuoles, bleb formation, chromatin loosening, nuclear condensation, perinuclear halos, nucleoli, karyotype, and karyolysis. These changes suggest cell death by apoptosis and reflect the cytotoxic capacity of AFN01 in all three Sk-Mel cell lines tested.
3.3. Apoptotic cell death in Sk-Mel cell lines
To investigate whether the AFN01-induced cytotoxicity is attribu-table to apoptosis or necrosis, AO/BE differential staining assay was performed (Fig. 2). In the negative control group, > 90% of cells had a normal viable morphology. Cells treated with AFN01, on the other hand, presented an increase in the number of apoptotic cells in a con-centration-dependent manner, with significant differences observed at all concentrations tested. The number of necrotic cells was not sig-nificantly different across any of the treatments, except in the Sk-Mel-28 cells (Fig. 2b), and this also occurred in a dose-dependent manner. In addition to the fact that all the cells treated with doxorubicin and da-carbazine presented significant necrotic cell death, AFN01 at 5.0μM caused more than double the percentage of apoptotic cells compared to doxorubicin in the cell lines Sk-Mel-19 and 28.
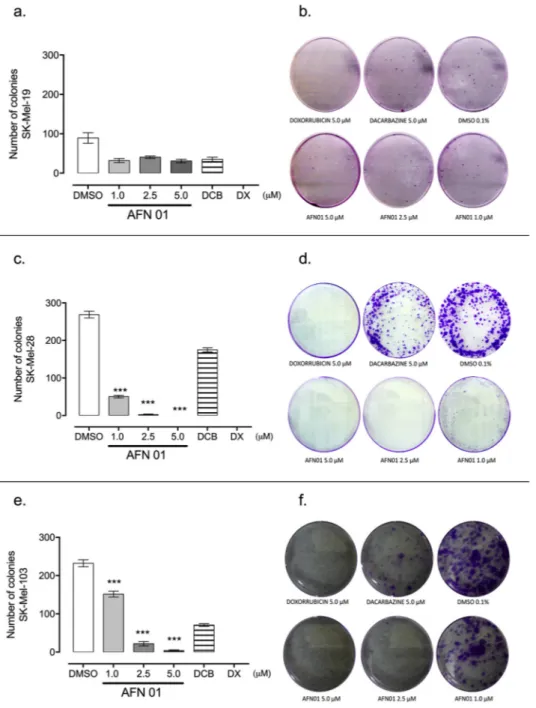
3.4. Clonogenic activity in the Sk-Mel cell lines
To evaluate the ability of a single cell to form a colony after AFN01 treatment, the clonogenic assay was performed (Fig. 3). AFN01 in-hibited colony formation in the human melanoma cells tested. The Sk-Mel-28 and Sk-Mel-103 cell lines showed a marked reduction in clo-nogenic formation after AFN01 treatment (Fig. 3c, d, e and f), which Fig. 2.a. Morphological alterations in the Sk-Mel cells treated with AFN01 for 24 h, as assessed by hematoxylin/eosin staining. Thefigures are representative of three independent experiments. The changes found at ×100 included (a) cell lysis, (b) intracellular vacuoles, (c) bleb formation, (d) chromatin loosening, (e) nuclear condensation, (f) perinuclear halos, (g) nucleoli, (h) karyotype, and (i) karyolysis. The red arrow represents the alterations. b. Time-dependent induction of apoptosis in human melanoma cells determined by acridine orange and ethidium bromide staining andfluorescence microscopy. The Sk-Mel-19, Sk-Mel-28 and Sk-Mel-103 cells after 24 h of treatment with AFN01. The vehicle, 0.1% dimethylsulfoxide (DMSO), was used as the negative control, and 5μM Dacarbazine (DCB) and 5μM
occurred in a dose-dependent manner, reaching up to zero colonies at the highest concentration (5μM) of AFN01. Although AFN01 did not demonstrate significant clonogenic inhibition in the Sk-Mel-19 cell line, nevertheless, the Sk-Mel-19 cells presented a low clonogenic capacity, even in the negative control (Fig. 3a, b). Doxorubicin completely in-hibited colony formation in contrast to Dacarbazine (5μM). AFN01 at 5.0μM inhibited melanoma cells colony formation in the Sk-Mel 28 and 103 cell lines with a similar intensity as doxorubicin at 5.0μM.
