Mestrado Integrado em Medicina Veterinária Ciências Veterinárias
Chewing Lice in Birds of Northern Greece
José Bernardo Pedroso Couto Soares
Orientador: Professor Doutor Ivan Literák Co-orientador: Professor Doutor Luis Cardoso
Universidade de Trás-os-Montes e Alto Douro Vila Real, 2017
Acknowledgements
To begin with, I would like to thank my supervisor Professor Doctor Ivan Literák for suggesting my master thesis topic, welcoming at his Department of Biology and Wildlife Diseases, at Brno’s University of Veterinary and Pharmaceutical Sciences. To Professor Sychra Oldřich for his warm welcoming to the Laboratory of Entomology and guidance on the laboratory work.
Also, I would like to thank Ass. Prof. MVDr. Anastasia Diakou, Dr. Alivizatos Charalampos and all the team members present in the field-base studies within Koronea and Volvi National Park, for the hospitality demonstrated in the simple things, such as the typical Greek pastry brought every morning, laughs and small talk that greeted me every early morning.
But most of all I would like to thank Professor Doutor Luis Cardoso for his empathy, advices and acknowledgement of my work.
Finally, I want to show my deep appreciation to my life partner, Jarmila Bednárová, for her emotional support and kind words, and above all, for the big adventures to the smallest of moments shared. May we continue growing-up together and learning from what life may bring us.
“Klaar is Kees” Jan-Willem Höppener
Table of Contents
Acknowledgements... II Abstract... V List of Abbreviations ... VII Index of Images ...VIII 1. Introduction... 10 2. Literature review... 11 2.1. Order Phthiraptera...112.1.1. Evolution and taxonomy... 11
2.1.2. Biology... 12
2.2. Chewing lice - Suborders Amblycera and Ischnocera...15
2.2.1. Taxonomy... 15
2.2.4. Morphology... 16
2.2.6. Biology... 20
2.3. Suborder Amblycera (avian lice)...21
2.3.1. Family Menoponidae... 22
2.3.3. Family Laemobothriidae... 22
2.3.4. Family Ricinidae... 23
2.4. Suborder Ischnocera (avian lice)...23
2.4.1. Family Philopteridae... 24
2.5. Birds...24
2.5.1. Birds as parasitic hosts... 24
2.5.1. National and migratory birds... 27
2.6. Chewing lice in birds of Greece...29
3. Materials and methods... 31
3.1 Field Study...31
3.2. Methods of laboratory work...33
3.2.1 Canada balsam technique... 33
3.3. Statistical analysis...35
3.4. Morphometric analysis... 36
3.4.1 Morphometric analysis of genus Philopterus... 36
4. Results ... 40
4.1. Occurrence of chewing lice in passerines in Northern Greece...40
4.2. Morphological comparison of Penenirmus longuliceps and Penenirmus albiventris...42
4.3. Morphological characteristics of specimens of the genus Philopterus...44
5. Discussion... 48
6. Conclusion ... 50
7. References ... 51
Abstract
In recent years, chewing lice have been the subject of extensive and innovative studies on host-parasite relationship and co-evolution (Smith, 2003). Furthermore, the fitness of the wild passerine population is an indicator of environmental changes as they sensitively react to pollution and other negative effects. Also, the knowledge concerning the infestation of passerine hosts by ectoparasites allows also the use of ectoparasites infestation as a sentinel of the overall ecological status of a biotope.
Within the Greek context this geographical area holds a great importance local and migratory European bird species by providing a great variety of habitats. With a recorded 442 bird species, of which, 242 are locally breeding, while the rest are migratory birds. Despite this data concerning louse fauna of birds living in Greece are practically lacking. Hence the main aim of this work was to provide the first parasitological survey conducted on ectoparasites of birds.
The field work was conducted, within several locations, inside Koroneia and Volvi National Park in Northern Greece. From 2013 to 2016, comprising 9 ornithological ringing sessions, where a total of 729 birds were examined and a total of 560 lice specimens were collected and further examined under laboratory conditions.
This parasitological study allows to a better understanding of the host-parasite relations and discover new associations. Moreover, this study allowed a review of the association between Acrocephalus melanopogon and Philopterus-complex, and a re-description of Philopterus acrocephalus.
The parasitological results also mimic other reports of bird’s chewing lice from Europe, that present a suggestive deterioration of habitats.
Ultimately the better knowledge of the complex parasite-host systems may also provide tools for designing successful measures for wildlife and environment conservation.
Resumo
Nos últimos anos, os malófagos mastigados têm-se tornado objecto de extenso e inovadores estudos sobre a relação entre hospedeiro-parasita e a sua co-evolução. Adicionalmente a condição da população de passarideos é um indicador de alterações ambientais, devido à sua sensibilidade a efeitos negativos, tal como a poluição. Paralelamente o conhecimento dos níveis de infestação de hospedeiros passarideos por ectoparasites permite o uso destes dados como representativos do bem-estar ecológico do biotipo.
No contexto da Grécia, esta área geográfica é de grande importância para espécies de passerideos migratórias e autóctones, pois proporcionando uma grande variedade de habitats. E com registros de 442 species de aves, das quais, 242 são autóctones, e as restantes migratórias. Apesar destes números, existe falta de dados relativamente à fauna de ftirápteros na Grécia. Logo, o objectivo principal deste trabalho é providenciar o primeiro estudo parasitológico de ectoparasites em aves.
O trabalho de campo foi conduzido, em diversos locais, dentro dos Parques Nacionais de Koroneia e Volvi no Norte da Grécia. De 2013 a 2016, compreendendo 9 sessões captura ornitológica, nas quais foram examinados 729 espécies de aves e um total de 560 espécimes de malófagos foram coletados e examinados em condições laboratoriais.
Este estudo parasitológico permitiu compreender melhor a relação entre hospedeiro-parasita e descobrir novas associações. Adicionalmente, a revisão da relação entre
Acrocephalus melanopogon e o complexo Philopterus sp., e re-descrever a espécie Philopterus acrocephalus.
Os resultados, também, demonstraram ser similares a outros trabalhos em malófagos mastigadores em aves na Europa, os quais parecem apresentar uma aparente deterioração dos habitats.
Em suma, o melhor conhecimento do complexo sistema de hospedeiro-parasita providencia ferramentas para o desenvolvimento de medidas de conservação ambiental e fauna selvagem.
Palavras-chave: Phthiraptera, Ischnocera, Amblycera, Philopterus, ectoparasites, birds, Greece.
