Vol.:(0123456789)
1 3
Medical Oncology (2018) 35:36
https://doi.org/10.1007/s12032-018-1092-9
SHORT COMMUNICATION
Immunosuppressive monocytes (CD14
+/HLA‑DR
low/−) increase
in childhood precursor B‑cell acute lymphoblastic leukemia
after induction chemotherapy
D. S. Lima1,2 · R. P. G. Lemes2 · D. M. Matos3
Received: 18 January 2018 / Accepted: 31 January 2018 / Published online: 10 February 2018 © Springer Science+Business Media, LLC, part of Springer Nature 2018
Abstract
In tumor microenvironment, immunosuppression is a common event and results from the inhibition of activated immune cells and generation of cells with immunosuppressive capacity, as some subtypes of monocytes. The aim of this study was to evaluate the presence of immunosuppressive CD14+/HLA-DRlow/− monocytes in pediatric patients with the diagnosis of B-cell acute lymphoblastic leukemia (B-ALL) and, moreover, verify whether the chemotherapeutic treatment has any effect on these cells. Peripheral blood (PB) and bone marrow (BM) samples were collected from 15 untreated pediatric patients. The presence of CD14+/HLA-DRlow/− monocytes was evaluated at diagnosis and in the end of induction chemotherapy by flow cytometry. CD14+/HLA-DRlow/− monocytes increase was observed in 60% (9/15) of the patients at the end of the induction therapy. We were able to detect an increase in CD14+/HLA-DRlow/− monocytes values in BM and PB samples of pediatric patients with B-ALL. This increase was observed in the end of induction chemotherapy, which leads us to believe that these changes probably could have been induced by the inflammatory process engendered by the cytotoxic treatment or by drugs used in the chemotherapy treatment. This finding may be useful to guide new therapeutic approaches contemplating immunomodulatory drugs that act in the depletion of immunosuppressive monocytes.
Keywords Acute lymphoblastic leukemia · Immunosuppressive monocytes · Flow cytometry
Introduction
Acute lymphoblastic leukemia (ALL) is an abnormal prolif-eration and accumulation of clonal progenitor cells compro-mised with the differentiation of lymphoid cells in the bone marrow [1]. The immunosuppressive state observed in tumor microenvironment plays an important role in cancer initia-tion, progression and therapeutic failure [2]. CD14+ /HLA-DRlow/− monocytes mediate immunosuppression through a range of mechanisms as, for example, release of interleu-kin-10 [3] and induction of T-cell regulatory populations [4].
The presence of immunosuppressive CD14+ /HLA-DRlow/− monocytes was previously described in malignant solid tumors as melanoma and prostate cancer [3, 5], and also in hematologic cancers as multiple myeloma [5], non-Hodgkin lymphoma [6] and chronic lymphocytic leukemia [7].
Here, we evaluated the presence of CD14+ /HLA-DRlow/− monocytes in pediatric patients with the diagnosis of precursor B-ALL and, besides, we sought for any effect of chemotherapeutic treatment with regard to this population of monocytes.
Patients and methods
Patients
From August 2015 to May 2017, peripheral blood (PB) and bone marrow (BM) samples were collected from 33 untreated pediatric patients who fulfill the World Health Organization diagnostic criteria for de novo precursor * D. M. Matos
dmazza@alumni.usp.br
1 Oncohematology Section, Albert Sabin Children Hospital,
Ceará, Brazil
2
Department of Clinical and Toxicological Analysis, Federal University of Ceará, UFC, Ceará, Brazil
3 Flow Cytometry Section, Clementino Fraga Laboratory,
B-ALL. Out of 33 initial patients recruited, 18 died or there was insufficient sample from bone marrow aspirate at the end of induction chemotherapy protocol. Thus, 15 patients were further stratified as high or low risk of relapse, based on the following criteria: age, initial white blood cells count, CNS involvement, cytogenetic and molecular profile [8].
