w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
article
A
cytotoxic
Petiveria
alliacea
dry
extract
induces
ATP
depletion
and
decreases
-F1-ATPase
expression
in
breast
cancer
cells
and
promotes
survival
in
tumor-bearing
mice
John
F.
Hernández
a,b,
Claudia
P.
Urue˜na
a,
Tito
A.
Sandoval
a,
Maria
C.
Cifuentes
a,
Laura
Formentini
b,
Jose
M.
Cuezva
b,
Susana
Fiorentino
a,∗aGrupodeInmunobiologíayBiologíaCelular,FacultaddeCiencias,PontificiaUniversidadJaveriana,Bogotá,Colombia
bDepartamentodeBiologíaMolecular,CentrodeBiologíaMolecularSeveroOchoa,ConsejoSuperiordeInvestigacionesCientíficas-UniversidadAutónomadeMadrid,Madrid,Spain
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received17May2016 Accepted20September2016 Availableonline9February2017
Keywords: Petiveriaalliacea
Breastcancer
-F1-ATPase Respiration ATPdepletion
a
b
s
t
r
a
c
t
Metabolicplasticityincancercellsassurescellsurvivalandcellproliferationundervariablelevelsof oxy-genandnutrients.Therefore,newanticancertreatmentsendeavortotargetsuchplasticitybymodifying mainmetabolicpathwaysasglycolysisoroxidativephosphorylation.InAmericantraditionalmedicine PetiveriaalliaceaL.,Phytolaccacea,leafextractshavebeenusedforleukemiaandbreastcancertreatments. Herein,westudycytotoxicityandantitumoraleffectsofP.alliaceaextractintumor/non-tumorigeniccell linesandmurinebreastcancermodel.BreastcancercellstreatedwithP.alliaceadryextractshowed reductionin-F1-ATPaseexpression,glycolyticfluxtriggeringdiminishedintracellularATPlevels, mito-chondrialbasalrespirationandoxygenconsumption.Consequently,adeclineincellproliferationwas observedinconventionalandthree-dimensionspheresbreastcancercellsculture.Additionally,invivo treatmentofBALB/cmicetransplantedwiththemurinebreastcancerTS/AtumorshowedthatP.alliacea extractviai.p.decreasestheprimarytumorgrowthandincreasessurvivalintheTS/Amodel.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Cancer cells may assure survival and proliferation under shiftinglevelsofoxygenand nutrientsthrough metabolic plas-ticitybetweenglycolysisandoxidativephosphorylation(OxPhos) metabolicpathways.Integratingthesepathwaysinglucose oxida-tionprovidesimportantsubstratesasATP,NADHandbiosynthetic precursorsforthecellhousekeepingprocesses(Formentinietal., 2010).MitochondrialactivityandspecificallyOxPhosplaya rele-vantroleinfacilitatingtheexecutionofcelldeath(Cuezvaetal., 2009).InthemitochondrialinnermembraneisfoundtheATPase orcomplexV,amulti-enzymaticcomplexwithtwodomains:a hydrophobicintramembranedomainF0andahydrophilicdomain F1 facing to the matrix leaflet. F1 domain has five sub-units ␣33␥1␦11andthreecatalyticsites(subunitand␣/ inter-face).Theelectrochemicalgradientgeneratedbythemitochondria REDOXreactions,makespossibletheprotoninfluxintothematrix through F0 domain providing the necessary energy for ADP
∗ Correspondingauthor.
E-mail:susana.fiorentino@javeriana.edu.co(S.Fiorentino).
phosphorylation (Stock et al., 1999; Gledhill et al., 2007).It is wellknownthatcancercells canhavestructuralandfunctional mitochondrial alterations. For instance, it has been shown in human carcinomas that selective repression of -F1-ATPase is inverselycorrelatedwithglyceraldehyde-3-phosphate dehydroge-nase(GAPDH)levelscausingdecreaseinmitochondrialactivityand increaseinglycolyticflux(Cuezvaetal.,2002).Moreover,human breastcancercellsoverexpressedlactatedehydrogenaseisoformA (LDH-A)leadingtoanincreaseinlactatesecretion(Koukourakis et al., 2008). Hence highlactate levels are associated to taxol resistanceand cell proliferation augmentation under a hypoxic microenvironment(Fantinetal.,2006;Zhouetal.,2010).
