1. M.S., Santos School of Physical Education – Fefis-Unimes. Ph.D. un-derway – School of Physical Education – Unicamp.
2. Ph.D. – School of Physical Education – Unicamp.
3. Ph.D. – Department of Sports – School of Physical Education and Sports – University of São Paulo.
Received in 22/3/04. 2nd version received in 24/11/04. Approved in 30/1/05. Correspondence to: Prof. Dr. Benedito Pereira, Departamento de Esporte, Escola de Educação Física e Esporte, Universidade de São Paulo, Av. Prof. Mello Moraes, 65, Butantã – 05508-900 – São Paulo, SP, Brazil. E-mail: benepe@ usp.br
Physical exercise and oxidative stress
Effect of intense physical exercise on the urinary
chemiluminescence and plasmatic malondialdehyde
Tácito Pessoa de Souza Jr.1, Paulo Roberto de Oliveira2 and Benedito Pereira3
O
RIGINALA
RTICLEKey words: Physical exercise. Oxidative stress. Metabolism. Reactive oxygen spe-cies.
ENGLISH VERSION
ABSTRACT
Several studies have demonstrated that intense physical exer-cise causes oxidative stress in animals and humans, being possi-bly related, for instance, to fatigue and tissue lesions. However, the effects of high intensity exercise or training performed by ath-letes on the occurrence of oxidative stress are not fully clear, pos-sibly due to methodological limitations. The objective of this study was to identify the occurrence of oxidative lesions in lipids due to physical training in athletes, through the quantification of the uri-nary chemiluminescence and plasmatic malondialdehyde (MDA). Post-exercise samples were collected after four training protocols: a) treadmill running (25-30 min); b) 20 km running performed by marathon runners; c) interval training accomplished by 400 m run-ners; d) soccer game with 50 min duration; and e) strength training with and without creatine supplementation. In the last four items, only the urinary chemiluminescence was evaluated. The conditions that presented elevation in urinary chemiluminescence after exer-cise completion were: a) 20 km running; b) soccer game; and c) strength training without creatine supplementation. The treadmill running increased plasmatic MDA concentration during and after its performance, and the plasmatic antioxidant capacity had an in-verse behavior compared to the increase in MDA. The exercise used in this work promoted oxidative stress in a different way and this may be related to the duration and the intensity performed by athletes, and not only to intensity. In this work it was also observed that creatine ingestion associated with strength training might work as antioxidant.
INTRODUCTION
Studies on oxidative stress conducted with experimental ani-mals and human beings have demonstrated that the increase on the metabolic activity furthers the occurrence of oxidative lesions in biomolecules(1-5). As sportive training and competition elevate
substantially the cellular metabolic activity, these lesions may present dimensions even larger in such conditions(6,7). The
oxida-tive stress involves increase on the formation of superoxide anion (O2•-), hydrogen peroxide (H
2O2) and hydroxyl radical (HO•), among
others, generically called as reactive oxygen species (ROSs), in disadvantage of the available chemical and enzymatic antioxidant defenses(8). There are evidences that the concentration of nitric
oxide (NO•) and oxidant derivatives (reactive nitrogen species, RNSs)
are high in the organism during physical exercise. However, once not many studies on the effect of physical exercise on the forma-tion of RNSs are available, these derivatives will not be menforma-tioned in this work any longer.
One may say, therefore, that intense physical activity may in-crease the ROSs intracellular formation, thus promoting oxidative stress. However, not many works on this problem involving hu-man beings can be found because hu-many procedures available to quantify oxidative lesions in biomolecules are invasive and of diffi-cult application, especially those involving muscle biopsy. In this context, the objective of this work was to study the acute and chronic effect of the physical exercise on the urinary chemilumi-nescence in athletes involved in different intense physical activi-ties (soccer game with 50 min duration, marathon runners, 400 m runners and strength training with and without creatine supple-mentation). Meanwhile, soccer players were submitted to tread-mill running, where the urinary chemiluminescence, the total plas-matic antioxidant capacity and the malondialdehyde concentration (MDA) were quantified. Both the urinary chemiluminescence and the plasmatic MDA are considered as indicative of oxidative le-sions in body lipids (lipid peroxidation).