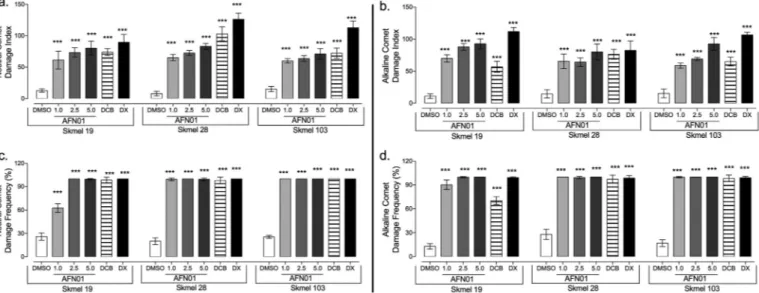
3.5. DNA fragmentation induction in the Sk-Mel cell lines
To determine and compare the induction of DNA fragmentation by AFN01 in the human melanoma cell lines Sk-Mel-19, 28 and 103, we determined the damage index and damage frequency using the alkaline and neutral comet assays (Fig. 4). First, we analyzed the AFN01
capacity to induce single-strand breaks, double-strand breaks and al-kali-labile sites using an alkaline comet assay (Fig. 4b, d). Then, we determined the particular induction of double-strand breaks using a neutral comet assay (Fig. 4a, c). After a 3 h incubation period, AFN01 induced statistically significant single and double DNA strand breaks that led to a significantly higher damage index in a dose-dependent manner compared to the negative control in all the Sk-Mel cell lines (Fig. 4a–d). In the alkaline comet assay, AFN01 at 5.0μM induced a damage index (DI) that was approximately three times greater than the index of the negative control.
No statistically significant difference was observed between the al-kaline and neutral damage index across the Sk-Mel cell lines. Therefore, the AFN01 at 5.0μM induced a similar DNA fragmentation capacity as doxorubicin in all the cell lines studied. Based on these results, AFN01 shows genotoxic activity in human melanoma cells.
Fig. 3.Clonogenic assay showing the effects after seven (07) days of treatment with AFN01 on the strains Sk-Mel-19 (a., b.), Sk-Mel-28 (c., d.) and Sk-Mel-103 (e., f.). AFN01 was tested at 1, 2.5 and 5μM. The vehicle, 0.1% dimethylsulfoxide (DMSO), was used as the negative control, and 5μM Dacarbazine (DCB) and 5μM
Fig. 4.Comet test showing the effect of AFN01 on the DNA strand. Comet Assay at a neutral pH showing the damage index (a.) and the frequencies of damage (c.). Comet Assay at an alkaline pH showing the damage index (b.) and the frequencies of damage (d.). The vehicle, 0.1% dimethylsulfoxide (DMSO), was used as the negative control, and 5μM Dacarbazine (DCB) and 5μM doxorubicin (DX) were used as positive controls. The data are presented as the mean values ± SE from three
independent experiments performed in independent biological replicates in triplicate. ***p< 0.01, compared to the negative control; ANOVA + Tukey's post-test.
Fig. 5.AFN01 inhibits cell migration. The extension of the space between the edges of the“scratch”in the monolayers of the Sk-Mel-19, 28 and 103 strains at 48 h (a.). Migration inhibition in the Sk-Mel-19 (b.), 28 (c.) and 103 (d.) cell lines at 24, 48 and 72 h after treatment with AFN01. The measurement of the scratch was done using the LAS EZ Image Acquisition Software–20X. Thefigures are representative of three independent experiments. The vehicle, 0.1% dimethylsulfoxide (DMSO), was used as the negative control, and 5μM Dacarbazine (DCB) and 5μM doxorubicin (DX) were used as positive controls. The data are presented as the
3.6. Evaluation of cell migration and invasion in Sk-Mel cell lines
To evaluate Sk-Mel cell migration after treatment with AFN01, the in vitro scratch assay was performed (Fig. 5a–d). In the Sk-Mel-19 and Sk-Mel-28 cells, AFN01 caused important migration inhibition mainly after 48 h at 2.5μM and 5μM. In the Sk-Mel-103 cells, the cytotoxic effect of AFN01 made the migration assay non-viable. In the negative control, the scratch closed completely after 48 h in all the cell lines tested.
The evaluation of the invasion capacity of the human melanoma cell lines was performed using the Boyden chamber invasion assay. AFN01 inhibited cell invasion in all the cell lines studied (Fig. 6a–f). The Sk-Mel-19 cell presented the highest invasion inhibition by AFN01 in all the concentrations tested (Fig. 6a, b). In addition, the invasion inhibi-tion was also statistically significant in the Sk-Mel 28 and 103 cells relative to the negative control in a dose-dependent manner (Fig. 6c–f).
