UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Sexual dimorphism and reproductive phenology of common
birds in São Tomé Island – conservation implications
Bárbara de Castro Marques Arez Madeira
Mestrado em Biologia da Conservação
Dissertação orientada por:
Doutor Ricardo Faustino Lima
Doutor Martim Pinheiro de Melo
II
AGRADECIMENTOS
Quero começar por agradecer aos meus orientadores por todo o apoio que me deram quer em São Tomé, quer no Porto e em Lisboa, nunca deixei de me sentir apoiada. Quero agradecer principalmente ao Ricardo Lima pela ajuda e orientação em São Tomé. Sem ele seria muito difícil orientar o trabalho de campo e toda a logística que isso envolve, pelo incansável apoio ao longo do ano e por me incentivar mesmo quando parecia difícil avançar. Quero também agradecer a duas pessoas impecáveis ao Martim Melo e à Rita Covas por me receberem em sua casa como se eu fosse família e me orientarem no laboratório para o tratamento das amostras de ADN, pela sua disponibilidade em fazer reuniões mesmo estando longe ou atarefados, agradeço imenso os vossos “inputs” para melhorar o desenvolvimento da tese e por vezes “desatar nós” quando parecia não haver solução.
Quero também agradecer a simpatia e a ajuda determinante de Carlos Pacheco, e a sua disponibilidade para viajar para São Tomé, onde ajudou na identificação de algumas características morfológicas das nossas espécies em estudo. Com a sua ajuda pudemos também confirmar algumas suspeitas e ideias.
O trabalho de campo foi parcialmente financiado por uma “Booster Grant” da “Rufford Foundation” (“The Role of Bird Seed Dispersal on São Tomé Forest Dynamics” - Ref.: 18618-B).
Quero agradecer em especial ao Eng. Arlindo Carvalho, Director Geral do Ambiente por apoiar as nossas actividades em São Tomé e nos dar as autorizações necessárias para o desenrolar das mesmas.
Agradeço ainda à Associação Monte Pico, pelo alojamento durante a minha estadia em São Tomé e pela ajuda no trabalho de campo.
Agradeço também aos meus incansáveis guias de campo, Octávio Veiga, Leonel Viegas e Sedney Samba que conseguiam levar quilos de carga para os acampamentos que ficavam a uns bons metros senão quilómetros das estradas, por terras para mim escorregadias que para eles eram do mais estável que se arranjava. Um especial obrigado ao Octávio pela sua hospitalidade, companhia nos finais de tarde no campo, pelas suas piadas e histórias e claro por ser um verdadeiro chefe, cozinhando as suas fantásticas refeições numa fogueira, não era de todo algo que estivesse habituada.
Tenho de agradecer a todas as pessoas que conheci em São Tomé, pois ajudaram-me a abrir os horizontes. Permitiram-me “espreitar” para as suas vidas e a experienciar durante dois meses a sua cultura, comida e costumes. Agradeço do fundo do coração à família que mais nos recebeu em sua casa e um imenso obrigada à Lucy, que nos recebeu em casa como se fossemos filhas, pelas refeições cinco estrelas, e sobretudo pelo apoio emocional, sem ti São Tomé não teria sido a mesma coisa, nunca esquecerei o teu sorriso terno.
Agradeço ao Gégé Lima por ser imparável e a pessoa mais prestável que conheci sempre pronto a ajudar na anilhagem, por nos levar a conhecer a as magnificas paisagens e cascatas escondidas da ilha e com uma simpatia do mais genuíno que poderemos encontrar deixei um verdadeiro amigo naquela ilha.
Obrigada ao Catoninho ao Lito e Lau pela sua boa disposição e carinho demonstrado ao longo da nossa visita.
III Um obrigado especial à minha mãe que sempre me apoiou em todas as minhas decisões. Quero agradecer ao Rui, que para além de me ajudar a perceber algumas coisas quando era preciso, teve de me aturar a falar de coisas que não entendia muito bem, e de dar a volta, no sentido de me ajudar, quer na resolução da tese, quer emocionalmente. De tal modo que já não quer ouvir falar de aves nos próximos meses. Obrigado por estares cá sempre para mim.
Finalmente gostaria de agradecer a todos os meus amigos e colegas de mestrado pelo apoio principalmente ao Fernando e Manuel, e um obrigado especial à Filipa e Martina pela companhia nesta aventura partilhada em São Tomé, e pelo ombro amigo em Portugal, vão estar sempre na minha memória.
IV
RESUMO ALARGADO
As ilhas são reconhecidas como importantes para a biodiversidade e como tal são frequentemente reconhecidas como áreas prioritárias para a conservação. Devido ao isolamento e à maior simplicidade das relações ecológicas, as ilhas também são frequentemente utilizadas como modelos para estudos ecológicos. Nesta tese tentamos complementar alguns estudos realizados ao longo dos anos sobre a importante comunidade de aves da ilha de São Tomé, caracterizada por um elevado grau de endemismo. Os dois capítulos investigam as diferenças de coloração e massa entre os machos e as fêmeas e a fenologia das espécies mais comuns de São Tomé, uma informação crucial tanto para pesquisas fundamentais sobre ecologia como para a conservação desta comunidade única.
São Tomé, com uma área de 857 km2, é a maior ilha da República Democrática de São Tomé e Príncipe, no Golfo da Guiné. Encontra-se a 255 km do continente africano e a 150 km da ilha do Príncipe. A 2 024 m de altitude o Pico é o ponto mais alto e explica o acentuado gradiente climático da ilha, caracterizado por altos níveis de humidade e chuvas frequentes no sudoeste da ilha, que contrastam com o ambiente semiárido do nordeste. Originalmente coberta por floresta, hoje em dia a floresta nativa está sobretudo circunscrita às áreas montanhosas do centro e sudoeste da ilha. Esta está envolta por floresta secundária, plantações de sombra e zonas não florestadas, que na sua maioria resultaram de uso agrícola e expansão urbana. São Tomé é extremamente importante para a conservação de aves a nível global, possuindo 17 espécies endémicas, além de três espécies partilhadas apenas com as outras ilhas do Golfo da Guiné. Com o rápido desenvolvimento económico da ilha nos últimos anos, tornaram-se ainda mais necessários esforços activos para a conservação das suas espécies e ecossistemas.
Determinar o sexo e a idade dos indivíduos em populações selvagens é importante para estudos ecológicos e para conservação. No entanto, para muitas espécies de aves, sexo e idade são difíceis de determinar, por isso decidimos abordar este tema no primeiro capítulo desta tese de forma a poder melhorar o conhecimento sobre as diferenças entre o sexo e a idade das espécies mais comuns existentes na ilha de São Tomé.
Os ambientes insulares oferecem oportunidades únicas para estudar a evolução do dimorfismo sexual porque as populações insulares frequentemente enfrentam diferentes pressões de predação, condições de alimentação, competição intraespecífica e interespecífica do que as populações continentais. O objectivo principal deste capítulo foi o de obter um melhor conhecimento do dimorfismo sexual das espécies de aves de São Tomé. Medições retiradas no campo, fotografias e sexagem molecular de 1046 indivíduos, permitiram identificar características relacionadas ao sexo e à idade para as 12 espécies mais frequentemente capturadas em redes de anilhagem. Oito dessas espécies eram sexualmente dimórficas, enquanto as quatro restantes não podiam ser sexadas com base em caracteres observáveis. O uso de modelos lineares generalizados baseados em biometria para desenvolver funções discriminantes baseadas em morfometria permitiu distinguir o sexo de três destas espécies. A incorporação de informações sobre altitude e tipo de habitat permitiu ainda distinguir o macho e a fêmea dos Tecelões de São Tomé Ploceus sanctithomae, enquanto melhorou a capacidade de distinguir o sexo da maioria das outras espécies. A coloração foi crucial para identificar a idade na maioria das espécies e contribuiu para a sexagem de adultos em seis espécies, em duas das quais apenas os machos adultos puderam ser identificados com um alto grau de confiança.
