http://dx.doi.org/10.1590/s2175-97902017000400229
Article
*Correspondence: L. J. Ping. College of Veterinary Medicine, Gansu Agri-cultural University. No.1 Ying Men Cun, Lanzhou, 730000, China. E-mail: liangjp100@sina.com
Relationship between oxidative stress and inflammation
in peripheral and cerebral system of oxonate-induced
hyperuricemic rats
Wu Jing
1,2, Jia Zhong
1,2, Liang Jian Ping
1*, Liu Hong Yan
21College of Veterinary Medicine, Gansu Agricultural University, LanZhou, 2Department of Pharmacy, Pulmonary Hospital of
Lanzhou, LanZhou, Gansu Province, China
To study what kind of role uric acid play on the relationship between oxidative Stress and inflammation in peripheral and cerebral system of oxonate-induced hyperuricemic rats. Twenty-six eight male Wistar rats were divided into two groups randomly. Potassium oxonate was used to establish hyperuricemic model for four weeks. In 2nd and 4th week, uric acid (UA) level, total superoxide dismutase (T-SOD),
Gu,Zn-SOD activity and interleukin-1beta (IL-1β) concentration in serum were determined respectively. In 4th
week, one hour after last PO treatment, five rats of every group were given Evans Blue to test blood–brain barrier (BBB) permeability. Other brains were obtained to analysis T-SOD, Gu,Zn-SOD activity and IL-1β concentration in cerebral system. Meanwhile, brain and kidney were stained with hematoxylin and eosin (H&E) to observe pathological change. In 2nd week, both of T-SOD and Gu,Zn-SOD activity
in serum increased obviously (P<0.05) in hyperuricemia rats. However, IL-1β content didn’t change remarkably. In the 4th week, T-SOD activity in model group had become similar with control group, and
at the same time IL-1β content in serum increased significantly (P<0.05). Pathological section showed the structural and functional unit of the kidney had been damaged. On the contrary, both of T-SOD and Gu,Zn-SOD activity in brain increased obviously (P<0.05), but IL-1β concentration was no significant difference between two groups. In addition, the results of Evans Blue and H&E suggested the integrity of BBB and structure of brain were not changed after PO treatment. The permeability of BBB and form of UA would be potential factors to decide what kind role UA play on keeping balance between anti-oxidative stress and induction of inflammatory response.
Keywords: Hyperuricemic. Blood brain barrier. Potassium oxonate. Oxidative stress. Inflammatory. Total-SOD. Gu,Zn-SOD. IL-1β. Pathological injury.
INTRODUCTION
Uric acid (UA) is the end product of purine metabolism in humans. High UA level caused by metabolic disturbance would lead to hyperuricemia, which turns into risk factors to chronic renal disease (Feig, 2009), metabolic syndrome (Choi, Ford, 2007), hypertension (Mazzali et al., 2010) and cardiovascular disease (Grayson et al., 2011).
Owing to redundant UA deposition, tissue injury triggers innate responses and early phases of host defence,
and promotes more pro-inflammatory cytokine producing,
initiating the inflammatory reaction. It was well known that pro-inflammatory cytokine has been closer link with nervous system disease, such as interleukin-1beta (IL-1β),
which could aggravate amyloid peptide deposits in brain
and promote development of Alzheimer’s disease (AD)
(François et al., 2013).
It seems UA is a risk factor to central nervous system disease. However, as Alvarez-Lario said (Alvarez, Macarrón, 2011), if UA was a waste or harmful product, it
poorly explained why our kidneys recover 90% of filtered
UA, instead of eliminating it. In fact, UA as a natural antioxidant plays an important role not only on induction of inflammatory response but also on anti-oxidative stress in peripheral and central tissue (Glantzounis et al., 2005). Especially in cerebral system, UA have displayed
with a reduced risk of some neurodegenerative diseases
including Parkinson’s disease (PK) (Andreadou et al., 2009) and AD (Cutler et al.,2015).