4. Discussion
The accumulated evidence demonstrates benzothiazole-based com-pounds are promising anticancer agents for different types of cancer. Benzothiazole compounds has moderate to excellent activity against human cancer cell lines by inducing significant amount of ROS pro-duction inside cells causing cell cycle arrest and/or apoptosis ( El-Damasy et al., 2016, 2015;Hegde et al., 2017;Hutchinson et al., 2003; Lawrence et al., 2009; Ma et al., 2014; Pietrancosta et al., 2006; Pushpavalli et al., 2011;Xia et al., 2014).
the induction of single and double DNA strand brakes. This apoptosis induction led to cell function alterations, inhibiting colony formation, migration and invasion in the melanoma cell lines. This is thefirst time AFN01 activity is related to a genetic background.
Initially, we treated a panel of melanoma cell lines to determine the effects of AFN01 on cell viability. AFN01 induced cell death in all the melanoma cell lines that contained different point mutations in com-ponents of the RAS-RAF-MEK-ERK and p53 pathway, such as B-RAF, NRAS and TP53, that play important roles in melanocytic cancers. AFN01 revealed IC50 concentrations below 10μM, showing an im-portant feature of this compound. This result corroborates with ben-zothiazole-based compounds with effective cytotoxicity at 4μM. These compounds displayed decrease in the phosphorylated forms of AKT, p38 MAPK and ERK1/2 which play a vital role in the cell proliferation (Pushpavalli et al., 2011).
The viability assays suggested that the Sk-Mel-28 (TP53R273H, B-RAFV600E,NRASwt) cell line was more sensitive to AFN01. However, the Sk-Mel-103 (TP53wt,B-RAFwtandNRASQ61R) cells show lower cytotoxic sensitivity to AFN01 than the Sk-Mel-19 cells (TP53wt, B-RAFV600E, NRASwt) after 24 h of treatment. Thus, these results suggest a higher susceptibility to AFN01 in the cells with the TP53mutation. Mutant TP53is one of the best“druggable”targets since normal cells mostly do not have mutations in theTP53gene.
The SK-Mel-28 cells have a point mutation in codon 145 of theTP53 gene, causing the overexpression of a mutant protein (Haapajãrvi et al., 1999;Haapajãrvi et al., 1999), which alters DNA contact and has been shown to positively regulate the genes crucial for tumor transformation and the maintenance of tumorigenicity in melanoma (Brohem et al., 2012). Several approaches targeting the p53 pathway for anti-cancer therapy are being investigated for pharmacological intervention in-cluding: rescue and reactivate the function of mutant or misfolded p53, induce depletion of mutant p53, inhibit downstream pathways of on-cogenic mutant p53 and/or inhibit the interaction of MDM2 and p53 to block wt-p53 degradation (Essmann and Schulze-Osthoff, 2012;Lane et al., 2010;Parrales and Iwakuma, 2015). The development of small-molecule MDM2 inhibitors to restore dysfunctional p53 activities re-presents an important approach for cancer treatment (Zhang et al., 2014).Thus, the cytotoxicity presented by AFN01 suggest future in-vestigations about specific mechanism of action of this benzothiazole in p53 pathway.
The AFN01 conformation of [(1: X = 2-OH; Y = 5-NO2) DMSO], with the OH group close to N2, accommodates intramolecular hydrogen bonding between the hydroxyl group, OeH, and the nitrogen atom, N2 (Nogueira et al., 2011). It creates a possible interaction with hydro-phobic pocket of MDM2 in a manner similar to p53 binding, it would thereby interfere with the interaction between MDM2 and p53 leading to cell cycle arrest and/or apoptosis (Devine and Dai, 2013). NSC 333003 is an example of benzothiazole-hydrazone compound that could potentially affect binding to MDM2-p53 (> 50% inhibition at 100μM and dose response studies gave an IC50 of approximately 20μM in the ELISA assay) (Lawrence et al., 2009). Continued research efforts is required to expand chemodiversity and identify potent and selective MDM2 antagonists with desirable in vitro and in vivo anticancer properties (Zhang et al., 2014).
The cytotoxicity of AFN01 corroborated the important cell mor-phology alterations, including cytoplasmic vacuolization, irregular cy-toplasmic borders (serrated), perinuclear halos, loosening of chromatin, no nuclear condensation, the formation of nucleoli and the formation of blebs, which suggested apoptotic induction. This was also observed by the AO/EB differential staining, where AFN01 increased the percentage of melanoma apoptotic cells. The Sk-Mel-28 cell line characteristics lead to a decrease in apoptotic effectiveness and a consequent increase in the oncological activity of this cell line (Brohem et al., 2012). The increased amount of apoptotic cells in the samples treated with AFN01 in the Sk-Mel human melanoma cells indicated that AFN01 was a more efficient cytotoxic drug than the positive controls. Novel benzothiazole
derivatives bearing an ortho-hydroxy N-carbamoylhydrazone had moderate to excellent activity againstfive human cancer cell lines (NCI-H226, SK-N-SH, MKN45, HT29, and MDA-MB-231) their strong pro-caspase-3 activation potency (15 g, EC50 = 1.42μM; 16b, EC50 = 0.25μM) suggested that they might inhibit the growth of tumor cells by activating procaspase-3 kinase. The analysis of SARs indicated that introduction of a lipophilic group (e.g., a benzyloxy group or heteroaryloxy group) at the 4-position of the 2-hydroxy phenyl ring could enhance in vitro cytotoxic activity, and the introduction of sub-stituents containing nitrogen that are positively charged at physiolo-gical pH, was beneficial to antitumor activity (Ma et al., 2014).