MATERIAL AND METHODS
The studies were approved by the Ethics Research Committee from the Biomedicine Sciences Institute – University of São Paulo and all participants signed a consent form agreeing with the per-formance of the experimental procedures (physical effort, sam-ples collecting, etc.). We emphasize that athletes who participate in this study do not smoke or use drugs forbidden by the Interna-tional Olympic Committee. This fact was verified through personal interview.
Urinary chemiluminescence
The ROSs oxidative action on lipids, especially on lipids with biological membranes, promotes the formation of hydroperoxides that might be cyclized, generating heterocyclic compounds called as dioxietanes. These compounds may undergo thermal cleavage producing excited-state carbonyls (singlet or triplet, depending on the substituents on the dioxetane ring). The reduction of these carbonyls into the fundamental state with energy release as pho-tons is the main factor responsible for the chemiluminescence ob-served in the urine. The luminescence intensity is, therefore, pro-portional to the concentration of excited carbonyls and reflects the extension of the tissue lipid peroxidation process.
were performed using 3 mL of urine supplemented with 1 mL of sodium phosphate 0.1 M, pH 6.2. Creatine was measured through procedure described by Heinegard and Tinderstrom(9) and its value
was used to correct the samples dilution. A previous absorbance measurement of the urine sample is required in order to avoid ex-cessive luminous absorption that could influence the luminescence quantification. In this work, whenever the absorbance of the cen-trifuged sample presented value above 0.3, it was diluted with Milli Q water up to this limit. The method was originally described by Lissi et al.(10) and the luminescence intensity measured in liquid
scintillator.
Malondialdehyde (MDA)
Aldehydes are frequently produced when lipoperoxides are metabolized by aerobic organisms. Their identification provides an indirect index of oxidative lesions as result of the lipid peroxida-tion. MDA is one of the most abundant aldehydes resulting from tissue lipid peroxidation, especially from arachidonic acid (20:4), eicosapentaenoic acid (20:5) and docosahexaenoic acid (22:6). The Yagi(11) procedure was adopted for MDA quantification by means
of fluorescence, in which the plasmatic MDA concentration is measured through sample excitement in 515 nm and quantifica-tion in 553 nm. The MDA quantity was expressed in nmol per mL of plasma. The procedure’s sensitiveness allows the utilization of the volume of 50 µL of blood samples collected from the earlobe. As these measurements concurred with the determination of the blood lactate obtained from the same site, it was decided for col-lecting of samples in blood vessel at the forearm cubital region, in which approximately 5.0 mL of blood was obtained from each col-lection, with a total of 1.0 mL of plasma after cooled centrifuga-tion. 0.5 mL of plasma were used in this experiment and 0.5 mL at the dosage of the total plasmatic antioxidant capacity.
Total antioxidant capacity
The plasmatic antioxidant capacity was determined through the Whitehead(12) method, using the chemiluminescence technique to
measure the antioxidant capacity of biological fluids. The light is-sue emission occurs when the chemiluminescent substrate (lumi-nol) is oxidized by H2O2 in reaction catalyzed by “horseradish per-oxidase”. The stabilities and intensities of the light emitted are high due to the addition of p-iodine phenol and its emission de-pends on the constant production of intermediate free radicals derivative of p-iodine phenol, luminol and O2. For this reason, the light emission is sensible to antioxidants but reestablished when these antioxidants are consumed in the reaction. As the genera-tion of intermediate free radicals is constant, the light suppression period is directly related to the amount of antioxidant in the sam-ple. The assay is sensible to measure the antioxidant capacity of biological samples, being expressed relatively to the 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), soluble anal-ogous of the vitamin E. The result is expressed as equivalent of trolox per liter of sample.