Recently, dichloroacetate (a pyruvate dehydrogenase kinase inhibitor)hasbeenproposedasanewanticancerdrugspecially forglycolytictumorsposinglimitedsideeffects(Papandreouetal., 2011)andbroadeningthespectrafornewOxPhosregulators.In thisregard,plantscouldbeasourceofcompoundsabletotarget cancermetabolicpathways.
In fact, Petiveria alliacea L., Phytolaccaceae, infusions from leafand roothave beenreportedto haveanti-spasmodic, anti-rheumaticandanti-inflammatoryproperties(Moralesetal.,2001) andparticularlyusedinleukemiaand breastcancertreatments
http://dx.doi.org/10.1016/j.bjp.2016.09.008
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314
(Garcia-Barriga, 1974; Gupta, 1995). It has been shown that P. alliacea extracts are cytotoxic on leukemia, lymphoma and melanomacelllines(Rossi,1990;Rossietal.,1993;Urue˜naetal., 2008), howeverit poseslow toxicity onhumanfibroblasts and peripheralbloodmononuclearcells(Urue˜naetal.,2008).Recently, wehaveproposedthatantitumoractivityofa dryextractfrom P.alliaceacanbepartlyexplainedbytheglycolyticfluxshifting ofcancercells,asshownon4T1breastcancermodel(Hernandez etal.,2014).
Herein, we demonstrated that a dry extract from P. alli-aceacauseschangesinmitochondrialactivitycharacterizedbya decreasein-F1-ATPaseexpressionandATPdepletionleadingto adecreaseinbreastcancercellproliferationinvitroandinvivo.
Materialsandmethods
Plantmaterialandextractionprocedure
Petiveria alliacea L., Phytolaccaceae, leaves and stems (local name“anamu”)werecollectedinCachipay,Cundinamarca, Colom-biaonApril2009andidentifiedbyCarlosParrafromtheColombian National Herbarium; voucher number COL 569765 (Colombian EnvironmentalMinistryagreementnumber 1927relatedtothe use of genetic resources and derivatives products). P. alliacea extractionprocedureand chemicalcharacterization were previ-ouslydescribed (Urue˜naet al.,2008).Briefly,dryground leaves and stemswereextracted with96%ethanol (15±5◦C),filtered andconcentratedunderreducedpressure.Ethanolicextractwas trapped on fumed silica, fractionated with ethyl acetate and extractedwithmethanol:wateryieldingadryextract(DER gen-uine:10000–11000:1).Thecompoundsidentifiedin dryextract fromP.alliaceawere:benzaldehyde,leridol,petiveral,myricetin, petiveral4-ethyl, pinitol,dibenzyldisulfideanddibenzyl trisul-phide. HPLC chromatographic fingerprint was acquired in a Jasco®PU2089plusequippedwitha UVdetector(254nm)using a C18 column and water/acetonitrile gradient as mobile phase (Hernandezetal.,2014).TomeetEMAguidelines,theactivemarker selectedwasdibenzyldisulfide,a reportedcytotoxiccompound (Cifuentesetal.,2009)foundata concentrationof2.6mg perg ofextract.
Celllines
4T1,TS/A,3T3andHS578TcelllineswereculturedinDMEM andHCT116celllineinMcCoy’s5Amedium,bothsupplemented withfetal calf serum (FCS) heat-inactivated (10%), l-glutamine
(2mM),penicillin (100U/ml), streptomycin (100g/ml), HEPES buffer(0.01M) andsodiumpyruvate(1mM)(EurobioToulouse, FR).MCF12Fcelllinewascultured inDMEM/F-12medium sup-plementedwithfetalhorseserum(5%),epidermalgrowthfactor (20ng/ml),humaninsulin(10g/ml),hydrocortisone(500ng/ml), choleratoxin(100mg/ml),penicillin(100U/ml)andstreptomycin (100g/ml).Celllineswereincubatedunderhumidified environ-mentat37◦Cand5%CO2.