List of Abbreviations
ADPL= anterior dorsal plate length (taken at middle line) ADPW=anterior dorsal plate width (taken at its widest point) ANW= width of anterior notch (taken between the bases of as3) AW= maximum width of the abdomen (taken at level of segment V) AL= abdomen length
EWG= external width of genital chamber HL= head length
IWG= internal width of genital chamber PAL= preantennal length
PAW= preantennal width from the bases of the coni
POL= preantennal length (taken from the base of the conus to the bases of as3, obliquely to the head axis)
PTW= pterothorax width PTL= pterothorax length PW= prothorax width TL= total length
TPVL= tergal plate V length TRL= trabecula length TRW= trabecula width TW= temple width F = female M = male IMM = immature L = left R = right
Index of Images
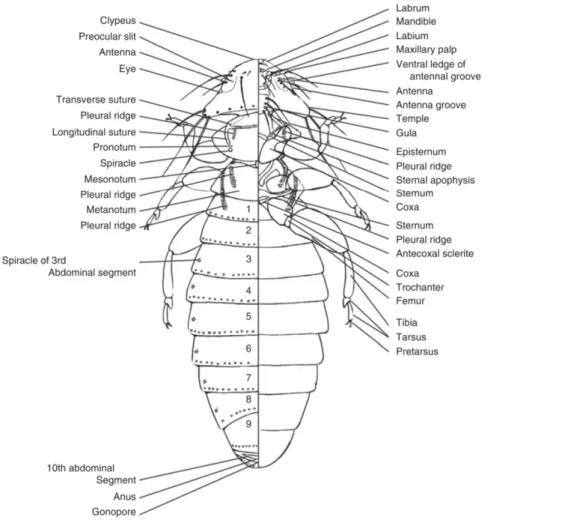
Figure 1 Diagram of a chewing louse with display of dorsal view (left) and ventral view
(right) (8) ... 12
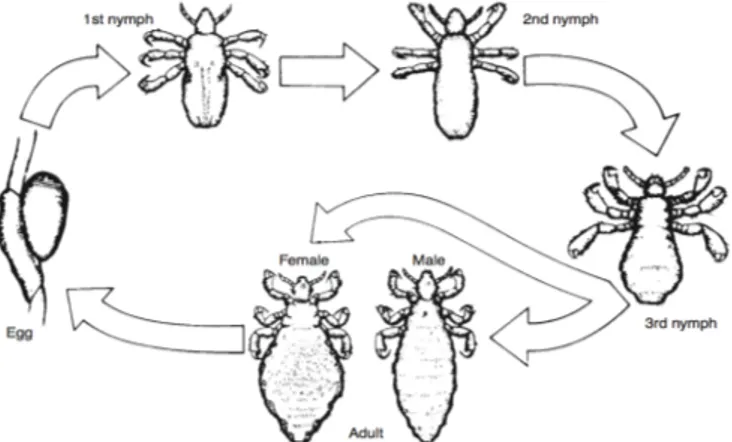
Figure 2 Life cycle of head lice, Pediculus humanus capitis (1) ... 14
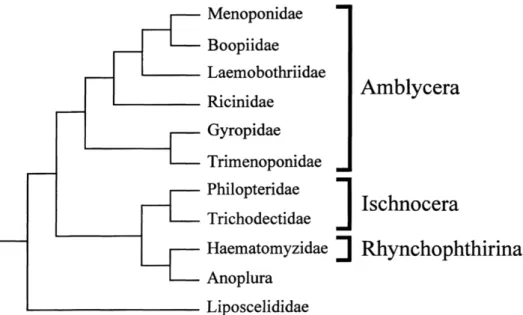
Figure 3 Taxonomic organization of suborders and families (4) ... 16
Figure 4 Internal features of chewing lice. Alimentary canal of Docophoroides brevis (Ischnocera: Philopteridae) ex Wandering Albatross (Diomedea exulans) (4)... 17
Figure 5 Internal features of chewing lice. Male reproductive tract of Craspedorrhynclus spathulatus (Ischinocera: Philopteridae) ex Black Kite (Milvus m. migrans) (4)... 18
Figure 6 Internal features of chewing lice. Female reproductive tract of Philopterus ocellarus (Ischnocera: Philopteridae) ex Carrion Crow (Corvus corona sharpii) (4) ... 19
Figure 7 Outline of head of suborder Ischnocera (A) and Amblycera (B) (13)... 21
Figure 8 Different types of feathers (37)... 25
Figure 9 Locations where birds were captured and examined in Greece. a: Loutra Volvis), b:Platia Volvis, c:Nea Agathoupoli Pieria, d:Dasos Apollonia, e:East Cost of Koronia Lake, f:Varvara). g:Porto Lagos... 31
Figure 10 Captured birds being subjected to fumigation to collect ectoparasites, Greece (own authorship)... 32
Figure 11 Fumigation chamber used for smaller birds (own authorship) ... 33
Figure 12 Fumigation chamber for the removal of lice from live birds (26) ... 32
Figure 13 Morphometric measurements of the head of the parasite (34) ... 37
Figure 14 Morphometric body measurements of the ectoparasite (34) ... 38
Figure 15 Drawing of the male (left) and female (right) Penenirmus albiventris (own authorship)... 42
Figure 16 Penenirmus albiventris (F) ... 43
Figure 17 Philopterus acrocephalus (F) (own ownership) ... 44
Figure 18 Philopterus acrocephalum (M) (own ownership) ... 45
Figure 20 Philopterus acrocephalum (F) (own ownership)... 45
Index of Tables
Table 1 Morphometric measurements of Penenirmus albiventris ... 43
Table 2 Morphometric measurements of Philopterus sp. ... 46
Table 3 Number of setae in male and female Philopterus sp... 47
Table 4 Number of captured birds at diverse locations in Northern Greece... 55
Table 5 Host-parasite association... 57
Table 6 Percentage of dominance. ... 62
Table 7 Percentage of dominance of ectoparasites in different bird species. ... 62
Table 8 Prevalence of ectoparasites in different bird species. ... 64
1. Introduction
The following thesis derives from cooperation between Prof. MVDr. Ivan Literák and Assoc. Prof. RNDr. Oldřich Sychra of the Department of Biology and Wildlife Diseases, Faculty of Veterinary Hygiene and Ecology of the University of Veterinary and Pharmaceutical Sciences of Brno and Ass. Prof. MVDr. Anastasia Diakou of the Laboratory of Parasitology and Parasitic Diseases, School of Veterinary Medicine, Faculty of Health Sciences of Aristotle University of Thessaloniki in a research project focused on the ecology and evolution of ectoparasites and wild birds in Northern Greece.
In collaboration with the administration of Koronea and Volvi National Park and Dr. Alivizatos Charalampos from the Greek Ornithological Society, member of the Hellenic Bird Ringing Centre, several field-base studies were conducted inside the national park, from 2013 to 2016, using mist nests to capture bird species. Every individual bird was identified, sexed and aged using appropriate books and chewing lice specimens were collected using fumigation chamber method and visual inspection of the host’s body.
Subsequent to the field work, collected samples were slide mounted in permanent preparations in the Entomological Laboratory of the Department of Biology and Wild Life Diseases from the University of Veterinary and Pharmaceutical Sciences of Brno and followed by the study of the specimens’ anatomy and taxonomic classification.
The aims of this thesis are (1) to broaden the knowledge on geographical distribution of chewing lice species on small passerine birds in Northern Greece; (2) to analyze parasitological parameters of lice infestations; (3) the morphological comparison of
Penenirmus longuliceps and Penenirmus albiventris; (4) to identify morphologically the
samples of the genera Philopterus collected from Acrocephalus melanopogon and to do the appropriate comparison with other close related hosts.