Simulated soccer game and strength training – The soccer training involved two groups of 12 players enrolled in the Physical Education and Sports Graduation School-USP, using the entire field, but in only 10 students the samples were collected. The duration was of 50 minutes and the strength training was composed of pulling and throwing; to elevate the value of the maximal strength in the respective exercises was the objective during the entire train-ing period (three times a week durtrain-ing three months). Two weeks later, the strength training always used load close to maximal (80-90% of the 1RM) with only a few repetitions with no interval (6-8 series with 3-6 repetitions with 3-5 minutes of interval between series).
Marathon and 400 m runners training – Athletes specialized in marathon ran 20 km (heart rate of 180-185 bpm) as part of their daily training that represents the general physical preparation
peri-od in which the main objective is to work with large volumes of weekly training. The group of 400 m runners performed one daily training session with specific exercises for the anaerobic power development (training with intervals), also corresponding to the general physical preparation period. The main part of the training was composed of a session of 3 x 4 series of 300 m with intervals of 3 minutes between each exercise and of 5 minutes between each series. The rhythm required was of 36-38 seconds in each exercise, and the exercise with intervals was performed in 400-m athletics track and the 20 km running, at the University of São Pau-lo campus.
Strength training and creatine supplementation – 18 students from the Santos School of Physical Education, who already pre-sented practical experience for about one year with exercises typ-ically performed in gymnastics academies, were divided into two groups of nine students, namely: groups A and B. These students were previously submitted to muscular hypertrophy training in tests battery and entered the first and second preparation weeks. After this period, they entered in the hypertrophy training weeks. The supplementation with monohydrated creatine (CrH2O) was used according to Volek et al.(13) protocol modified by Souza Jr.(14).
After 1RM tests, anthropometrical measurements were per-formed and during the two preparation weeks, the daily CrH2O intake of individuals was of 30 g divided into six equal doses of 5 g with intervals of 3 and 4 hours with a daily total of 30 g in the first supplementation week (30 g/day for 7 days, total of 210 g) or pla-cebo (maltodextrin), corresponding to the third training week. Af-ter the initial intake period, the groups received a maintenance quan-tity of 5 g/day for 42 days, total of 210 g, corresponding to the last 5 training weeks.
The substance CrH2O was supplied by Protech Systems and the placebo substance (maltodextrin) was purchased from a store of nutritional supplements. The substances were conditioned into tablets by Pedrosa & Delsin – Farmácia de Manipulação Ltda., CGC 00243338/0001-74. Both substances CrH2O and maltodextrin were equally conditioned into tablets containing 0.5 g in order to avoid participants of knowing who would be taking creatine tablets. For this experiment, the training protocol used was the protocol pro-posed by Souza Jr.(14), applied during eight weeks. The first two
weeks (phase A) were used for the neuromuscular adjustments of the individuals and the next six weeks (phase B) were aimed at increasing the maximal muscular strength and the muscular mass (hypertrophy).
The preparation week (phase A) was composed of exercises performed with 50% of the 1RM with intervals of 120 seconds between them. The hypertrophy training (phase B) was composed of the use of 80% of the maximal load using the 1RM test as ref-erence, 4 series of 8-10 repetitions with 120 seconds of intervals between series and of 120 seconds between exercises for anoth-er muscular group. The creatine and placebo supplementation be-gan in the third week along with the beginning of the hypertrophy training.
The training protocol consisted of the division of the muscular groups and exercises for the respective groups as well as the spe-cific training days. In the two first weeks, the volunteers performed 3 series with 12 repetitions in all exercises proposed, only chang-ing the abdominal exercises, which were performed twice a week with 5 series of 20 repetitions each series and with 50% of the 1RM. For further details on the training procedure adopted in this experiment, see Souza Jr.(14).