Invitrocytotoxicityassays
Cytotoxic effects were evaluated using methylthiazol tetra-zolium (MTT, Sigma-Aldrich,Saint Louis, MO) and trypan blue dye assays. Cells (5×103 cells/well) were seeded in 96-wells plateswithdifferentconcentrationsofdryextractfromP.alliacea (250–0.95g/ml)orethanol(0.02%)asnegativecontrolfor48h. ProliferationwasestimatedbyMTTassayaccordingtoprocedure previouslydescribed(Urue˜naetal.,2008).TheIC50valuewas esti-matedwithnonlinearregressionanalysis(GraphPadPrism5for Windows).
Westernblots
Cellsweresuspendedinlysisbuffercontaining25mMHEPES, 2.5mM EDTA,0.1% TritonX-100,1mM PMSFand 5mg/ml leu-peptin.Extractswerecentrifugedat11,000×gfor15minat4◦C. Supernatantsprotein concentrationwasdeterminedwith Brad-fordproteinassay.Cellularproteins(7–20g)werefractionated bySDS/PAGE (12%)andtransferred ontoPVDFmembranes. Pri-marymonoclonalantibodieswere: anti--F1-ATPase (1:50,000) (Cuezvaetal.,2002),anti-Hsp60(1:10,000)andanti-NADH9 (com-plexI39kDa)(1:1000)(Aceboetal.,2009),anti-ComplexIIIsubunit Core 2 (1:1000) from Abcam; anti-SDH (succinate dehydroge-nase)(1:500)fromLifeTechnologies;anti--actin(1:20,000)and anti-tubulin(1:5000)fromSigma-Aldrich.Peroxidase-conjugated anti-mouseoranti-rabbitIgG(NordicImmunology,1:3000)were usedassecondaryantibodies.Blotsrevealedwithluminol electro-chemiluminescence(ECL)reagent(AmershamPharmaciaBiotech, LittleChalfont,UK).
Oxygenconsumptionestimation
CellularoxygenconsumptionratesweredeterminedinanXF24 ExtracellularFluxAnalyzer(SeahorseBioscience).Cells(4×104) wereseededinXF24-wellcellculturemicroplates(Seahorse Bio-science), treated with dry extract from P. alliacea or ethanol, incubatedat37◦Cand5%CO2 for6or24h.Aftertreatmentthe followingsubstanceswereconsecutivelyinjectedtoachieve the indicatedfinalconcentration:oligomycin(OLIGO,6M), dinitro-phenol(DNP)0.5mM,rotenone1Mandantimycin1M.
Glycolysisfluxestimation
Cells(1.5×105)wereseededandallowedtogrowuntil60%of confluence.Todetermineglycolyticrates,cells weretreatedfor 3,6,24or48hwithdryextractfromP.alliaceaorethanolwith orwithoutOLIGO6M.Aftertreatmentmediumwasreplacedby freshone(FCS0.5%)andcellswereallowedtorestduring2h. Sam-plemedium(100l)wasprecipitatedwithperchloricacid(6%), neutralized(KOH20%),andglycine–hydrazine–EDTAbuffer(1M: 0.4M:1.3mM)containingLDH(RocheDiagnosticsGmbH)and -NADHhydrate (Sigma-Aldrich) wasadded (Govindarajan etal., 2007).Lactatelevelswereestimatedat340nminaShimadzu spec-trophotometer.ProteincontentwasestimatedbyBradfordprotein assay(Sigma-Aldrich).
IntracellularATPdetermination
Intracellular ATP was measured using ATP Bioluminescence AssaykitHS II(RocheDiagnostics). Briefly,1.5×105 cells were platedin6-wellplates,treatedwithdryextractfromP.alliacea, DOG/metforminHCl (Sigma-Aldrich)orethanolduring6h, har-vested, counted, and lysedwithlysis buffer(50l) at 20◦C for 5min.Adilution(1:100)fromsampleorstandard(50l)was trans-ferredtoa96-wellplate,andluciferasereagent(50l)wasadded. Theemittedlightwasmeasuredimmediatelyandintegratedusing aPlateChameleonVModel425-156(Hidex)for10s.Blankvalue (noATP)wassubtractedfromeachsample’srawdataandATP con-centrationswerecalculatedfromthestandardcurveandexpressed asmolper1×105cells.