Os organismos evoluíram para coordenar os seus esforços reprodutivos com o pico de recursos necessários para criar as suas crias. Em zonas temperadas, isso levou a épocas de reprodução curtas
V coincidentes com uma sazonalidade marcada na disponibilidade de recursos. Em contraste, as áreas tropicais próximas ao Equador são alguns dos ambientes mais estáveis da Terra, com pouca sazonalidade na disponibilidade de recursos. Isso sugere que as espécies da floresta tropical se possam reproduzir durante a maior parte do ano. Por outro lado, a variação na precipitação pode levar a mudanças na disponibilidade de recursos que afectam as decisões reprodutoras. A insuficiência de estudos detalhados sobre a época de reprodução das espécies da floresta tropical, não permitiu testar isso efectivamente, mesmo em aves - um dos grupos de vertebrados mais bem estudados. Neste segundo capítulo realizou-se um estudo detalhado sobre a sazonalidade de reprodução e muda das espécies de aves comuns de São Tomé, e a sua associação com variáveis ambientais e características de espécies. As épocas de reprodução e muda foram identificadas para cada espécie com base em dados de anilhagem para as 12 espécies com o maior número de registos. A época de reprodução das espécies de aves comuns de São Tomé ocorreu principalmente durante a estação das chuvas, atingindo o pico durante a estação seca curta (gravanito). A muda seguiu-se á época de reprodução, e ainda assim ocorreu antes do início da longa estação seca, a gravana. As espécies mais pesadas, com períodos de reprodução mais longos, tendem a reproduzir-se mais cedo de forma a garantir que as eclosões coincidam com o pico de alimento. Os nossos resultados também sugerem que a muda, e portanto a reprodução, são mais tardias em altitudes mais elevadas.
Este estudo melhorou muito a nossa capacidade de identificar o sexo das aves endémicas de São Tomé no campo, uma informação crucial tanto para pesquisas fundamentais sobre ecologia e evolução como para a conservação desta comunidade única. Contribuir para uma melhor compreensão da sazonalidade em São Tomé é fundamental para apoiar estratégias de conservação de aves, nomeadamente no que se refere à caça e outras actividades extractivistas. Assim forneceremos informações fundamentais para orientar acções de conservação baseadas em evidências tão necessárias para a ilha e para ecossistemas de ilhas tropicais semelhantes, uma vez que não se sabe muito sobre como as aves tropicais lidam com a variação da sazonalidade reprodutora. Também mostra que, apesar da reduzida variabilidade ambiental, o ciclo de vida anual das aves tropicais pode ser determinado pelas variações na precipitação que deverão controlar a disponibilidade de recursos.
Palavras-chave: África, endemismo, estratégias reprodutoras, florestas tropicais, funções
discriminantes
ABSTRACT
The island of São Tomé is the largest of the Democratic Republic of São Tomé and Principe, in the Gulf of Guinea, central Africa. Home to a wide variety of endemic and endangered species, it is often recognized as an important area for the conservation of biodiversity worldwide, and for birds in particular. The main goal of this study was to gain a better knowledge of the sexual dimorphism and breeding seasonality of São Tomé bird species. To do so, we used and extended a bird ringing dataset, which was created in 2002. Biometrics, photographs and molecular sexing, allowed identifying characteristics related to sex and age in 12 species. Eight of these were sexually-dimorphic, while the remaining could not be confidently sexed based on observable characters. Coloration was crucial to identify age in most species, and contributed to sex adults in six species of which in two only adult males could be identified with a high degree of confidence. The development of morphometric-based discriminant functions using generalized-linear models based on biometrics allowed a better sex differentiation of three of the eight species. The incorporation of information on altitude and habitat
VI type further allowed sexing the São Tomé Weavers Ploceus sanctithomae, while improving the ability to distinguish the sex of most other species. The data on brood patches and moulting of primary flight feathers allowed understanding the seasonality of breeding in the common birds of São Tomé. The breeding activity occurred mostly during the rainy season, peaking during the short dry season of
gravanito. Moult followed breeding activity, and still occurred mostly before the start of the long dry
season, the gravana. Heavier species tended to breed earlier, probably due to having longer breeding seasons and therefore needing to ensure that hatching coincides with peak food availability. Results show that, despite reduced environmental variability, the annual life cycle of tropical birds might be structured around precipitation-driven changes in resource availability. Our results also suggest that moult, and thus breeding, is delayed at higher altitudes. This study helped improve the knowledge of the sexual dimorphism of São Tomé bird species which is crucial for fundamental research on ecology and evolution and for the conservation of this unique community. Our phenology data are key to support bird conservation strategies, namely regarding hunting and other extractive activities and contributes towards a better understanding of seasonality on São Tomé and for similar tropical island ecosystems - since not much is known on how tropical birds cope with seasonal variations in their environment.
VII
TABLE OF CONTENTS
GENERAL INTRODUCTION……….…1
CHAPTER 1: Coloration and morphology of endemic birds for sex and age identification…………...5
INTRODUCTION……….…..5 METHODS……….….…7 Study Area………..7 Data Collection……….……..…………8 Data analysis……….………..9 RESULTS………9
Morphometrics and sex………10
Distinguishing sex and age: Individual species accounts……….12
Morphometric-based sex discriminant functions……….18
DISCUSSION………21
Age identification……….21
Coloration………...………..…21
Moult………22
Sexing ……….……….22
Sexual dimorphic species ………23
Non sexual dimorphic species………..24
Sex-discriminant functions analyses………24
Morphometrics ………25
Conclusions………..……....25
CHAPTER 2: Seasonality in the tropics: Breeding and moulting of the common birds of São Tomé Island……….…..27
INTRODUCTION……….……27
METHODS………29
Study Area………....29
VIII
Data analysis……….30
RESULTS………..31
Breeding and moulting activity………33
Species traits and seasonality………...35
Environmental characteristics and seasonality……….35
DISCUSSION………37
Breeding seasonality……….37
Moulting seasonality………38
Phenological determinants: environmental characteristics and species traits………..39
Implications for conservation………...39
FINAL CONSIDERATIONS……….41
REFERENCES………...42
IX
LIST OF TABLES
Table 1.1 - List of São Tomé common birds species……….10
Table 1.2 - Measurements of São Tomé common bird species……….17
Table 1.3 - Sex and age distinctiveness of São Tomé common bird species, based on morphometrics and coloration……….18
Table 1.4 - Sex discriminant functions using morphometric-based GLMs for the São Tomé common bird species……….19
Table 1.5 - Sex discriminant functions using environmental variables-based GLMs for the São Tomé common bird species………..20
Table 2.1- List of the common birds of São Tomé Bird characteristics………31
Table 2.2 – Chi-square test results from stepwise backward elimination procedure on the full model and respective AIC………..36
LIST OF FIGURES Figure 1.1- Map of São Tomé Island in relation to mainland Africa……….……….8
Figure 1.2-Morphometrics in São Tomé common bird species……….11
Figure 1.3- Photos of Newton Sunbird………..12
Figure 1.4- Photos of Lemon Dove………12
Figure 1.5 - Photos of Principe Seedeater………..13
Figure 1.6 - Photos of Giant Sunbird……….13
Figure 1.7 - Photos of Common Waxbill………...13
Figure 1.8 - Photos of Giant Weaver……….14
Figure 1.9 – Photos of São Tomé Weaver……….14
Figure 1.10 - Photos of São Tomé Prinia………..……….15
Figure 1.11 - Photos of São Tomé Paradise-flycatcher……….15
Figure 1.12 - Photos of São Tomé Thrush……….16
Figure 1.13 - Photos of São Tomé white-eye………...……….16
X
Figure 2.1 - Map of São Tomé Island with sample location………..32 Figure 2.2 - Link between rainfall, breeding and moulting seasonality in São Tomé………...33 Figure 2.3 - Breeding and moulting activity of the 12 common bird species of São Tomé…………..34 Figure 2.4 - Correlation between the timing of breeding/moulting, and species traits………..35 Figure 2.5- The monthly proportion of moults in altitude for the eight species with most records…..35 Figure 2.6 - All possible linear models used to explain the proportion of moulting individuals……..36 Figure S1. - Bi-plots of five morphological measurements for 11 endemic bird species of São Tomé
and for one non-endemic………...49-50
Figure S2. - São Tomé Thrush differences in iris colour between males and females of molecularly
tested individuals………....51
Figure S3. - Brood patch sample effort distribution, throughout the year……….52
Figure S4. - Moult sample effort distribution, throughout the year………..53 Figure S5. - Breeding and moulting activity of sexed females of the 12 common bird species of São
Tomé………...54
LIST OF ABBREVIATIONS AND ACRONYMS
LC Least Concern VU Vulnerable NT Near threatened
Ananew Anabathmis newtonii Newton Sunbird
Collar Columba (larvata) simplex Lemon Dove
Criruf Crithagra rufobrunnea Príncipe Seedeater
Dretho Dreptes thomensis Giant Sunbird
Estast Estrilda astrild Common Waxbill
Plogra Ploceus grandis Giant Weaver
Plosan Ploceus sanctithomae São Tomé Weaver
Primol Prinia molleri São Tomé Prinia
Teratr Terpsiphone atrochalybeia São Tomé
Paradise-flycatcher
Turoli Turdus olivaceofuscus São Tomé Thrush
Zosfea Zosterops feae São Tomé White-eye
1 GENERAL INTRODUCTION
Reproduction is a fundamental feature of life. When food resources are scarce, the climate becomes hostile, or individual survival is jeopardized by some other adverse change in living conditions, species have to employ strategies to ensure the survival of their progeny (Hau et al. 2008). To study these strategies we first need to know how to differentiate the sexes of the study species.