Treadmill exercise – The training exercise in motorized tread-mill was performed according to protocol developed especially to assess the athlete’s maximal anaerobic capacity (Mart test = max-imal anaerobic running test)(15). The intensity was progressively
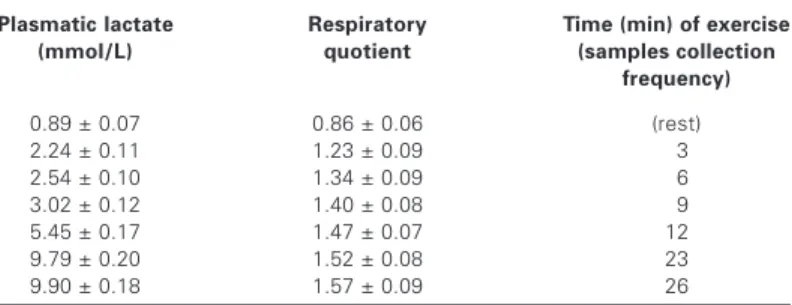
The athletes were submitted to treadmill test with concurrent determination of O2 intake, heart rate, respiratory quotient and blood lactate. The blood samples collecting frequencies for lactate and other parameters dosage are found in tables 1 and 2. Blood for lactate dosage was collected from the left earlobe with the aid of heparinized capillaries with capacity of 25 µL of blood. The plas-matic MDA and antioxidant capacity determinations were per-formed in plasma obtained as described above, in other words, blood collected from forearm cubital region at the same time inter-vals. Heparinized disposable syringes were used for these blood collections and hemolyzed samples were rejected. The individual results were compared with results in rest. In other words, the athletes have been their own control group for the parameters an-alyzed.
RESULTS
Data with regard to the effects of the treadmill exercise on the parameters used with the objective of monitoring its intensity are described in table 1. One observes from the analyses that the phys-ical exercise was effective in promoting great physphys-ical requirement in function of the blood lactate concentration obtained at the end of the test and the respiratory quotient value. All results compared to rest values in table 1 are significantly higher (p < 0.01). Data with regard to the effect of the exercise on the urinary chemilumi-nescence are described in table 2. One observes in this table that the only significant effect obtained for this parameter was the re-duction (28%) three hours after the end of the exercise.
Table 2 shows significant increase on the plasmatic MDA, espe-cially at 6 min (33%), 23 min (714%) and 26 min (761%) of physical exercise. It is also observed in this experiment that the plasmatic MDA was reduced between 9 and 12 min times, being significant-ly lower (24%) at 12 min of running. The total plasmatic antioxi-dant capacity (table 2) increased significantly at 9 min (34%) and 12 min (36%) of exercise, while decreased significantly at 23 min (52%) and 26 min (59%) of exercise.
The results obtained with marathon runners group are described in table 3. Both ptraining (31.5%) and post-training (41.5%) re-sults were obtained for this group, being significantly higher when compared to the inactive individuals group. In the 400 m runners group (table 3), no significant difference was found in the pre- and post-training urinary chemiluminescence when compared to the inactive individuals group. In fact, some reduction tendency is ver-ified in this parameter for this group relatively to the inactive indi-viduals.
TABLE 1
Blood and respiratory parameters of athletes (n = 10) indicative of treadmill exercise intensity
Plasmatic lactate Respiratory Time (min) of exercise
(mmol/L) quotient (samples collection
frequency)
0.89 ± 0.07 0.86 ± 0.06 (rest)
2.24 ± 0.11 1.23 ± 0.09 03
2.54 ± 0.10 1.34 ± 0.09 06
3.02 ± 0.12 1.40 ± 0.08 09
5.45 ± 0.17 1.47 ± 0.07 12
9.79 ± 0.20 1.52 ± 0.08 23
9.90 ± 0.18 1.57 ± 0.09 26
All results compared with rest situation are significantly higher (p < 0.01). Results are expressed as average ± standard deviation.