Above-mentioned diseases have been reported to contribute to a high oxidative stress condition in internal organism environment. It is confused, in peripheral circulatory system, why high UA level complicated with those diseases can not exert antioxidant activity for organic tissues, but become inducing factor associated with inflammatory reaction. Up to now, it is not clear that how UA pretend neuron and cerebral tissue from superoxide and hydroxyl free radicals, instead of inducing
inflammatory factors. Pakpoor reported the overall risk of
subsequent multiple sclerosis (MS), PD and motor neuron disease (MND), which was significantly attributable to an increased risk observed in the early years after
hospitalization for gout. Moreover after five years, the
increased risk of neurological disease did not remain (Pakpoor et al., 2015).
Therefore, we postulated that it may exist some ways to assist UA to keep a balance between anti-oxidative stress and induction of inflammatory response. This researcher observed what kind of variety of oxidative
stress and inflammation in peripheral and cerebral system
under high UA condition, which would reveal the potential factors to destroy the balance.
MATERIAL AND METHODS
Material
Potassium oxonate and Evans Blue dye was
purchased from Sigma Chemical Co. (St Louis, MO, USA); Uric acid and super oxide dismutase (SOD) assay
kits were from Jiancheng Biotech, Nanjing, China; IL-1β ELISA assay kit was obtained from Meilian Biotech,
Shanghai, China. All other reagents used were from of analytical grades.
Animal preparation
Twenty-six eight male Wistar rats (8-10 weeks of age, weighing 200-220 g) were obtained from Experimental Animals Center in GanSu University of Chinese Medicine. They were fed with a commercial laboratory diet and allowed food and water ad libitum for an acclimatization period of 1 week prior to the experiment. Housing conditions and experimental procedures were set to be in accordance with international standards. All animals were maintained on a 12 h day-night cycle and the temperature and humidity were
kept at 23 ± 1 °C and 50%, respectively. The animal
model was made as described previously. Briefly, rats
were divided into two groups randomly. One group as model group was injected with potassium oxonate (PO) 250 mg/kg intraperitoneally(i.p.), dissolved in normal saline (NS). And control group was administrated with equal volume NS. Treatments were carried out for four weeks after hyperuricemia induction (Haidari, Rashidi,
Mohammad, 2012). Then all rats were sacrificed 1 h after
last PO treatment.
Assay for serum uric acid (SUA)
Blood samples were obtained for SUA analysis in 2nd and 4th week. Whole blood samples were centrifuged for 5 min at 12 000 rev/min to get serum samples. SUA levels were determined using enzymatic colorimetric methods,
and measured by a spectrometer (λ= 690 nm).
Assay for Total superoxide dismutase (SOD) and Gu,Zn-SOD activity in brain and serum
Blood samples were obtained for SOD analysis in
2nd and 4th week. When rats were sacrificed, left cerebral hemisphere were removed quickly, cleaned with cold
phosphate buffer, and homogenized with NS (w/v, 1: 9) in
a glass homogenizer. After centrifuging the homogenized brain tissue at 2500 rev/min for 15 min, SOD activityof
supernatant was measured by a spectrometer (λ= 550 nm),
and corrected by the protein content of per gram. Total SOD and Gu,Zn-SOD activity in serum were tested by the same way, which were expressed in U/ml serum.
ELISA to determine IL-1β concentration in brain
and serum
All samples prepared as described previously, ELISA assay experiments were conducted according to
the manufacturer’s instructions.
Leakage of Evans Blue from brain vessels
One hour after last PO treatment, Evans Blue (80 mg/kg) was injected into the rats’ tail vein. Brain
samples were taken one hour later. Five samples were
needed. Brain cortex weighing 0.25 g was homogenized
in 50% trichloroacetic acid and centrifuged for 20 min at 1000 rev/min. The resulting supernatants of the samples were diluted with ethanol, and the fluorescence of
emission wavelength 680 nm). The concentrations of Evans Blue were calculated by standard curve. The results were expressed in microgram Evans Blue per
gram cortex (Wu et al., 2009).
Histological analysis
W h e n r a t s w e r e s a c r i f i c e d , r i g h t c e r e b r a l
hemisphere and renal tissues were fixed in 10% (V/V) neutral buffered formalin for 24 h, embedded in paraffin wax, cut into 4 μm thicknesses, deparaffinized in xylene,
and processed with graded ethanol series. Sections were stained with Hematoxylin and Eosin (H&E) and observed by light microscopy (Leica DM500) at 200x
magnification.