Spheresnumberandareaestimation
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314
streptomycin (100g/ml). Cells were daily treated during 6 dayswithethanol(negativecontrol),dryextractfromP.alliacea (11g/ml),deoxyglucose(DOG,0.24mM)anddoxorubicin(DOX, 0.08M).After7days,sphereswerecountedbytwoindependent observersusinganopticalmicroscopeOlympus(10×).Cellculture mediumwas recovered,centrifuged(100×g)during 3min and spheres pelletsuspended in phosphate buffer saline(PBS) and placed onmicroscope slide. Spheres’ areawas measuredusing Axiovision®software(CarlZeiss).
Animals
Female BALB/c mice,6–12 weeks old were purchased from CharlesRiversLaboratoriesInternational,Inc.(Boston,MA), and housedinouranimalresearchfacilityfollowingtheestablished protocols of the Ethics Committee of the Science Faculty and National and International Legislation for Live Animal Experi-mentation(Colombia Republic, Resolution 8430/1993; National
AcademyofSciences, 2010).Micewerehousedinpolyethylene cageswithfoodandwateradlibitum,controlledtemperature,anda 12-hlight/darkcycle.Beforetreatments,themicewereacclimated foroneweekunderstandardconditions.Thisprojectwasapproved bytheEthicsCommitteeoftheScienceFacultyon29/04/2009.
Tumormodel
TS/Acells(1×104)suspendedin100lofPBSwereinjected intotherightmammaryfatpad(subcutaneously[SC])onday0 and then randomly assigned to PBS control group (n=9), DOX group (3mg/kg Al Pharma®, n=9) or P. alliacea extract group (250mg/kg, n=8).After5 days ofinoculation, treatmentswere injectedintraperitoneally(i.p.)onceaweekforDOXandtwicea weekforP.alliaceaextractuntil56dayspost-inoculation.Tumors weremeasuredwithVerniercalipersthreetimesaweek,andtumor volumewascalculatedusingthefollowingformula:tumorvolume (mm3)=[(width)2
×length]/2Gallotannin-richCaesalpiniaspinosa
100
80
60
40
20
0
0 25 50 75 100 125 150
P. alliacea dry extract (µg/ml)
Control
125 µg/ml 62.5 µg/ml P. allieacea
Control Doxorubicin
5 µM 2.5 µM
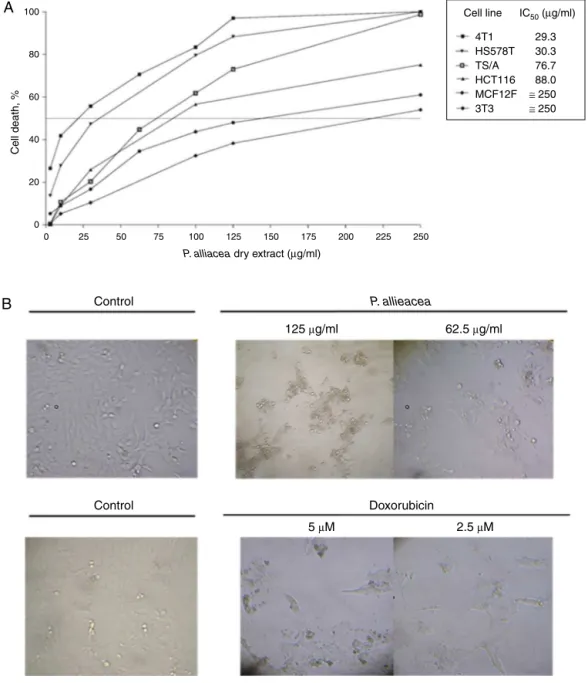
Cell line
4T1 HS578T TS/A HCT116 MCF12F 3T3
29.3 30.3 76.7 88.0
≅ 250
≅ 250 IC50 (µg/ml)
Cell death, %
175 200 225 250
A
B
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314
20
A
C
D
E
B
16
12
8
4
0
20
16
12
8
4
0
Control Oligo
∗∗ ∗∗
∗∗
∗∗
∗∗∗
3h 6h 24h
P. alliacea extract 3 µg/ml
48h Control
nmol lactate/
µ
g protein/h
±
SEM
nmol lactate/
µ
g protein/h
±
SEM
nmol lactate/
µ
g protein/h
±
SEM
nmol lactate/
µ
g protein/h
±
SEM
nmol lactate/
µ
g protein/h
±
SEM
Oligo 3h 6h 24h
P. alliacea extract 3 µg/ml 48h
20
16
ns