2. Literature review
2.1. Order Phthiraptera
2.1.1. Evolution and taxonomy
The first written records of lice date back to Ancient Egypt, i.e. 3,000 to 400 BC, and physical evidence were found in mummies, such as a 10,000 years-old human head from northeastern Brazil. And although there is no scientific consensus on the age and origins, various authors suggest that Phthiraptera lice date back to the Late Carboniferous and the end of the Cretaceous, 66-320 million years ago, and derive from Psocoptera lice (bark lice), which each share several important morphological synapomorphies. Phthiraptera lice are highly adapted and successful ectoparasites of mammals and birds, being comprised of 5,800 species of insects and whose name derives from the Greek words “phtheir” (louse), plus “a” (without), and “pteron” (wing). (1-4)
Phthiraptera was traditionally classified in two orders the Mallophaga (chewing lice) and the Anoplura (sucking lice). This classification, although convenient, has been suggested to not reflect the phylogenetic relationship between the suborders. Following morphology, Lyal (5) and molecular studies, Barker (6), the Mallophaga (Ischnocera, Amblycera, Rhynchophthirina) were found to be paraphyletic in respect to the Anoplura and each of the three suborders (Ischnocera, Amblycera and Rynchophthirina) monophyletic. These studies were conclusive to the fact that chewing and sucking lice are plesiomorphic rather than synapomorphic. (1,6,7)
Currently, modern louse systematic agree that Phthiraptera taxonomic order is comprised of four suborders: Amblycera, Ischnocera, Rhynchophthirina, and Anoplura. All species of Anoplura and Rhynchophthirina are restricted to mammals, whereas species of Ischnocera and Amblycera are known from both mammals and birds. (2,8)
2.1.2. Biology
Lice are dorso-ventrally flattened, wingless insects with a body segmented into head, thorax and abdomen. The shape of the body and head varies considerable, particularly in the avian chewing lice, in adaptation to the different ecological microhabitats on the host’s body. Size ranges from 0.8 mm to 11 mm has a strong correlation between the size of the host and the size of their lice as described by the Harrison’s Rule, and despite the considerable variation among taxa (9). There’s also an influence from the sexual dimorphism, in which females are typically 10-20% larger than males. (2, 8, 10)
Highly modified mouthparts can be distinguished in the head, which are involved in feeding, and play a vital secondary role in anchoring the louse to the host. A pair of short antennae (divided into 3-5 short segments) and reduced or absent eyes. (2,3)
The shape of the head and body varies considerably between the taxonomic groups. In the sucking lice, the head is characteristically smaller than the prothorax and possesses highly modified mouthparts adapted for piercing the skin of their hosts. In the chewing lice the head is wider then their prothorax with exception of Rynchophthirina, the sister group to Anoplura. Their size of the head allows accommodating their diet specifications and mouthparts with mandibles adapted for biting and grasping. (1,3,10)
The insect thorax is divided into three segments: prothorax, mesotorax, metathorax. The dorsal exoskeletal plates, mesonotum or metanonum, may be fused into the ptherotorax and in Anoplura the three plates are fused into a single segment. Legs are well developed and modified for clinging to their host’s feathers or pelage. The tarsus is subdivided into two tarsomeres and the pretarsus bears two tarsal claws in bird lice and one claw in lice of mammals. Also in the thorax, there is one pair of thoracic breathing pores (spiracles). (3,10)
The elongated abdomen adorned with numerous setae is composed of sclerotized dorsal (tergite), ventral (sternite) and lateral (pleurite) plates which provide rigidity to the abdomen and allow this to distend after a meal. Typically there’s between 8-11 visible segments (numbered by Roman numerals) and six abdominal spiracles borne from segments II-VIII. In the adult the abdominal segments terminate in genitalia. In females the genital lobe is homologous to segment IX and in males the genital opening is posterior to sternite IX. (10,11)
As a permanent ectoparasite lice display variety of behavioral and morphological adaptations to remain attached to the host and ensure survival. The dorso-ventrally flattened body allows them to lay flat against the hair shaft or between feathers, thus reducing the chance of becoming dislodged during grooming. Furthermore, the three pairs of jointed legs have claws for clinging tightly to fur, hair and feathers. Plus, when escaping grooming, lice use their mandibles to grip to feather barbs or hair shafts. (2,3)
Coloration varies from pale beige to dark grey and cryptic coloration is sometime present allowing the louse to match the host’s plumage or pelage. Color may also darken considerably on feeding of epidermal tissue debris, part of feathers, sebaceous or blood. (2,3)
Diet and feeding ecology also contribute as a major factor for the taxonomic division of the Phthiraptera. While the Anoplura feed exclusively on mammalian blood the chewing lice, (Amblycera, Ischnocera) feed primarily on hair and feathers, but also occasionally sebaceous secretions, mucus, shedding epidermis and if available blood exuding from scratched skin. (1,2)
Lice display remarkable host specificity, and most individuals infest just one host during their hemimetabolous life cycle which compromises three stages egg, nymph and adult. Mating occurs on the host and mature female lice generally deposit one to two eggs per day, and considering adults commonly live for up to a month, this means that she will lay an average of 50 to 150 eggs. On oviposition eggs are directed, one at a time, through two pairs of finger-like gonopods into a precise location and orientation while a cement substance is secreted around the egg. They are laid in single or in clumps and cement into the host’s feathers and hairs. Eggs are usually simple ovoid, with a terminal cap, the operculum and depending on the habitat and temperature require 4 to 10 days of incubation before hatching. (1,11,12)
Development proceeds through three to five nymphal instars which last between 3 to 12 days each depending on the specie and influenced by environment conditions. The hatching of the first-stage nymph occurs due to the passage of air through the alimentary canal that then accumulates behind the nymph. When sufficient pressure is reached, the operculum, on the non-cemented end of the egg is forced open. In some louse species this first-stage nymph, although not sexually mature, may resemble a small adult with fewer setae and lacking genitalia but in others cases it undergoes a development stage in which through each nymphal instar the nymph more closely will resemble the adult. (1, 2, 10-12).
Along one to three weeks the nymph feed and molt through the several stages, finalizing on the molting to a sexually mature adult. The entire life-cycle lasts between 4 to 6 weeks. (3)
Direct contact, is the primary route of transmission, for louse occurring through two pathways: firstly vertical transmission mother-to-child while nesting; and secondly horizontal transmission between two adults in shared nest holes, mating and other means. Less common ways of transmission occur predator-prey interactions and mixed species use of dust baths and phoresy, in which lice attach to other, larger and more mobile, arthropods and are carried from one host to another (2, 11, 13).
Lice respond to warmth, humidity and chemical odours through receptors located on the antennae but also heat and humidity receptors present over the entire body. Lice have a tightly defined band of humidity and temperature preferences which can be described as a microclimate which lead for several species of lice to coexist on the same area of the host species (microhabitat). These microhabitats are more evident on birds and compartmentalised by the different feather types present on the head, wing, back and rump. (2, 3)
With a worldwide parasite distribution, lice mirrors, with few exception, the distribution of the host. Its prevalence and abundance are often associated with host’s annual activities but in cases of sick animals, such as birds with damage bills enabling them to groom or preen effectively, an abnormally high parasitism can be present on an individual. (2)
2.2. Chewing lice - Suborders Amblycera and Ischnocera
2.2.1. Taxonomy
Modern classification divides the taxonomic order Phthiraptera into four suborders, three of which constitute suborders with chewing lice: Amblycera, Ischnocera and Rhynchophthirina. The Amblycera and Ischnocera compose the larger suborders containing families whose species parasitise birds and mammals while Rhynchophthirina is comprised of two species, one of which is parasite of warthogs and the other of elephants. (3, 4)