Statistical analysis
Data were represented as mean±standard deviation (SD), and analyzed by multifactorial analysis of variance,
Student’s t-test.
RESULTS
Hyperuricemia model
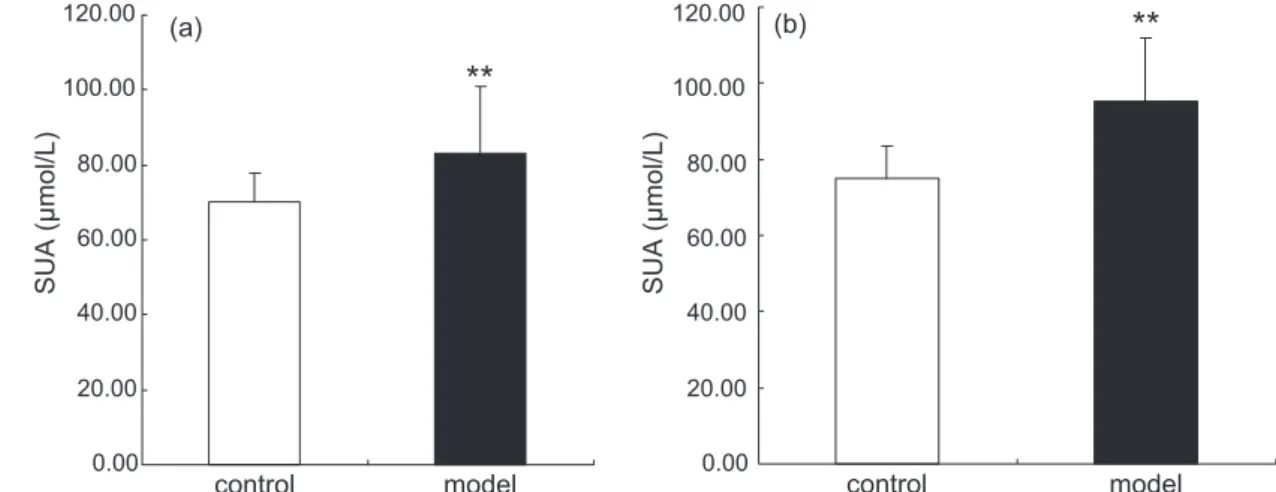
Treated with PO for 2 weeks, SUA level of rats was
70.15 ±7.64 μmol/L in control group and 83.22 ±17.56 μmol/L in model group (n=8). Compared with control
rats injected with vehicle alone, PO made SUA level
significantly increased by 18.6% (P<0.01) (Figure 1(a)). In 4th week, SUA level of rats was 75.02 ±8.48
μmol/L in control group and 95.18±16.74 μmol/L in model group (n=8). As the same time, PO made SUA level significantly increased by 26.7% (P<0.01) (Figure 1(b)).
Effect of hyperuricemia on T-SOD and Gu,Zn-SOD activity
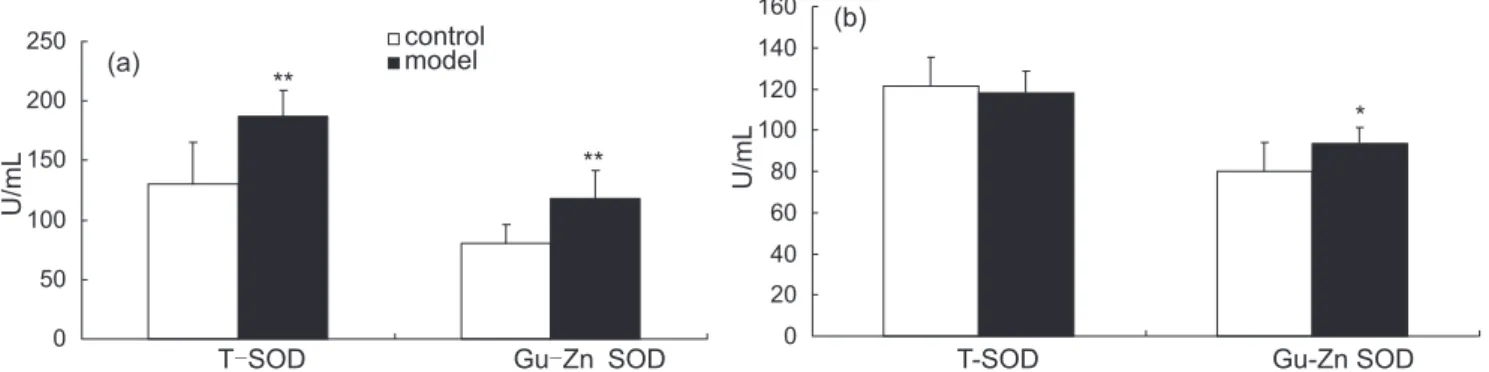
T- S O D a n d G u , Z n - S O D a c t i v i t y i n s e r u m were measured in 2nd and 4th week, which evaluated antopxidation. In 2nd week, T-SOD activity was 130.44±34.71 U/mL in control group and 187.21±21.31 U/mL in model group respectively. Gu,Zn-SOD activity was 80.40±34.71 U/mL in control group and 117.91±23.71
U/mL in model group respectively(n=8).Compared with
control group, high UA level increased both T-SOD and
Gu,Zn-SOD activity significantly (P<0.01) (Figure 2(a)). In 4th week, T-SOD activity was 121.34±12.17 U/mL in control group and 118.07±10.55 U/mL in model group
respectively (n=8). There was no significant difference
between two groups. However, compared with control group (79.95±14.21 U/mL), the Gu,Zn-SOD activity
in model group (93.46±8.04 U/mL) went up obviously
(P<0.05) (Figure 2(b)).
T-SOD and Gu,Zn-SOD activity in brain were measured in 4th week. T-SOD level was 154.10±25.81 U/g
in control group and 179.69±40.19 U/g in model group respectively (n=8). Gu,Zn-SOD activity was 44.55±12.72
U/g in control group and 111.24±31.47 U/g in model group
respectively (n=8). Compared with control group, both T-SOD and Gu,Zn-SOD activity increased significantly
(P<0.05, P<0.01) (Figure 3).