TABLE 2
Blood and urinary parameters of athletes (n = 10) indicative of oxidative lesions in body lipids during and after the treadmill exercise
Urinary MDA Plasmatic Time
chemiluminescence (nmol/mL antioxidant (min) of
(cpm/mg of creatine) of plasma) capacity exercise
(µµµµµmol of Trolox – Eq/L)
910.0 ± 10.0 0.21 ± 0.012 483 ± 88 (rest)
0.23 ± 0.013 470 ± 65 03
0.28 ± 0.011* (↑ 33%) 465 ± 50 06 0.19 ± 0.011 650 ± 90* (↑ 34%) 09 0.16 ± 0.012* (↓ 24%) 655 ± 88* (↑ 36%) 12 1.50 ± 0.009* (↑ 714%) 233 ± 12* (↓ 52%) 23 895.8 ± 9 1.60 ± 0.013* (↑ 761%) 200 ± 14* (↓ 59%) 26
657.8 ± 8* (↓ 28%) 3 h after
* p < 0.01 compared with rest situation.
MDA = malondialdehyde; cpm = counting per minute.
Results are expressed as average ± standard deviation. ↑ increase; ↓ drop.
The motorized treadmill especially developed to assess human beings used is the Quinton 65 model, Quinton Instruments, Seatle-WA, and the blood lactate analysis occurred using automated ana-lyzer (model 2300 stat plus, Yellow Springs Instruments, Yellow Springs, OH). The metabolic analysis system used in the respirato-ry quotient study was the Teen-100 – Aeroesport Inc. USA, all equip-ments from Ladesp (Sportive Performance Laboratory – Depart-ment of Sports-USP). The fluorimeter employed in the MDA and the total plasmatic oxidant capacity analyses was the Yellow Springs model YSI 53 (Biochemistry Laboratory – Department of Biochem-istry – IQ-USP) and the scintillation counter used was the Packard Tri-carb system (Department of Histology – ICB-USP). All reagents were obtained from Sigma and Merck. The statistical analysis used the Student’s t test for non-paired means after the two-way analy-sis of variance.
TABLE 3
Urinary chemiluminescence (cpm/mg of creatine) of athletes before and after physical exercise and intense training
Groups Before After
Inactive individuals 0.9260. ± 39 –
Marathon runners 1,3520. ± 23 (↑ 31.5%)# 1,582.0 ± 69 (↑ 41.5%)#
400 m runners 0.900.0 ± 97 0.890.0 ± 97
Soccer (1) 0.965.5 ± 34 1,465.0 ± 37 (↑ 58%)# (↑
52%)*
Soccer (2) 0.955.5 ± 30 1,640.4 ± 26 (↑ 77%)# (↑
71%)*
Strength training (1) 0.955.0 ± 29 1,630.0 ± 23 (76%)# (71%)*
Strength training (2) 0.956.5 ± 28 1,135.4 ± 20 (↓ 43%)**
# Compared with inactive individuals; p < 0.01. * After compared with before; p < 0.01.
** Strength training 2 (after) compared with strength training 1 (after); p < 0.01.
Results are expressed as average ± standard deviation. Marathon runners: 284 samples in 10 athletes were analyzed after 20 km running at heart rate of 180-185 bpm. 400 m runners: 10 athletes and 10 inactive individuals, all male (average of 24 analyses each group). Soccer (1): simulated soccer game with 50 min (average of 24 analyses of 10 players); Soccer (2): strength training of soccer players (average of 24 analyses of 10 players). Strength training (1): before and after with placebo; strength training (2) before and after with creatine with 9 students each group. ↓ drop; ↑ increase.
The results obtained for soccer players (table 3) in simulated game with 50 min duration revealed that in this condition the phys-ical exercise may elevate significantly the urinary chemilumines-cence, when compared with the rest situation (52%) and with in-active individuals (58%). In other words, despite the fact that the urinary chemiluminescence does not increase significantly after treadmill test at maximal intensity, it is possible that it would occur after the game. The same situation is observed in the strength training period for the same athletes (table 3) in which a significant increase on the urinary chemiluminescence was observed when compared with inactive individuals (77%) and with rest situation (71%).
results suggest the existence of protective effects of the creatine on the urinary chemiluminescence. Indeed, it was verified, com-paratively to the group with placebo supplementation, that the uri-nary chemiluminescence is significantly lower (43%) in group treat-ed with creatine.
