ContentslistsavailableatScienceDirect
Microbiological
Research
jo u r n al ho m e p ag e :w w w . e l s e v i e r . c o m / l o c a t e / m i c r e s
Gibberellins
in
Penicillium
strains:
Challenges
for
endophyte-plant
host
interactions
under
salinity
stress
Ana
Lúcia
Leitão
a,∗,
Francisco
J.
Enguita
baMEtRICs,DepartamentodeCiênciaseTecnologiadaBiomassa,FaculdadedeCiênciaseTecnologia,UniversidadeNOVAdeLisboa,CampusdeCaparica, 2829-516Caparica,Portugal
bFaculdadedeMedicina,UniversidadedeLisboa,Av.Prof.EgasMoniz,Lisboa1649-028,Portugal
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received9November2015 Accepted14November2015 Availableonline1December2015 Keywords: Penicillium Plants Salinity Gibberellins Symbioticinteractions
a
b
s
t
r
a
c
t
ThegenusPenicilliumisoneofthemostversatile“mycofactories”,comprisingsomespeciesableto pro-ducegibberellins,bioactivecompoundsthatcanmodulateplantgrowthanddevelopment.Although plantshavetheabilitytosynthesizegibberellins,theirlevelsarelowerwhenplantsareundersalinity stress.Ithasbeenrecognizedthatdetrimentalabioticconditions,suchassalinestress,havenegative effectsonplants,beingtheavailabilityofbioactivegibberellinsacriticalfactorfortheirgrowthunder thisconditions.ThisreviewsummarizestheinterplayexistingbetweenendophyticPenicilliumstrains andplanthostinteractions,withfocusonbioactivegibberellinsproductionasafungalresponsethat allowsplantstoovercomesalinitystress.
©2015ElsevierGmbH.Allrightsreserved.
Contents
1. Introduction...8
2. Gibberellinsasmodulatorsofplant-endophyteinteractions...9
2.1. Fungalendophytes ... 9
2.2. Gibberellinsasphytohormones...9
2.3. Moleculardetailsofgibberellinaction...9
3. Fungiasgibberellinproducers:biosyntheticgeneclusters...10
4. GenusPenicillium...11
5. Salinestress,gibberellinsandPenicillium...13
5.1. Salinity ... 13
5.2. InteractionsbetweenPenicilliumandplantsthroughgibberellins...14
5.3. PutativegibberellinbiosyntheticgenesinPenicillium...15
6. Conclusionsandfutureperspectives...16
References...16
1. Introduction
Fungiareimportantmicrobialfactoriesofbioactive
extracel-lularmetabolitesor “extrolites”,namelysecondarymetabolites.
Enzymes, immunosuppressive agents, antitumor agents,
antibi-otics,vitamins,andpigments,areexamplesoftherepresentative
panoplyofproductswithincreasinginteresteitherforscientific
∗ Correspondingauthor.Fax:+351212948543. E-mailaddress:aldl@fct.unl.pt(A.L.Leitão).
communityorindustrialsector(Brakhage,2013;Correaetal.,2014;
Kimetal.,2014;LeitãoandEnguita,2014;Quangetal.,2014).
Thegibberellins,hormonessynthesizedbyplants,are
interest-ingextrolitesalsoproducedbysomestrainsoffungilikeFusarium
sacchari,Fusariumkonzum,Fusariumsubglutinans,Aspergillus
fumi-gatus,PenicilliumjanthinellumandPenicilliumresedanum,among
others(Troncosoetal.,2010;Khanetal.,2011b,2015a,b).The
gib-berellinsarerelatedbytheirchemicalstructure (resultingfrom
isoprenepolymerization)andtheirbiosynthesispathway(origin
inhydroxymethyl-glutarylcoenzymeA),butonlyfewofthemare
bioactive.Oneofthesebioactivegibberellinsisthegibberellicacid,
oftencalledGA3;curiouslysomefungalstrainsareabletoproduce
http://dx.doi.org/10.1016/j.micres.2015.11.004 0944-5013/©2015ElsevierGmbH.Allrightsreserved.
higherquantitiesofGA3whencomparedtoplants(Heddenetal., 2001).
Theuseof fungalendophytesand theirextrolites canbean
excellentopportunitytominimizethenegativeeffectofabiotic
factors,suchassalinity,oncropyield.Theterm
“plant-growth-promoting-fungi”wasestablishedtodesignatesomerhizosphere
fungiabletopromoteadirecteffectonplantgrowthuponroot
col-onizationorbythetreatmentwiththeirmetabolites(Hossainetal.,
2014).Recentstudies have revealedthat Penicillium endophyte
couldsupplygibberellinstoplanthost,whichisparticularly
impor-tantwhenplantisunderbioticorabioticstress.Inthisreview,we
summarizerecentdiscoveriesontheendophyticPenicilliumstrains
andplanthostinteractions,withemphasisonbioactivegibberellins
asresponsetosaltstress.
2. Gibberellinsasmodulatorsofplant-endophyte interactions
2.1. Fungalendophytes
Fungalendophytesrefertothefungiwhichinvadeorliveinside
the tissues of plants without causing apparent harm to them
(Chandra,2012).Theyweredescribedbythefirsttimein1904in
thedarnel,Loliumtemulentum(Freeman,1904),buttheydidnot
receivemuchattentionuntiltherecentdevelopmentofscreening
technologiesthatrevealedtheirgreatpotentialasamainsourceof
extroliteswithpromisingagriculturalandpharmaceutical
appli-cations(Tanand Zou, 2001; Kusari etal., 2012).Therefore,the
relationshipbetweentheendophyteandtheplantisgenerally
con-sideredmutualisticbecausetheendophytesignificantlyimproves
hostplanttolerancetoabioticstressessuchasdroughtand
water-deficitorbioticfactorssuchasinsects,vertebrateherbivoresand
nematodes,alongwithincreasedresistanceandpromotingplant
growth,nutrientsuptake,andwaterresourceuse;andinturnthe
plantprovidesthemicroorganismwithnutrients,protection,and
efficientdissemination(Schardlet al.,2004).However,theidea
thattherearenoneutralinteractionsbutratherthat
endophyte-hostrelationshipisabalancedsymbioticcontinuumrangingfrom
mutualismthroughcommensalismtoparasitism,isgaining
follow-ers(Alyetal.,2011).Infact,wheninsidetheplant,fungiassume
aquiescentstateuntilenvironmentalconditionsarefavorablefor
theirgrowth.Thefungihavetheabilitytocolonizetheplantmostly
byassociationwithbut insomecasescan liveinsidetheplant
eitherpenetratinginsidetherootcortexorintheaerialpartsof
theplant,duetotheirextracellularenzymaticsystem(Waqasetal.,
2012;Khanetal.,2013a).Aftercolonizationfungigrowwellinthe
apoplasticwashingfluidofthehost(Chandra,2012).
The fungal endophyte-plant host relationship seems to be
tightly dependent ongenetic, physiological and environmental
control(Kogeletal.,2006).Despiteofthat,thereisnodoubtthat
inthecaseof mutualisticinteractionthepresence ofthe
endo-phytehelpstomitigatetheeffectsofplantstresses,whichrequires
acontinualmetabolicinteractionbetweenfungusandplanthost.
Endophyticfungihaveastrongtolerancetowardsplant´ıs
metabo-litesduetotheirabilitytotransformanddetoxifythemwiththe
concomitant production ofextrolites, some of themwith great
pharmaceuticalpotentialas bioactivecompounds(Kusari etal.,
2012;Khanetal.,2015b).
In some cases from endophyte-host relationship results
metabolitesthatareproducedsimultaneouslybytheplantandthe
fungus,likethephytohormonesgibberellins(Takedaetal.,2015).
Twooppositetheoriestriedtoexplainthiscuriousphenomenon.
Onesupportstheideathatendophyteevolvedgibberellins
biosyn-thetic pathwaysindependently fromplants, based on the high
conservationofgibberellinsclusterorganizationinPhaeosphaeria
spp. and Sphaceloma manihoticola, two distantly related fungal
species. Thedifferences betweenplants andfungi at
biochemi-cal and geneticlevels strengthensthat higher plants and fungi
haveevolvedtheirbiosyntheticpathwaystogibberellins
indepen-dently(MacMillan,1997;Heddenetal.,2001;Yamaguchi,2008;
BomkeandTudzynski,2009).Theotheronepointoutthatduring
theco-evolutionofmicroorganismsandtheirhostplants,
endo-phytesundergogeneticmodification,for instanceby hostgene
transfer,thatallowthemtoadaptsuccessfullytotheplant
microen-vironments,whichcouldbealsocorroboratedbythelackofplant
responseagainstthepresenceofendophytes(ChapmanandRagan,
1980;Germaineetal.,2004).
2.2. Gibberellinsasphytohormones
Gibberellinswerefirstidentifiedasphytohormonesinthe1930s
basedonanover-growthriceseedlingduetoinfectionsbyFusarium
fujikuroi(teleomorphGibberellafujikuroi)a pathogenicrice
fun-gus(Ogas,2000).Thesefungalsecondarymetaboliteshavebeen
reportedtoplayapivotalroleinplantgrowthanddevelopment
processes,suchasregulationofgeneexpressioninthecereal,seed
germination,stemelongation,floweringandfruitdevelopment.In
thepresenceofgibberellins,plantsareabletoaltertheir
physiol-ogyandbiochemistryinrapidresponsetoenvironmentalchanges
(Olszewskiet al., 2002).Gibberellins were merely isolated and
identifiedasplanthormonefromextractsofhigherplantsinthe
mid-50sbyBritishscientists(Lang,1956;Radley,1956).The
knowl-edgeofgibberellicacid(GA3)structurefromG.fujikuroiopened
thewindowfornewstudiesthatculminatedwiththediscovery
thatgibberellinswerediterpenoidcompounds(Birchetal.,1958;
Crossetal.,1959).Furtherstudiesweredone,mostofthemwith
the mutant BI-41a (GA-deficientmutant of G. fujikuroi blocked
atanearlystepofthepathway), andbringtolight the
biosyn-theticpathwayofgibberellicacidintheG.fujikuroi(Bearderetal.,
1974;Bearder,1983).During severalyears, gibberellinpathway
wasonlyreportedintheF.fujikuroi.Detailedcharacterizationat
chemical,biochemicaland geneticlevelsinF.fujikuroihasbeen
reported(Cerda-Olmedoetal.,1994;Tudzynski,2005;Bomkeand
Tudzynski,2009).TheGA3biosynthesis,forexample,involvetwo
early cyclizationreactions, from geranylgeranyl diphosphate to
ent-kaurene,followedbyseveraloxidativereactionscatalyzedby
cytochrome P450monooxygenases torender thefinal product,
19–10␥-lactone(Kellerand Hohn,1997;TudzynskiandHolter,
1998).