Several chemical compounds have been reported to damage the DNA, either directly or indirectly slowing down the cancer cell pro-gression by causing a cell cycle arrest. Direct or indirect reactive oxygen species formation causes DNA damage leading to cell cycle arrest and subsequent cell death (Hegde et al., 2017). AFN01 caused global DNA damage, producing single and double DNA breaks and breakage of the labile alkaline sites on the DNA strands of the melanoma cells. In the Sk-Mel-19 and Sk-Mel-103 cell lines, AFN01 demonstrated a production of alkaline labile breaks that exceeds that of the alkylating agent da-carbazine, indicating a higher DNA fragmentation by AFN01. This sensitivity may be caused by, for example, the wild-typeTP53 gene action and the p14 protein normal expression, which promote the triggering of the apoptotic processes in cells subject to irreversible DNA damage (Nowsheen and Yang, 2012).
In the Sk-Mel-28 cell, the triggering of apoptosis through irrever-sible DNA damage was deficient. Important genes for cell homeostasis, such asTP53andTP14, may accelerate cell repair (Mirzayans et al., 2012). Accelerated cell repair conditions through specific genes may have contributed to cell repair and, consequently, the lower damage rate presented by the Sk-Mel-28 cells (Fig. 4a, c). This result added to the higher sensitivity of the Sk-Mel-28 cells, and the cytotoxic action of AFN01 demonstrated that the pathway of death triggered by its me-chanism of action may not be directly linked to DNA strand breaks but rather to damaging the repair processes that make cell signaling un-feasible.
In order to understand the capacity of AFN01 to induce functional alterations in human melanoma cells, we performed clonogenic, wound healing and invasion assays, considering that the ability to form new colonies, migrate and invade are important mechanisms that provide cancer cells with the ability metastasize in vivo (Seyfried and Huysentruyt, 2013). Importantly, AFN01 inhibited colony formation, migration and invasion in all human melanoma cells tested.
AFN01 significantly inhibited both colony formation and invasion in the Sk-Mel-28 (TP53R273H, B-RAFV600E, NRASwt) and Sk-Mel-103 cell lines (TP53wt, B-RAFwt and NRASQ61R). In the clonogenic assay, the inhibition was more significant in the Sk-Mel 28 and 103 cell lines than in the Sk-Mel-19 cell line (TP53wt, B-RAFV600E,NRASwt). However, in the invasion assay, the Sk-Mel 19 cells presented the highest invasion inhibition by AFN01, even when using the lower AFN01 concentration (1.0μM). It has already been shown that the accumulation of mutated p53 protein in the cancer cell facilitates metastasis (Muller et al., 2009) and provides new pro-oncogenic functions (Lang et al., 2004; Olive et al., 2004). Furthermore, melanoma patients withRASmutations are more resistant to combination therapies than theB-RAFmutants, which means that the negative impacts of AFN01 activity on the Sk-Mel 19 and 103 cells has the potential to improve chemotherapy (Held et al., 2013).
In the wound healing assay, AFN01 was more effective for in-hibiting cell migration in Sk-Mel-19 (TP53wt,B-RAFV600E,NRASwt) and Sk-Mel-28 (TP53R273H,B-RAFV600E,NRASwt) (Fig. 5d) and less effective in Sk-Mel-103 (TP53wt,B-RAFwtandNRASQ61R) (Fig. 5a, d). These re-sults corroborate the AFN01 cytotoxicity, mainly for the Sk-Mel 103 results, where AFN01 inhibited cell proliferation and, consequently, cell migration.
as theBRAFV600E/Kinhibitor PLX4032, play an important role in tar-geted therapy. PLX4032 inhibits ERK1/2 in the highly sensitive BRAFV600E/Kbut also induces the proliferation of resistant B-RAFWTcell population via RAF1activation, regardless of the status of the muta-tions inNRASorPTEN, which confer an advantage toB-RAFWTprimary and metastatic tumor cells in vivo (Halaban et al., 2010). The capacity of AFN01 to act in both genetic landscapes promotes a therapy ad-vantage, even though AFN01 seems to act mainly throughTP53R273H.