Determining the sex of individuals in natural populations is a useful tool for studying population dynamics, population structure, habitat use, behavior and mating systems, species vulnerability to extinction, distribution shifts and therefore for making appropriate management decisions, both at the habitat and species level (Shaw et al. 2003; Schwartz 2012). Sexual dimorphism is a common, and often substantial form of intraspecific phenotypic differentiation, such as in colour, shape, size, structure or behavior. It can occur in any group of living beings, from protists to plants and animals (Punzalan & Hosken 2010). When interspecific competition is small, sexes tend to diverge morphologically. In some plants, the dissimilarities are only functional, not competitive (McGee & Wainwright 2013). In birds, as in other vertebrates, the sexes usually differ in size and colour, and sometimes also in the proportion of body parts (Selander 1966). Variation in the extent of sexual dimorphism among bird species is traditionally attributed to divergences in social mating system (Owens & Harley 1998). Many birds show some dimorphism in terms of coloration, usually the female being cryptically coloured to remain hidden on the nest, while the more-colourful male uses it to for territorial and courtship behaviors (McGee & Wainwright 2013). Unfortunately, for many bird species it is difficult to determine sex based solely on morphometrics and coloration (Calabuig et al. 2011). Molecular sexing is one of the simplest and most used DNA-based techniques applied to wild birds. These are usually applied to blood samples, although feather samples have also been used (Harvey et al. 2006). Morphometric-based sexing depends on measuring variances in body size between sexes, but its reliability can be limited and it decreases when sex dimorphism is reduced (Volodin et al. 2015). With a better knowledge of differences between sexes we can study the seasonality of breeding.
Almost all organisms experience seasonal variations in their environment conditions, such as rainfall, temperature, food abundance, disease and social factors (Nelson & Demas 2002). For birds, rainfall regimes and associated environmental changes are common cues to determine breeding seasonality (Karr 1976). Bird populations may present very different breeding seasonality depending on the region, habitat, and more specifically on the climate variations of the region they occupy (Hau et al. 2008). Breeding can be detected by changes in behavior, plumage and physiology, but the presence of a brood patch is one of the easiest and most reliable means to confirm breeding activity (Tucker 1943). The brood patch is a featherless, reddish and wrinkled skin with many blood vessels visible on the underparts of breeding female in most bird species, usually developing in the ventral region before incubation to improve heat transfer to the eggs (Demongi 2016).
Breeding and moulting, are the primary periods of the annual cycle in most birds and they are usually connected. Both actions are costly in terms of energy, and therefore they usually do not overlap in time (Demongin 2016; Freed & Cann 2011). Feather moulting is a regular replacement of all or parts of the plumage. It occurs in all species to ensure that plumage function is maintained, and it is also used to adjust plumage characteristics to specific life cycle needs of the bird (Jenni & Winkler, 1994). It should not be confused with plumage replacement, which occurs sporadically when some feathers are lost or damaged.
2 Compared to mainland counterparts, island birds tend to have lower fecundity, greater reproductive investment and extended developmental periods (Covas 2011). Little is known about the extent and variability of the breeding season. Despite these parameters being vital to understand population dynamics and to assess the causes behind demographic changes (Burr et al. 2016), the effort to comprehend the variation of seasonality within tropical populations and among species in tropical assemblages has been quite small (Stouffer et al. 2013).
There is now compelling evidence that the ongoing climate change is having ecological impacts on both animals and plants, from polar to tropical environments (Bellard 2012). Climate change itself is expressed in a different way across and even within tropical islands, but several factors can be recognized in tropical island ecosystems such as: climate is changing rapidly for tropical islands and that it can be expressed as a wide diversity of threats, including altered rainfall, the frequency and intensity of big storms, warming, and interactions with disturbances such as fire and invasive species (Keener et al. 2012). The responses of both flora and fauna span an array of ecosystems and organizational hierarchies, from molecular to global levels (Walter et al. 2002). The implications of climate change for birds have only recently begun to be addressed (Crick 2004). Climate affects not only the metabolic rate of birds, but also behavior, either directly or indirectly. Reproduction is a vulnerable phase of life cycle, and is thus being particularly affected by climate change. For example, it can influence the ability to carry out essential behaviors, such as courtship, while also impacting on breeding success through food scarcity, earlier breeding, changes in timing of migration and differential selection between components of a population (Crick 2004). The effects of climate change are particularly severe in tropical islands, where a large part of the species is endemic (Keener et al. 2012).
Compared to the mainland, island ecosystems are in many ways simplified (Whittaker & Palacios 2007). Islands have a smaller number of species compared with the mainland regions and have been studied for their capacity to offer insights into evolutionary and ecological processes (Roulin & Salamin 2010). This study will focus on São Tomé Island, with the goal of improving our knowledge on its birds so that insular patterns can be inferred and compared with the mainland communities.
São Tomé is an 857 km2 oceanic island about 255 km from mainland Africa. Together with Príncipe, it constitutes the Democratic Republic of São Tomé and Príncipe, in the Gulf of Guinea, Central Africa. It is a volcanic island, never connected to the mainland. Volcanic activity persisted until recently which explains the rugged topography. The highest elevation point at 2024 m a.s.l. is the “Pico de São Tomé” (Salgueiro & Carvalho 2001). The geographic location of São Tomé has allowed the development of high levels of endemism, being far enough to be isolated, while at the same time allowing a fairly high number of potential colonizers (Peet & Atkinson 1994). The mountainous center of the island halts the dominant southwest winds, creating a very strong rainfall gradient across the island: annual precipitation varying from less than 600 mm in the northeast to over 7,000 in the southwest (Bredero et al. 1977). The montane rivers have plenty of waterfalls, carving deep valleys in the center of the island, and slowing down near the coast to create small estuaries, occasionally bordered by mangroves.