Effect of hyperuricemia on IL-1β concentration
IL-1β concentration in serum was measured in 2nd and 4th week, which estimated inflammatory response. In 2nd week, IL-1β concentration was 60.79 ±15.71 ng/mL
in control group and 64.22 ±18.01 ng/mL in model group respectively (n=8). There was no significant difference
between two groups (P>0.05) (Figure 4(a)). In 4th week,
IL-1β concentration was 59.34 ±19.40 ng/mL in control group and 79.18 ±17.20 ng/mL in model group respectively (n=8).
Compared with control group, high UA level increased
IL-1β concentration significantly (P<0.05) (Figure 4(b)).
IL-1β concentration in brain was measured in 4th
week. It was 976.49 ±272.26 pg/g in control group and 984.91 ±231.27 pg/g in model group respectively (n=8). There was no significant difference between two groups
(P>0.05) (Figure 5).
Effect of hyperuricemia on permeability of the blood–brain barrier in rats
Evans Blue dye was used as a blood-brain barrier
indicator for examining the extravasation from the blood to
the brain. The assay for leakage of Evans Blue from brain
vessels showed that the dye concentration was 8.91 ±2.45
μg/g in control group and 8.54 ±2.02 μg/g in model group (n=5). There was no significant difference between the two groups, which suggested the integrity of the blood–brain
barrier was not changed after PO treatment.
Effect of hyperuricemia on pathological section
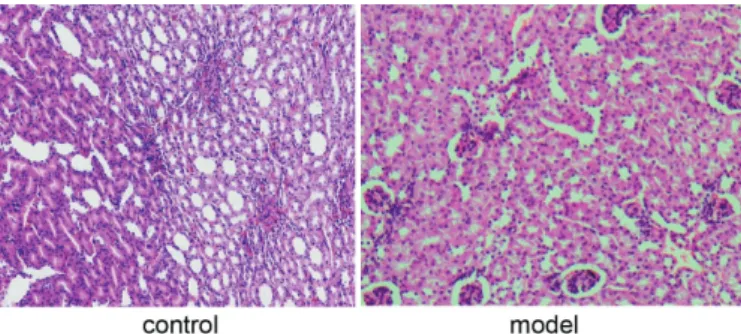
In control group, glomerular showed a sharply circumscribed boundary of tissue. Meanwhile the structure of renal tubular epithelium was clear and
holonomic. No inflammatory exudate could be observed
in renal interstitium. In model group, the structural and functional unit of the kidney was damaged. Glomerular contraction occurred, and the most striking change in the tubules was hydropic degeneration of the epithelium. Renal interstitium was collapsed and surrounded by
inflammatory exudate with epithelial cells necrosis and abscission in nephron (Figure 6).