The induction chemotherapy protocol was performed according to the recommendations of the Brazilian Coop-erative Group for Childhood ALL Treatment (GBTLI-2009) [8]. Briefly, the induction chemotherapy for low risk B-ALL patients includes: dexamethasone (6 mg/m2), prednisone (60 mg/m2), vincristine (1.5 mg/m2), daunorubicin (25 mg/ m2), L-asparaginase (5000 U/m2) and, in presence of
clini-cal indication, MADIT (intratheclini-cal methotrexate, cytarabine and dexamethasone) (10–12 mg/m2, 20–24 mg/m2, 2 mg/m2, respectively). Alternatively, for high-risk B-ALL patients, the induction chemotherapy includes: prednisone (60 mg/ m2), vincristine (1.5 mg/m2), daunorubicin (40 mg/m2 per day), L-asparaginase (10,000 U/m2), cyclophosphamide
(500 mg/m2) and, in presence of clinical indication, MADIT (intrathecal methotrexate, cytarabine and dexamethasone) (10–15 mg/m2, 20–30 mg/m2, 2 mg/m2, respectively).
Informed consent was obtained from the parents of all the patients included, and, moreover, the study was approved by the Albert Sabin Hospital local ethical committee.
Cytogenetics and molecular analysis
The G-band cytogenetic analysis was performed according to the recommendations elsewhere [9]. The molecular analy-sis of fusion genes expression was performed by reverse transcriptase-polymerase chain reaction (RT-PCR) technique using SuperScript™ III One-Step RT-PCR System with Platinum™ Taq High Fidelity DNA Polymerase kit (Invit-rogen™), following the recommendations described by the manufacturer. All patients were evaluated for the presence of AF4/MLL, BCR/ABL1 p190 and p210, E2A/PBX1 and ETV6/RUNX1 fusion genes.
CD14+/HLA‑DRlow/− monocytes detection
The presence of CD14+/HLA-DRlow/− monocytes was evalu-ated—at diagnosis (D0, day zero) and in the end of induction chemotherapy protocol (D35, day thirty five of treatment)— in PB and BM samples, by flow cytometry, using the fol-lowing anti-human monoclonal antibodies conjugated with fluorochromes: CD14 (PE), CD19 (FITC), CD45 (PerCP) and HLA-DR (FITC). All monoclonal antibodies were pur-chased from BD Biosciences® (San Jose, CA, USA).
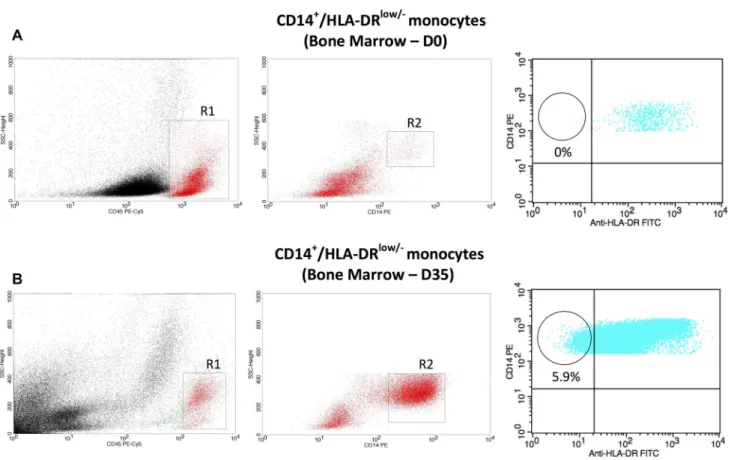
The acquisition was performed using FACSCalibur™ flow cytometer, and the analysis was done using the Cell-Quest Becton–Dickinson® software. Initially, a region (R1) was defined in CD45 versus internal complexity (SSC)
dot-plot to determine the cells of lymphomonocytic lineage. A second region (R2) was defined in CD14 versus inter-nal complexity (SSC) dot-plot to specifically delineate the monocytic lineage. The next step was to evaluate the dot-plot HLA-DR versus CD14 with the purpose of quantifying the CD14+/HLA-DRlow/− monocytes (Fig. 1). For all analysis, 100,000 events were acquired. Based on minimal residual disease studies performed in ALL patients and the accurate quantitation of rare events strategy, we used a minimum of 40 clustered events characterized by both CD14 positivity and HLA-DR with low/negative expression in order to con-sider a sample containing an immunosuppressive monocyte population [10].