Thegibberellinsaresmallmoleculesofalargegroupof
tetra-cyclicditerpenoidcarboxylicacids,beingdefinedbytheirchemical
structure based on the ent-gibberellane carbon skeleton and
assignedgibberellin“numbers”dependingonchronologicalorder
oftheiridentification.Nowadays,thereare136knowngibberellins
producedbyfungi,plantsandevenbacteria.Nevertheless,onlya
smallnumberofthem,suchasGA1,GA3,GA4andGA7are
promi-nentbioactive(Davies,2004).
2.3. Moleculardetailsofgibberellinaction
Asphytohormones, gibberellins regulatecritical stepsin the
plantlife cycle. Theirphysiological action is mainlyexertedby
counteractingtheinhibitoryeffectofDELLAproteins,afamilyof
nuclearnegativeregulatorsthatrestrictplantgrowthprobablyby
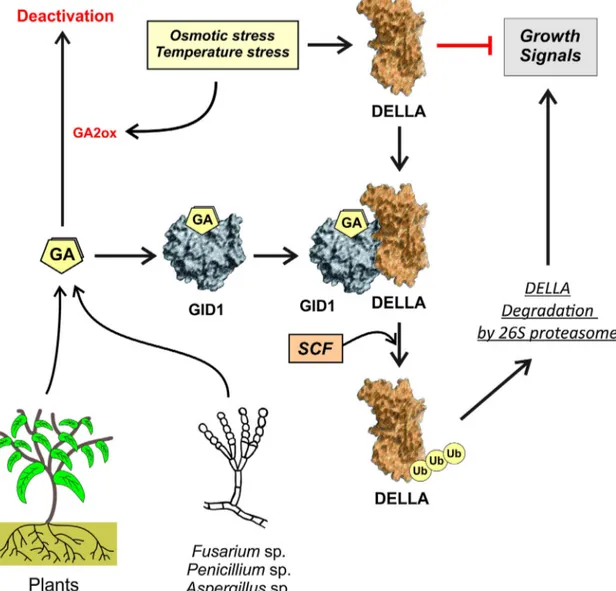
transcriptionalreprogramming(Fig.1)(Sun,2011).DELLAproteins
areexpressedunderosmoticortemperaturestress,repressingthe
plantgrowth.ExposureofArabidopsisthalianatosaltstress
trig-gersareductioninbioactivegibberellins,promotesDELLA(groupof
transcriptionalregulators)accumulationandconsequently
DELLA-mediatedgrowthrestriction(Achardetal.,2006).Althoughitis
Fig.1. Actionmechanismofgibberellins.Gibberellins(GA)actionisexertedbybindingtotheGID1nuclearreceptor,andsubsequentrecruitmentofDELLAproteins.The formationoftheternarycomplex,GA-GID1-DELLA,facilitatestheactionoftheubiquitintransferaseSCF,whichactsoverDELLAproteinsandinducestheirdegradationvia 26Sproteasome.Gibberellinscanbesynthesizeddirectlybyplantsorbyendophyticmicroorganisms,namelyfungi,counteractingtheinhibitoryeffectsofDELLAproteins overtheplantgrowingsignals.Underabioticstressconditions,severalgibberellin-inactivatingenzymesareproduced,suchasgibberellin-oxidases(GA2ox).
cascadeofinteractionsinvolvinggibberellinsandotherhormone
signalling pathways, the regulationof expressionor activity of
transcriptionsfactors involved in gibberellinmetabolism genes
couldrepresentonemechanismofstresstoleranceinplants.At
thesametime,theabioticformofstressisabletoinduceaseries
ofenzymeswhichareinvolvedintheinactivationofgibberellins,
namelygibberellin-oxidases(Rieuetal.,2008).Activeformsof
gib-berellins(namedGA1,GA3,GA4andGA7)actviaGID1proteins,a
familyofspecificnuclearreceptorsthatplayanimportantrolein
regulatingdifferentdevelopmentalprocessesinplants(
Ueguchi-Tanakaetal.,2007;Voegeleetal.,2011).Gibberellinbindingto
GID1receptorinducesaconformationalchangeintheproteinthat
makesitpronetointeractwiththeN-terminaldomainofDELLA
repressors.Gibberellinsoffungaloriginsharethesamefunctional
characteristicsoftheplantgibberellins,sincetheyareidenticalin
theirchemicalstructure(Khanetal.,2013b).Aftertheinteraction
withgibberellins,theGID1-DELLAcomplexissubsequently
ubiq-uitinatedbytheubiquitin-transferaseSCF,andthustargetedfor
proteindegradationmediatedbythe26Sproteasome.In
conse-quence,thegibberellinactionresultsinreducedlevelsoftheDELLA
repressorandastimulationofplantgrowth(Fig.1).
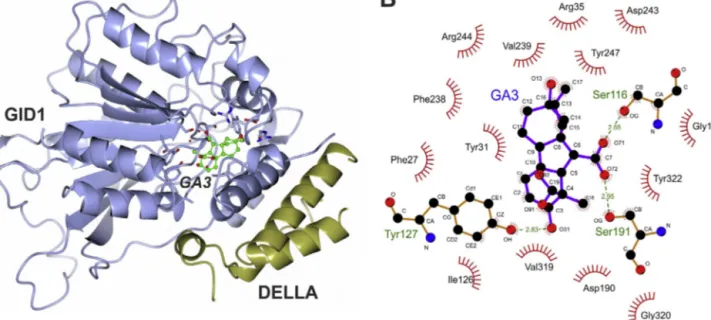
The detailed molecular mechanism of the
gibberellin-GID1-DELLAinteractionhasbeencharacterizedbyX-raycrystallography
studies(Fig.2)(Muraseetal.,2008;Shimadaetal.,2008).
Com-plexescontaininggibberellinsGA3andGA4,theGID1receptorand
theN-terminalDELLAdomainhavebeenresolvedathigh
resolu-tion,showinganintimateinteractionbetweenthereceptorand
thegibberellinmolecule,establishedinadeepproteinpocketand
basedmainlyonhydrophobicinteractions(Fig.2)(Muraseetal.,
2008).However,thelackofthestructuralinformationonthe
apo-receptorpreventedtounderstandthedynamicsofitsinteraction
withthegibberellinligandandthesubsequentbindingtothe
N-terminalDELLAdomain.
3. Fungiasgibberellinproducers:biosyntheticgene clusters
Several species of fungi belonging to the geni Fusarium,
Aspergillus and Penicilliumhave beencurrentlycharacterizedas
gibberellinproducers(Tudzynski,2005).Thecanonicalpathway
for gibberellinbiosynthesisin fungiwasoriginallydescribed in
F. fujikuroi and their molecular details and involved enzymes
Fig.2. StructuraldeterminantsforthegibberellinactionasdeterminedbyX-raycrystallography.(A)ribbonrepresentationoftheGA3-GID1-DELLA(PDBcode:2ZSH), showingtheGID1structurewiththebindingpocketwheregibberellinGA3islocated,andalsotheN-terminaldomainofDELLA.Thegraphwaspreparedbyusingthe CCP4MGsoftware(McNicholasetal.,2011).(B)planardiagramofmolecularinteractions(Ligplotdiagram)occurringinthesubstratebindingpocketofGID1receptor involvedintherecognitionofgibberellinGA3.Atomsinvolvedinhydrogenbondsbetweenthereceptorandtheligandaredepictedforeachaminoacidandrepresentedby dottedlines.Hydrophobicinteractionsaredepictedonlybythenumberandtypeofresidueinvolved.ThepanelwasdesignedandeditedbytheLigplot+software(Laskowski andSwindells,2011).
2009). Gibberellins, like other diterpenoid compounds, are
synthesized starting from geranyldiphosphate (GDP), farnesyl
diphosphate(FDP)andgeranylgeranyldiphosphate(GGDP).This
lastcompound isaprecursor forgibberellinsandalsofor some
carotenoidsandubiquinones.Infungiandplants,GGDPiscyclized
to produce ent-kaurene, the first gibberellin-specific precursor,
whichwillsuffersequentialoxidationstogenerateGA12-aldehyde
(Fig.2).Fungalgibberellinbiosyntheticpathwaywillconvert
GA12-aldehydeintoGA14-aldehydebyanoxidationreactioncatalyzed
bya cytochromeP450protein.Furtheroxidation and
desatura-tionreactionswillproducethegibberellinsGA1,GA3,GA4andGA7
(Tudzynski,2005;BomkeandTudzynski,2009).
Despitethebiochemicalcharacterizationofgibberellin
biosyn-theticpathwayinfungi,thegeneticbackgroundiscomparatively
lessknown.In F.fujikuroithegibberellinbiosyntheticclusteris
comprisedbysevenclusteredgenesencodingfourcytochrome
P-450oxidoreductases(P450-1, P450-2,P450-3and P450-4), two
GGDPsynthases(Ent-kaur-16-enesynthase,CPS/KS,and
geranyl-geranyldiphosphatesynthase,GGS2),andaGA4desaturase(DES).
BesidesspeciesbelongingtotheFusariumgenus,thereareonlytwo
documentedcasesofthegeneticcharacterizationofagibberellin
biosyntheticgeneclusterinSphaceloma(Bomkeetal.,2008)and
Phaeosphaeria(Kawaideetal.,1997,2000).Theevolutionary
mech-anismsbywhichthesefungiacquiredthegibberellinbiosynthetic
geneclustersarenotyetclear.Anincreasingnumberofevidences
pointedoutthat thepresenceofhomologousbiosyntheticgene
clustersindistantlyrelatedfungicanprobablyresultfrom
horizon-talgenetransfer(SlotandRokas,2011).Interestingly,recentdata
alsoindicated thepresenceofdefectiveortruncatedgibberellin
biosyntheticclustersinsomeFusariumspecies(Wiemannetal.,
2013).Theseincompleteclustersareprobablyrelatedwith
adap-tivephenomenainthesefungalspecies,whichexertedselection
pressureforspecificgenedeletion(Maloneketal.,2005).In
Fusar-iummangiferae,FusariumcircinatumandsomestrainsofFusarium
oxysporum,thegibberellingbiosyntheticclusterispresentbutits
expression prevented by a non-functional promoter (Wiemann
et al.,2013).Silentbiosynthetic clusterslikethose observed in
someFusariumspecies arevery commonamong fungi,andcan
opennewpossibilitiesofgeneticmanipulationfortheproductionof
secondarymetaboliteslikegibberellins(LeitãoandEnguita,2014).