Despite its proximity to the Equator, São Tomé has two clear main seasons: the dry season, between June and August, called “gravana”, and a rainy season, from September to May. There is also the “gravanito”, a smaller and less marked dry season between December and January (Tenreiro 1961). The average temperature is mostly constant throughout the year, ranging between 18°C and 33°C across the island (Bredero et al. 1977), decreasing with rainfall, altitude and in the dry season.
3 The humidity levels and cloud cover are also very high throughout the year and mostly towards the southwest (Carvalho et al. 2004).
The human occupation of São Tomé started in the late 15th century, when the Portuguese discovered the island, allegedly uninhabited and almost entirely covered in forest, except for tiny areas of sand dune (Peet & Atkinson 1994). The forests have a dense canopy, with trees often exceeding 30 m of height, plentiful lianas and epiphytes, like mosses, ferns and orchids (Peet & Atkinson 1994). There are three key distinct types of forest: Lowland, which extends from the sea level up to 800 m,; Montane, which extends from 800 m to 1,400 m, is composed by a dense canopy and by tall trees covered by epiphytes, and it is currently threatened by agricultural development; and Mist, which extends from 1,400 m to 2.024 m (Exell 1944), has an open canopy, the lowest temperatures and a difficult access, which has kept it protected from most human activities (de Lima et al. 2013). The drier northeast of the island is subject to frequent fires, and has many deciduous tree species (Leventis and Olmos 2009); most of which has been extensively cleared for agriculture and turned into savannah-type habitat (Oliveira 2002, Carvalho et al. 2004). These dry coastal lowland forests have suffered the most, being largely cleared for sugar cane starting in the 16th century (Tenreiro 1961). During the 19th and 20th century, large extents of intensive cocoa and coffee shade plantations, known as “roças” (Oliveira 1993; Frynas 2003) led to deforestation up to 1200 m. Subsequent agricultural abandonment driven by falls in production and global prices of cocoa and coffee led to significant regeneration (Tenreiro 1961; Oliveira 2002; Carvalho et al. 2004). The resulting secondary forests, have lower canopy, higher proportion of introduced species and less biomass (Exell 1944).
The biodiversity of São Tomé is unique and fascinating to many researchers around the world. It is extremely rich in endemic species, with its forests having been identified as the third most important for the conservation of forest bird species worldwide (Buchanan et al. 2011). The endemics are strongly dependent on the native forest (Rocha 2008; de Lima 2012; Soares 2017), most of which is now included in the 295 km2 Obô Natural Park (STONP) (Dallimer et al. 2009; Soares 2017). This park was created in 2006 to protect native fauna and flora, as well as their habitats (Direcção Geral do Ambiente 2006). A buffer zone was also envisioned but never officialized. The STONP action and management plans have been created and revised (Albuquerque et al. 2008; Albuquerque et al. 2014), but implementation remains weak (de Lima et al. 2015).
The avifauna of São Tomé is particularly remarkable, including at least 17 single-island endemic species, three endemic species shared with other islands in the Gulf of Guinea, and eight endemic subspecies of widespread species (Jones & Tye 2006). These high levels of endemism have led to São Tomé having multiple Important Bird Areas and to be one of the smallest high priority Endemic Bird Area at a global level (Dallimer & King 2008). Twenty out of the 50 terrestrial bird species that breed on the island are endemic, nine of which are globally threatened, including three Critically Endangered, one Endangered, and five Vulnerable, plus two Near Threatened (IUCN 2017). Notably the most threatened species are those that are most dependent on native forest (Dallimer et al. 2009). The right amount of isolation allowed many species to evolve in environments distinct from those found in the mainland (Miller et al. 2012). São Tomé Island also holds many bird species that exhibit several island syndromes, such as dwarfism and giantism. In islands, small species tend to get bigger, while large species tend to get smaller (Meiri et al. 2006). The Giant Sunbird Dreptes thomensis, the Giant Weaver Ploceus grandis, and the São Tomé Grosbeak Neospiza concolor are all the largest within the respective genera, while the São Tomé Speirops Zosterops lugubris and the São Tomé Thrush Turdus olivaceofuscus are the largest African representatives within the respective genera. On the other hand, the largest endemic bird species in São Tomé, the Dwarf Ibis Bostrychia bocagei is smaller than the mainland counterparts (Melo 2006; Melo et al. 2017).
4 Previous studies on São Tomé have emphasized that a significant number of island endemics also use human-modified habitats, such as secondary forests and shade plantations (Rocha 2008; Dallimer et al. 2012; de Lima et al. 2012; Soares 2017). Still, there are limits to how much change endemic species can withstand and so, although there is still much forest left on São Tomé, the fast growing human population and resulting urbanization will lead to an increase in natural resource use. These critical changes are likely to lead to a critical decrease of population size or even extinction of most endemic birds (Dallimer et al. 2009). Enforcing strong hunting regulations, like establishing hunting periods and specific quotas for abundant species, is a key conservation need. As is raising awareness, since most São Tomé hunters and game consumers are not aware of the difference between endemic, native or exotic species, or the conservation problems resulting from overhunting and logging (Carvalho et al. 2016). One of the best strategies to avoid this particular threat is to involve the population in conservation efforts, namely by sharing their knowledge and putting in use to support conservation.
This thesis has two main goals, relating to São Tomé common bird species. In the first chapter, we study the coloration and biometrics to determine age and sex. In the second chapter, we assess breeding and moulting seasonality, relating it to species traits and local environmental conditions. Improving our knowledge on these aspects of bird reproduction is key to develop effective conservation strategies.
5
CHAPTER 1: Coloration and morphology of endemic birds for sex and age
identification
Abstract: Determining the sex and age of individuals in natural populations is important for
ecological studies and for conservation. However, for many bird species, sex and age are difficult to determine. Island environments offer unique opportunities to study the evolution of sexual dimorphism because insular populations frequently face different predation pressures, feeding conditions, intraspecific and interspecific competition than continental populations. The main goal of this chapter is to gain a better knowledge of the sexual dimorphism of São Tomé bird species. Field measurements, photographs and the molecular sexing of 1046 individuals, allowed identifying sex and age related characteristics for the 12 species most often captured in mistnets. Eight of these were sexually-dimorphic, while the remaining four could not be sexed based on observable characters. Using generalized-linear models based on biometrics to develop morphometric-based discriminant functions allowed a better sex distinction of three species. The incorporation of information on altitude and habitat type further allowed distinguishing male and female São Tomé Weavers Ploceus
sanctithomae, while improving the ability to distinguish the sex of most other species. Coloration was
crucial to identify age in most species, and contributed to sex adults in six species of which two, only adult males could be identified with a high degree of confidence. This study greatly improved our ability to sex the endemic birds of São Tomé in the field, a crucial information both for fundamental research on ecology and evolution and for the conservation of this unique community.
Keywords: conservation, discriminant functions, endemism, morphometrics, sex identification INTRODUCTION
Sexual dimorphism, where sexes have distinct phenotypes, is a common and often substantial form of intraspecific phenotypic differentiation, such as in colour, shape, size, structure or behavior. It can occur in any group of living beings, from protists to plants and animals (Punzalan & Hosken 2010). The differences in coloration found between sexes can be explained by the different climates, the presence of predators and of parasites (Covas 2011). When intra-specific competition for resources is high in environments where interspecific competition is small sexes tend to diverge morphologically for ecological reasons: unequal-sized males and females can exploit different sizes of food leading to a decrease in competition for resources. In some plants, the dissimilarities are only functional, not competitive (McGee & Wainwright 2013). Many birds show some dimorphism in terms of coloration, usually the female being cryptically coloured to remain hidden on the nest while the more-colourful male uses it to demonstrate territorial and courtship behaviours (McGee & Wainwright 2013). The function of sexual dimorphism, in many cases, is related to the competition of individuals for access to reproduction, using such characters to attract or fight for a partner (Hamilton 1961). The differences may be extreme, as in the variety seen in the exotic plumages and colours of the male species or in the adaptations for protection (Johnsen et al. 2003).