Results
Sixty percent (9/15) of patients were stratified as high risk and 40% (6/15) as low risk. Forty-six percent (7/15) of patients showed aberrant karyotype that included numeri-cal and/or structural alterations, and 53% (8/15) of patients presented positivity for one of the three fusion genes E2A/ PBX1, ETV6/RUNX1 or AF4/MLL. None of the patients was positive for BCR/ABL1 fusion gene.
Table 1 shows patients’ clinical and biological data, as well as the percentages and absolute values of CD14+ /HLA-DRlow/− monocytes in peripheral blood and bone marrow, at diagnosis (D0) and after the initial chemotherapy protocol (D35).
There was an expansion of CD14+/HLA-DRlow/− mono-cytes observed in 60% (9/15) patients when the values of CD14+/HLA-DRlow/− cells at the final of induction chem-otherapy (D35) were compared to those of the diagnosis (D0). Out of this total, approximately 56% (5/9) presented the expansion in BM and PB concomitantly, while 44% (4/9) presented the expansion in BM or PB only. Three patients demonstrated CD14+/HLA-DRlow/− monocytes at diagnosis that were confined to PB samples.
Discussion
CD14+/HLA-DRlow/− monocytes have important modula-tory action on the interaction between immune system and malignant cells [11].
Medical Oncology (2018) 35:36
1 3
Page 3 of 5 36included in the chemotherapy protocol, or by both. Thus, in a previous study, Mougiakakos et al. [12] showed that the frequency of circulating CD14+/HLA-DRlow/− monocytes was significantly increased after allogeneic hematopoietic stem cell transplantation, especially in patients with more severe acute graft-versus-host disease.
In animals models, the treatment with cyclophosphamide has induced the expansion of monocytic immunosuppressive myeloid cells (CD11b+Ly6ChiCCR2hi), which are not equal, but still equivalent to human immunosuppressive CD14+/ HLA-DRlow/− monocytes, in mice with advanced B-cell lym-phoma or lung metastasis of colon cancer [13]. Whether cyclophosphamide or possibly other cytotoxic drugs used in treatment protocols of pediatric patients with B-ALL could be responsible for the expansion of CD14+ /HLA-DRlow/− monocytes observed in our patients and, moreover,
whether this expansion would contribute to tumor progres-sion and/or therapeutic failure is uncertain, and, thus, more studies with longer time of follow-up are required.
In our study, three patients (number 8, 12 and 13) (Table 1) demonstrated CD14+/HLA-DRlow/− monocytes at diagnosis that were confined to PB samples. We have no
definitive explanation for this phenomenon. However, we can speculate that the non-detection of the CD14+ /HLA-DRlow/− monocytes in BM samples of these three patients can probably be justified by the fact that, notwithstanding the CD14+/HLA-DRlow/− monocytes accumulate at the tumor site (medullar microenvironment), these cells are rapidly recruited by tumor cells and enter in a process of differ-entiation which transform them in a specific type of mac-rophages, namely tumor-associated macrophages (TAMs), as suggested by recent data [14].
Intriguingly, we verified uncommon CD14+ /HLA-DRlow/− monocytes kinetics in two patients. Namely, the
values of CD14+/HLA-DRlow/− monocytes observed at diag-nosis in patients 8 and 13 declined after initial induction therapy. Curiously, a similar finding was detected in patients with the diagnosis of chronic lymphocytic leukemia, where a reduced frequency of CD14+/HLA-DRlow/− monocytes was seen after the chemotherapeutic treatment [7].