4. GenusPenicillium
Penicillium belongs tothe phylum Ascomycota, however its
taxonomic characterization is still a matter of discussion and
the difficulties in identifying most Penicillium species requires
multidisciplinaryapproaches.Clarificationofspeciesconceptsin
the genus Penicillium was supported mainly by morphological
characteristics.RaperandThom,forexample,basedPenicillium
tax-onomyclassificationonthecombination ofmacroscopical(such
as colony texture and color) with micromorphological features
(RaperandThom,1949).InRaperandThomclassification,Penicillia
thatproducemonoverticillateconidiophoreswereincludedinthe
Monoverticillatagroupandthisgroupwasdividedintonineseries
(genussubdivision).LaterinPitt’sclassificationmodificationson
serieswereperformedandsectionswereintroducedinsubgenus
basedonthepresenceofaswellingatthestipeapex(Pitt,1979).
Despite ofdirectidentificationof purePenicilliumspecies being
possiblebyimageanalysis(Dorgeetal.,2000);conidialcolor,
pro-ductionofascomataandascospores,shapeandornamentationof
conidiaandgrowthratesonsolidmediaremainrelevant
parame-tersforspeciesidentification(Houbrakenetal.,2012).Houbraken
etal.(2011)basedonamultigeneapproachredefinedthegenus
Penicilliumusing singlenamenomenclature and includingboth
asexualandsexualreproducingspecies.Theyproposedasectional
classificationandsubdividedPenicilliumintotwosubgeneraand25
sections(Houbrakenetal.,2011).
ThegenusPenicilliumhasreceivedmuchattentionduetohave
the bestknown producerof the antibioticpenicillin,P.
chryso-genum.Later,mycotoxinsbecamefocuseduponastheyappeared
intheprocessingorripeningofhumanfoods,beingamajorrisk
forhumanhealthduetotheircytotoxicity(KellerandHohn,1997).
However,Penicilliumspeciesarealsowellknownaspotentialtool
intheenvironmentfield,sincetheyhavetheabilitytodegrade,or
toremoveawidevarietyofcompoundsandheavymetals(Leitão,
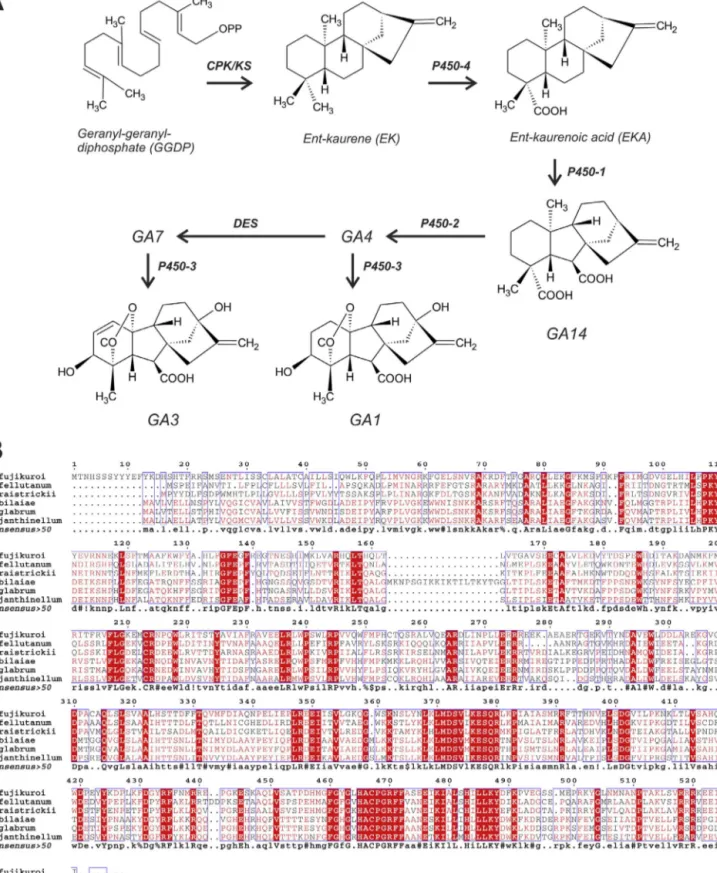
bio-Fig.3.GibberellinbiosyntheticpathwayinFusariumfujikuroiandputativegibberellinbiosyntheticgenesinseveralPenicilliumspecies.A,gibberellinbiosyntheticpathway ascharacterizedinF.fujikuroiincludingtheinvolvedenzymes;GGDP,geraryl-geranyldiphosphate;EK,ent-kaneurin;EKA,ent-kaneuroicacid;GA14-ald,GA-14aldehyde. B,sequencealignmentofP450-1proteinfromF.fujikuroiwithitsputativeorthologsinseveralPenicilliumspecies.Conservedresiduesalongthesequencearedepictedinred boxes.(Forinterpretationofthereferencestocolorinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
logicalactivityofPenicilliumstrainscanreducethegenotoxicity
inducedbyseveraltoxiccompounds(Pereiraetal.,2014;
Romero-Aguilaretal.,2014).
ThesuccessofPenicilliumstrainsismainlyduetotheir
occur-renceinvariousfoodandfeedstuffs(Santinietal.,2014),indoor
environmentssuchasair,dustanddampbuildingmaterials(Chang
etal.,1995;Scottetal.,2004;McMullinetal.,2014;Visagieetal.,
2014), as wellas in the marine (Gong et al., 2014; Kim et al.,
2014;Liaoetal.,2014;Quangetal.,2014;Guoetal.,2015;Park etal.,2015)andsoil(Leitãoetal.,2007; Alyet al.,2011;Gong et al., 2014; Moore-Kucera et al., 2014; Tansakul et al., 2014)
environments.Moreover,thecataboliccapacityofthese
microor-ganismsduetotherelativeunspecificityoftheirenzymestogether
withtheirlimited growth requirements, diversity of secondary
metabolitesproductionandhighabilitytoformextendedmycelial
networksallowthemtosurviveinaninhospitableenvironment.
It hasbeensuggested thattheproducts originatedfromfungal
metabolicmachinerysupply themwitha chemicalarsenal that
increases itsfitness under challenging ecological conditions. In
fact, these characteristics are shared by several fungal species
and couldbea seriousadvantage interms ofnatural selection.
Ontheotherhand,symbioticinteractionswithotherorganisms
co-occuringinthesamehabitathaveasignificantimpactinthe
ecosystem.Forinstance,itisknownthatPenicilliumspeciesare
importantphosphate-solubilizingmicroorganisms; this capacity
allowedPenicilliumoxalicumI1topromote maizegrowthwhen
funguswasinoculatedintheplant(Gongetal.,2014).
SomePenicilliumspeciesareconsideredtobeplantpathogens
duetotheircapacitytopotentiallyproducemycotoxinsthatare
then consumed by humans and animals. For instance,
Penicil-liumexpansum,PenicilliumitalicumandPenicilliumdigitatum,major
postharvestpathogens ofpomeandcitrus, producethe
polyke-tidelactone,namelypatulin(Lietal.,2015);howeverpatulinis
notrequiredbyP.expansumtosuccessfullyinfectapples(Ballester
etal.,2015;Lietal.,2015).Interesting,cell-freefiltrateof
Penicil-liumGP15-1increasedsystemicresistanceagainstcucumberleaf
infectionby theanthracnose pathogenColletotrichum orbiculare
(Hossainetal.,2014).OtherexampleisthepenicisteroidAisolated
from the culture extracts of the Penicillium chrysogenum
QEN-24Sstrainthatcolonizesanunidentifiedmarineredalgalspecies
belonging to the genus Laurencia. This polyoxygenated steroid
showedmoderateantifungalactivityagainstAlternariabrassicae
and potent activity against Aspergillus niger (Gao et al., 2011).
Recently,astudyconductedwithaP.janthinellumstrainshowed
thatitsinoculationintolerantSolanumlycopersicumreduced
cel-lularsuperoxideanionsinaluminumstress(Khanetal.,2015c).
Additionally,theeffectoffungalstraininthetomatoplantwas
com-paredtoexogenousgibberellicacidandasimilarbio-prospective
potentialwasdescribed.Basedontheseresults,theapplicationof
biochemicallyactiveendophyte wasproposedtoincreasemetal
phytoextractionandensurecropphysiologicalhomeostasis.
5. Salinestress,gibberellinsandPenicillium
5.1. Salinity
Salinityisthewordthatdescribessoilsthatenclosehigh
concen-trationsofwater-solublesalts,mainlyNaCl,whichcausingserious
agriculturalyieldlosses.Itisestimatedthat20%oftheworld’s
cul-tivatedfieldsandapproximatelyhalfofthearablesoilareaffected
bysalinity(SairamandTyagi,2004).Ifweconsiderthatin2050
thepopulationwillincrease2.3billion,representinganincreaseof
70%offoodcropproductiondemandsanewapproachfor
threat-eningfoodsecurityworldwideis essential(FAO, 2009).Salinity
is hostiletomost formsof life becauseit is responsiblefor an
imbalanceof cellularion homeostasis,which requiresa quickly
osmoticadjustmentviamorphologicalflexibilityandbiosynthesis
ofsecondarymetabolitessuchascompatiblesolutes,which
accu-mulationinplantsatthemillimolarrangeplayanimportantrole
inplanttolerancetosaltstress(ChenandMurata,2011;Nounjan
etal.,2012).Despitethat,theabilityofPenicilliumstrainstotolerate
highconcentrationsofNaClisknown.Infact,thegeneraPenicillium
isrepresentativeofthepan-globalstablemycobiotainhypersaline
environment(Butinaretal.,2011).