DISCUSSION
The exercise’s intensity and duration are factors that determine the type of energetic substrate used. In the case of treadmill exer-cise (table 1) performed until exhaustion, the main energetic sub-strate used was the muscular glycogen. This conclusion is based on the fact that both the plasmatic lactate and the respiratory quo-tient increased significantly when compared to the pre-exercise values. Therefore, this type of exercise is characterized as of high intensity. In this condition, a decrease on the plasmatic MDA con-centration was verified (table 2) after the beginning of the exercise and a drop on the urinary chemiluminescence (table 2) during and after exercise; these effects may reflect the presence of antioxi-dant capacity sufficient to protect the organism at the initial exer-cise moments but not after this phase.
Indeed, a decrease on the plasmatic MDA is verified in the be-ginning of the exercise in agreement with the presence of high plasmatic antioxidant content (table 2). On the other hand, the plas-matic MDA elevation after 12 min of exercise occurs concurrently to the drop on its total antioxidant capacity. It is important to em-phasize that the high lactate plasmatic concentration verified in the blood of individuals who performed this type of exercise is propitious to the plasmatic antioxidant capacity, once it was already verified that it presents antioxidant properties(1,7). Still, one may
say that the plasmatic elevation of lipid peroxidation products dur-ing extended intense physical exercise may reveal the lack of ca-pacity of the organism to support the long-term continuous oxida-tive stress.
The reduced urinary chemiluminescence values detected after the end of the exercise support the previous statement that the organism presents antioxidant capacity sufficient to be protected at the beginning of the exercise, but it is not in agreement with the high MDA content found after 12 min of exercise. In fact, despite the drop on the urinary chemiluminescence (table 2) shortly after the exercise has not been significant, this becomes more evident when it is verified that this parameter presents drop even higher three hours after the end of the exercise. Therefore, it is possible that the organs present at the splenic region may have sufficient antioxidant capacity to protect themselves against the exercise effects on their ROSs production systems, what may reflect in lower chemiluminescence index detected in the urine(15).
Lipoperoxides may be transferred from one organ or tissue to another and metabolized by those with high antioxidant capacity (16-18). This is particularly important during intense exercise because
the splenic region presents blood flow strongly diminished in such condition, while it is increased in the skeletal muscles. Thus, be-sides the possibility of lipoperoxides being metabolized by organs at the splenic region, hemodynamic factors make the transporta-tion of these lipoperoxides towards this region difficult, elevating their concentration in plasma and in other tissues where lipoperox-ides are produced during intense physical exercises. These state-ments are based on data obtained by Maugham et al.(19), who
dem-onstrated that lipoperoxides present their concentrations increased in plasma until six hours after intense exercise like the exercise performed in this work. Therefore, due to the recovery of the renal blood flow in this time interval, the evaluation of the urinary chemi-luminescence of athletes submitted to intense exercise in a time interval longer than that used in this work becomes necessary.
Although the statements previously made on treadmill exercise performed by the athletes, an elevation on the urinary chemilumi-nescence is verified in table 3 for soccer players after the
perfor-mance of a single training session in simulated game (50 min) as well as the urinary chemiluminescence after strength training ses-sions in their general physical preparation phase (table 3). The uri-nary chemiluminescence measurement occurred before and after single training session and the training did not present suppres-sive effect on the urinary chemiluminescence, which remained high during all this period, probably because the objective was to in-crease the players’ maximal strength. In other words, this type of physical training must not have provided significant effect on stim-ulating antioxidant defenses in order to protect the athlete’s organ-ism during the training period.
The average-duration intense physical exercise used in this ex-periment as well as marathon and ultramarathon(20,21) promote
in-creases on the plasmatic MDA, but the effect of the extended exercise on the urinary chemiluminescence remains unclear. In-deed, besides the high plasmatic MDA detected by Kanter et al.(20,21)
group, in studies involving ultramarathon runners, athletes special-ized in marathon events present increased urinary chemilumines-cence after training with intense physical exercise (table 3). Data presented in table 3 suggest that long-duration intense physical activities performed by human beings promote more oxidative stress than short or average-duration physical activities performed at high intensities. Furthermore, data show that the daily physical training does not develop the antioxidant capacity of marathon run-ners in such way to protect them from oxidative lesions due to ROSs, especially when their pre-training urinary chemiluminescence results are compared with results from inactive individuals and run-ners in general (table 3).