In conclusion, our results show that AFN01, a benzothiazole deri-vative, decreased the viability of melanoma cell lines despite the pre-sence of the main mutations in theNRAS,B-RAFandTP53genes. Under a morphological evaluation, AFN01 induced cytotoxicity via apoptosis, inducing DNA fragmentation and leading to decreased proliferation, colony formation, migration and invasion. In summary, the present study contributes to a better understanding of the anticancer effects of AFN01 as a way to promote its future use in cancer therapy. Furthermore, our research raises new questions that still need to be answered regarding the drug mechanisms and proteins involved in this process.
Transparency document
The Transparency document associated with this article can be found, in online version.
Acknowledgements
The authors gratefully thank the National Council for Scientific and Technological Development (CNPq) (485433/2012-5), the Coordination of Improvement of Higher Level Personnel (CAPES), the Amazonas State Research Support Foundation (FAPEAM) (N.020/ 2013), the Federal University of Amazonas (UFAM), the Rio de Janeiro State Research Support Foundation (FAPERJ) and the Post-Graduate Program in Pharmaceutical Sciences for providing thefinancial support and the necessary infrastructural facilities for carrying out this work.
References
Ahmed, S.A., Gogal, R.M.J., Walsh, J.E., 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170, 211–224. Akhtar, T., Hameed, S., Al-Masoudi, N.A., Loddo, R., La Colla, P., 2008. In vitro antitumor
and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione deriva-tives. Acta Pharma. 58, 135–149.http://dx.doi.org/10.2478/v10007-008-0007-2. Brohem, C.A., Cardeal, L.B.D.S., Tiago, M., Soengas, M.S., Barros, S.B.D.M., Maria-Engler,
S.S., 2011. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res. 24, 35–50.http://dx.doi.org/10.1111/j.1755-148X.2010.00786.x. Brohem, C.A., Massaro, R.R., Tiago, M., Marinho, C.E., Jasiulionis, M.G., de Almeida,
R.L., Rivelli, D.P., Albuquerque, R.C., de Oliveira, T.F., de Melo Loureiro, A.P., Okada, S., Soengas, M.S., de Moraes Barros, S.B., Maria-Engler, S.S., 2012. Proteasome inhibition and ROS generation by 4-nerolidylcatechol induces melanoma cell death. Pigment Cell Melanoma Res. 25, 354–369.http://dx.doi.org/10.1111/j. 1755-148X.2012.00992.x.
Burger, A., Sawhney, S.N., 1968. Antimalarials. III. Benzothiazole amino alcohols. J. Med. Chem. 11, 270–273.http://dx.doi.org/10.1021/jm00308a018.
Collins, A.R., Dusinská, M., Horská, A., 2001. Detection of alkylation damage in human lymphocyte DNA with the comet assay. Acta Biochim. Pol. 48, 611–614. Devine, T., Dai, M.-S., 2013. Targeting the ubiquitin-mediated proteasome degradation of
p53 for cancer therapy. Curr. Pharm. Des. 19, 3248–3262.
El-Damasy, A.K., Seo, S.H., Cho, N.-C.C., Kang, S.B., Pae, A.N., Kim, K.-S.S., Keum, G., 2015. Design, synthesis, in-vitro antiproliferative activity and kinase profile of new picolinamide based 2-amido and ureido quinoline derivatives. Eur. J. Med. Chem. 101, 754–768.http://dx.doi.org/10.1016/j.ejmech.2015.07.025.
El-Damasy, A.K., Lee, J.-H., Seo, S.H., Cho, N.-C., Pae, A.N., Keum, G., 2016. Design and synthesis of new potent anticancer benzothiazole amides and ureas featuring pyr-idylamide moiety and possessing dual B-RafV600E and C-Raf kinase inhibitory ac-tivities. Eur. J. Med. Chem. 115, 201–216.http://dx.doi.org/10.1016/j.ejmech.2016. 02.039.
Essmann, F., Schulze-Osthoff, K., 2012. Translational approaches targeting the p53 pathway for anti-cancer therapy. Br. J. Pharmacol. 165, 328–344.http://dx.doi.org/ 10.1111/j.1476-5381.2011.01570.x.
Facchinetti, V., Reis, R. da R., Gomes, C.R.B., Vasconcelos, T.R.A., 2012. Chemistry and biological activities of 1,3-benzothiazoles. Mini-Rev. Org. Chem. 9, 44–53.http://dx. doi.org/10.2174/157019312799079929.
Ferreres, J.R., Moreno, A., Marcoval, J., 2009. Multiple primary melanoma. In: Actas Dermo-Sifiliográficas, English Ed. 100. pp. 414–419.http://dx.doi.org/10.1016/ S1578-2190(09)70087-3.