In plantshighsalinityinhibitsthegrowthof rootand shoot
systemsbylimitingtheavailabilityofwaterandmicronutrients
causingcellulardamageandmodulatingseveralprocesses.Besides
thegreateffortcanalizedtothecompatiblesolutes(forexample
betaine(Gaoetal.,2004),glycinebetaine(ChenandMurata,2011),
trehalose(Nounjanetal.,2012)andproline(Strizhovetal.,1997;
Nounjan et al.,2012)), phytohormone(abscisic acid(ABA),
jas-monate(JA),brassinosteroid(BR)andgibberellicacid(GA)(Geng
etal.,2013;Ismailetal.,2014;JulkowskaandTesterink,2015))
and enzymes(as ascorbate peroxidase, glutathione peroxidase,
catalase,polyphenoloxidase(Sofoetal.,2015))biosynthesis,the
salinityhasadditionalnegativeeffectsonthecellularenergy
sup-ply, photosynthesis and redox homeostasis, since plants must
assimilate Na+ and Cl− (Zhu et al., 2010; Jacoby et al., 2011;
Mulleretal.,2014).Whenplantsareundersalinityconditions,the
decreaseinphotosynthesiscanbemainlyattributedtolowerCO2
availabilitythroughstomatalclosure,beingthecontrolof
respira-tionratesdependonsubstratesupplyandbiochemicalregulation.
Itissuggestedthatthevariabilityinrespiratoryresponsesmayvary
significantlybetweenspecies(Jacobyetal.,2011).
Theinfluxofsodiumionsbyrootepidermalandcorticalcells
through nonselectivecation channels (NSCCS) induces
depolar-ization of theplasma membrane, reducing potassium ions(K+)
channels uptake through inward-rectifying (Shabala and Cuin,
2008).To preventadditional influx of sodiumions two
deacti-vationmechanismsmaybeinvolved:byNSCCchannelsthrough
cAMP/cGMP-dependent signals or by high affinity potassium
transporter(HKAT)channel.Asaconsequenceofosmoticstress,
activationofmechanosensitivecalciumchannelsresultsinan
addi-tionalinfluxofprotonsandcalciumions(Ismailetal.,2014).The
cytosolicconcentrationofcalcium(Ca2+)increaseinducingreactive
oxygenspecies(ROS)productionthroughNADPHoxidase
stimula-tionandactivatingCa2+calmodulim-dependentkinases.TheCa2+
calmodulim-dependentkinasesstimulatestheplasma-membrane
H+-ATPasesactivityamongothersenzymes,restoringmembrane
voltageandinhibitingdepolarization-activatedNSCCS(Klobusand
Janicka-Russak, 2004; Shabala et al., 2006; Ismail et al., 2014; JulkowskaandTesterink,2015).Ontheotherhand,Ca2+andROS
modulatesthereleaseofabscisicacid(ABA),aphytohormonethat
regulatesseveralplantbiologicalprocessessuchasgrowth,
biosyn-thesisofcompatiblesolutes,controlofstomatalclosure,among
others(Ismailetal.,2014).
Inarecentandveryinterestingreview,itwasproposedthat
dependingonthetimingoftheeventstriggeredbythesodiumion
anadaptive/acclimationresponsesorthesodiumaccumulationin
thecytoplasmmightoccur.Theadaptiveresponsescouldinvolve
mechanismsofsequestrationintovacuoleandextrusionofsodium
aswellastheconstraintofjasmonate(JA)signalling.Meanwhile,a
delayintheactivationand,consequently,alsointhedeactivation,
of“salinitysignalling”throughthegenerationanddissipationof
triggeredcalcium-dependentsignalrelativetoasignaltransmitted
byROSwilloriginatetheactivationofJAsignalingandthusleading
tocelldeath(Ismailetal.,2014).
Itisoutofthescopeofthisreviewtodescribetheplant
cellu-larmechanismsandmolecularresponsestohighsalinity,butfor
thosereaderswhoareinterestedinthisaspectwerecommendthe
Table1
GibberellinsproductionbyPenicilliumstrainsandeffectonplantgrowthundersalinitystress.
Penicilliumstrain Gibberellin(GA) Plantgrowtheffectundersalinitystress References P.citrinumKACC43900 GA11.95ng/ml CanpromoteIxerisrepensgrowth(shoot
length,plantlength)
Khanetal.(2008) GA33.83ng/ml GA46.03ng/ml GA50.365ng/ml GA72.35ng/ml GA90.65ng/ml GA120.11ng/ml GA150.72ng/ml GA190.67ng/ml GA200.30ng/ml GA241.40ng/ml
P.funiculosumLHL06 GA11.53ng/ml Canpromotesoybeangrowth(shootlength, shootfresh/drybiomass,chlorophyllcontent, photosynthesisrate,leafarea)
Khanetal.(2011a)
GA49.34ng/ml GA81.21ng/ml GA937.87ng/ml
P.minioluteumLHL09 GA412.84ng/ml Canpromotesoybeangrowth(shootlength, shootfresh/drybiomass,chlorophyllcontent, leafarea)
Khanetal.(2011b)
GA748.912ng/ml
Penicilliumsp.SJ-2-2 GA11.185ng/ml Canpromotecucumbergrowth(shootlength, plantheight,chlorophyllcontent,leafarea)
Youetal.(2012) GA31.255ng/ml GA43.497ng/ml GA71.357ng/ml GA90.530ng/ml GA120.335ng/ml GA190.011ng/ml GA200.033ng/ml GA240.838ng/ml GA340.049ng/ml
Penicilliumsp.LWL3 GA15.33ng/ml Canpromotesitiensgrowth(shootlength, shootfreshbiomass,photosynthesisrate)
Waqasetal.(2012) GA33.42ng/ml
P.janthinellumLK5 GA31.2ng/ml Khanetal.(2013a,b)
GA410.19ng/ml GA70.71ng/ml GA1213.98ng/ml
P.resedanumLK6 GA17.1ng/ml Canpromotepeppergrowth(shootlength, shootdryweight,photosynthesisrate)
Khanetal.(2015a,b,c) GA313.9ng/ml GA419.159ng/ml GA71.12ng/ml GA92.2ng/ml GA121.93ng/ml GA201.68ng/ml
Tyagi,2004;Ismailetal.,2014;Julkowskaand Testerink,2015).
Despite ofplant salinitystress researchadvancesin the recent
years,anunderstandingofthetemporaldynamicnatureof
tran-scriptionaleventsisstilllacking.Gibberellinsareanexampleof
classicalgrowthpromotinghormone;however,saltstressinduced
repressionofthegibberellinsignalingpathwaysresultinginlower
cellcycle(Westetal.,2004).GAbiosynthesisandsignalinghave
recentlybeenshowntobenecessaryduringthelatephasesofthe
saltresponsetopromoterecovery(Gengetal.,2013).Therefore,GA
presenceisalsoacriticalfactorundersaltstress,justifyingan
alter-nativeapproachtopreventitsabsence.Cansymbioticinteraction
plant-fungussupplygibberellins?
5.2. InteractionsbetweenPenicilliumandplantsthrough
gibberellins
Although various Penicillium species have been reported as
endophytics(SpurrandWelty,1975;Colladoetal.,1999;Larran
etal.,2001;Caoetal.,2002;Petersonetal.,2005),earlierthan2008
verylittlewasknownaboutthesesymptomlessmicroorganisms
livinginsidehostplantandgibberellinsproductionunder
salin-itystress.AstrainofPenicilliumcitrinumisolatedfromduneplant
Ixerisrepeneswasdescribedforthefirsttimeasapossible
advan-tageforplantsatsalineenvironment(Khanetal.,2009).P.citrinum
KACC43900promotedI.repenesgrowthbytheproductionof
bioac-tivegibberellinsintherhizosphere(Khanetal.,2009).Later,several
studieswithdifferentPenicilliumstrainshavebeenreportedusing
alowgibberellinsbiosynthesis mutantricecultivar,Waito-Cfor
plantgrowth-promotingverification.Waito-CisaGA-deficientrice
mutant,whichlacksGA3-hydroxylase,andconsequentlyis
hin-deringGA1synthesisfromGA20(Ahmadetal.,2010).Inallofthese
studieswereconfirmedthatfungalstrainssuppliedplantgrowth
promotiontoWaito-C.InthecaseofPenicilliumfuniculosumLHL06
andPenicilliumminioluteumLHL09,isolatedfromGlycinemax.L.,
stimulatedWaito-Cgrowthbysecretionofbioactivegibberellins.
Table2
PutativegibberellinbiosyntheticenzymesidentifiedbyhomologywiththeproteinsfromF.fujikuroi.
Species Putativegibberellinbiosyntheticenzyme Mycocosm ProteinID
Hitlenght %Identity E-value PenicilliumbilaiaeATCC20851v1.0 Ent-kaureneoxidase(P450-4) Penbi1|369049 163 34.90% 6.72E-65
CPS-KSEnt-kaur-16-enesynthase(CPS/KS) Penbi1|369053 243 40.30% 8.86E-107 CytochromeP450monooxygenase(P450-3) Penbi1|375870 110 35.37% 3.89E-51
GA14-synthase(P450-1) Penbi1|375870 150 41.10% 3.95E-74
GA20oxidase(P450-2) Penbi1|375870 150 42.25% 8.78E-78
Geranylgeranyldiphosphatesynthase(ggs2) Penbi1|416244 90 48.13% 4.96E-53
GA4desaturase(des) Penbi1|481848 36 39.56% 5.69E-09
PenicilliumfellutanumATCC48694v1.0 Ent-kaureneoxidase(P450-4) Penfe1|374742 103 34.33% 9.80E-47 CPS-KSEnt-kaur-16-enesynthase(CPS/KS) Penfe1|374742 113 35.42% 1.72E-54 CytochromeP450monooxygenase(P450-3) Penfe1|403578 171 45.72% 3.95E-81
GA14-synthase(P450-1) Penfe1|403578 82 41.84% 1.35E-50
GA20oxidase(P450-2) Penfe1|417921 47 43.12% 1.76E-12
Geranylgeranyldiphosphatesynthase(ggs2) Penfe1|424093 216 42.27% 7.29E-72
GA4desaturase(des) Penfe1|424093 172 42.57% 1.12E-90
PenicilliumglabrumDAOM239074v1.0 Ent-kaureneoxidase(P450-4) Pengl1|107178 112 37.46% 6.11E-52 CPS-KSEnt-kaur-16-enesynthase(CPS/KS) Pengl1|107178 144 42.99% 3.76E-76 CytochromeP450monooxygenase(P450-3) Pengl1|345507 96 47.76% 3.36E-60
GA14-synthase(P450-1) Pengl1|374226 64 38.55% 7.71E-16
GA20oxidase(P450-2) Pengl1|400029 126 40.38% 8.45E-60
Geranylgeranyldiphosphatesynthase(ggs2) Pengl1|401725 142 36.79% 3.39E-62
GA4desaturase(des) Pengl1|436008 177 39.51% 6.39E-71
PenicilliumjanthinellumATCC10455v1.0 Ent-kaureneoxidase(P450-4) Penja1|284697 35 34.65% 1.46E-07 CPS-KSEnt-kaur-16-enesynthase(CPS/KS) Penja1|427667 154 45.16% 1.99E-82 CytochromeP450monooxygenase(P450-3) Penja1|427667 149 43.44% 4.65E-88
GA14-synthase(P450-1) Penja1|434515 115 44.92% 4.97E-54
GA20oxidase(P450-2) Penja1|445613 203 45.62% 9.72E-84
Geranylgeranyldiphosphatesynthase(ggs2) Penja1|447197 145 37.56% 6.09E-60
GA4desaturase(des) Penja1|459586 87 48.07% 2.11E-46
PenicilliumraistrickiiATCC10490v1.0 Ent-kaureneoxidase(P450-4) Penra1|287118 191 43.51% 8.32E-115 CPS-KSEnt-kaur-16-enesynthase(CPS/KS) Penra1|287118 171 40.05% 2.71E-110 CytochromeP450monooxygenase(P450-3) Penra1|348500 99 37.79% 2.87E-48
GA14-synthase(P450-1) Penra1|352910 139 34.32% 1.17E-57
GA20oxidase(P450-2) Penra1|355675 83 41.50% 1.06E-26
Geranylgeranyldiphosphatesynthase(ggs2) Penra1|363110 112 47.66% 3.51E-58
GA4desaturase(des) Penra1|376286 194 35.93% 1.57E-60
GA4andGA7weredetected,respectively,showingthecapacityof thesestrainstoproducebioactivegibberellinsundersalinitystress (Ahmadetal.,2010;Khanetal.,2011c).Inanotherreportan
endo-phyticfungus,Penicilliumsp.LWL3,wasisolatedfromrootsoffield
growncucumberplantsandsecretedGA1andGA3(Waqasetal.,
2012).Bioactivegibberellins,GA3,GA4andGA7,wereisolatedfrom
P.janthinellumLK5,anendophyticfungusinhabitingtherootsofS.