The suborder Amblycera is divided into 6 taxonomic families with a total of 1,334 lice species from which the ones composing the taxonomic families Menoponidae, Laemobothriidae, Ricinidae and the genus Therodoxus from Boopiidae parasitise birds. Although family level classification is relatively stable only limited molecular phylogenetic analysis to the relationship between genera and monophyly of genera has been done. (4)
Contrary to Amblycera, the classification of the Ischnocera has been difficult and classification of the avian Ischnocera has been problematic. This is suborder is divided into
two families (Philopteridae and Trichodectidae) with a total of 3,060 lice species from which the taxonomic family Philopteridae, except the genus Trichophilopterus, parasitise birds. (4)
The term “chewing lice”, referring to the chewing mouth parts, although outdated, previously used to describe the Mallophaga, is still commonly used for the purpose of zoological and veterinary practice to identify the suborders Amblycera, Ischnocera and Rhynchophthrina and therefore it will be used to describe, in the following paragraphs, as a general term for the avian ectoparasites species present within the Amblycera and Ischnocera suborders. (13)
Figure 3 Taxonomic organization of suborders and families (4)
2.2.4. Morphology
An adult chewing louse has a dorsally flattened body varying in length from 0.8 mm to 11 mm and in most species the female is larger than the male, often by 20%. On their large head, broader then their prothorax, are visible mandibular mouthparts on the underside of the head which are composed of a labrum, a pair of mandibles and a pair of maxillae attached laterally to the labium which is reduced to a simple broad plate. Antennae have between 3 to 5 segments and the eyes are absent or, exceptionally in a few species, reduced which function as light sensors. (3, 4, 10)
The prothorax is distinctly separated from other thoracic segments but mesothorax and metathorax may be divided (Amblycera) or fused, forming a pterothorax (Ischnocera). The
And each abdominal segment is composed by dorsal (tergite), ventral (sternite) and lateral (paratergite) plates. (4, 10)
Lice sense organs are their mouths, antennae, eyes (in some species) and sensory hairs, or setae, distributed over the body, which also protect lice from being dislodged by the host grooming. (4, 10)
Avian chewing lice feed mostly on feather but also on blood and other fluids, which are obtained by gnawing the skin or from the central pulp of a developing feather. Their alimentary canal fills most of their internal anatomy and is composed by a esophagus, a well-developed crop and midgut, a smaller hindgun, four malpighian tubules, and a rectum with six papillae. (10)
Figure 4 Internal features of chewing lice. Alimentary canal of Docophoroides brevis (Ischnocera: Philopteridae) ex Wandering Albatross (Diomedea exulans) (4)
When feeding the mandibles sever small pieces of feather, which drop onto the labrum and are forced inside the mouth from which progress through the oesophagus until the crop. Depending on the suborder, and reflecting the species diet, the crop may be a simple sweeping between the oesophagus and midgut or a diverticulum from the oesophagus. (4, 10)
By the arrival of feather fragments the crop initiates pulsating movements inducing the breaking up of the food into smaller particles by rubbing them against comb-like teeth in the crop walls. Beside the mechanical force, to digest the feather’s keratin, the feather-eating lice species rely on powerful gut enzymes and endosymbiotic bacteria. Furthermore, haematophagous, and facultative haematophagous, lice species also develop symbiotic relationship with haemolytic bacteria that reside in specialised cells called mycetocytes. (1, 4, 10)
In many Ischnocera and some Amblycera species, which aren’t partly blood-feed have an efficient water-vapour uptake system that extracts water from the atmosphere. For this process, they use the lingual sclerites which are posted vertically between the labrum and labium, enabling this way for the lice to feed solely on feathers and dry flakes of dead skin and other debris. (2, 4)
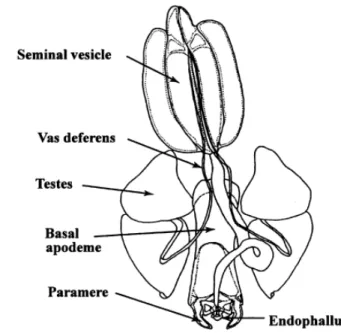
Figure 5 Internal features of chewing lice. Male reproductive tract of Craspedorrhynclus spathulatus (Ischinocera: Philopteridae) ex Black Kite (Milvus m. migrans) (4)
The reproductive tract is composed by the genitalia which in the male encompass up to half of the length of their abdomen with a typical configuration comprised of, a pair of mesomeres, a pair of parameres, and a flattened or rod-like basal apodeme, which supports an endophallus, testes, seminal vesicle and vas deferens. The parameres are sickle-shaped structures that lie laterally or ventrally to the endophallus and help to locate the female genital opening and protect the delicate endophallus during copulation. The pair of mesomeres lie laterally or dorsally to the endophallus, and like the parameres, may be fused. The apodeme support the endophallus and associated scleratized structures (parameres and mesomeres) which get everted during copulation. The testes are connected to the vas deferens, which, in turn, coalesce to form the seminal vesicle, which stores the spermatozoa.
(4, 5)
Figure 6 Internal features of chewing lice. Female reproductive tract of Philopterus ocellarus (Ischnocera: Philopteridae) ex Carrion Crow (Corvus corona sharpii) (4)
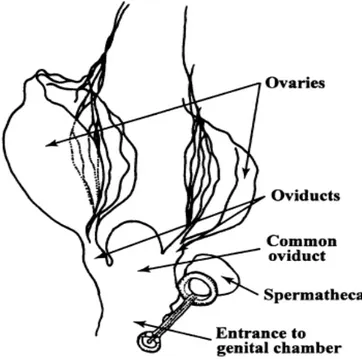
In the female, the reproductive tract is composed of several ovaries, with eggs in various stages of development, which are connected by the oviducts to the common oviduct (or uterus), leading to the entrance of the genital chamber and spermatheca. The male lice deposit a spermatophore in the spermatheca during insemination, which may be in continuous use until it is replaced at a future insemination. Female lice have no ovipositor, but instead finger-like monopods that help position the eggs during laying. Most species of lice attach
their eggs individually or in clumps on the basal regions of the hair or feathers with glandular cement. (4)
2.2.6. Biology
The relationship between the lice and its host is defined by the interaction of biotic and abiotic parameters. The biotic parameters relate or originate from the host and can be translated by the host’s environment, behaviour and microclimates (variations of temperature and humidity near the skin) defining this way the host specificity of the parasite. Due to this factors the lice microhabitat and location for oviposition is influenced by a favourable microclimate and an area that protect from the host preening, such as the scruff of the neck, or between the barbs of feathers. (4, 12, 14)
A adult louse lives around a month, and a female produces on average one egg per day, which corresponds to a total of 12 to 20 eggs. The eggs are adhered to the host’s feathers with the cement substance produced on the female glands, forming nits. And have an incubation period of 4 to 10 days depending on the species. After the hatching the nymph goes through a process of incomplete metamorphosis, hemimetabolism, with three nymphal instars. In some species the nymphs look like small adults but in other there’s a considerable difference on appearance. (4, 12)
As permanent parasites, chewing lice have a prominent role in the study of cospeciation, the joint speciation of both host and parasite, tracking their host’s evolutionary history with varying fidelity. Also in some species occurs the synchronisation of the parasite-host life cycles with described report date of increased population during parasite-hosts breeding season.
(12, 15)
Population density is influenced by several factors such as hosts size, preening ability (hots’s defences) and season. The parasitic effect on the host and is mostly function of their density and not due to direct damage. In which heavy louse infestation, pediculosis, is usually associated with young animals or older animals in already poor health. (3, 4)
2.3. Suborder Amblycera (avian lice)
Chewing lice in the suborder Amblycera differ anatomically from other chewing lice through the following characteristics: four antennae segments, with a pedunculate third segment; antennae recess in the lateral grooves; mandibles articulate horizontally from the head and lie parallel to its ventral surface; maxillary palpi may have two to five joints; mesothorax and metathorax are divided. (2-4, 13)
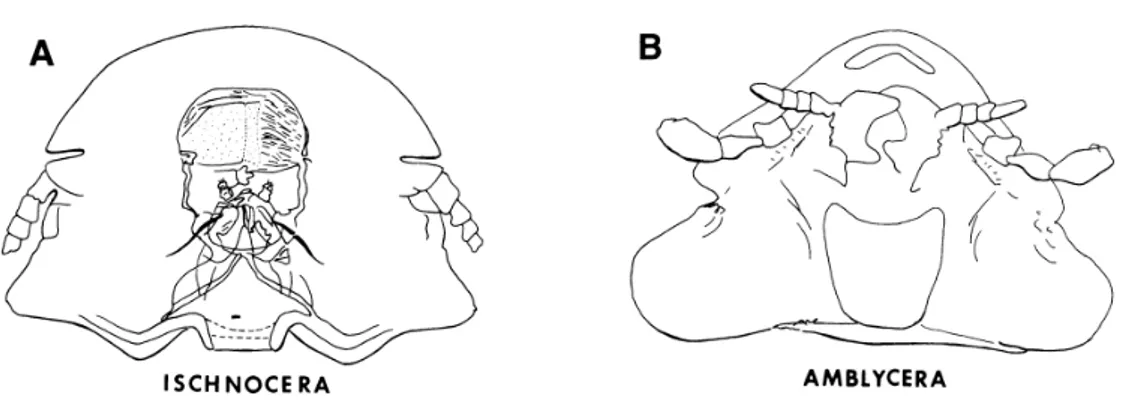
Figure 7 Outline of head of suborder Ischnocera (A) and Amblycera (B) (13)
Amblycera are the most generalised and least host specific chewing lice and more closely resemble the Psocoptera ancestor, by their biology and morphology characteristics. Amblycera species have a generalist feeding habits, in addition to feathers, also feed in flakes of dead skin and skin secretions. The alimentary canal has well-developed comb-like structures in the crop, which prevent undigested feather parts or other particles from progressing to the midgut. On blood-feedings species the crop is a mer enlargement of the oesophagus. The male genitalia haves three pairs of testes. (2, 4, 10)