12
8
4
0
20
16
12
8
4
0
20
16
12
8
4
0
Control Control Oligo Control Oligo P. alliacea
3 µg/ml P. alliacea extract 10 µg/ml
After wash 24h
After wash 48h
3h 6h 24h
Oligo 3h
P. alliacea 12 µg/ml
Fig.2. Petiveriaalliaceaextractdecreasesglycolyticfluxinbreastcancercelllines.(A)4T1,(B)HS578T,(C)MCF12F,(D)HCT116,(E)HS578T.Celllinesweretreatedwitha
P.alliaceadryextract(IC50/10)during3,6,24or48h.Aftertreatmentlactateconcentrationwasevaluatedbyenzymaticassay.Datarepresentthemean±S.E.M.ofatleast
threeindependentexperiments*p<0.05,**p<0.001,***p<0.0001comparedtocontrolusingStudent’sttest.
fractiondecreasestheprimarytumorandfactorsassociatedwith poorprognosisina murinebreast cancermodel (Urue˜naetal., 2013).Astudyofsurvivaldefiningtheendpointofeach individ-ualaccordingtothecriteriaof toxicityand animalwelfarewas conducted.TheanimalswereeuthanizedinaCO2chamberwhen achieveoneormoreendpointcriteria.
Statisticalanalysis
Resultsareexpressedasmean±S.D.Foroxygenconsumption estimations and mammosphere analyses two-way ANOVAwas usedandunpairedttestforremaininganalyses.Survivalcurves obtainedbytheKaplan–Meiermethodwerestatisticallyanalyzed usingtheLog-ranktest.StatisticalanalysesweredoneusingGraph PadPrism5withap<0.05significance.
Results
Petiveriaalliaceadryextractismorecytotoxictobreastcancer celllineswhilesparingtofibroblastsandepithelialbreastcells
Dryextract from P.alliacea is cytotoxicto breast and colon tumorcell lines in a dose-dependent mannerwhile sparingto fibroblasts(3T3)and non-tumorigenicepithelial breastcell line (MCF12F)(Fig.1A).ThecorrespondingIC50is30g/mlforhuman HS578Tandmurine4T1breastcancercells,77g/mlformurine TS/Abreastcancercelllineand88g/mlforcolontumorcellline HCT116(Fig.1A).Cytotoxicityobservedinbreastcancercelllines wasassociatedtomorphologicalchangeslikeincreaseofcellular volume,theappearanceofrefringentvesiclesanddetachmentfrom theculturesurface(Fig.1B).
Reductionintheglycolyticfluxwasobservedaftertreating4T1 andHS578Tcelllines(3and6h)withP.alliaceaextract,at sub-cytotoxicconcentrations–IC50/10th–(Fig.2,panelAandB).The extracteffectisearlyandtransientdisappearingafter24h, regard-lesswhethertheextractremainsornotinthecellculture(Fig.2, panelE).Noeffectisobserved onMCF12F orHCT116celllines (Fig.2,panelCandD,respectively)after24htreatment,neither intheexpressionofglycolyticenzymesasGAPDH,pyruvatekinase (PK)andLDH(datanotshown).Thelattersuggeststhattheextract compoundsmaybindanyglycolyticenzymeinareversibleway promotingthetransientdecreaseintheglycolyticflux.