lycopersicumMill(tomatoplant)fromfieldslocatednear
Kyung-pookNationalUniversity.P.janthinellumLK5improvesgrowthof
Waito-C,aswellasofABA-deficienttomateundersalinity,
reduc-ingsodiumiontoxicityandincrementingcalciumcontentsinits
rootascomparedtocontrol(Khanetal.,2013b).Recently,ithas
beenreportedthatendophytescouldhaveeffectscomparableto
thoseofexogenousgibberellins.WhentheendophyticP.resedanum
LK6,isolatedfromCapsicumannuumL.,andexogenousgibberellic
acidtreatmentswereappliedonpepperplantssignificantly
ame-lioratedthenegativeeffectofsalt stress.Ahigherbenefiteffect
wasobservedbyapplicationofcombinedLK6strainplus
gibberel-licacidtreatment.Moreover,italsoshowedthatLK6strainhadthe
abilitytoincreasebiomass,shootlength,chlorophyllcontentand
photosynthesisratecomparedwiththeuninfectedcontrolunder
salinitystress,suchasoccurredinotherPenicilliumstrains(Table1)
(Khanetal.,2015b).However,theprocessbywhichthese
phyto-hormonesaresecretedintoplanttissuesisnotknown(Khanetal.,
2015c).
Comparative study on gibberellins production of Fusarium
fujikuroi and Penicillium sp., curiously revealed that Penicillium
strainscapacity isgenerally similarorhigher than wildtype F.
fujikuroi.Early,ithasbeenreportedthatbioactiveGAproduction
capacityofaP.citrinumstrainwasmuchhigherthanF.fujikuroi
(Khanetal.,2008).ThePenicilliumsp.SJ-2-2,ahalophyteofhealthy
rootscollectedfromasaltmarshofSuncheonBayinSouthKorea,
synthesizedasmuchGA1andGA3thanF.fujikuroi,and
synthe-sizedmuchmoreofGA4andGA7(Youetal.,2012).Similarly,the
P.resedanumLK6wasalsoreportedtoproducesignificantlyhigher
amountsofGA1andGA4thantheF.fujifuroi,andGA3contentwas
atlowerlevel(Khanetal.,2015a).
Ithasbeenreportedthatplantsreacttomycorrhizationby
GAs-secretingPenicilliumstrains,alteringtheABA,JAandsalicylicacid
levels,aswellastheaccumulationofisoflavonesandtheenzymes
activitiesinvolvedintheremovalofROS,catalasesandperoxidases.
Additionally,endophytetreatmentimproveplantnutritionbalance
asaconsequenceofhighernitrogenandphosphorussolubilization
andK+,Mg2+andCa2+levels,whichinturnmightlimit/inhibitthe
uptakeofNa+.Despiteofantagonisticbehaviordescribedinthe
lit-eratureinwhatconcerntoJA,ABAandenzymes,whichneedsto
beclarifiedat“omics”levels,endophyticassociationhasnotonly
re-programmedtheplantforhighergrowthbutalsosignificantly
amelioratedtheeffectofsaltinducedstress(Khanetal.,2011a;
Khanetal.,2011b;Waqasetal.,2012;Khanetal.,2013a).The
mech-anismbywhichendophytetreatmentaugmentshostresponseto
salinitystressisstillnotclearlyunderstood(Khanetal.,2015a).
5.3. PutativegibberellinbiosyntheticgenesinPenicillium
InF.fujikuroigenomethegenesinvolvedinthemainstepsof
gibberellinbiosynthesisareclusteredtogether(Linnemannstons
com-posed by seven genes: four genes encoding cytochrome P450
oxidoreductases(namedfrom1to4)involvedindifferent
hydrox-ylationstepsofthegibberellinnucleus,twoGGDPsynthasegenes
locatedintandem(Ent-kaur-16-enesynthase,CPS/KS,and
geranyl-geranyldiphosphatesynthase,ggs2),andaGA4desaturasegene
(des),encodingtheenzyme which convertsGA4 toGA7.
Avail-ablegenomicdatafromJGIMycocosmgenomicresource(Grigoriev
etal.,2014)allowedustolocalizeputativegibberellinbiosynthetic
genesin5outofthe14availablecompletegenomesbelongingto
thePenicilliumgenus(Table2).Interestingly,oneoftheanalyzed
genomesbelongstoP.janthinellumwhichhasbeenpreviously
char-acterizedasagibberellinproducer(Khanetal.,2013;KhanandLee,
2013).Thesequencehomologyoftheputativegibberellin
biosyn-theticenzymesindifferentPenicillium speciesshoweda higher
degreeofhomologyinthegroupofthecytochromeP450enzymes,
astheent-kaureneoxidase(P450-4)andtheGA14-synthase
(P450-1)(Fig.3).AlsoasdepictedinTable2,putativeCPS/KSproteinsfrom
differentPenicilliumspeciesshowedahighhomologywiththe
orig-inalenzymefromF.fujikuroi,whereasthedesaturaseenzymes(des)
arecomparativelylessconserved.
6. Conclusionsandfutureperspectives
Thereisincreasinginterestinthediscoveryofnaturally
occur-ring chemical molecules for stimulating plant growthin order
toincreaseagriculturalcropsyield.Endophyticfungiarewidely
foundinalmostallkindsofplants,andtheirspeciescomposition
andnumberseemstobeaffectedbyagesofplantsand
environ-mentalamongotherfactors.FungilikePenicilliumspeciescanbe
usedasa readilyrenewable and inexhaustiblesourceof
extro-litecompoundsthatcanimproveplantsundernegativebioticas
wellasabioticconditions.Itisnowcommonlyacceptedthatthis
phenomenonisobservedwhenendophyticfungi-plantsareunder
salinestress.Inthisenvironmentalconditiongibberellinsare
pro-ducedbyPenicilliumstrains.StructuresGA1,GA3,GA4andGA7
havebeenidentifiedwithfunctionasgrowthhormonesproduced
byPenicilliumstrainsassalinitystressresistanceresponse.
Inter-esting,therhizobacteriumPseudomonasputidaH-2-3isalsoableto
secretegibberellinwhensoybeanisundersalineanddroughtstress
conditions,improvingtheplantgrowth(Kangetal.,2014).Onthe
otherhand,ithasbeenshownthatplantsreducegibberellins
pro-ductionintheresponsetoabioticstress,reducinggrowthinorder
thatplantcanfocusitsenergyandcarbonresourcesonresisting
thestress(Colebrooketal.,2014).Ifwe consideredthat all
liv-ingorganismhaveasprioritygrowthandsurvival,thecapacityof
microorganismstoproducemetabolitesthatareplantsecondary
metabolitescouldbeawaythatmicroorganismsfoundto
embar-rassthehostplants?. Endophytic Penicilliumstrainsdrawfrom
plantthewater,foodandphysicalprotectionagainstbioticand
abi-oticadverseconditions,whichallowthemtolivewithintheplant
hoppingbyfavorableconditionstocompletelycolonizethe
host-plant.Meanwhile,endophyticsymbiosisresultedinsignificantly
higherassimilationofnutrientlikephosphorus,sulfur,magnesium,
calciumandpotassiumascomparedtocontrolplants;besides
sec-ondarymetabolitesthatendophytemayproduce.Ithasbeenalso
reportedthatendophyticfungibiomasscouldconstitutean
inter-estingnitrogensourceforplant.Furthermore,severalPenicillium
culturesrevealedthepresenceofindoleaceticacid,otherimportant
phytohormone (Khan et al., 2011b; Waqas et al., 2012).
Addi-tionally,itwasdescribedthatPenicilliumendophytescanhelped
planttore-programits responsestosalinestressbyregulating
theendogenousphytohormonesandenzymestominimizecellular
toxicity(Khanetal.,2013a;Khanetal.,2015c).Suchinteractions
betweennativeendophyticfungiandplanthostcouldbea
reli-ablemethodologytotheplantsaltstress,sincechemicalsolutions
couldbeharmfultoorganismsinthesoil,reducingthe
biodiver-sityofecosystems.Themajorconcernistopredictenvironmental
changes,endophyte-hostinteractions,aswellasplant-microflora
systemdevelopment.Nevertheless,webelievethatapplicationof
bioactivegibberellins Penicilliumstrainsproducers isa promise
environmentalfriendlystrategy of improvingplantgrowthand
amelioratingdamagecausebysaltstressincultivationcrops.