The Amblycera includes six families (Menoponidae, Boopiidae, Laemobothriidae, Ricinidae, Gyropidae, Trimenoponidae) of which Menoponidae, Laemobothriidae, Ricinidae and the genus Therodoxus from Boopiidae parasitise birds. Species of the family Menoponidae, containing for example the genera Menacanthus and Menopon, which are of major veterinary importance on birds. (3)
2.3.1. Family Menoponidae
Family Menopoidae is the largest of the families of Amblycera and with a worldwide distribution. A key with 47 genera was established on 1969 but currently it’s believed that there may be around 60 genera and species are found on a wide variety of birds. (13)
Specimens from this taxonomic family have a characteristic broadly triangular head expanded behind the eyes. The maxillary palpi have four segments and the labial palpus has usually one segment with five distal setae. Antennae have between four to five distal segments. In cases in which the antennae have four segments there is two adjacent sensible on the last segment. In five segmented antennae there is one sensillum on segment 4 and 5. The mesothorax and metathorax are not fused and are separated from tergum one. The mesonotum doesn’t have protuberances with setae. And in the legs two and three there are two tarsal claws. (13)
Species from genus Menopon are small, 1.5 to 2.5 mm, do not possess spine-like processes on the underside of the head and only one row of setae along the dorsal margins of the abdominal segments. Menoponids differentiates themselves from members of the family Boopiidae by the spikelike processes on the underside of the head that are reduced or absent and the antennae have not strongly clubbed. (13)
Genus Menacanthus is the largest and the most economically important genus of the family Menoponidae and can be distinguished from the genus Menopon, which also parasitises chicken, through two short spine-like processes on the underside of the head. The terminal segment of the antennae is not decided has numerous ridges. (13)
2.3.3. Family Laemobothriidae
Established in 1910, the current classification is divided between the subgenus
Laemobothrion, species parasitising birds of prey, and a Eulaemobothrion subgenus species
parasitising water birds in the orders Ciconiiformes and Gruiformes. (13)
The family distinguishes itself from other Amblycera through a ventral area of microtrichia in the femur III and terminal dorsal patches of microtrichia in the distal ends of tibia II and III and also through a sculptured area on the temples (posterolateral margins of the head) with outer row of piglike projection. (13)
2.3.4. Family Ricinidae
With three recognised genera in which genus Ricinus is the one with most species, with a worldwide distribution and genus Trochiloecetes and genus Trochiliphagus parasitise hummingbirds in the New World. (13)
Species from the genus Ricans have modified mouthparts for bloodsucking, do not possess labial palpi and mandibles have pointy tips suited for piercing the skin. Dorsal sclerites of the mesothorax, metathorax and first abdominal segment are fused on a single sclerite. Head is usually elongated with a bluntly rounder apex. (13)
2.4. Suborder Ischnocera (avian lice)
Chewing lice in the suborder Ischnocera differ anatomically from other chewing lice through the following characteristics: three to five antennae segments, with a filiform shape; antennae are not hidden in grooves; mandibles articulate ventrally from the head and move in a vertical plane; the maxillary palpi are absent; mesothorax and metathorax are fused forming a pterothorax. (2, 3, 13, 16)
Ischnocera are more specialised, more host specific, usually limited to a particular region of their host’s body or to a certain part of a feather. Feeding preferences are more limited, confined to feather barbules. In the alimentary canal many Inschnocera, and some Amblycera, have lingual sclerites located vertically between the labrum and labium, which are part of the water vapour uptake system. The crop is a diverticulum of the oesophagus. Rickettsia-like bacteria are present in many avian Ischnocera gut residing on specialised cells, mycetocytes, sometimes concentrated in structures called mycetomes. The bacteria migrate into developing eggs in the female louse, undergoing transovarial transmission. The male genitalia haves two pairs of testes. (4, 17)
The Ischnocera includes two families (Philopteridae and Trichodectidae). The Philopteridae contains for example the genera Cuclotogaster, Lipeurus, Goniodes and Goniocotes, which are important ectoparasites of domestic birds. The Trichodectidae contains the genus Bovicola (referred to in some literature as Damalinia), Felicola and Trichodectes, which are ectoparasites of mammals. (3)
2.4.1. Family Philopteridae
Largest family of the Mallophaga with highly specialisation therefore in many avian orders the lice species found in a host may be classified by the morphological types that occupy different microhabitats (locations) in the host’s body. Head and neck of birds usually occupied by small and round louse species with rather large heads due to the strong mandibles. In the broader and longer feathers (back and wing) louse species have a slender and flattened body. (17)
In contrast with Amblycera, that walk on the host’s skin, Philopteridae species remain immobile on the plumage using their strong mandibles to attach themselves. Also feeding habits defer from Amblycera, feeding primarily on barbs and barbules and wing species mainly on hooklets of feathers. Philopteridae have antennae with five segments and legs with a pair of claws. (17)
2.5. Birds
2.5.1. Birds as parasitic hosts
Birds are endothermic animals as their body temperature is largely influenced by the heat produced by their own metabolism rather than from the environment. And for thermal regulation, birds possess a dense insulating layer, feathers, that allow them to live in a wide range of ecological and climatic conditions, locomotion (flying), communication and create the physical environment for ectoparasites. Feathers are epidermal growths composed mostly by beta-keratin, a fibrous protein polymer with forms microscopic filaments, which provide great strength and minimal weight. (12)
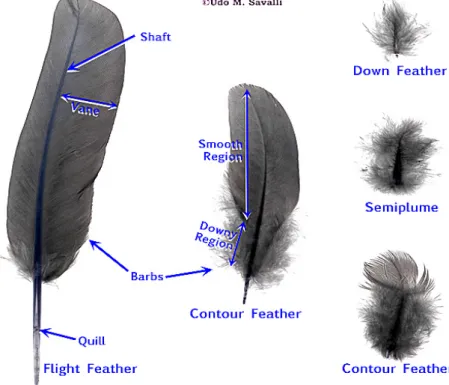
Figure 8 Different types of feathers (37)
(a) Contour feathers: biggest feathers on the body, creating the outlines of the tail, wings and body of the bird. They are providing waterproof layer and are composed of a firm vane supported by a shaft. The vane consists of a large number of barbs and barbules, which are held together by interlocking hooks or humuli. (12)
(b) Semiplumes: are a derivation of the contour feathers and their structure is completely intergraded with them. They have a part called rachis with a down web without hooks and so there is no firm vane present. These feathers are distributed in-between, and hidden under the contour feathers, mainly present on the edge of pterylae and in the apteria. (12)
(c) Filoplumes: highly specific hair-like feathers, can be possibly distributed through the whole surface of the body. (12)
(d) Down: little soft feathers which only have a very short rachis and there is no vane present. They normally cover the gobies of newly-born birds, but are also present in adults, close to the skin and less often in the apteria. (12)
Their body covering is quite differentiated this way providing a greater variety of niches for ectoparasites. (12, 18)
The stable temperature and relative humidity which is provided by this environment gives lice the ideal conditions for survival on mammals and birds. Even though this seems to be a uniform environment, in reality it is a collection of microhabitats which are connected with each other on many levels, and therefore each species of lice have adapted their morphology and behavior to being able to exploit these niches on the host. (19)
This characteristic of the environment is allowing multiple species of lice to inhabit one host at the same time and coexist without problems. The division into microhabitats is much easier to observe on birds and is presented by different types of feathers covering the wings, rump, back and the head of the host. The fact that birds are not able to groom all parts of their bodies in the same way and intensity, puts a lot of pressure on the ability of louse to adapt their morphology, and has an immense influence on the choice of habitat the louse occupy. Majority of lice species are host specific to such degree that they are only able to inhabit one single host species or a small group of closely related hosts. (2)
Due to all the important functions of the feathers it is necessary to maintain them in a suitable condition by a continual grooming and by periodic molting.
There were more than 40 species of chewing lice identified on birds. Birds try to get rid of lice during grooming, by using their feet to scratch their head and freeing their whole body with the bill. Infestation can become a serious cause of severe irritation, which leads to excessive damage of the feather, restlessness, inability to feed or anorexia and badly affected birds can pluck their feathers. Inevitable result of such state is loss of weight and possible death. (19) The host is usually controlling the population of the ectoparasites.