Francisco Nogueira, A., Carvalho Azevedo, E., Francisco Ferreira, V., Jersia Araujo, A., Alves dos Santos, E., Pessoa, C., Veras Costa-Lotufo, L., Carvalho Montenegro, R., Odorico de Moraes, M., Rocha Alves Vasconcelos, T., 2010. Synthesis and antitumor evaluation of (E)-2-benzothiazole hydrazones. Lett. Drug Des. Discovery 7, 551–555. http://dx.doi.org/10.2174/157018010792062740.
Freshney, R.I., 2011. Culture of animal cells: a manual of basic technique and specialized applications, Sixth Edition.http://dx.doi.org/10.1002/9780470649367.
Haapajãrvi, Tarja, Pitkãnen, Kimmo, Laiho, M., 1999. Human melanoma cell line UV responses show independency of p53 function. Cell Growth Differ. 10, 163–171. Halaban, R., Zhang, W., Bacchiocchi, A., Cheng, E., Parisi, F., Ariyan, S., Krauthammer,
M., McCusker, J.P., Kluger, Y., Sznol, M., 2010. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and pro-liferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 23, 190–200.http:// dx.doi.org/10.1111/j.1755-148X.2010.00685.x.
Hegde, M., Vartak, S.V., Kavitha, C.V., Ananda, H., Prasanna, D.S., Gopalakrishnan, V., Choudhary, B., Rangappa, K.S., Raghavan, S.C., 2017. A benzothiazole derivative (5 g) induces DNA damage and potent G2/M arrest in cancer cells. Sci. Rep. 7, 2533. http://dx.doi.org/10.1038/s41598-017-02489-3.
Held, M.A., Langdon, C.G., Platt, J.T., Graham-Steed, T., Liu, Z., Chakraborty, A., Bacchiocchi, A., Koo, A., Haskins, J.W., Bosenberg, M.W., Stern, D.F., 2013. Genotype-selective combination therapies for melanoma identified by high-throughput drug screening. Cancer Discov. 3, 52–67.http://dx.doi.org/10.1158/ 2159-8290.CD-12-0408.
Hutchinson, I., Bradshaw, T.D., Matthews, C.S., Stevens, M.F., Westwell, A.D., 2003. Antitumour benzothiazoles. Part 20: 3′-cyano and 3′-alkynyl-substituted 2-(4′ -ami-nophenyl)benzothiazoles as new potent and selective analogues. Bioorg. Med. Chem. Lett. 13, 471–474.http://dx.doi.org/10.1016/S0960-894X(02)00930-7.
Kasibhatla, S., Amarante-Mendes, G.P., Finucane, D., Brunner, T., Bossy-Wetzel, E., Green, D.R., 2006. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb. Protoc. 2006, 4493.http://dx.doi.org/10.1101/pdb. prot4493.
Krause, W., Jordan, A., Scholz, R., Jimenez, J.L.M., 2005. Iodinated nitroimidazoles as radiosensitizers. Anticancer Res. 25, 2145–2151.
Lane, D.P., Cheok, C.F., Lain, S., 2010. p53-based cancer therapy. Cold Spring Harb. Perspect. Biol. 2, a001222.http://dx.doi.org/10.1101/cshperspect.a001222. Lang, G.A., Iwakuma, T., Suh, Y.-A., Liu, G., Rao, V.A., Parant, J.M., Valentin-Vega, Y.A.,
Terzian, T., Caldwell, L.C., Strong, L.C., El-Naggar, A.K., Lozano, G., 2004. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872.http://dx.doi.org/10.1016/j.cell.2004.11.006.
Lawrence, H.R., Li, Z., Yip, M.L.R., Sung, S.-S., Lawrence, N.J., McLaughlin, M.L., McManus, G.J., Zaworotko, M.J., Sebti, S.M., Chen, J., Guida, W.C., 2009. Identification of a disruptor of the MDM2-p53 protein-protein interaction facilitated by high-throughput in silico docking. Bioorg. Med. Chem. Lett. 19, 3756–3759. http://dx.doi.org/10.1016/j.bmcl.2009.04.124.
Liang, C.-C., Park, A.Y., Guan, J.-L., 2007. In vitro scratch assay: a convenient and in-expensive method for analysis of cell migration in vitro. Nat. Protoc. 2, 329–333. http://dx.doi.org/10.1038/nprot.2007.30.
Lindgren, E.B., De Brito, M.A., Vasconcelos, T.R.A.A., de Moraes, M.O., Montenegro, R.C., Yoneda, J.D., Leal, K.Z., 2014. Synthesis and anticancer activity of (E)-2-benzothia-zole hydrazones. Eur. J. Med. Chem. 86, 12–16.http://dx.doi.org/10.1016/j.ejmech. 2014.08.039.