References
Achard,P.,Cheng,H.,DeGrauwe,L.,Decat,J.,Schoutteten,H.,Moritz,T.,VanDer Straeten,D.,Peng,J.,Harberd,N.P.,2006.Integrationofplantresponsesto environmentallyactivatedphytohormonalsignals.Science311(5757),91–94. Ahmad,N.,Hamayun,M.,Khan,S.A.,Khan,A.L.,Lee,I.J.,Shin,D.H.,2010.
Gibberellin-producingendophyticfungiisolatedfromMonochoriavaginalis.J. Microbiol.Biotechnol.20(12),1744–1749.
Aly,A.H.,Debbab,A.,Proksch,P.,2011.Fungalendophytes:uniqueplant inhabitantswithgreatpromises.Appl.Microbiol.Biotechnol.90(6), 1829–1845.
Ballester,A.R.,Marcet-Houben,M.,Levin,E.,Sela,N.,Selma-Lazaro,C.,Carmona,L., Wisniewski,M.,Droby,S.,Gonzalez-Candelas,L.,Gabaldon,T.,2015.Genome, transcriptome,andfunctionalanalysesofPenicilliumexpansumprovidenew insightsintosecondarymetabolismandpathogenicity.Mol.PlantMicrobe Interact.28,232–248.
Bearder,J.R.,1983.InvivoditerpenoidbiosynthesisinGibberellafujikuroi:the pathwayafterent-kaurene.In:Crozier,A.(Ed.),TheBiochemistryand PhysiologyofGibberellins,vol.1.Praeger,NewYork,pp.251–387. Bearder,J.R.,MacMillan,J.,Wels,C.M.,Chaffey,M.B.,Phinney,B.O.,1974.Position
ofthemetabolicblockforgibberellinbiosynthesisinmutantBI-41aof Gibberellafujikuroi.Phytochem13,911–917.
Birch,A.J.,Richards,R.W.,Smith,H.,1958.Thebiosynthesisofgibberellicacid. Proc.Chem.Soc.,192–193.
Bomke,C.,Tudzynski,B.,2009.Diversity,regulation,andevolutionofthe gibberellinbiosyntheticpathwayinfungicomparedtoplantsandbacteria. Phytochemistry70(15–16),1876–1893.
Bomke,C.,Rojas,M.C.,Gong,F.,Hedden,P.,Tudzynski,B.,2008.Isolationand characterizationofthegibberellinbiosyntheticgeneclusterinSphaceloma manihoticola.Appl.Environ.Microbiol.74(17),5325–5339.
Brakhage,A.A.,2013.Regulationoffungalsecondarymetabolism.Nat.Rev. Microbiol.11(1),21–32.
Butinar,L.,Frisvad,J.C.,Gunde-Cimerman,N.,2011.Hypersalinewaters-a potentialsourceoffoodbornetoxigenicaspergilliandpenicillia.FEMS Microbiol.Ecol.77(1),186–199.
Cao,L.X.,You,J.L.,Zhou,S.N.,2002.EndophyticfungifromMusaacuminataleaves androotsinSouthChina.WorldJ.Microbiol.Biotechnol.18,169–171. Cerda-Olmedo,E.,Fernandez-Martin,R.,Avalos,J.,1994.Geneticsandgibberellin
productioninGibberellafujikuroi.AntonieVanLeeuwenhoek65(3),217–225. Chandra,S.,2012.Endophyticfungi:novelsourcesofanticancerleadmolecules.
Appl.Microbiol.Biotechnol.95(1),47–59.
Chang,J.C.S.,Foarde,K.K.,VanOsdell,D.W.,1995.Growthevaluationoffungi (PenicilliumandAspergillusspp.)onceilingtiles.Atmos.Environ.29, 2331–2337.
Chapman,D.J.,Ragan,M.A.,1980.Evolutionofbiochemicalpathways:evidence fromcomparativebiochemistry.Annu.Rev.PlantPhysiol.31,639–678. Chen,T.H.,Murata,N.,2011.Glycinebetaineprotectsplantsagainstabioticstress:
mechanismsandbiotechnologicalapplications.PlantCellEnviron.34(1),1–20. Colebrook,E.H.,Thomas,S.G.,Phillips,A.L.,Hedden,P.,2014.Theroleofgibberellin
signallinginplantresponsestoabioticstress.J.Exp.Biol.217(pt.1),67–75. Collado,J.,Platas,G.,González,I.,Peláez,F.,1999.Geographicalandseasonal
influencesonthedistributionoffungalendophytesinQuercusilex.New Phytol.144,525–532.
Correa,R.C.,Rhoden,S.A.,Mota,T.R.,Azevedo,J.L.,Pamphile,J.A.,deSouza,C.G., PolizeliMde,L.,Bracht,A.,Peralta,R.M.,2014.Endophyticfungi:expandingthe arsenalofindustrialenzymeproducers.J.Ind.Microbiol.Biotechnol.41(10), 1467–1478.
Cross,B.E.,Grove,J.F.,MacMillan,J.,Moffatt,J.S.,Mulholland,T.P.C.,1959.Arevised structureforgibberellicacid.Proc.Chem.Soc.,302–303.
Davies,P.J.(Ed.),2004.KluwerAcademicPublishers,Dordrecht,Netherlands,pp. 63–94.
Dorge,T.,Carstensen,J.M.,Frisvad,J.C.,2000.Directidentificationofpure Penicilliumspeciesusingimageanalysis.J.Microbiol.Methods41(2),121–133. FAO,2009.HighLevelExpertForum—HowtoFeedtheWorldin2050.Economic
andSocialDevelopment.FoodandAgriculturalOrganizationoftheUnited Nations,Rome.
Freeman,E.M.,1904.Theseed-fungusofLoliumtemulentum,L.,theDarnel.Phil. Trans.R.Soc.B196,1–27.
Gao,X.P.,Pan,Q.H.,Li,M.J.,Zhang,L.Y.,Wang,X.F.,Shen,Y.Y.,Lu,Y.F.,Chen,S.W., Liang,Z.,Zhang,D.P.,2004.Abscisicacidisinvolvedinthewaterstress-induced betaineaccumulationinpearleaves.PlantCellPhysiol.45(6),742–750. Gao,S.S.,Li,X.M.,Li,C.S.,Proksch,P.,Wang,B.G.,Penicisteroids,A.,2011.andB,
antifungalandcytotoxicpolyoxygenatedsteroidsfromthemarine alga-derivedendophyticfungusPenicilliumchrysogenumQEN-24S.Bioorg. Med.Chem.Lett.21(10),2894–2897.
Geng,Y.,Wu,R.,Wee,C.W.,Xie,F.,Wei,X.,Chan,P.M.,Tham,C.,Duan,L.,Dinneny, J.R.,2013.Aspatio-temporalunderstandingofgrowthregulationduringthe saltstressresponseinArabidopsis.PlantCell25(6),2132–2154.
Germaine,K.,Keogh,E.,Garcia-Cabellos,G.,Borremans,B.,Lelie,D.,Barac,T., Oeyen,L.,Vangronsveld,J.,Moore,F.P.,Moore,E.R.,Campbell,C.D.,Ryan,D., Dowling,D.N.,2004.Colonisationofpoplartreesbygfpexpressingbacterial endophytes.FEMSMicrobiol.Ecol.48(1),109–118.
Gong,M.,Du,P.,Liu,X.,Zhu,C.,2014.TransformationofinorganicPfractionsof soilandplantgrowthpromotionbyphosphate-solubilizingabilityof PenicilliumoxalicumI1.J.Microbiol.52(12),1012–1019.
Grigoriev,I.V.,Nikitin,R.,Haridas,S.,Kuo,A.,Ohm,R.,Otillar,R.,Riley,R.,Salamov, A.,Zhao,X.,Korzeniewski,F.,Smirnova,T.,Nordberg,H.,Dubchak,I.,Shabalov, I.,2014.MycoCosmportal:gearingupfor1000fungalgenomes.NucleicAcids Res.42,D699–D704(Databaseissue).
Guo,W.,Li,D.,Peng,J.,Zhu,T.,Gu,Q.,Penicitols,A.-C.,Penixanacid,A.,2015.from theMangrove-DerivedPenicilliumchrysogenumHDN11-24.J.Nat.Prod. Hasegawa,P.M.,Bressan,R.A.,Zhu,J.K.,Bohnert,H.J.,2000.Plantcellularand
molecularresponsestohighsalinity.Annu.Rev.PlantPhysiol.PlantMol.Biol. 51,463–499.
Hedden,P.,Phillips,A.L.,Rojas,M.C.,Carrera,E.,Tudzynski,B.,2001.Gibberellin biosynthesisinplantsandfungi:acaseofconvergentevolution?J.Plant GrowthRegul.20(4),319–331.
Hossain,M.M.,Sultana,F.,Miyazawa,M.,Hyakumachi,M.,2014.Plant growth-promotingfungusPenicilliumspp:GP15-1enhancesgrowthand confersprotectionagainstdamping-offandanthracnoseinthecucumber.J. OleoSci.63(4),391–400.
Houbraken,J.,Frisvad,J.C.,Samson,R.A.,2011.Fleming’spenicillinproducing strainisnotPenicilliumchrysogenumbutP.rubens.IMAFungus2(1),87–95. Houbraken,J.,Frisvad,J.C.,Seifert,K.A.,Overy,D.P.,Tuthill,D.M.,Valdez,J.G.,
Samson,R.A.,2012.Newpenicillin-producingPenicilliumspeciesandan overviewofsectionChrysogena.Persoonia29,78–100.
Ismail,A.,Takeda,S.,Nick,P.,2014.Lifeanddeathundersaltstress:sameplayers, differenttiming?J.Exp.Bot.65(12),2963–2979.
Jacoby,R.P.,Taylor,N.L.,Millar,A.H.,2011.Theroleofmitochondrialrespirationin salinitytolerance.TrendsPlantSci.16(11),614–623.
Julkowska,M.M.,Testerink,C.,2015.Tuningplantsignalingandgrowthtosurvive salt.TrendsPlantSci.20(9),586–594.
Kang,S.M.,Radhakrishnan,R.,Khan,A.L.,Kim,M.J.,Park,J.M.,Kim,B.R.,Shin,D.H., Lee,I.J.,2014.Gibberellinsecretingrhizobacterium,PseudomonasputidaH-2-3 modulatesthehormonalandstressphysiologyofsoybeantoimprovethe plantgrowthundersalineanddroughtconditions.PlantPhysiol.Biochem.84, 115–124.