FIGURE 3 - Effects of hyperuricemia on T-SOD and Gu,Zn-SOD activity in brain in 4th week. Data are mean ±SD, n =8. *P<0.05, **P<0.01 compared with control group
FIGURE 2 - Effects of hyperuricemia on T-SOD and Gu,Zn-SOD activity in serum in different time. Data are mean ±SD, n =8. (a): in 2nd week and (b): in 4th week. *P<0.05,**P<0.01 compared with control group
However, no pathological injuries could be observed in brain of two groups (Figure 7).
DISCUSSION
In peripheral system, our data show in the 2nd week, compared with control group, both of T-SOD and Gu,Zn-SOD activity in serum increased obviously (P<0.05) in hyperuricemia rats. However, IL-1β content
didn’t change remarkably. From 2nd week, rats in model group drank more water and excreted more urine (Data
not show), which meant the function of rats’ kidney
had been damaged. In the 4th week, T-SOD activity in model group had become similar with control group,
and meanwhile IL-1β content increased significantly
(P<0.05). Pathological section showed basement membrane of kidney tubules and glomerulus had been destroyed under hyperuricemia condition. It predicted that organism produced anti-response to stimulation effect caused by initial high UA level and enhanced immune responses, while UA mainly played a role of
antioxidant activity rather than induction of inflammatory
response. Nevertheless, when high UA level sustained
in peripheral system, UA would have displayed effects on induction of inflammatory response. Especially UA
concentration in serum was high enough to form sodium
urate crystals which produced a chronic inflammatory response with interstitial fibrosis and brought luxuriant pro-inflammatory cytokine such as IL-1β (Johnson et al., 1999).
It was worth to note that in the 4th week, Gu,Zn-SOD activity in model group was still higher than control.
However, UA didn’t take on adequate antioxidation against inflammatory reaction induced by IL-1β.
In Cerebral system, T-SOD activity increased obviously (P<0.05) in the brain of rats with hyperuricemia.
Yet there was no difference between two groups in IL-1β content. The assay for leakage of Evans Blue dye from
brain vessels and pathological section of brain showed
that the blood–brain barrier (BBB) permeability was not damaged in the model group, which signified that UA had
not mad microvascular endothelial cell in brain injured.
It conjectured that BBB kept IL-1β originated from
peripheral system out, and defended cerebral tissue from
inflammatory response. Therefore, UA as an antioxidant
protected brain tissue and neurons in the early stage. It is known there are three types of mitochondrial antioxidant enzyme, including copper, zinc superoxide dismutase (Cu,Zn-MOD), manganese superoxide dismutase (Mn-SOD) and iron superoxide dismutase (Fe-SOD). All of them are crucial in maintaining cellular and organism homeostasis, which are tightly regulated during inflammatory challenges. Qiu X found that
induction of Mn-SOD gene expression by IL-1β had been
mediated through a complex intronic enhancer element (Tong et al., 2005). T-SOD and Cu,Zn-MOD activity can reflect action of proinflammatory cytokine indirectly. From our researches, both in peripheral and cerebral
system SOD activity was influenced mainly by UA instead of IL-1β, which exhibited protection.
According to our data, mechanism of central protection may involve two ways. One was permeability
of BBB, and another was the form of UA. It is well known the BBB is important for the maintenance of
water and protein balance between the intravascular and extravascular compartments. Any injuries in FIGURE 7 - Pathological section of brain in 4th week (HE×100).
FIGURE 6 - Pathological section of renal tissue in 4th week (HE×200).
endothelial barrier function implicated in the genesis and/or progression of a variety of pathological conditions would lead to neurodegenerative disorders, angioedema, sepsis and cancer (Rodrigues, Granger, 2015).