Islands have a smaller number of species compared with the mainland regions and have been studied for their capacity to offer insights into evolutionary processes (Grant 1998; Whittaker & Palacios 2007; Roulin & Salamin 2010). A reduction in interspecific competition, predation and other factors might allow island organisms to achieve optimal morphological adaptation resulting in a different degree of sexual dimorphism in comparison to the more complex mainland assemblages (Greenberg & Danner 2013). When a taxon colonizes a new habitat it starts the process of adaptation
6 to this new environment. In the case of islands, which are often characterized by reduced interspecific competition, and a different distribution of resources, species tend to have wider ecological niches, resulting in: (A) sub-niches being occupied by all individuals; (B) intraspecific sub-niches linked to sex; or (C) specific sub-niches unrelated to sex (Greenberg & Olsen 2010). These patterns in islands are associated with rapid morphological divergence, often involving strong morphological divergence from their continental relatives, sometimes resulting in large radiations (Pratt 2005; Grant 2008; Alström et al. 2015). In birds, some genera like Terpsiphone and Zosterops, have a high propensity to colonize islands and to speciate rapidly once there (Moyle et al. 2009; Fabre et al. 2012). Island environments can lead to changes in morphological divergence as a result of changes in social interactions (Robinson-Wolrath & Owens 2003). Mating and parental care strategies may change after island colonization (Robinson-Wolrath & Owens 2003; Covas 2011). In birds it is frequently believed that insular taxa develop dull coloration and are less sexually dichromatic likely due to a decrease in the intensity of sexual selection but supporting evidence remains limited (Doutrelant et al. 2016). This indicates that we have opposite predictions in islands depending on whether the ecological or sexual factors play a dominant role.
To date there is no generally recognized method to record age in birds. Juveniles tend to have their first complete moult as soon as they leave the nest (Demongin 2016). However, they still have some distinctive signs easily to recognize like feathers that tend to be weaker and looser than the adults; growth bars on tail and wing feathers; the shape of the tail feathers that tend to be narrower and more pointed than the adults. Also iris and bill coloration can vary with age or sex, generally duller and darker in juveniles than in adults and in adult females when compared to adult males (Stevensson 1992, Demongin 2016). Often gape flanges are also used to identify juveniles but it should be noted that some species have flanges as adults (Stevensson 1992). The adult plumage is definitely acquired and does not change the appearance with age and successive moults, except between seasons e.g. breeding (Demongin 2016).
Determining the sex and age of individuals in natural populations is a useful tool for studying population dynamics, population structure, habitat use, behavior and mating systems, species vulnerability to extinction, distribution shifts and therefore for making appropriate management decisions, both at the habitat and species level (Shaw et al. 2003); Schwartz 2012). Accurate and easy methods to determine the sex of individuals are thus valuable for ornithological studies. Unfortunately, for many bird species it is difficult to determine sex based solely on morphometrics and coloration (Calabuig et al. 2011). The sex of birds is commonly determined by laparoscopy, cloacal examination, genetic analyses and morphometrics. All of these techniques require capturing birds and the use of procedures that compromise the wellbeing of birds. Non-invasive sexing can be inferred by differences in coloration, morphology and behavior, but are overall less accurate than more invasive techniques (Volodin et al. 2015). Molecular sexing is one of the simplest and most used DNA-based techniques applied to wild birds. For obtaining genetic material, the most common sampling method for sexing is the removal of blood samples, because blood is fairly easy to take from captured individuals and have a large amount of DNA (Harvey et al. 2006). Feather samples have been used less often for molecular sexing, but they too represent an appealing sampling method, because removing a single feather is one of the least invasive methods of obtaining a genetic sample (Harvey et al. 2006). Genetically-based sexing is very reliable, but it is relatively expensive and time-consuming. Additionally, incorrect settings of genetic analyses may, to some extent, decrease the reliability of this method (Volodin et al. 2015).
Morphometric-based sexing depends on measuring variances in body size between sexes. It requires minimal equipment and staff training, and provides immediate results. However, its reliability
7 can be limited and it decreases when sex dimorphism is reduced (Volodin et al. 2015). Discriminant functions analyses (DFA) are a popular statistical tool because it can classify individuals of unknown origin into groups, classes or categories of the same type, using a discriminant function (DF) generated from a data set composed of individuals of known origin (White & Ruttenberg 2007). The use of DFA using morphological measurements has proved useful to determine sexes (Berkunsky et al. 2009). However, the skill of the researcher for sex estimation using DFA plays an important role (Moore 2013). DFA may incorporate not only characteristics of the birds, but also the locations where they are captured and/or the time of year. Molecular sexing is a very good complement to DFA in species with unclear sexual dimorphism, since the human ability to always take the same measurements for some morphometric-based sexing plays a big role in the percentage of human error (Calabuig et al. 2011), although mass is clearly less dependent on who is measuring than other measures.
The island of São Tomé is one of the 218 Endemic Bird Areas identified worldwide (Dallimer et al. 2009). It is home to twenty endemic bird species, of which three are considered Critically Endangered, one Endangered, six Vulnerable, two Near Threatened and eight Low Concern (IUCN 2017). The main goal of this chapter is to gain a better knowledge of the sexual dimorphism of São Tomé common bird species. Specifically, we intend to:
• Identify characteristics that allow identifying sex and age;
• Use morphometrics to create functions that allow discriminating sexes; • Assess if habitat and altitude can improve the ability to distinguish sexes.
We will thus verify and complement the knowledge on the sexual dimorphism of this endemic-rich community (HBW Alive 2017; Naurois 1994; Jones & Tye 2006).
METHODS Study area
The 857 km2 oceanic island of São Tomé (0º25’N-0º01’S, 6º28’E-6º45’E) is the largest island of the Democratic Republic of São Tomé and Príncipe. Located in the Gulf of Guinea, it is part of the Cameroon line (Fig. 1.1). It lies 255 km west of mainland of Africa (Gabon) and 150 km south-southwest of Príncipe Island (Peet & Atkinson 1994). Its location has allowed the development of high levels of endemism, being far enough to be isolated, while at the same time allowing a fairly high number of potential colonizers (Melo 2007; Melo 2012). The highest elevation point is “Pico de São Tomé” at 2,024 m a.s.l.. The mountainous center of the island halts the dominant southwest winds, originating a very strong rainfall gradient across the island. Annual precipitation varies from less than 600 mm in the northeast to over 7,000 in the southwest. Despite its proximity to the equator, São Tomé has two clear main seasons: the dry season, between June and August, called “gravana”, and a rainy season, from September to May. There is also the “gravanito”, a smaller dry season between December and January (Leventis & Olmos 2009). The average temperature is regularly constant throughout the year, oscillating between 18°C and 33°C across the island (Coelho 2016).
São Tomé was originally covered in forest, except for tiny areas of rocky outcrops (Peet & Atkinson 1994). There are three key distinct types of forest: Lowland forest, which extends from the
8 sea level up to 800 m, and most of which is nowadays cultivated or savanna in the north of the island where rainfall is very low; Montane forest, which extends from 800 m to 1,400 m, composed by a dense canopy and by tall trees covered by epiphytes, and currently threatened by agricultural development; and Mist forest, extending from 1,400 m to 2.024 m (Exell 1944) it has an open canopy, the lowest temperatures and is of difficult access, which has kept it protected from most human activities (de Lima et al. 2013).
Data collection
We compiled the bird ringing data that has been collected in São Tomé Island since 2002 by Martim Melo and colleagues. These were collected throughout the year, in 30 sampling locations across the island. We collected additional data between mid-January and the end of March 2017, respecting the same locations used in the years before. We used vertical understory mist-nets to capture the birds (De Beer et al. 2001). The location and number of nets was decided on the spot, to maximize diversity and number of birds captured, while taking into consideration our capacity to process them and external factors, such as weather. Whenever possible, nets were opened just before dawn and kept open until we could make sure that the birds could be safely released on the same day. Each location was sampled for three days, which has be shown to be the ideal period to minimize bird mist-net avoidance ((Marques et al. 2013), tested locally).