Petiveriaalliaceadryextracttreatmentcausesdecreasein mitochondrialrespiration,ATPsynthaseexpressionand intracellularATPconcentrationonbreastcancercelllines
MitochondrialOxPhosproteinexpressionofNADH9(complex I), succinatedehydrogenase (complexII30kDairon–sulfur sub-unit),cytochromeb-c1subunit2(complexIIICoreIIsubunit)and -F1-ATPase (complexV)weredetermined toassess P.alliacea extractactivity.Wefoundthattheexpressionof-F1-ATPase pro-teinwasaffectedbythetreatmentinbothbreastcancercelllines (Fig.3).
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314 Control 1
A
B
30 48 52 52 42 30 39 48 52 52 42 SDH 3.5 3 2.5 2 1.5 1 0.5Control 24 h
0
2 7 20 12
10 8 6 4 2 0 15 10 5 0 6 5 4 3 2 1 0 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 3.5 3 2 1 0 3 2.5 2 1.5 1 0.5 Control Core II/α-tubulin
SDH/α-tubulin β-F1/β-actin
∗
∗
24 h Control 24 h
Control 24 h Control 24 h
Control 24 h Control
NADH9/α-tubulin SDH/α-tubulin Core II/α-tubulin β-F1/β-actin
24 h 0 Core II β-F1-ATPse α-tubulin β-actin SDH NADH9 Core II β-F1-ATPse α-tubulin β-actin
2 3 1 2 3
P. alliacea extract (3 µg/ml)
Control
1 2 3 1 2 3
P. alliacea extract (3 µg/ml)
Fig.3. Petiveriaalliaceaextractdecreases-F1-ATPaseexpressioninbreastcancercelllines.(A)4T1and(B)HS578TcelllinestreatedwithaP.alliaceadryextractfor24h. NADH9,SDH,andCOREIIproteinexpressionwerealsodeterminedshowingnoeffectaftertreatment.RepresentativeWesternblotsandtheircorrespondinghistogramsare shownofthreeindependentpreparations(lanes1–3).Theleftsideofblotshowsproteinmolecularmassin(kDa).Blackbarsrepresentnormalizedbandswith␣-tubulinor
-actininarbitraryunits.Resultsarethemean±S.E.M.ofthreeindependentexperiments.*p<0.05comparedtocontrolusingStudent’sttest.
100 80 60 40 20 0 0 50 100 150
0 10 20
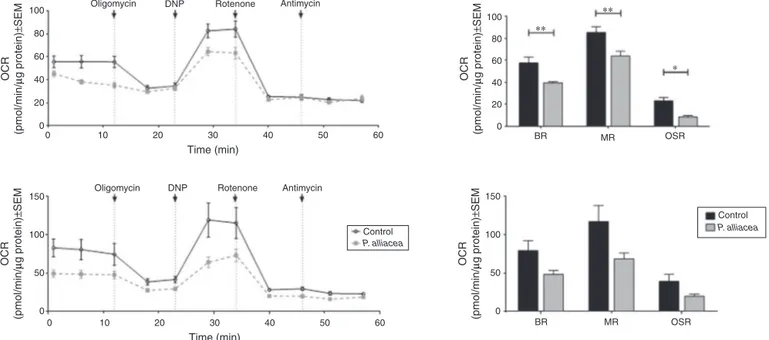
Rotenone Rotenone Antimycin Antimycin Control P. alliacea Control ∗ ∗∗ ∗∗ P. alliacea Oligomycin Oligomycin DNP DNP 30 Time (min) OCR (pmol/min/ µ g protein) ± SEM OCR (pmol/min/ µ g protein) ± SEM OCR (pmol/min/ µ g protein) ± SEM OCR (pmol/min/ µ g protein) ± SEM
40 50 60
0 10 20 30
Time (min)
40 50 60 BR MR OSR
BR MR OSR
150 0 20 40 60 80 100 100 50 0
Fig.4.Petiveriaalliaceaextracttreatmentreducesbasalrespiration,maximalrespirationandoxygenconsumptionrate(OCR)associatedtoATPproductionin4T1cells. After6h(upperpanel)and24htreatment(lowerpanel)theOCRwasmeasuredfollowingtheadditionoftheindicatedagents(left).Histogramsshowthecomparisonin basalrespiration(BR),maximalrespiration(MR)andoligomycinsensitiverespiration(OSR)betweencontrolandP.alliaceaextractcellstreated.Resultsareexpressedasthe mean±SEMoftwoindependentexperiments.*p<0.05,**p<0.001comparedtocontrolusingatwo-wayANOVA.