Grooming is a process in which the birds re-arrange their feathers and remove any dirt, they wipe off grease and loose skin particles of the epidermis. The secondary function of booming is the elimination of ectoparasites. The most important and most often exhibited behavior in birds is direct preening with the beak, during this process the bird nibbles on individual feathers and draws them through the mandibles, which help with restoring the original structure of the vane, and also facilitates distribution of oil on the feathers from the oil gland. Since birds are unable to reach their head in other means, they use their feet to groom the head area. (19)
non-of the process which includes: anting, water-bathing, oiling, preening. During anting the birds anoint their feathers. (16)
Host body size was a major factor in establishing louse house specificity. Lice could not survive on bird species smaller than their natural hosts unless the host was prevented from preening effectively. In reciprocal transfers, louse species from smaller birds did not survive on large host species regardless of preening ability. The results suggested that in the case of both body and wing lice on birds, host switching is most likely to occur between host species of similar sizes. (16)
2.5.1. National and migratory birds
Each year, during spring and fall, millions of birds set out for migration to take advantage of seasonal opportunities for better feeding and nesting areas. To certain extend, the biggest, most frequent migration routes are influenced by the topography of the continents and therefore their orientation is from north to south in North America and from east to west in Europe. Several of the routes used for migration are retracing the historical trails of expansion of the species. (18)
Scientists have been asking themselves why in the same species of birds, we can find migratory as well as sedentary populations, for a very long time. The migration pattern may emerge in new populations of previously non-migratory bird species or on the other hand, the habit can disappear in populations, which colonize new areas, even though they are created by migratory species. (18)
The length of the migration distance of individual birds is determined by the balance between the cost and the benefits of the trip. Shorebirds that migrate are widely distributed in correlation with the availability of the food in wetlands at the coast, where they spend the winter. It appears that they don’t suffer any extra damage by traveling such long distances to be able to save energy. The flight taken by several migratory species, which travel long distance, requires extreme physical ability and endurance. (18)
Many of the migratory bird species are on the decline due to the bird populations being faced by extensive losses of their habitats and also wintering grounds in Central America and the Caribbean, or the locations used for breeding in North America and at crucial sites where the migrating population needs to stop on their way alongside the migration corridors. (18)
If we suppose that parasitism and capability of resisting parasites have direct effect on migration of the birds, then we must suppose measures of migratory performance to be related to parasitism and immunity. Several studies conducted in Denmark, Italy and Spain on Barn Swallows, have previously shown a possibility to predict spring arrival date to the breeding grounds by the numbers of chewing lice, feather mites and blood parasites in the male part of the population, but without such big significance in females which do not have to enter the competition for early arrival. (20)
Additionally, the date of arrival of males, not so much females, can be presupposed by T-cell mediated immune response. The correlation was proved by an experiment in which the intensity of hematophagous mite infection was artificially rised and it had an effect on arrival date the following Spring. (20)
Other studies, with lower number of data for different species also confirmed the hypothesis that the timing of spring migration is affected by parasite load. This was especially true in individuals, who are forced to contest for fast migration. (20)
In addition, migratory connectivity is expected to have a role in determination of the load of parasites in individuals, and in this way they affect the physical condition of the bird and directly influence their ability to migrate. (20)
The cause of this is that individuals which spread through wider areas and therefore contribute to disruption of migratory connectivity, will come in contact with new types of parasites (or parasite strains) to which their immunity system can’t respond effectively, and subsequently reduces the physical abilities of the individual with further negative effect on the endurance for physically demanding activities such as migration for long distances. Von Ro¨nn (2010) recently conducted studies about Barn Swallows, which have shown that heterogeneity in stable isotopes of feathers, which were grown in the African winter quarters is in close association with bigger diversity and prevalence of blood parasites including Plasmodium. This points to the fact that individuals who spend winter in different areas and/or habitats can be infected with Plasmodium parasites, which are usually not encountered by the population of Barn Swallows. The results of the studies confirmed the hypothesis about winter habitat specificity and parasite-mediated selection against dispersal during wintering season. (20)
Because birds as parasitary hosts with migratory divides have longer dispersal distances than hosts without a divide, it can be expected that hosts will have advantage over
though, that by breaking local adaptations by bigger dispersal, parasites will have advantage over the hosts in species dispersed on longer distances. This latter case could explain the observation that prevalence of parasites and bigger variety of species are greater in birds with migratory divides. There is observed similarity between patterns for directly transmitted and vector-transmitted parasites, what suggests that it is the behaviour of the host rather than the parasite that is causing differences between hosts with and without migratory divides.(20)
In addition, it has been observed that two of the three components of immunity were different between species with and without divides, even though this was only noted in adult hosts, as we found for dispersal.A result of differences in parasitism between hosts with and without migratory divides is further selection for maintenance of migratory divides, in case the genetic bases for resistance to specific strains of parasites are present, and if parasites differ in their virulence among host populations on the two sides of a migratory divide. (20)
This way of maintaining migratory divides based on parasite-mediated selection could be a direct result of assortative mating based on the direction of migration, which would result in partial genetic isolation. At some point in the future, this kind of differentiation can lead to speciation in which parasites would be the selective force promoting isolation. (21)
Hence, there can concluded that species of birds, which have migratory divides, are very common and include almost one fourth of all European passerine birds. Migratory divides create several consequences for many aspects of the bird population, such as dispersal, size range and population size, with effects on local adaptation. Evidence was found, for several levels of local adaptation in host species with and without migratory divides, caused by the fact that species with migratory divides had increased parasite loads in comparison with species without divides, and the difference was also noted between immunity levels of adult hosts. (21)
2.6. Chewing lice in birds of Greece
The topic of lice infestation in birds in Greece is a very important study which will help us explore the correlation between the hosts, parasites and the habitat in which they live. However, very few articles were published and the research for this area is practically non-existent. (22)
Greece is situated on the eastern part of the Mediterranean area and it has an important geographical position for both, local and migratory birds, as it is on the junction of three different continents and provides habitats of great diversity. High mountains and uneven relief in alternating with narrow valleys and farmland mostly fill the mainland. Freshwater lakes and lagoons at the coast form an important network of wetlands. And of course, the island archipelagos; more than 2,000 islands, some of them bigger, some only small rocky islets, together create a home for nesting seabirds and Eleonora's Falcons. (22)
Records show 442 bird species in Greece, 242 of them are locally breeding, the rest are migratory birds which spend the winter or fly over the area during migration, or some of them could be randomly found in Greece. (22) Even though more than 900 species of lice have been found and identified on the birds species present in the area (with almost 1800 host-louse associations) (4), the knowledge and scientific data on the louse fauna of birds living in Greece are insufficient.
3. Materials and methods
3.1 Field Study
The field study was conducted, in several field trip from 2013 to 2016, in conjunct with the member of the Hellenic Bird Ringing Centre on several locations inside the Koroneia and Volvi National Park, Northern Greece, which resulted in the examination of 728 birds and collection of 560 samples of chewing lice.
The exact locations of the sampling sites were:
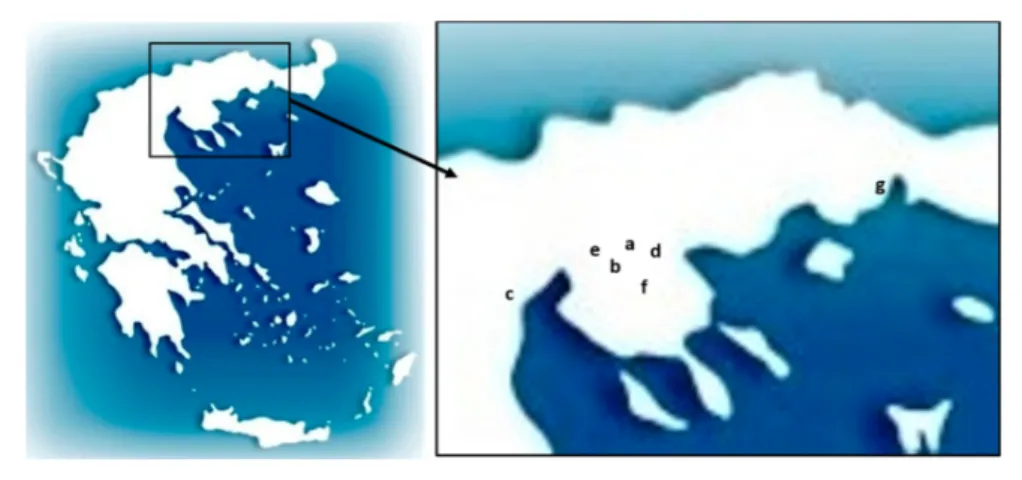
Figure 9 Locations where birds were captured and examined in Greece. a: Loutra Volvis), b:Platia Volvis, c:Nea Agathoupoli Pieria, d:Dasos Apollonia, e:East Cost of Koronia Lake, f:Varvara). g:Porto Lagos.