Kawaide,H.,Imai,R.,Sassa,T.,Kamiya,Y.,1997.Ent-kaurenesynthasefromthe fungusPhaeosphaeriasp.L487.cDNAisolation,characterization,andbacterial expressionofabifunctionalditerpenecyclaseinfungalgibberellin biosynthesis.J.Biol.Chem.272(35),21706–21712.
Kawaide,H.,Sassa,T.,Kamiya,Y.,2000.Functionalanalysisofthetwointeracting cyclasedomainsinent-kaurenesynthasefromthefungusPhaeosphaeriasp. L487andacomparisonwithcyclasesfromhigherplants.J.Biol.Chem.275(4), 2276–2280.
Keller,N.P.,Hohn,T.M.,1997.Metabolicpathwaygeneclustersinfilamentous fungi.FungalGenet.Biol.21(1),17–29.
Khan,A.L.,Lee,I.J.,2013.EndophyticPenicilliumfuniculosumLHL06secretes gibberellinthatreprogramsGlycinemaxL.growthduringcopperstress.BMC PlantBiol.13,86.
Khan,S.A.,Hamayun,M.,Yoon,H.,Kim,H.Y.,Suh,S.J.,Hwang,S.K.,Kim,J.M.,Lee, I.J.,Choo,Y.S.,Yoon,U.H.,Kong,W.S.,Lee,B.M.,Kim,J.G.,2008.Plantgrowth promotionandPenicilliumcitrinum.BMCMicrobiol.8,231.
Khan,S.A.,Hamayun,M.,Kim,H.Y.,Yoon,H.J.,Seo,J.C.,Choo,Y.S.,Lee,I.J.,Kim,S.D., Rhee,I.K.,Kim,J.G.,2009.AnewstrainofArthriniumphaeospermumisolated fromCarexkobomugiOhwiiscapableofgibberellinproduction.Biotechnol. Lett.31(2),283–287.
Khan,A.L.,Hamayun,M.,Kim,Y.H.,Kang,S.M.,Lee,I.J.,2011a.Ameliorative symbiosisofendophyte(PenicilliumfuniculosumLHL06)undersaltstress elevatedplantgrowthofGlycinemaxL.PlantPhysiol.Biochem.49(8), 852–861.
Khan,A.L.,Hamayun,M.,Kim,Y.H.,Kang,S.M.,Lee,J.H.,Lee,I.J.,2011b.Gibberellins producingendophyticAspergillusfumigatussp.LH02influencedendogenous phytohormonallevels,isoflavonoidsproductionandplantgrowthinsalinity stress.ProcessBiochem.46,440–447.
Khan,A.L.,Hamayun,M.,Ahmad,N.,Hussain,J.,Kang,S.M.,Kim,Y.H.,Adnan,M., Tang,D.S.,Waqas,M.,Radhakrishnan,R.,Hwang,Y.H.,Lee,I.J.,2011c.Salinity stressresistanceofferedbyendophyticfungalinteractionbetweenPenicillium minioluteumLHL09andGlycinemaxL.J.Microbiol.Biotechnol.21(9),893–902. Khan,A.L.,Waqas,M.,Hamayun,M.,Al-Harrasi,A.,Al-Rawahi,A.,Lee,I.J.,2013a.
Co-synergismofendophytePenicilliumresedanumLK6withsalicylicacid helpedCapsicumannuuminbiomassrecoveryandosmoticstressmitigation. BMCMicrobiol.13,51.
Khan,A.L.,Waqas,M.,Khan,A.R.,Hussain,J.,Kang,S.M.,Gilani,S.A.,Hamayun,M., Shin,J.H.,Kamran,M.,Al-Harrasi,A.,Yun,B.W.,Adnan,M.,Lee,I.J.,2013b. FungalendophytePenicilliumjanthinellumLK5improvesgrowthof ABA-deficienttomatoundersalinity.WorldJ.Microbiol.Biotechnol.29(11), 2133–2144.
Khan,A.L.,Waqas,M.,Lee,I.J.,2015a.ResilienceofPenicilliumresedanumLK6and exogenousgibberellininimprovingCapsicumannuumgrowthunderabiotic stresses.J.PlantRes.128(2),259–268.
Khan,A.L.,Hussain,J.,Al-Harrasi,A.,Al-Rawahi,A.,Lee,I.J.,2015b.Endophytic fungi:resourceforgibberellinsandcropabioticstressresistance.Crit.Rev. Biotechnol.35(1),62–74.
Khan,A.L.,Waqas,M.,Hussain,J.,Al-Harrasi,A.,Hamayun,M.,Lee,I.J.,2015c. Phytohormonesenabledendophyticfungalsymbiosisimprovealuminum phytoextractionintolerantSolanumlycopersicum:anexamplesofPenicillium janthinellumLK5andcomparisonwithexogenousGA3.J.Hazard.Mater.295, 70–78.
Kim,D.C.,Lee,H.S.,Ko,W.,Lee,D.S.,Sohn,J.H.,Yim,J.H.,Kim,Y.C.,Oh,H.,2014. Anti-inflammatoryeffectofmethylpenicinolinefromamarineisolateof Penicilliumsp.(SF-5995):inhibitionofNF-kappaBandMAPKpathwaysin lipopolysaccharide-inducedRAW264.7macrophagesandBV2microglia. Molecules19(11),18073–18089.
Klobus,G.,Janicka-Russak,M.,2004.Modulationbycytosoliccomponentsof protonpumpactivitiesinplasmamembraneandtonoplastfromCucumis sativusrootsduringsaltstress.Physiol.Plant121(1),84–92.
Kogel,K.H.,Franken,P.,Huckelhoven,R.,2006.Endophyteorparasite—what decides?Curr.Opin.PlantBiol.9(4),358–363.
Kusari,S.,Hertweck,C.,Spiteller,M.,2012.Chemicalecologyofendophyticfungi: originsofsecondarymetabolites.Chem.Biol.19(7),792–798.
Lang,A.,1956.InductionofflowerformationinbiennialHyoscyamusbytreatment withgibberellin.Naturwissenschaften43,284–285.
Larran,S.,Monaco,C.,Alippi,H.E.,2001.EndophyticfungiinleavesofLycopersicon esculentumMill.WorldJ.Microbiol.Biotechnol.17,181–184.
Laskowski,R.A.,Swindells,M.B.,2011.LigPlot+:multipleligand-proteininteraction diagramsfordrugdiscovery.J.Chem.Inf.Model.51(10),2778–2786. Leitão,A.L.,2009.PotentialofPenicilliumspeciesinthebioremediationfield.Int.J.
Environ.Res.PublicHealth6(4),1393–1417.
Leitão,A.L.,Enguita,F.J.,2014.Fungalextrolitesasanewsourcefortherapeutic compoundsandasbuildingblocksforapplicationsinsyntheticbiology. Microbiol.Res.169(9–10),652–665.
Leitão,A.L.,Duarte,M.P.,SantosOliveira,J.,2007.Degradationofphenolbya halotolerantstrainofPenicilliumchrysogenum.Int.Biodeterior.Biodegrad.59, 220–225.
Li,B.,Zong,Y.,Du,Z.,Chen,Y.,Zhang,Z.,Qin,G.,Zhao,W.,Tian,S.,2015.Genomic characterizationrevealsinsightsintopatulinbiosynthesisandpathogenicityin Penicilliumspecies.Mol.PlantMicrobeInteract.
Liao,L.,Lee,J.H.,You,M.,Choi,T.J.,Park,W.,Lee,S.K.,Oh,D.C.,Oh,K.B.,Shin,J., 2014.PenicillipyronesAandB,meroterpenoidsfromamarine-derived Penicilliumsp.fungus.J.Nat.Prod.77(2),406–410.
Linnemannstons,P.,Voss,T.,Hedden,P.,Gaskin,P.,Tudzynski,B.,1999.Deletions inthegibberellinbiosynthesisgeneclusterofGibberellafujikuroibyrestriction enzyme-mediatedintegrationandconventionaltransformation-mediated mutagenesis.Appl.Environ.Microbiol.65(6),2558–2564.
MacMillan,J.,1997.Biosynthesisofthegibberellinplanthormones.Nat.Prod.Rep. 14,221–244.
Malonek,S.,Rojas,M.C.,Hedden,P.,Hopkins,P.,Tudzynski,B.,2005.Restorationof gibberellinproductioninFusariumproliferatumbyfunctional
complementationofenzymaticblocks.Appl.Environ.Microbiol.71(10), 6014–6025.
McMullin,D.R.,Nsiama,T.K.,Miller,J.D.,2014.Secondarymetabolitesfrom Penicilliumcorylophilumisolatedfromdampbuildings.Mycologia106(4), 621–628.
McNicholas,S.,Potterton,E.,Wilson,K.S.,Noble,M.E.,2011.Presentingyour structures:theCCP4mgmolecular-graphicssoftware.ActaCrystallogr.DBiol. Crystallogr.67(pt.4),386–394.
Moore-Kucera,J.,Cox,S.B.,Peyron,M.,Bailes,G.,Kinloch,K.,Karich,K.,Miles,C., Inglis,D.A.,Brodhagen,M.,2014.Nativesoilfungiassociatedwithcompostable plasticsinthreecontrastingagriculturalsettings.Appl.Microbiol.Biotechnol. 98(14),6467–6485.
Muller,M.,Kunz,H.H.,Schroeder,J.I.,Kemp,G.,Young,H.S.,Neuhaus,H.E.,2014. DecreasedcapacityforsodiumexportoutofArabidopsischloroplastsimpairs salttolerance,photosynthesisandplantperformance.PlantJ.78(4),646–658. Murase,K.,Hirano,Y.,Sun,T.P.,Hakoshima,T.,2008.Gibberellin-inducedDELLA
recognitionbythegibberellinreceptorGID1.Nature456(7221),459–463. Nounjan,N.,Nghia,P.T.,Theerakulpisut,P.,2012.Exogenousprolineandtrehalose
promoterecoveryofriceseedlingsfromsalt-stressanddifferentiallymodulate antioxidantenzymesandexpressionofrelatedgenes.J.PlantPhysiol.169(6), 596–604.
Ogas,J.,2000.Gibberellins.Curr.Biol.10(2),R48.