Pro-inflammatory cytokine from peripheral system might affect the function of the central nervous system (CNS) by crossing the BBB for direct interaction with CNS
tissue. Saturable transport systems from blood to the CNS have been described for interleukin-1 alpha
(IL-1α), IL-1β, IL-1 receptor antagonist (IL-1ra), and tumor necrosis factor-alpha (TNF-α). There is a large number of blood-to-brain uptakes of IL-1, IL-1β, and IL-1ra
located in the most brain sites, which make incidence
rate of inflammatory reaction reduced (Banks, Kastin, Broadwell, 1995). It is reported a pro-inflammatory
cytokine response is associated with greater clinical
severity, BBB permeability, and neuroimaging damage
in encephalitis (Michael et al., 2015). Sadowska found
Blood-to-brain transport of IL-1β was higher across
brain regions in fetuses exposed to ischemia-reperfusion than non-ischemic fetuses. Therefore, hypoxic-ischemic injury could accentuate systemic cytokine transfer
across the fetal BBB (Sadowska et al., 2015). Tong
XK reported oxidative stress and structural alterations
in the cerebrovascular dysfunctions were associated with AD (Qiu et al., 2008). If permeability of BBB had been damaged, it would become more convenient for
redundant pro-inflammatory cytokine cross BBB to enter
cerebrospinal fluid and interstitial fluid spaces of the brain and spinal cord.
So we considered that UA could play a role on neuron protection in brain instead of induction of
inflammatory response, when overfull UA hadn’t made permeability of BBB damaged. How does UA work? The
form of UA may be the crucial factor.
In summary, BBB is a significant barrier to limit cytokine (e.g.IL-1β) invading into brain. Furthermore, BBB also restricts UA level in brain. In other words, whether UA can cross BBB and show antioxidation has
been decided by the form itself. Firstly, only water-soluble and low molecular weight (LMN) compounds, such
as morphine, are known to cross the BBB in sufficient amounts (Banks, Kastin, Broadwell, 1995). Free UA with
LMN is slightly soluble in water. Only a small quantity
of UA should be allowed to cross BBB and enter brain
from peripheral system, which improves SOD activity in order to clean more reactive oxygen species (ROS). In the
meanwhile, it still significantly decreased apoptosis via ROS formation and inflammatory cytokine production in CNS (Barichello et al., 2011). Even though UA formed
crystal, holonomic BBB would be tight enough to keep
them away from brain and prevent crystal deposition on tissue surfaces. In phagocytes, UA crystals have also been shown to trigger stress signals, including generation of free
radicals, potassium efflux and cathepsin B release from
fractured liposomes, which may be a key in the activation
of the inflammasome (Martinon, 2010).
Secondly, both UA and metabolite of UA own antioxidation. Haberman reported the synthetic 1,7-dimethyl derivative of UA reported previously to be protective in models of brain focal ischemia in mice retained the protective effects of its parent compound (Haberman et al., 2007). But not all catabolites evoke
antioxidative effect. In Guerreiro’s researches, xanthine
the immediate precursor of UA, which was produced via the degradation pathway of adenosine or that of guanosine,
failed to afford neuroprotection. Likewise, hypoxanthine
and guanine, the two purines bases, which gave rise to xanthine via catabolism of adenosine and guanosine, respectively, were ineffective and failed to reduce intracellular oxidative stress too (Guerreiro et al., 2009). And they found there was no positive correlation between UA concentration and antioxidation. Neuroprotection by
UA was reproduced by desferrioxamine (10 μM) which
predominantly chelates ferric iron and by catalase (500
IU/mL). Nevertheless, the survival effect of UA (200 μM)
was not improved by any of these antioxidants (Guerreiro et al., 2009).
CONCLUSION
In the pathological process of hyperuricemia, UA level mainly played a role on antioxidant activity rather
than induction of inflammatory response in the beginning.
As high UA level sustained in peripheral system until organ tissue damaged by sodium urate crystals, UA would display effects of induction of inflammatory response.
However, in Cerebral system, if BBB kept integrity, UA
would protect brain tissue and neurons as an antioxidant. In view of the above, no matter in peripheral or cerebral system, UA could play an important role both on
anti-oxidative stress and induction of inflammatory response. The permeability of BBB and form of UA would be
potential factors to decide which action of UA occupied predominant position and keep balance between protection and damage.