Studies involving marathon and ultramarathon runners just like the studies conducted by Kanter et al.(20,21) found significant
corre-lation between the lipoperoxide plasmatic concentrations and the creatina kinase enzyme and the muscular lactate dehydrogenase (LDH-M). This group observed that the lipoperoxide concentrations and the activity of these enzymes increase in plasma after 50 miles of running. The researchers concluded that oxidative lesions oc-curred in the muscular fibers of these individuals during exercise in function of the increased plasmatic concentration of these en-zymes. Thus, both the increased plasmatic MDA, as suggested by Kanter et al. studies, and the elevated urinary chemiluminescence in the present study may indicate the occurrence of oxidative tis-sue injuries in individuals who perform extended intense physical activities, showing the need of dietary supplementation with chem-ical antioxidants for these individuals.
With regard to the strength training and oxidative stress, only a few studies are available. One of them has verified that when iso-metric contraction prevails in the strength training, oxidative le-sions may occur in biomolecules as demonstrated through lipoper-oxides blood measurements(22). Thus, it is possible that the strength
training would also induce the oxidative stress. Besides, it was verified in studies involving strength training associated to creat-ine intake as energetic supplement (table 3) that the lower urinary chemiluminescence detected in these individuals suggests the existence of protective antioxidant effects from this substance. This probably occurs because the creatine ingested along the strength training may maintain the intramuscular ATP values long-er without favoring high activation of the purine degradation cycle, main O2-independent catabolic process responsible for the ROSs intramuscular production(23).
Not many data on the quantification of metabolic markers of the purine degradation cycle activation in intense physical exercise associated to the creatine ingestion are available. Therefore, fur-ther experiments are required in order to evaluate whefur-ther this hypothesis is plausible. However, it has been verified that the phys-ical performance is elevated during repeated sessions of maximal exercise associated to the ingestion of 20 g/day for 5-7 days of creatine(23). Furthermore, a drop was verified both in the ammonia
preceded by creatine ingestion(24). Although the effect of creatine
supplementation and oxidative stress during intense exercise has not yet been systematically studied, it is possible speculating that the low hypoxanthine production as result of this procedure may reduce its catabolism into xanthine and urate with lower concur-rent production of O2•-, H
2O2 and HO
•. Therefore, it is possible that
the additional creatine ingestion before intense physical exercise performed in short period of time involving strong muscular con-tractions may act as antioxidant.
CONCLUSION
Treadmill running performed at high intensity by human beings shows that the average-duration physical activity (20-30 min), rath-er than the short (strength training) and the long duration physical activities (20 km running and 50 min soccer game) reduces the urinary chemiluminescence. The first type of exercise, on the oth-er hand, increases the plasmatic MDA concentration. Thoth-erefore, the intense exercise stimulates the oxidative stress in human be-ings in a different way, depending on the duration. Although the
strength/hypertrophy training elevates the urinary chemilumines-cence values, the creatine ingestion associated to this type of train-ing may be beneficial as antioxidant protection.
ACKNOWLEDGMENTS
The authors are thankful to Prof. Dr. Etelvino José Henriques Bechara (IQ-USP) for allowing the use of the biochemistry laboratory in which most of the analyses were performed and for the suggestions in the text edit-ing. To Professors Marília Seelaender and Luiz Fernando Bicudo (Depart-ment of Histology – ICB-USP), for allowing the use of the equip(Depart-ment for the urinary chemiluminescence analysis (scintillation counter). To Profes-sors Marcelo, Valéria and Edson (Ladesp), for the execution of the tread-mill tests and for collaborating in the blood samples collection for lactate and respiratory quotient measurements. To Sports and Physical Education graduation students who directly or indirectly collaborated in experiments conducted in this study.