All birds were identified to species level, and all first captures were ringed. Whenever possible, sex and age were determined in the field, even if tentatively, using size, plumage, moult, breeding marks and any other physical traits such as iris and bare parts colour. To determine sex-specific morphometrics we measured: the length of the wing (distance between the carpal joint and the tip of the longest primary, measured on the closed wing), the length of the tail to the nearest half-millimeter, using a ruler, the length of the tarsus measured to the 10th of a millimeter using a digital caliper, and the weight (measured using a scale or a dynamometer, appropriate to the weight of the bird).
Figure 1.1- Map of São Tomé Island in relation to mainland Africa (Small inset on the bottom right). The sampling locations are shown by the orange dots. The initial 37 sampling locations were grouped in 23 map locations to allow visualization, since some of them were in close proximity of each other.
9 Some birds were sampled for genetic material, by collecting blood samples or two or three plucked feathers. The blood samples were obtained from the brachial vein in the wing and we collected approximately one or two drops of blood in capillary tubes via brachial venipuncture with a sterile hypodermic needle. Blood samples were immediately transferred to tubes containing 0.5 ml of blood lysis buffer. Feather samples were obtained from the individuals by pulling two or three feathers one at a time from the breast of each bird. Feather samples were then kept in individual paper envelopes. The blood and feather samples were labelled for molecular sexing, and subsequent association with field measurements. Molecular sexing followed the protocol of Griffiths et al. (1998). We combined non-invasive techniques with one invasive technique (DNA collection) to improve our ability to correctly sex individuals of each study species and therefore reduce the error of each technique and to look for sets of traits in discriminant functions analyses (DFA) capable of identifying sex for those species whose sexual dimorphism is small or still poorly understood e.g. confusion with age as in São Tomé Weaver or with moult patterns as in São Tomé Paradise-flycatcher Terpsiphone atrochalybeia.
Whenever possible we took photos from multiple perspectives of the birds in the hand, and took notes on individual morphologic characteristics, such as feather, eye and beak coloration and moult scores, for subsequent association with sex and age. These photos were organized and categorized by species, and then by sex and if possible age. The files were named using capture date and ring code, to make it easy to link with the information on the database. Our records, including those obtained from molecular sexing, were added to the São Tomé bird ringing database, which was then scanned for errors, and standardized.
Data analysis
To ensure we could perform robust statistical analyses, we selected for subsequent analyses only the 12 species that had over 100 records from the initial 39 species. We discuss sexual dimorphism for all these 12 species, but focus on those for which the sexual dimorphism is unknown or not clear. To assess sexual dimorphism we used boxplots, scatterplots and Kruskal-Wallis tests to compare field measurements (weight, except possible females with egg; wing, tail and tarsus length) of each sex (as determined by molecular analyses) for each species. We took into account the possibility of abrasion of the flight-feathers and tail while measuring birds on the field.
Information on morphometric and other external characteristics that allow sex and age identification was compiled for each São Tomé common bird species. The photographs of molecularly-sexed individuals were checked to identify additional characteristics that could allow sex determination. Finally, we used generalized linear models (GLM) with binomial error distribution to create sex discriminant functions based on morphometrics. Habitat were categorized as forest or plantation and altitude as montane or lowland, based on whether they were located above or below 800 m a.s.l., respectively. Habitat and altitude were subsequently added to the GLMs, to evaluate if they increased the ability to discriminate sexes.
RESULTS
We obtained 6125 ringing records, belonging to 39 species. These were collected since 2002, and include 761 new records from 2017. There were 12 species that had over 100 records, henceforth referred to as São Tomé common bird species (Table 1.1). For some species we already had an idea of
10 the sexual dimorphism and for others we were almost certain from the field observations but to test this, 1046 individuals were molecularly sexed from blood and feather samples. We photographed 759 individuals, 258 of which were sexed using molecular techniques, which allowed confirming external morphological characters associated with sex.
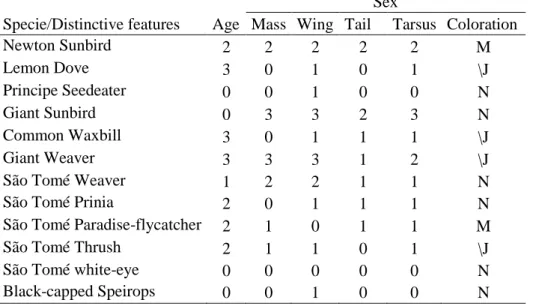
Table 1.1- List of São Tomé common birds, including the number of valid records (n), the number of individuals that were sexed using molecular techniques (n sexed – percentage of the “n” shown in parenthesis), the number of sexed individuals that also had photos (n photos – percentage of “n sexed” shown in parenthesis) and the knowledge of sexual dimorphism prior to this studyin colour, size our both; those that do not appear to have any sex dimorphism (not known) and others that seem to have almost certainly sex dimorphism especially in morphometrics (in bolt) and others in which there were doubts for different reasons (?). The species are ordered alphabetically, according to the scientific name.
Morphometrics and sex
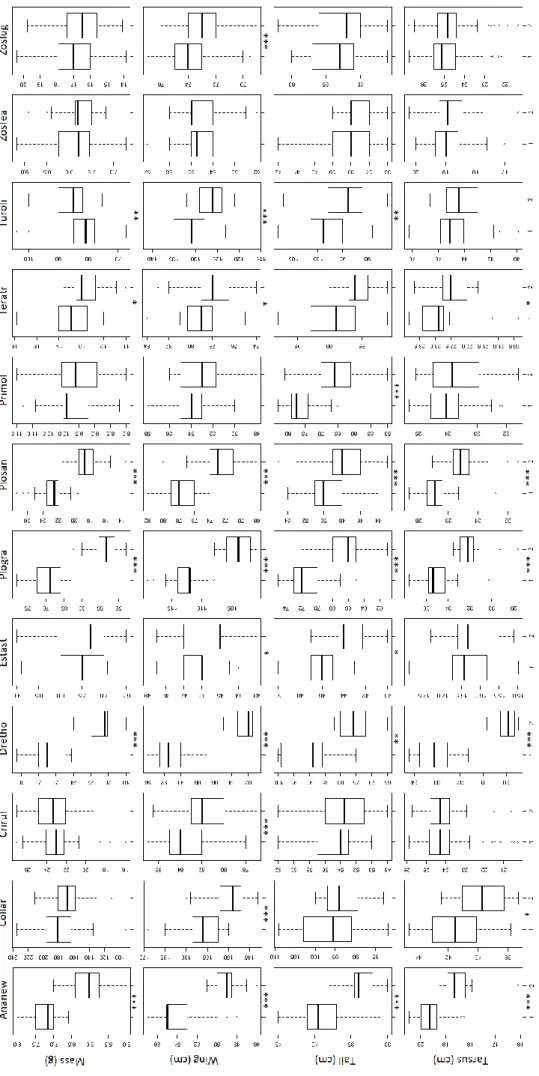
Except for the São Tomé White-eye species, there were significant morphometric differences between sexes for all species (Fig. 1.2 & Fig. S1). The Newton Sunbird, the Giant Sunbird, the Giant Weaver and the São Tomé Weaver showed significant differences between males and females for all measurements (Fig. S1). When there were differences, males were larger than females, except for the weight of the São Tomé Thrush. Out of the four measurements, wing length differed between sexes in 10 out of 12 species, whereas weight and tarsus length differed only in six species.