Liu, Z., Semenza, G.L., Zhang, H., 2015. Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B 16, 32–43.http://dx.doi.org/10.1631/jzus. B1400221.
Lochter, A., Srebrow, A., Sympson, C.J., Terracio, N., Werb, Z., Bissell, M.J., 1997. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accom-panies acquisition of stromelysin-1-dependent invasive properties. J. Biol. Chem. 272, 5007–5015.http://dx.doi.org/10.1074/jbc.272.8.5007.
Ma, J., Chen, D., Lu, K., Wang, L., Han, X., Zhao, Y., Gong, P., 2014. Design, synthesis, and structure–activity relationships of novel benzothiazole derivatives bearing the ortho-hydroxyN-carbamoylhydrazone moiety as potent antitumor agents. Eur. J. Med. Chem. 86, 257–269.http://dx.doi.org/10.1016/J.EJMECH.2014.08.058. Maldonado, J.L., Fridlyand, J., Patel, H., Jain, A.N., Busam, K., Kageshita, T., Ono, T.,
Albertson, D.G., Pinkel, D., Bastian, B.C., 2003. Determinants of BRAF mutations in primary melanomas. J. Natl. Cancer Inst. 95, 1878–1890.http://dx.doi.org/10.1093/ jnci/djg123.
Mirzayans, R., Andrais, B., Hansen, G., Murray, D., 2012. Role of p16(INK4A) in re-plicative senescence and DNA damage-induced premature senescence in p53-defi -cient human cells. Biochem. Res. Int. 2012, 951574.http://dx.doi.org/10.1155/ 2012/951574.
Mohamed, L.W., Taher, A.T., Rady, G.S., Ali, M.M., Mahmoud, A.E., 2017. Synthesis and cytotoxic activity of certain benzothiazole derivatives against human MCF-7 cancer cell line. Chem. Biol. Drug Des. 89, 566–576.http://dx.doi.org/10.1111/cbdd. 12879.
Muller, P.A.J., Caswell, P.T., Doyle, B., Iwanicki, M.P., Tan, E.H., Karim, S., Lukashchuk, N., Gillespie, D.A., Ludwig, R.L., Gosselin, P., Cromer, A., Brugge, J.S., Sansom, O.J., Norman, J.C., Vousden, K.H., 2009. Mutant p53 drives invasion by promoting in-tegrin recycling. Cell 139, 1327–1341.http://dx.doi.org/10.1016/j.cell.2009.11. 026.
Nogueira, A., Vasconcelos, T.R.A., Wardell, J.L., Wardell, S.M.S.V., 2011. Crystal struc-tures of hydrazones, 2-(1,3-benzothiazolyl)-NHeN]CHeAr, prepared from
Nowsheen, S., Yang, E.S., 2012. The Intersection Between DNA Damage Response and Cell Death Pathways. 34. NIH Public Access, pp. 243–254.
Olive, K.P., Tuveson, D.A., Ruhe, Z.C., Yin, B., Willis, N.A., Bronson, R.T., Crowley, D., Jacks, T., 2004. Mutant p53 gain of function in two mouse models of Li-fraumeni syndrome. Cell 119, 847–860.http://dx.doi.org/10.1016/j.cell.2004.11.004. Palmer, P.J., Trigg, R.B., Warrington, J.V., 1971. Benzothiazolines as antituberculous
agents. J. Med. Chem. 14, 248–251.
Parrales, A., Iwakuma, T., 2015. Targeting oncogenic mutant p53 for cancer therapy. Front. Oncol. 5, 288.http://dx.doi.org/10.3389/fonc.2015.00288.
Paulai, F.R., Serrano, S.H.P., Tavares, L.C., 2009. Aspectos mecanísticos da bioatividade e toxicidade de nitrocompostos. Quim Nova 32, 1013–1020.http://dx.doi.org/10. 1590/S0100-40422009000400032.
Pietrancosta, N., Moumen, A., Dono, R., Lingor, P., Planchamp, V., Lamballe, F., Bähr, M., Kraus, J.-L., Maina, F., 2006. Imino-tetrahydro-benzothiazole derivatives as p53 in-hibitors: discovery of a highly potent in vivo inhibitor and its action mechanism. J. Med. Chem. 49, 3645–3652.http://dx.doi.org/10.1021/jm060318n.
Plumb, J.A., 1999. Cell sensitivity assays: clonogenic assay. In: Cytotoxic Drug Resistance Mechanisms. Humana Press, New Jersey, pp. 17–24. http://dx.doi.org/10.1385/1-59259-687-8:17.