CD14+/HLA-DRlow/− cells are a subtype of human mye-loid-derived suppressor cells (MDSCs) which are mainly described as the monocytic subpopulation [15]. Recently, Liu et al. [16], analyzing another subtype of human MDSC
Fig. 1 Flow cytometric analysis in BM of patient number 1 at D0 and D35, respectively. a The first dot-plot (CD45 vs. SSC) delimited the lymphomonocytic region (gate R1). The second dot-plot (CD14 vs. SSC) delimited the monocytic region (gate R2). The third dot-plot (HLA-DR vs. CD14) delimited the population of interest, namely
CD14+/HLA-DRlow/− monocytes (circle). b The same strategy of
analysis was performed, but now in the end of induction therapy (D35). Notice the presence of 5.9% of CD14+/HLA-DRlow/−
M
edical Onc
ology (2018) 35:36
Page 4 of 5
Table 1 Patients data and CD14+/HLA-DRlow/− monocytes population in PB and BM
D0 = Day zero of treatment
D35 = Day 35 of treatment (final of induction therapy) AM Absence of metaphases
a Death
Patient Id Sex Age (years) Risk stratification Cytogenetics analysis RT-PCR Peripheral blood CD14+/HLA-DRlow/− monocytes Bone marrow CD14+/HLA-DRlow/− monocytes Follow-up (months) D0 (%)/(mm3) D35 (%)/(mm3) D0 (%)/(mm3) D35 (%)/(mm3)
1 M 9y High risk 47,XY,t(1;19)(q23;q13),+8[16] E2A/PBX1 positive 0%/(0 cells/mm3) 13%/(2.860 cells/mm3) 0%/(0 cells/mm3) 5.9%/(944 cells/mm3) 22
2 M 9y High risk 46,XY,der(19)t(1;19)(q23;p13) [18]
E2A/PBX1 positive 0%/(0 cells/mm3) 2.3%/(62 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 21
3 F 15y High risk 46,XX,t(4;11)(q21;q23)[12] AF4/MLL positive 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 20
4 M 3y Low risk 55,XY,+X,+4,+6,+8,+9,+10, +13,+21+22[9]/46,XY[3]
Negative 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 20
5 M 1y High risk 80,XXY,+X,+1,+2,+3,t(4;11) (q21;q23),+8,+12,+17,−18, +19,+21,+22,+mar1,+mar2 [9]/46,XY[3]
AF4/MLL positive 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 2a
6 M 17y High risk AM Negative 0%/(0 cells/mm3) 2.1%/(73 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 15
7 F 7y High risk 46,XX[20] Negative 0%/(0 cells/mm3) 23.6%/(5.475 cells/ mm3)
0%/(0 cells/mm3) 16%/(848 cells/mm3) 14
8 M 6y High risk 46,XY,inv(9)(p12q13)c[20] E2A/PBX1 positive 7.9%/(126 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 12
9 F 1y High risk AM ETV6/RUNX1 positive 0%/(0 cells/mm3) 4.7%/(583 cells/mm3) 0%/(0 cells/mm3) 2.2%/(143 cells/mm3) 10
10 M 3y High risk 54,XY,+X,+6,+14,+17,+18,+ 21,+21,+mar[10]/46,XY[10]
Negative 0%/(0 cells/mm3) 60.6%/(16.241 cells/ mm3)
0%/(0 cells/mm3) 25.4%/(6.959 cells/ mm3)
10
11 F 3y Low risk 46,XX[14] ETV6/RUNX1 positive 0%/(0 cells/mm3) 7.5%/(1.260 cells/mm3) 0%/(0 cells/mm3) 5.7%/(296 cells/mm3) 10
12 F 2y Low risk 46,XX[16] Negative 9.9%/(118 cells/mm3) 6.9%/(227 cells/mm3) 0%/(0 cells/mm3) 9.9%/(1.603 cells/mm3) 7
13 F 1y Low risk 46,XX[18] Negative 6.9%/(110 cells/mm3) 0.9%/(48 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 6
14 F 5y Low risk 59,XX,+1,+4,+5,+6,+9,+11, +12,+14,+16,+17,+18,+21, +22[12]/46,XX[3]
Negative 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 0%/(0 cells/mm3) 6
Medical Oncology (2018) 35:36
1 3
Page 5 of 5 36(granulocytic-MDSC), showed that these cells were signifi-cantly elevated in both the peripheral blood and bone mar-row of patients with B-ALL. Taken together, Liu et al. and our findings suggest that immunosuppressive cells, being of granulocytic or monocytic lineage, are present in patients with B-ALL and, hence, we can speculate that the increased numbers of MDSCs might contribute to the immunosuppres-sive microenvironment inside the bone marrow.