(a) Loutra Volvis (40.65´ N, 23.41´ E, 53m altitude, from 4th to 6th of June 2013) (b) Platia Volvis, (40.62´ N, 23.34´ E, 517m altitude, 6th and 7th of July 2013)
(c) Nea Agathoupoli Pierias (40.46´ N, 22.58´ E, 2m altitude, from 26th to 31st of July 2013 and 11th to 14th of December 2013)
(d) Dasos Apollonias (40.65´ N, 23.49´ E, 43m altitude, from 26th to 29th of September 2013, 6th to 9th September 2014, 9th and 10th of June 2015 and 15th to 18th of October 2015)
(e) East Coast of Koronia Lake (40.67’N 23.21’E, 54m altitude, 15th and 16th of April 2015)
(f) Varvara - Stratoniko Mt. (40.57’ N 23.65’ E, 650 m altitude, 10th to 13th of June 2015)
The small passerines were captured by mist-netting and every individual was identified and sexed, using the identification guide by Svensson (24) and Baker (25) followed by the host’s body visual inspection for ectoparasites and submission to fumigation chamber method. (26)
The method of chewing lice collection used, fumigation chamber, consisted on a wide-mouthed plastic jar fitted with a plastic bag containing a cotton ball socked in chloroform. The individual neck would pass through the opening on the screw-cap lid and proper fixation was done by using a heavy rubber band. (23)
Figure 10 Captured birds being subjected to fumigation to collect ectoparasites, Greece (own authorship)
The method’s principal consists on the fact that it takes a mere 5 minutes for the lice to detach and therefore reducing the handling time of birds. In this case the birds would be kept in the fumigation chamber for a period of 10 minutes. The plastic bag would be minutely inspected and the specimens collected were stored in 96% ethanol.
Ruffling of the bird over a collecting surface was done to increase the probability of lice recovery on birds with small infestations. (23)
Figure 11 Fumigation chamber used for smaller birds (own authorship)
3.2. Methods of laboratory work
3.2.1 Canada balsam technique
Subsequently, under laboratory conditions, collected samples were slide-mounted in Canada balsam as permanent preparations, following the technique by Palma (27), to allow the proper study of their anatomy and taxonomic classification following Price et al. (4), Price (13), Zlotorzycka (28-32), Sychra et al. (33)
The technique used for preparation of slides involves several steps:
At first a suitable specimen is selected. This depends on the condition of the specimens, which were collected. Naturally we try to select those with complete set of legs and antennae in extended position, and without and overly dark gut contents. (27)
All specimens of a new or rarely collected species should be used for slide preparation, if their number is not too large. If we have collected well known and frequently collected species a minimum of 6 to 12 male and female pairs needs to be selected to provide adequate representation of the infestation. (27)
Selected specimens are then properly identified and treated with 20% aqueous solution of potassium hydroxide for a time period of 15-35 hours at room temperature. We need to inspect them regularly to monitor progress and avoid any possible damage, caused by overtreatment. (27)
Figure 12 Fumigation chamber for the removal of lice from live birds (26)
The most important effects of KOH are the maceration of all the soft (non-chitinous) tissues, descoloration of the sclerotin, and softening and distending of the whole body. The maceration permits easier elimination of internal organs, leaving the exoskeleton as empty as possible. This can be facilitated by gentle puncture of the abdominal cavity and applying pressure to facilitate the removal of internal organs. (27)
Following step is neutralisation. After the previous steps are finished, the KOH solution is replaced by water for 30 minutes. Later the water is carefully replaced by 10% aqueous solution of acetic acid for at least 30-40 minutes. The role of the acid is to neutralize the remaining alkaline solution, stop maceration and avoid destruction of the sample by overtreatment. (27)
After completing the first three steps of the process, the sample or a desired segment of the collected specimen can be stained, using an aqueous solution with high concentration of acid fuchsin (acid magenta, rubin S) for a period of 8-16 hours at room temperature. When desired staining has been obtained, small amount of 70% ethanol is mixed into the acid fuchsin; after some time the mixture is replaced by 70% ethanol without any other additives, which facilitates the removal of any excessive stain on the inside of the exoskeleton.
Staining of the samples is in many cases necessary and desirable, as acid fuchsin primarily stains the parts of the exoskeleton, which are important for the systematic study and classification of the species. (27)
All specimens, those, which were stained as well as the non-stained ones, must be gradually dehydrated, what we achieve by multiple changes of ethanol solution with increasing concentration. (27)
When dehydration is finished, the 96% or absolute ethanol is drained and the next medium used is pure clove oil. The sample must be submerged in the oil for minimum of 24 hours, but it can stay indefinitely in this medium. Stained specimens slowly lose their stain when left in clove oil for longer periods of time. This presents itself ad a good opportunity to correct any mistakes, such a excessive coloration and contrast if the specimens have been over-stained. (27)
Conventionally, writing the information on the left side of the slide identifies the specimens and all the collecting data, which refer to the host, are recorded on the right side. We use neutral Canada balsam in xylene as a mounting medium. The ability to dilute the balsam with xylene allows us to obtain the desired thickness of the medium. A thin layer is
arranged on the center and the coverslip is placed flat on top. Small air bubbles present in the balsam will be eliminated by the heat of the oven later in the process. Convention dictates than whenever possible, a male and female specimens of the same species should be mounted next to each other with the male on the left. (27)
Drying and hardening of the balsam is necessary to prevent movement of the specimens. Slides need to be dried in the oven for 3 weeks at the temperature of 50-55°C, since drying at room temperature would take too long. The heat of the oven helps to harden the medium and at the same time eliminate all air bubbles, which might be present. It is important to put weight on top of the coverslips and let the balsam set for at least 24 hours before drying in the oven. This is done to avoid uneven positions of a coverslip, caused by shrinking of the balsam as it hardens. (27)
Any spilled drops of balsam can be easily cleaned after drying, with a sharp blade. (27)
3.3. Statistical analysis
We have analysed several parameters which help us better understand the intensity of parasitic infestation in populations of birds, which were examined. These results were grouped in tables, to facilitate comparison and evaluation of the values.
From collected samples, and counts of captured birds and parasitic infestation, we were able to calculate several parameters. (35)
Prevalence represents how common or prevalent certain characteristic is. In our study, that is the proportion of hosts who were infected, among all the hosts who underwent examination. We calculated percentage of prevalence to express how often certain species of ectoparasites were found in the captured bird species. (35)
Another parameter that is calculated and expressed in the tables, is mean intensity. Mean intensity is a value expressing mean number of parasites, which were found in the infected hosts (we do not include the zeros of uninfected hosts in the calculations). As the size of our sample and percentage of prevalence are known, mean intensity offers the quantity of parasites found in the sample of examined birds. (35)
In some cases it is preferable to use mean abundance instead of mean intensity. This is the mean number of parasites found in all hosts (including the zero values of hosts who weren’t infected). (35)
The percentage of dominance (see table 6) is a value expressing how dominant certain ectoparasites were, in samples included in out study. In the results section, we can see which genus of the parasites was dominant in collected and examined samples, which gives us important information on dominance of the species in the population of birds.
3.4. Morphometric analysis
On the morphometric analysis, the dimensions are given in millimetres and all specimens was done light microscope CX31RBSF (Olympus, Tokyo, Japan) and drawings were made by fitted with a drawing tube, then edited using Adobe Illustrator CS6 on a tablet PTZ-930 (Wacom, China).