Despite the large number of significant differences between sexes, most morphometrics revealed a wide overlap, making it difficult to use them as diagnostic characteristics. The exceptions to this were the Giant Sunbird and the Giant Weaver. On the other hand, despite having some sexual dimorphism in coloration, sexes of the São Tomé Paradise-flycatcher are very difficult to distinguish using morphometrics.
English name Scientific name n n sexed n photos prior
Newton Sunbird Anabathmis newtonii 662 66 (10%) 17 (26%) colour
Lemon Dove Columba (larvata) simplex 139 52 (38%) 18 (35%) not known Príncipe Seedeater Crithagra rufobrunnea 462 160 (35%) 25 (16%) not known Giant Sunbird Dreptes thomensis 136 27 (20%) 10 (37%) size
Common Waxbill Estrilda astrild 254 55 (22%) 6 (11%) colour Giant Weaver Ploceus grandis 126 61 (48%) 18 (30%)
colour & size
São Tomé Weaver Ploceus sanctithomae 347 149 (43%) 79 (53%) ?
São Tomé Prinia Prinia molleri 340 62 (18%) 10 (16%) ?
São Tomé
Paradise-flycatcher Terpsiphone atrochalybeia 485 53 (11%) 16 (30%) colour
São Tomé Thrush Turdus olivaceofuscus 882 88 (10%) 12 (14%) not known São Tomé
White-eye Zosterops feae 109 46 (42%) 2 (4%) not known
Black-capped
11 Fi g u re 1 .2 -M o rp h o m et ric s in S ã o To m é c o m m o n b ir d s p ec ie s. Ea c h c o lu m n r e p re se n ts a s p ec ie s, in d ic a te d b y a c o m b in a tio n o f th e th re e firs t lette rs o f th e g en e ric n a m e a n d th e fi rs t th re e l ette rs o f t h e s p ec ific n a m e o r d e re d a lp ha bet ic a ll y . Ea ch l in e re p r es e n ts a d iffe re n t m o rph o m e tr ic , fo llo w ed b y th e m e a su r e m e n t u n ity i n p a re nth es is. Ea ch g ra p h sho w s th e ra n g e o f v a lu es fo r m a le s (1 – o n th e left sid e) a n d fe m a le s (2 – o n th e rig h t si d e ): th e b o ld li n e re p re se n ts th e m ed ia n , th e b o x th e q u a rtil es a n d th e w h isk e rs th e 9 5 % in te rv a l co n fid en ce , a ssum in g a n o r m a l d ist rib u tio n o f th e d a ta . Th e a ste ris k s u n d er t h e g ra p h s sho w th e lev el o f sig n ifi ca n c e fo r th e d iff er en ce b etw ee n se xe s: “ *” – P ≤ 0 .0 5, “ ** ” – P ≤ 0 .0 1 an d “* ** ” – P ≤ 0. 00 1.
12
Distinguishing sex and age: Individual species accounts
Newton Sunbird Anabathmis newtoni. Adult has a clear sexual dimorphism (Fig. 1.3): the male
has a metallic blue throat and sunny yellow breast and belly, while the female has as a brownish throat and dull yellow underparts. Males are larger than females for all morphometric traits (Table 1.2). The coloration pattern of the juvenile is identical to that of the females (Fig. 1.3), and can only be distinguished by the overall weaker quality of the feathers or other general characteristics of juvenile birds, such as a bright, well-developed, gape.
Lemon Dove Columba larvata simplex. Sexual dimorphism is present but much reduced in adults:
the eye ring (bare skin) is red in males and grey in females; wings are often longer in males (Table 1.2). The juveniles are much browner, less iridescent, than adults (Fig. 1.4), with the wing coverts being fringed with golden orange bars; the iris is dark reddish-brown and the bill is light pinkish grey, compared to the red iris and dark bill of the adults.
Príncipe Seedeater Crithagra rufobrunnea. No sexual dimorphism was detected (Fig. 1.5). The
length of the wings is the only measurement that can distinguish male and female, but only for extreme values (Table 1.2). Juveniles can only be distinguished by the overall weaker quality of the feathers and other general characteristics of juvenile birds. Although we suspect that there is a possible difference in the coloration of the iris of adults and juveniles, this was difficult to demonstrate due to the influence of light conditions in our assessment of colour.
Figure 1.4 - Photos of Lemon Dove. From left to right: adult male, adult female and fledgling. Figure 1.3- Photos of Newton Sunbird. From left to right: adult male, adult female and juvenile.
13
Giant Sunbird Dreptes thomensis. Strong sexual dimorphism in morphometrics but not on colour:
females are smaller than males with no overlap in any of the measurements, except in a few occasions on the tail (Fig. 1.2, Table 1.2). The bill of females is visibly shorter than that of males, in proportion to body size. The coloration pattern of the juvenile is identical to that of adults (Fig. 1.6) and can only be distinguished by the overall weaker quality of the feathers or other general characteristics of juvenile birds, such as a protruding gape.
Common Waxbil Estrilda astrild. The sexes of adult Common Waxbill can be identified by the
undertail coverts, which are dark brown to completely black in the males and paler and visibly barred in the females (Fig.1.7). Males also have a pinkish-red flush along the belly, which is paler on females (Fig. 1.7). For extreme values, the sexes can be distinguished based on tail length (Table 1.2). Recently fledged juveniles are easily distinguished by the black bill and white gape flange which turns red with hints of brown in older juveniles; the bill of the adults is reddish orange and has a very small dark gape (Fig.1.7).
Figure 1.6 - Photos of Giant Sunbird. From left to right: adult male and female.
Figure 1.7 - Photos of Common Waxbill. From left to right: adult male, adult female and juvenile. The female of this photo have dirt on its bill.
14
Giant Weaver Ploceus grandis. Species with a clear sexual dimorphism in adults in all
morphometric characters (Table 1.2) and colouration. The head of males is black, down to the chin and throat, blending into chestnut-brown on the rear of the crown, neck and breast; the mantle and upper back are greenish yellow to olive, ending with black feathers with yellow tips, which continue to the wings; the primary feathers are all black; the belly and flanks are bright sunny yellow, as is the iris; the uppertail coverts are dark yellow, the tail is dark olive brown, and the bill is black and massive (Fig.1.8). The females have a brown head with a pale throat; the bill is also strong and completely black; the iris is a dull yellow; the breast is dark yellow to orange mottled with light brown, and blending into a brownish flank; the belly is white bending with the flanks; the wings have dark feathers with yellow tips; the mantle to the upper back is dark yellow to brownish (Fig. 1.8). The juveniles can be told apart from the females by: a non-black bill (dark brown upper mandible and pinkish brown lower mandible) with yellow and enlarged gape flanges in very young birds; a dark brown iris that becomes lighter with age; light flanks and a white belly; a paler breast with a yellowish brown colour; lighter olive brown head and upper back. The wings of the juveniles are similar to those of female, but are more dark brown than black.
São Tomé Weaver Ploceus sanctithomae has no clear sexual dimorphism. Individuals of this
species show a wide range of plumage colour intensity: from well-marked individuals, with a black crown and bright orange plumage, to dull individuals with a very faint crown. The locals associate these extremes with male and female plumages and so do some authorities (Craig 2017). Using molecular sexing, this study demonstrates that there is no colour dimorphism in the adults. The colourful individuals are adults, the dull ones juveniles. In the youngest juveniles there is no distinguishable crown, the bill is pink, since the upper mandible has no light brown (Fig. 1.9). The gradation in colour intensity in the adults is more likely related to wear, as suggested by Christy and Clarke (1998). In the adults, morphometric traits can distinguish the large males from the small females in every trait (Table 1.2).
Figure 1.8 - Photos of Giant Weaver. From left to right: adult male, adult female and juvenile.