All the authors declared there is not any potential conflict of inter-ests regarding this article.
REFERENCES
1. Jenkins RR. Exercise and oxidative stress methodology: a critique. Am J Clin Nutr 2000;72:670-4.
2. Polidori MC, Mecocci P, Cherubini A, Senin U. Physical activity and oxidative stress during aging. Int J Sports Med 2000;21:154-7.
3. Goldfarb AH. Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol 1999;24:249-66. 4. Ashton T, Rowlands CC, Jones E, Young IS, Jackson SK, Davies B, et al.
Elec-tron spin resonance spectroscopic detection of oxygen-centered radicals in hu-man serum following exhaustive exercise. Eur J Appl Physiol 1998;77:498-502.
5. Benzi G. Aerobic performance and oxygen free radicals. J Sports Med Phys Fitness 1993;33:205-22.
6. Powers SK, Ji LL, Leewenburgh C. Exercise training-induced alterations in skel-etal muscle antioxidant capacity: a brief review. Med Sci Sports Exer 1999;31: 987-97.
7. Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med 1999; 222:283-92.
8. Halliwell B, Gutteridge JMC. Free radical in biology and medicine. 3rd ed.
Ox-ford: University Press, 1999.
9. Heinegard D, Tinderstrom G. Determination of serum creatinine by a direct col-orimetric method. Clin Chem Acta 1973;43:395-410.
10. Lissi EA, Hanna-Salim M, Videla LA. Spontaneous urinary visible luminescence: characteristics and modification by oxidative stress-related clinical conditions. Braz J Med Biol Res 1994;27:1491-505.
11. Yagi K. Assay for blood plasma or serum. Methods Enzymol 1984;105:328-31. 12. Whitehead T, Thorpe GHG, Maxwell S. Enhanced chemiluminescent assay for
antioxidant capacity in biological fluids. Anal Chem Acta1992;226:265-77. 13. Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gomes AL, et al.
Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sport Exerc 1999;31:1147-56.
14. Souza Junior TP. Suplementação de creatina e treinamento de força: alteração da resultante de força máxima maximorum, hipertrofia muscular e variáveis
an-tropométricas [Dissertação de mestrado em Ciências do Esporte]. Campinas: Faculdade de Educação Física, Universidade Estadual de Campinas, 2002.
15. Kearney JT, Rundell KW, Wilber RL. Measurement of work and power in sport. In: Garret WE, Kirkendall DT, editors. Exercise and sport science. Philadelphia: Lippincott Williams & Wilkins, 2000.
16. Lew H, Pyke S, Quintanilha AT. The effects of physical exercise on the antioxida-tive capacity of the liver. Bioelectrochem Bioenerg 1987;18:231-46.
17. Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Kortoiuis RJ. Skele-tal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol 1990;68:2337-43.
18. Drapper HH, Hadley M. A review of recent studies on the metabolism of exog-enous malondialdehyde. Xenobiotica 1990;20:901-7.
19. Maughan RJ, Donnelly AR, Gleeson M, Whiting PH, Walker KA. Delayed-onset muscle damage and lipid peroxidation in man after a downhill run. Muscle Nerve 1989;12:332-6.
20. Kanter MM, Lesmes GR, Nequin ND, Kaminsky LA, Saeger JM. Serum lipid levels and lipid peroxidation in ultramarathon runners. Ann Sports Med 1986;3: 39-41.
21. Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ. Effect of exercise training on antioxidative enzymes and cardiotoxicity of doxorubicin. J Appl Phys-iol 1985;59:1298-303.
22. Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Gen-eration of reactive oxygen species after exhaustive aerobic and isometric exer-cise. Med Sci Sports Exerc 2000;32:1576-81.
23. Casey A, Greenhaff PL. Does dietary creatine supplementation plays a role in skeletal muscle metabolism and performance? Am J Clin Nutr 2000;72(Suppl): 607-17.