Pujar, V.D., Reddy, V.V.K., Rasal, V.P., Koti, B.C., 2005. Synthesis and antidiabetic ac-tivity of 2-amino[5(4-sulphonylbenzylidine)-2,4-thiazolidinedione]-7-chloro-6-fl uor-obenzothiazole 44, 2404–2408.
Pushpavalli, S., Ramaiah, M.J., Srinivas, C., Mukhopadhya, D., Aditya, J.L., Kumbhare, R.M., Bhadra, U., Bhadra, M.P., 2011. Effect of benzothiazole based conjugates in causing apoptosis by regulating p53, PTEN and MAP kinase proteins affecting miR-195a and miR-101-1. Cancer Cell Int. 11, 36. http://dx.doi.org/10.1186/1475-2867-11-36.
Ratnikov, B.I., Scott, D.A., Osterman, A.L., Smith, J.W., Ronai, Z.A., 2017. Metabolic rewiring in melanoma. Oncogene 36, 147–157.http://dx.doi.org/10.1038/onc.2016. 198.
Serratì, S., De Summa, S., Pilato, B., Petriella, D., Lacalamita, R., Tommasi, S., Pinto, R., 2016. Next-generation sequencing: advances and applications in cancer diagnosis. Onco. Targets. Ther. 9, 7355–7365.http://dx.doi.org/10.2147/OTT.S99807. Seyfried, T.N., Huysentruyt, L.C., 2013. On the origin of cancer metastasis. Crit. Rev.
Oncog. 18, 43–73.
Shinozaki, M., Fujimoto, A., Morton, D.L., Hoon, D.S.B., 2004. Incidence of BRAF onco-gene mutation and clinical relevance for primary cutaneous melanomas. Clin. Cancer
Res. 10, 1753–1757.
Siddiqui, N., Alam, M., Siddiqui, A.A., 2004. Synthesis and analgesic activity of some 2-[4-(alkyl thioureido) phenyl sulphonamido]-6-substituted benzothiazoles. Asian J. Chem. 16, 1005.
Siddiqui, N., Pandeya, S.N., Khan, S.A., Stables, J., Rana, A., Alam, M., Arshad, M.F., Bhat, M.A., 2007. Synthesis and anticonvulsant activity of sulfonamide derivatives-hydrophobic domain. Bioorg. Med. Chem. Lett. 17, 255–259.https://doi.org/10. 1016/j.bmcl.2006.09.053.
Singh, N.P., McCoy, M.T., Tice, R.R., Schneider, E.L., 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184–191.http://dx.doi.org/10.1016/0014-4827(88)90265-0.
Singh, M., Singh, S.K., Gangwar, M., Nath, G., Singh, S.K., 2014. Design, synthesis and mode of action of some benzothiazole derivatives bearing an amide moiety as anti-bacterial agents. RSC Adv. 4, 19013.http://dx.doi.org/10.1039/c4ra02649g. Sullivan, R.J., Flaherty, K.T., 2013. Resistance to BRAF-targeted therapy in melanoma.
Eur. J. Cancer 49, 1297–1304.http://dx.doi.org/10.1016/j.ejca.2012.11.019. Suresh, C.H., Rao, J.V., Jayaveera, K.N., Subudhi, H.K., 2011. Synthesis and anthelminitc
activity of 3 (2-hydrazino benzothiazoles)-substituted indole-2-one. IJPR 2, 257–261. Wajapeyee, N., Serra, R.W., Zhu, X., Mahalingam, M., Green, M.R., 2008. Oncogenic
BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132, 363–374.http://dx.doi.org/10.1016/j.cell.2007.12.032. Wojewódzka, M., Buraczewska, I., Kruszewski, M., 2002. A modified neutral comet assay:
elimination of lysis at high temperature and validation of the assay with anti-single-stranded DNA antibody. Mutat. Res. Toxicol. Environ. Mutagen. 518, 9–20.http://dx. doi.org/10.1016/S1383-5718(02)00070-0.
Xia, Y., Lei, Q., Zhu, Y., Ye, T., Wang, N., Li, G., Shi, X., Liu, Y., Shao, B., Yin, T., Zhao, L., Wu, W., Song, X., Xiong, Y., Wei, Y., Yu, L., 2014. SKLB316, a novel small-molecule inhibitor of cell-cycle progression, induces G2/M phase arrest and apoptosis in vitro and inhibits tumor growth in vivo. Cancer Lett. 355, 297–309.http://dx.doi.org/10. 1016/j.canlet.2014.09.042.
Zhang, X.-Y., Zhang, P.-Y., 2016. Genetics and epigenetics of melanoma (Review). Oncol. Lett. 3041–3044.http://dx.doi.org/10.3892/ol.2016.5093.