3.4.1 Morphometric analysis of genus Philopterus
In the morphometric analysis, we followed the article from Valim & Palma (36) but takes into consideration the alterations of the abbreviators of the several measurements suggested by Najer (34). In the description of the Philopterus-complex, this two articles provided the guidelines for the measurements and parameters to allow to identify the genus. All measurements are provided in millimeters and the detailed list will be provided within the results. (34, 36)
Within the head chaetotaxy follows Clay (1951), as modified by Mey (1994) for the
pos and mts 1–5; the head sensilla follow Valim & Silveira (2014). Parameters which were
analysed are the following: PAW = preantennal width; PAL = preantennal length; HL, head length (excluding hyaline membrane); POL, preantennal length (taken from the base of the conus to the bases of as3, obliquely to the head axis); PMCL = premarginal carina length; TW, temporal width; TRW, trabecula width; TRL, trabecula length, described within image D. In addition, the following setae dimensions were measured: as3, length of the anterior setae 3; dsms = length of dorsal submarginal setae; pas = length of preantennal setae; pcs =
Figure 13 Morphometric measurements of the head of the parasite (34)
On the dorsal plate, image B, the parameters are: ADPW, anterior dorsal plate width (taken at its widest point); ANW, width of anterior notch (taken between the bases of as3); ADPL, anterior dorsal plate length (taken at middle line); ADPLL, anterior dorsal plate lateral length (taken from the base of anterior dorsal setae – ads, to lateral apices of the plate). (34, 36)
The female terminology (inner genital sclerite, subvulval sclerites) follows Clay (1958), as shown in image C. The sub genital plate the SGPW = subgenital plate width. The male genitalia, in image A, the described parameters are GW, male genitalia width (taken at the basal plate) and GL, male genitalia length. (34, 36)
Within figure 14 the following additional morphometrics parameters are described: PW, prothorax width; PTW, pterothorax width; HL, head length (excluding hyaline membrane); TPVL, tergal plate V length; AL = abdomen length; TL, total length AW, maximum width of the abdomen (taken at level of segment V). Furthermore the length of several setae are taken as differential parameters. All Ischenoceran lice have fused mesothorax and metathorax, hence it is considered an single segment, pterothorax, as the terminology. Within this segment two setae l and r (meaning left and right side respectively) are measured. (34, 36)
Within the abdomen the following sternal setae are measured: tergocentral setae II anterior on the left and right side, II posterior l and r, then III, IV, V, VI, VII, VIII and IX+X, each of them on the left and right side; paratergal setae II – VIII, each of them on the left and right side, Sutural setae II-VIII each of them l and r. (34, 36)
The sternal abdominal setae are analysed, from the left to the right side of the specimen, and differentiated by the letters “S” for short and spine-like and “L” for long and flexible. The chaetotaxy of the abdominal tergocentral setae does not include the postspiracular setae, except for tergite II where postspiracular setae, if present, can not be distinguished from the remaining setae. (34, 36)
3.4.2 Morphometric analysis of genus Penenirmus
In genus Penenirmus, review within the article from Sychra (33), that describes the chaetotaxy characteristics proposed by Clay & Hopkins (1951) characteristics: head with postantennal setae, post-nodal and post-temporal setae, marginal temporal setae, anterior dorsal setae, tergites II – VI, postspiracular setae, posterocentral tergal setae II-VIII, sternal setae II-VII, and paratergal setae II-IX. Furthermore, the measurements of TW, temporal width; HL head length; PW, prothorax width; MW, AW, maximum width of the abdomen (taken at level of segment V); and TL, total length. (33)
4. Results
4.1. Occurrence of chewing lice in passerines in Northern Greece
During the research, several collections of samples were conducted in areas of Northern Greece. Together, there were 729 birds examined. The different species of birds, which were captured and examined on several occasions, and their number, are listed in the Table 4 in the annex.
From 4th until 7th of June 2013, 101 birds were captured at two locations, Lake Volvi (altitude 53m) and Platia (altitude 517m), represented in the table in the columns 1 and 2.
From 26th until 31st of July 2013, we worked at location Nea Agathoupoli Pierias (altitude 2m), where we captured 132 birds; this is represented in the table as column 3.
From 26th until 29th of September 2013, we’ve examined 133 birds caught at location Dassos Apollonias (altitude 43m), results are marked in the column 4 in the Table 4.
In December 2013 from 11th until 14th, 37 birds were captured and examined at a location Nea Agathoupoli, results are written in the column 5.
From April 23rd until April 27th 2014 we captured birds in Porto Lagos, with 22 birds examined, results shown in the column 6.
In September 2014, on 6th and 9th, 86 birds were captured and examined in Apollonia Forest, as represented by column 7 of the Table 4.
On 15th and 16th of April 2015 the research continued at the location Koronia East, where we caught 47 birds, what is represented by column 8.
Research continued at the location of Apollonia Forest in June 2015 from 9th until 10th
represented by column 9, and from 10th until 13th in Varvara (Stratoniko Mt.) where we
captured together 63 birds, results are marked in column 10.
Last collection of samples was conducted in October 2015, from 15th until 18th at the location of Apollonia Forest, with 108 birds captured and examined, which is represented by the column 11 of the Table 4.
The last column in the Table 4 represents a total count of each bird species captured and examined, during the whole process of research. We can see that the most common species of birds, which were captured, is Sylvia atricapilla, with a total count of 79 birds examined, that represents more than 10% of the overall count of captured birds.
From all the birds captured, 80 were found, to be infested with lice, representing 11%. There were several, previously known host-louse associations found, but we have also discovered five new associtions: Menacanthus agilis ex Cettia cetti, Menacanthus curuccae ex Acrocephalus melapogon, Menacanthus sinuatus ex Poecile lugubris, Myrsidea sp. ex
Acrocephalus schoenobaenus, Philopterus citrinellae ex Spinus spinus. In the Table 5 (Annex
I), we see a list of common names, as well as the scientific name for the bird species, and each of these birds is associated with a list of ectoparasites of those species.
Two species of Acrocephalus menalopogon were infested by specimens from the genus Philopterus. Within Philopterus-complex, one of the groups concerned is also
Philopterus s. str. occurring in reed-warblers (Acrocephalidae). Up to date, there are
published records of seven host-louse associations containing five Philopterus species within this host family; nevertheless, practically no description of the species can be considered as complete. They are either based on single sex only (38, 39) or their relevance may be disputable as the presented diagnostic features are (40), according to our view, tentative and not sufficient to separate new species.
Within one of the captured Cettia cetti a specimen of Penenirmus sp. was found. Due to the inability to determine axonomic classification through the available materials a morphometric analysis was required. Due to the close resemblemence between Penenirmus albiventris and Penenirmus longuliceps, specie still not identified in Cettia cetti, there was the possibility of a new host-parasite association.
We have also found 11 birds of co-especiation, infested with more than one species of lice. The most common intensity of infestation was one louse per bird –noted in 26 cases. The bird specimen with highest number of lice was infested with 101 specimens.
Furthermore, the percentage of dominance was calculated for each genus of the parasites, as well as total dominance for each suborder. Ischnocera showed much higher total dominance of 60.9%, compared to only 39.1% in Amblycera. These results are represented in the Table 6 (in annex). In Table 7 we see the correlation between bird species and, ectoparasites, and their percentage of dominance. Overall 560 specimens of ectoparasites were examined and identified.
Prevalence is another important marker that allows us to see how common certain parasites are in the samples examined during our research. Presence and number of parasites is noted in correlation with the gender as female, male or immature (nymph) in Table 8 in annex. Furthermore, we proceeded with the calculation of percentage of prevalence, mean
abundance and mean intensity, to better represent the state of the population in relation to the infestation by lice. These results are noted in Table 9 (Annex I).
4.2. Morphological comparison of Penenirmus longuliceps and Penenirmus
albiventris
The following figures represent a drawing and photos of a male (on the left) and female (on the right) specimens of Penenirmus albiventris. Both of the specimens were found on the same bird Cettia cetti (Cettidae) in Apollonia Forest. The date of capture was 17th
October 2015.