However,itmustbetakenintoaccountthattreatmentsignificantly lowers basal respiration explaining the no change in respira-tion.DNP,isaprotonionophorethatinduceselectrontransport chaintofunctionatitsmaximumratebycollapsingmitochondrial
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314
2.0
1.5
1.0
0.5
0.0
ETOH P. alliacea
(30 µg/ml)
P. alliacea
(3 µg/ml)
DOG (1.2mM)+ MET (0.5mM)
Treatment
∗∗∗ ∗∗ ∗∗
A
T
P [
µ
M]/1
×
10
5 cel
Fig.5. PetiveriaalliaceaextracttreatmentdecreasesintracellularATP.4T1cellswere treatedwithP.alliaceadryextractordeoxyglucoseplusmetformin(DOG+MET) during6h.ATPwasmeasuredbyabioluminescenceassay.ATPconcentrationis expressedasmolper1×105cells.Thefigurerepresentsonefromthree
indepen-dentexperiments.Resultsareexpressedasthemean±S.D.**p<0.001,***p<0.0001 comparedtocontrolusingStudent’sttest.
expressionlevelorfunctionalityof-F1-ATPaseisaltered(Fig.4, upperpanel).Similarresultswereobtainedafter24htreatment (Fig.4,lowerpanel).
IntracellularATPlevelin4T1 cellswasmeasuredafterusing DOG/metformin (1.2mM/0.5mM) as a severe ATP synthesis inhibitor,whichcausesa5-foldreductioninintracellularATP.Our treatmentshoweda 2.5-folddecrease(Fig.5)in 4T1cells, con-firmingthatmitochondrialATPsynthesisisdecreasebyP.alliacea extracttreatment.
AdryextractfromPetiveriaalliaceadecreases4T1spheresand conventionalcellcultureproliferation
Anoutstandingmodelfordrugscreeningarethespheressince theyaremidwaybetweenconventionalculturesandinvivotumors (Pampaloniet al., 2007).Aftersix days of P.alliacea treatment atsub-cytotoxicconcentrations,asignificantdecreaseinspheres’ numbernearly74%and75%inareawasobserved(Fig.6AandB).A comparablebehaviorwasobservedwithDOGandDOXtreatments. Decreaseinviablecellsnumber(60%)wasobservedin4T1 conven-tionalcellcultureafter6daystreatmentwithDOGorP.alliacea extractatsub-cytotoxicconcentrations(Fig.7).