15
São Tomé Prinia Prinia molleri. Species without clear sexual dimorphism. The males have a
chestnut to orange face and a rufous-brown crown merging into grey on nape, a pattern that is more faded on females (Fig. 1.10). Males also tend to have a longer tail, which allows sexing for extreme values (Table 1.2). The coloration pattern of the juvenile is almost identical to that of adult females but with a yellow wash in the throat and the breast (Fig. 1.10), and can be distinguished by the overall weaker quality of the feathers; the iris is greyer than in the adults which is warm brown. The inner lower mandible is more yellow pink in juveniles and pink in adults.
São Tomé Paradise Flycatcher Terpsiphone atrochalybeia. The adult São Tomé
Paradise-flycatcher has a clear sexual dimorphism in plumage colour. The adult males have an entirely dark-blue glossy dark-dark-blue plumage; the eye has a light-dark-blue featherless eye ring; with two central streamers that can reach up to 11.5 cm beyond the remaining tail feathers (Fig.1.11). The females have a rusty red mantle and back; the nape, lores and throat are light grey; they have a glossy/ iridiscent blue crown; the light-blue featherless eye ring is less developed and duller than that of males; the breast, belly and flanks are pale grey; the primaries and secondaries are dark brown; the tail is rusty-red with a dark patch at the end (Fig. 1.11). The juveniles are difficult to sex as they are very similar to adult females. Nevertheless, they are duller, especially in the rusty-red plumage and in the sky-blue eye ring. Fledglings have grey faces and small dull eye rings, and the bill is yellowish with a black tip (Fig.1.11). It appears that juvenile males may undergo more than one moult before reaching the full black adult plumage. Such putative immatures or young adult males have a few dark-blue iridescent feathers scattered across a female-looking plumage pattern. The sexes can be distinguished when the induviduals present extreme values of weight and tarsus length (Table 1.2).
Figure 1.11 - Photos of São Tomé Paradise-flycatcher. From left to right: adult male, adult female and juvenile. Figure 1.10 - Photos of São Tomé Prinia. From left to right: adult male, adult female and juvenile.
16
São Tomé Thrush Turdus olivaceofuscus. Species with reduced sexual dimorphism. Adult males
have a red iris, whereas the females have a chocolate brown iris (Fig. 1.12 & Fig. S2). Morphometric characters allow distinguishing males and females at extreme values of weight, tail length and especially wing length (Table 1.2). The coloration pattern of the juvenile is identical to that of adults, although they tend to have more white feathers in their chest and depending on the age the iris can be brownish-grey, brown or brownish-red. Young juveniles have the wing coverts tipped with light rusty-brown as typical of the Turdus genus.
São Tomé White-eye Zosterops feae. Species with no sexual dimorphism (Table 1.2). Juveniles
are indistinguishable from adults (Fig. 1.13), except for the overall weaker quality of the feathers and, depending on the age of the juvenile, they can have some of their orbital feathers still growing. The tip of the bill is generally lighter grey in juveniles.
Black-capped Speirops Zosterops lugubris. Species with very little sexual dimorphism. Sexes can
only be distinguished using morphometric characters in extreme cases (Table 1.2). Juveniles’ coloration pattern is identical to that of adults (Fig. 1.14). Juveniles can be distinguished by the same characteristics as the juveniles of São Tomé White-eye.
Figure 1.12 - Photos of São Tomé Thrush. From left to right: adult male, adult female and juvenile.
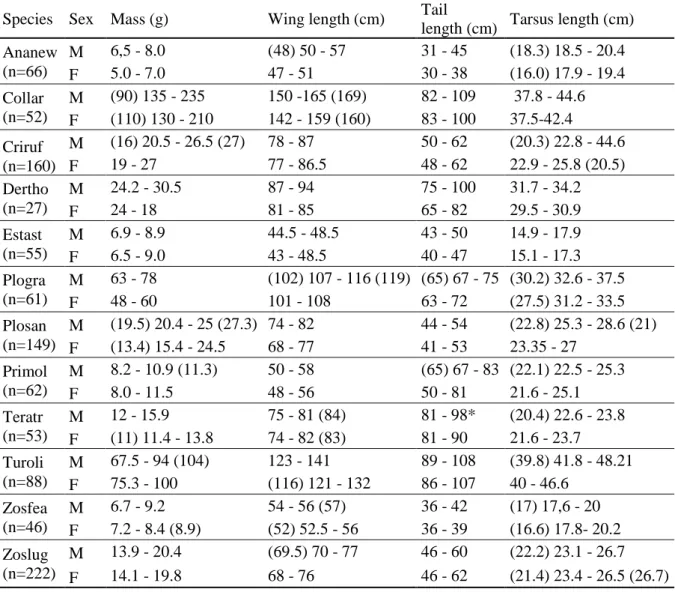
17 Table 1.2 – Measurements of São Tomé common bird species. These values refer to all individuals that were measured, and then sexed using molecular techniques. The values show the range of measurements that fall within a 95% confidence interval, assuming a normal distribution, and additional maximum and minimum values in parenthesis when there are outliers that fall outside that range. The species are ordered alphabetically, according to the scientific name and are indicated by a combination of the three first letters of the generic name and the first three letters of the specific name (see Table 1.1). The letter “M” represents the males and the letter “F” the females.
Species Sex Mass (g) Wing length (cm) Tail
length (cm) Tarsus length (cm) Ananew (n=66) M 6,5 - 8.0 (48) 50 - 57 31 - 45 (18.3) 18.5 - 20.4 F 5.0 - 7.0 47 - 51 30 - 38 (16.0) 17.9 - 19.4 Collar (n=52) M (90) 135 - 235 150 -165 (169) 82 - 109 37.8 - 44.6 F (110) 130 - 210 142 - 159 (160) 83 - 100 37.5-42.4 Criruf (n=160) M (16) 20.5 - 26.5 (27) 78 - 87 50 - 62 (20.3) 22.8 - 44.6 F 19 - 27 77 - 86.5 48 - 62 22.9 - 25.8 (20.5) Dertho (n=27) M 24.2 - 30.5 87 - 94 75 - 100 31.7 - 34.2 F 24 - 18 81 - 85 65 - 82 29.5 - 30.9 Estast (n=55) M 6.9 - 8.9 44.5 - 48.5 43 - 50 14.9 - 17.9 F 6.5 - 9.0 43 - 48.5 40 - 47 15.1 - 17.3 Plogra (n=61) M 63 - 78 (102) 107 - 116 (119) (65) 67 - 75 (30.2) 32.6 - 37.5 F 48 - 60 101 - 108 63 - 72 (27.5) 31.2 - 33.5 Plosan (n=149) M (19.5) 20.4 - 25 (27.3) 74 - 82 44 - 54 (22.8) 25.3 - 28.6 (21) F (13.4) 15.4 - 24.5 68 - 77 41 - 53 23.35 - 27 Primol (n=62) M 8.2 - 10.9 (11.3) 50 - 58 (65) 67 - 83 (22.1) 22.5 - 25.3 F 8.0 - 11.5 48 - 56 50 - 81 21.6 - 25.1 Teratr (n=53) M 12 - 15.9 75 - 81 (84) 81 - 98* (20.4) 22.6 - 23.8 F (11) 11.4 - 13.8 74 - 82 (83) 81 - 90 21.6 - 23.7 Turoli (n=88) M 67.5 - 94 (104) 123 - 141 89 - 108 (39.8) 41.8 - 48.21 F 75.3 - 100 (116) 121 - 132 86 - 107 40 - 46.6 Zosfea (n=46) M 6.7 - 9.2 54 - 56 (57) 36 - 42 (17) 17,6 - 20 F 7.2 - 8.4 (8.9) (52) 52.5 - 56 36 - 39 (16.6) 17.8- 20.2 Zoslug (n=222) M 13.9 - 20.4 (69.5) 70 - 77 46 - 60 (22.2) 23.1 - 26.7 F 14.1 - 19.8 68 - 76 46 - 62 (21.4) 23.4 - 26.5 (26.7) Figure 1.14 - Photos of Black-capped Speirops. From left to right: adult male, adult female and juvenile.