In summary, we demonstrated the presence and expansion of CD14+/HLA-DRlow/− monocytes, in the bone marrow and peripheral blood, of patients with the diagnosis of B-cell acute lymphoblastic leukemia. As far as we know, this is the first study investigating the presence of CD14+ /HLA-DRlow/− monocytes in patients with the diagnosis of B-cell acute lymphoblastic leukemia and, moreover, the influence of chemotherapy treatment on these cells, provided that we studied the CD14+/HLA-DRlow/− population in two different moments of treatment, namely at the diagnosis and at the end of induction chemotherapy regimen.
This finding may be useful to guide new therapeutic approaches in the future, since that, in recent years, MDSCs are considered as a potential target in hematological malig-nancies to enhance the effects of currently used immune modulating agents [15].
Acknowledgements The authors thank the Clementino Fraga Labora-tory for funding the research. The authors thank Débora Yasmin de Sousa and Thayna Nogueira dos Santos for technical assistance with flow cytometry.
Compliance with ethical standards
Conflict of interest The authors declare no conflict of interest.
References
1. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in chil-dren. N Engl J Med. 2015;373:1541–52.
2. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–64.
3. Vuk-Pavlović S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate. 2010;70(4):443–55.
4. Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of
myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenter-ology. 2008;135(1):234–43.
5. Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clini-cal implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61(2):255–63. 6. Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz
AB. Immunosuppressive CD14+HLA-DR(low)/− monocytes in
B-cell non-Hodgkin lymphoma. Blood. 2011;117(3):872–81. 7. Gustafson MP, Abraham RS, Lin Y, Wu W, Gastineau DA,
Zent CS, Dietz AB. Association of an increased frequency of CD14+HLA-DRlo/neg monocytes with decreased time to
progres-sion in chronic lymphocytic leukaemia (CLL). Br J Haematol. 2012;156(5):674–6.
8. Brandalise SR, Pinheiro VR, Lee ML. GBTLI. Grupo Brasileiro para o Tratamento de Leucemia Infantil. Protocolo de tratamento da leucemia linfoide aguda em crianças. Sociedade Brasileira de Oncologia Pediátrica; 2011.
9. Chauffaile MLL, Coutinho V, Yamamoto M, Kerbauy J. Com-bined method for simultaneous morphology, immunopheno-type and karyoimmunopheno-type (MAC) in leukemias. São Paulo Med J. 1997;115(1):1336–42.
10. Theunissen P, Mejstrikova E, Sedek L, van der Sluijs-Gelling AJ, Gaipa G, Bartels M, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leu-kemia. Blood. 2017;129(3):347–57.
11. Zhang ZJ, Bulur PA, Dogan A, Gastineau DA, Dietz AB, Lin Y. Immune independent crosstalk between lymphoma and myeloid suppressor CD14+HLA-DRlow/neg monocytes mediates
chemo-therapy resistance. Oncoimmunology. 2015;4(4):996470. 12. Mougiakakos D, Jitschin R, von Bahr L, Poschke I, Gary R,
Sund-berg B, Gerbitz A, Ljungman P, Le Blanc K. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following
allogeneic hematopoietic stem cell transplantation. Leukemia. 2013;27(2):377–88.
13. Ding Z, Lu X, Yu M, Lemos H, Huang L, Chandler P, Liu K, Wal-ters M, Krasinski A, Mack M, Blazar BR, Mellor AL, Munn DH, Zhou G. Immunosuppressive myeloid cells induced by chemo-therapy attenuate antitumor CD4+ T cell responses through the
PD-1/PD-L1 axis. Cancer Res. 2014;74(13):3441–53.
14. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–20.
15. De Veirman K, Van Valckenborgh E, Lahmar Q, Geeraerts X, De Bruyne E, Menu E, Van Riet I, Vanderkerken K, Van Ginder-achter JA. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front Oncol. 2014;4:349. 16. Liu YF, Chen YY, He YY, Wang JY, Yang JP, Zhong SL,