Olszewski,N.,Sun,T.P.,Gubler,F.,2002.Gibberellinsignaling:biosynthesis, catabolism,andresponsepathways.PlantCell14(Suppl),S61–S80. Park,M.S.,Fong,J.J.,Oh,S.Y.,Houbraken,J.,Sohn,J.H.,Hong,S.B.,Lim,Y.W.,2015.
Penicilliumjejuensesp.nov.,isolatedfromthemarineenvironmentsofJeju Island,Korea.Mycologia107(1),209–216.
Pereira,P.,Enguita,F.J.,Ferreira,J.,Leitão,A.L.,2014.DNAdamageinducedby hydroquinonecanbepreventedbyfungaldetoxification.Toxicol.Rep.1, 1096–1105.
Peterson,S.W.,Vega,F.E.,Posada,F.,Nagai,C.,2005.Penicilliumcoffeae,anew endophyticspeciesisolatedfromacoffeeplantanditsphylogenetic relationshiptoP.fellutanum,P.thiersiiandP.brocaebasedonparsimony analysisofmultilocusDNAsequences.Mycologia97(3),659–666. Pitt,J.I.,1979.ThegenusPenicilliumanditsteleomorphicstatesEupenicilliumand
Talaromyces.AcademicPress,London.
Quang,T.H.,Ngan,N.T.,Ko,W.,Kim,D.C.,Yoon,C.S.,Sohn,J.H.,Yim,J.H.,Kim,Y.C., Oh,H.,2014.TanzawaicacidderivativesfromamarineisolateofPenicilliumsp.
(SF-6013)withanti-inflammatoryandPTP1Binhibitoryactivities.Bioorg.Med. Chem.Lett.24(24),5787–5791.
Radley,M.,1956.Occurrenceofsubstancessimilartogibberellicacidinhigher plants.Nature178,1070–1071.
Raper,K.B.,Thom,C.,1949.ManualofthePenicillia.WilliamsandWilkinsCo., Baltimore.
Rieu,I.,Eriksson,S.,Powers,S.J.,Gong,F.,Griffiths,J.,Woolley,L.,Benlloch,R., Nilsson,O.,Thomas,S.G.,Hedden,P.,Phillips,A.L.,2008.Geneticanalysis revealsthatC19-GA2-oxidationisamajorgibberellininactivationpathwayin Arabidopsis.PlantCell20(9),2420–2436.
Romero-Aguilar,M.,Tovar-Sanchez,E.,Sanchez-Salinas,E.,Mussali-Galante,P., Sanchez-Meza,J.C.,Castrejon-Godinez,M.L.,Dantan-Gonzalez,E.,Trujillo-Vera, M.A.,Ortiz-Hernandez,M.L.,2014.Penicilliumsp.asanorganismthatdegrades endosulfanandreducesitsgenotoxiceffects.Springerplus3,536.
Sairam,R.K.,Tyagi,A.,2004.Physiologyandmolecularbiologyofsalinitystress toleranceinplants.Curr.Sci.86,407–421.
Santini,A.,Mikusova,P.,Sulyok,M.,Krska,R.,Labuda,R.,Srobarova,A.,2014. PenicilliumstrainsisolatedfromSlovakgrapeberriestaxonomyassessmentby secondarymetaboliteprofile.MycotoxinRes.30(4),213–220.
Schardl,C.L.,Leuchtmann,A.,Spiering,M.J.,2004.Symbiosesofgrasseswith seedbornefungalendophytes.Annu.Rev.PlantBiol.55,315–340. Scott,J.,Untereiner,W.A.,Wong,B.,Straus,N.A.,Malloch,D.,2004.Genotypic
variationinPenicilliumchysogenumfromindoorenvironments.Mycologia96 (5),1095–1105.
Shabala,S.,Cuin,T.A.,2008.Potassiumtransportandplantsalttolerance.Physiol. Plant133(4),651–669.
Shabala,S.,Demidchik,V.,Shabala,L.,Cuin,T.A.,Smith,S.J.,Miller,A.J.,Davies,J.M., Newman,I.A.,2006.ExtracellularCa2+amelioratesNaCl-inducedK+lossfrom
ArabidopsisrootandleafcellsbycontrollingplasmamembraneK+-permeable
channels.PlantPhysiol.141(4),1653–1665.
Shimada,A.,Ueguchi-Tanaka,M.,Nakatsu,T.,Nakajima,M.,Naoe,Y.,Ohmiya,H., Kato,H.,Matsuoka,M.,2008.Structuralbasisforgibberellinrecognitionbyits receptorGID1.Nature456(7221),520–523.
Slot,J.C.,Rokas,A.,2011.Horizontaltransferofalargeandhighlytoxicsecondary metabolicgeneclusterbetweenfungi.Curr.Biol.21(2),134–139.
Sofo,A.,Scopa,A.,Nuzzaci,M.,Vitti,A.,2015.Ascorbateperoxidaseandcatalase activitiesandtheirgeneticregulationinplantssubjectedtodroughtand salinitystresses.Int.J.Mol.Sci.16(6),13561–13578.
SpurrJr.,H.W.,Welty,R.E.,1975.Characterizationofendophyticfungiinhealthy leavesofNicotianaspp.Phytopathol65,417–422.
Strizhov,N.,Abraham,E.,Okresz,L.,Blickling,S.,Zilberstein,A.,Schell,J.,Koncz,C., Szabados,L.,1997.DifferentialexpressionoftwoP5CSgenescontrolling prolineaccumulationduringsalt-stressrequiresABAandisregulatedbyABA1, ABI1andAXR2inArabidopsis.PlantJ.12(3),557–569.
Sun,T.P.,2011.ThemolecularmechanismandevolutionoftheGA-GID1-DELLA signalingmoduleinplants.Curr.Biol.21(9),R338–45.
Takeda,N.,Handa,Y.,Tsuzuki,S.,Kojima,M.,Sakakibara,H.,Kawaguchi,M.,2015. Gibberellinregulatesinfectionandcolonizationofhostrootsbyarbuscular mycorrhizalfungi.PlantSignal.Behav.10(6),e1028706.
Tan,R.X.,Zou,W.X.,2001.Endophytesarichsourceoffunctionalmetabolites.Nat. Prod.Rep.18,448–459.
Tansakul,C.,Rukachaisirikul,V.,Maha,A.,Kongprapan,T.,Phongpaichit,S., Hutadilok-Towatana,N.,Borwornwiriyapan,K.,Sakayaroj,J.,2014.Anew phenalenonederivativefromthesoilfungusPenicilliumherqueiPSU-RSPG93. Nat.Prod.Res.28(20),1718–1724.
Troncoso,C.,Gonzalez,X.,Bomke,C.,Tudzynski,B.,Gong,F.,Hedden,P.,Rojas, M.C.,2010.Gibberellinbiosynthesisandgibberellinoxidaseactivitiesin Fusariumsacchari,FusariumkonzumandFusariumsubglutinansstrains. Phytochemistry71(11–12),1322–1331.
Tudzynski,B.,2005.Gibberellinbiosynthesisinfungi:genes,enzymes,evolution, andimpactonbiotechnology.Appl.Microbiol.Biotechnol.66(6),597–611. Tudzynski,B.,Holter,K.,1998.GibberellinbiosyntheticpathwayinGibberella
fujikuroi:evidenceforagenecluster.FungalGenet.Biol.25(3),157–170. Ueguchi-Tanaka,M.,Nakajima,M.,Katoh,E.,Ohmiya,H.,Asano,K.,Saji,S.,Hongyu,
X.,Ashikari,M.,Kitano,H.,Yamaguchi,I.,Matsuoka,M.,2007.Molecular interactionsofasolublegibberellinreceptor,GID1,withariceDELLAprotein, SLR1,andgibberellin.PlantCell19(7),2140–2155.
Visagie,C.M.,Hirooka,Y.,Tanney,J.B.,Whitfield,E.,Mwange,K.,Meijer,M.,Amend, A.S.,Seifert,K.A.,Samson,R.A.,2014.Aspergillus,PenicilliumandTalaromyces isolatedfromhousedustsamplescollectedaroundtheworld.Stud.Mycol.78, 63–139.
Voegele,A.,Linkies,A.,Muller,K.,Leubner-Metzger,G.,2011.Membersofthe gibberellinreceptorgenefamilyGID1(GIBBERELLININSENSITIVEDWARF1) playdistinctrolesduringLepidiumsativumandArabidopsisthalianaseed germination.J.Exp.Bot.62(14),5131–5147.
Waqas,M.,Khan,A.L.,Kamran,M.,Hamayun,M.,Kang,S.M.,Kim,Y.H.,Lee,I.J., 2012.Endophyticfungiproducegibberellinsandindoleaceticacidand promoteshost-plantgrowthduringstress.Molecules17(9),10754–10773. West,G.,Inze,D.,Beemster,G.T.,2004.Cellcyclemodulationintheresponseofthe
primaryrootofArabidopsistosaltstress.PlantPhysiol.135(2),1050–1058. Wiemann,P.,Sieber,C.M.,vonBargen,K.W.,Studt,L.,Niehaus,E.M.,Espino,J.J.,
Huss,K.,Michielse,C.B.,Albermann,S.,Wagner,D.,Bergner,S.V.,Connolly,L.R., Fischer,A.,Reuter,G.,Kleigrewe,K.,Bald,T.,Wingfield,B.D.,Ophir,R., Freeman,S.,Hippler,M.,Smith,K.M.,Brown,D.W.,Proctor,R.H.,
Munsterkotter,M.,Freitag,M.,Humpf,H.U.,Guldener,U.,Tudzynski,B.,2013. Decipheringthecrypticgenome:genome-wideanalysesofthericepathogen Fusariumfujikuroirevealcomplexregulationofsecondarymetabolismand novelmetabolites.PLoSPathog.9(6),e1003475.
Yamaguchi,S.,2008.Gibberellinmetabolismanditsregulation.Annu.Rev.Plant Biol.59,225–251.
You,Y.H.,Yoon,H.,Kang,S.M.,Shin,J.H.,Choo,Y.S.,Lee,I.J.,Lee,J.M.,Kim,J.G., 2012.Fungaldiversityandplantgrowthpromotionofendophyticfungifrom sixhalophytesinSuncheonBay.J.Microbiol.Biotechnol.22(11),1549–1556. Zhu,J.,Lee,B.H.,Dellinger,M.,Cui,X.,Zhang,C.,Wu,S.,Nothnagel,E.A.,Zhu,J.K.,
2010.Acellulosesynthase-likeproteinisrequiredforosmoticstresstolerance inArabidopsis.PlantJ.63(1),128–140.