ACKNOWLEDGEMENT
CONFLICT OF INTEREST
The Author(s) declare(s) that they have no conflicts
of interest to disclose.
REFERENCE
Alvarez-Lario B, Macarrón-Vicente J. Is there anything good in uric acid? QJM. 2011;104(12):1015-1024.
Andreadou E, Nikolaou C, Gournaras, Rentzos M, Boufidou F, Tsoutsou AF, et al. Serum uric acid levels in patients with Parkinson’s disease: their relationship to treatment and disease duration. Clin Neurol Neurosurg. 2009;111(9):724-728.
Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2(4):241-8.
Barichello T, Lemos JC, Generoso JS, Cipriano AL, Milioli GL, Marcelino DM, et al. Oxidative stress, cytokine/chemokine and disruption of blood-brain barrier in neonate rats after meningitis by Streptococcus agalactiae. Neurochem Res. 2011;36(10):1922-1930.
Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120(5):442-447.
Cutler RG, Camandola S, Malott KF, Edelhauser MA, Mattson MP. The role of uric acid and methyl derivatives in the prevention of age-related neurodegenerative disorders. Curr Top Med Chem. 2015;15(21):2233-8.
Feig DI. Uric acid: a novel mediator and marker of risk in chronic kidney disease? Curr Opin Nephrol Hypertens. 2009;18(6):526-530.
François A, Terro F, Janet T, Rioux Bilan A, Paccalin M, Page G. Involvement of interleukin-1β in the autophagic process of microglia: relevance to Alzheimer’s disease. J Neuroinflammation. 2013;10:151.
Glantzounis G, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145-4151.
Grayson PC, Kim SY, Lavalley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res. 2011;63(1):102-110.
Guerreiro S, Ponceau A, Toulorge D, Martin E, Alvarez-Fischer D, Hirsch EC, et al. Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. J Neurochem. 2009;109(4):1118-1128.
Haberman F, Tang SC, Arumugam TV, Hyun DH, Yu QS, Cutler RG, et al. Soluble neuroprotective antioxidant uric acid analogs ameliorate ischemic brain injury in mice. Neuromol Med. 2007;9(4):315-323.
Haidari F, Rashidi MR, Mohammad-Shahi M. Effects of orange juice and hesperetin on serum paraoxonase activity and lipid profile in hyperuricemic rats. BioImpacts. 2012;2(1):39-45.
Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB. Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis. 1999;33(2):225-34.
Martinon F. Update on biology: uric acid and the activation of immune and inflammatory cells. Curr Rheumatol Rep. 2010;12(2):135-141.
Mazzali M, Kanbay M, Segal MS, Shafiu M, Jalal D, Feig DI, Johnson RJ. Uric acid and hypertension: cause or effect? Curr Rheumatol Rep. 2010;12(2):108-117.
Michael BD, Griffiths MJ, Granerod J, Brown D, Keir G, Wnęk G, et al. The interleukin-1 balance is associated with clinical severity, blood-brain barrier permeability, neuroimaging changes and outcome in encephalitis. J Infect Dis. 2015;213(10):1651-1660.
Pakpoor J, Seminog OO, Ramagopalan SV, Goldacre MJ. Clinical associations between gout and multiple sclerosis, Parkinson’s disease and motor neuron disease: record-linkage studies. BMC Neurol. 2015;15(16):1-6.
Qiu X, Aiken KJ, Chokas AL, Beachy DE, Nick HS. Distinct functions of CCAAT enhancer-binding protein isoforms in the regulation of manganese superoxide dismutase during interleukin-1beta stimulation. J Biol Chem. 2008;283(38):25774-25785.
W. Jing, J. Zhong, L. J. Ping, L. H. Yan
Sadowska GB, Chen X, Zhang J, Lim YP, Cummings EE, Makeyev O, et al. Interleukin-1β transfer across the blood-brain barrier in the ovine fetus. J Cereb Blood Flow Metab. 2015;35(9):1388-1395.
Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J Neurosci. 2005; 25(48):11165-11174.
Wu J, Ji H, Wang YY, Wang, Li YQ, Li WG, Y et al. Glutathione depletion upregulates P-glycoprotein expression at the blood-brain barrier in rats. J Pharm Pharmacol. 2009;61(6):1-6.