Petiveriaalliaceadryextractdecreasestheprimarytumorand promotessurvivalinaTS/Amurinebreastcancermodel
ToassesstheantitumoreffectofP.alliaceaextract,a murine modelofmetastaticbreastcancerwasused.Previously,an esti-matedlethaldose50 (LD50) of1545mg/kg for theextract was reported(Hernandezetal.,2014),thustherapeuticdoseevaluated was6-foldlowertoassurelowtoxicity.FemaleBALB/cmicewere inoculatedwithSCinjectionof1×104TS/Acells.Afterfivedays, thetumorswerepalpable,andthemiceweretreatedwithP. alli-aceaextract(250mg/kg,twiceaweek),vehicle(PBS)orpositive control(DOX, 3mg/kgonce a week).Fig.8A shows thatP. alli-aceaextractsignificantlyreducedtumorgrowthcompared with thecontrol,becomingincreasinglymarkedfromday32,atday42 thetumorachievesavolumeof881mm3incontrolgroup com-pareto343mm3inP.alliaceatreatment.Likewise,DOXtreatment reducestumorvolumeinasignificantlymannershowingavolume of87mm3atday42witha67%ofanimalsfreeoftumor.Duetoa
15
A
B
C
800 000 ∗∗∗
∗∗∗ ∗∗∗ ∗∗∗
∗∗∗
∗∗∗
600 000
400 000
200 000
0 10
5
0
Control DOG
Treatment
Spheres n
umber
±
SD
Area (
µ
m
2)±
SD
DOX
P. alliacea
Control P. alliacea (6 µg/ml) DOG (0.24 mM) DOX (0.08 µM)
Control DOG
Treatment
DOX
P. alliacea
Fig.6. Petiveriaalliaceaextracttreatmentdecreasesthespheresinnumberandarea.4T1singlecellscultureonultra-lowattachmentplatesweretreatedwithaP.alliacea
dryextract(IC50/5),deoxyglucose(DOG,0.24mM))ordoxorubicin(DOX,0.08M)duringsixdays.(A)Sphereswerecountedbytwoindependentobserversonday7thusing
anopticalmicroscopyOlympus(10×).(B)SpheresareawasdeterminedusinganopticalmicroscopyOlympus(10×)andimagesanalyzedwithsoftwareAxiovision®.(C)
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314
500 000
400 000
300 000
200 000
100 000
0
ETOH ∗∗∗
∗∗∗ ∗∗∗
DOG Treatment
Number of viab
le cells
±
SD
DOX P. alliacea
Fig.7.ContinuoustreatmentwithPetiveriaalliaceadryextractusingsub-cytotoxic concentrationsdecreases4T1cellviability.4T1cellsweretreatedwithP.alliacea
dryextract(IC50/5),deoxyglucose(DOG,0.24mM))ordoxorubicin(DOX,0.08M)
duringsixdays.Atday7thviablecellswerecountedusingtrypanbluedye.Results areexpressedasmean±S.D.fromthreeindependentexperiments.***p<0.0001 comparedtocontrolusingatwo-wayANOVA.
markeddecreaseinthecontrolgrouppopulationbeforeday50th (77%),weevaluatethesurvivaloftreatmentsgroupsfollowingthe endpointcriteriaoftoxicityandanimalwelfareforeach individ-ual.AsshowninFig.8BP.alliaceaextractincreasessurvivalto48.5 dayscomparedto38daysin controlgroup(p=0.0055Log-rank test)whileDOXtreatmentimprovessurvivalto70dayscompared tocontrol(p=0.0004Log-ranktest).
1200
A
B
800
400
0
100
150
0
0 15 30 45 60 75 90
0 2 4 7 9 11 14 16 18 21
Time (days)
Time (days)
PBS
∗ ∗ ∗
∗∗∗
∗∗∗
∗
∗ DOX (3 mg/kg) (n=9) P. alliacea (250 mg/kg) (n=8)
PBS (n=9) DOX (3 mg/kg) (n=9)
P. alliacea (250 mg/kg) (n=8)
Sur
viv
al, %
T
u
mor v
olume (mm
3)
23 25 28 30 32 35 37 39 42
Fig.8.TumorgrowthinhibitionandincreaseinsurvivalinBALB/cmicebyPetiveria alliaceaextract.BALB/cmiceimplantedSCwith1×104TS/Acellsforfivedaysand
randomlydividedintothreegroups.Group1wastreatedwithPBS(vehicle),group 2wastreatedwith3mg/kgofdoxorubicin,andgroup3wastreatedwith250mg/kg ofP.alliaceaextract.(A)Meantumorvolume.Thegraphrepresentsthemeantumor volume(mm3)
±S.D.fromeachgroupwith8–9animalspergroup.***p<0.0001 comparedtocontrolusingStudent’sttest.(B)Kaplan–Meiersurvivalcurves rep-resenting%survivalofmicebearingsubcutaneousTS/Abreasttumors.Statistical analysiswasdoneusinglogranktest.**p<0.001,***p<0.0001comparedtocontrol.
Discussion
OurgroupaimstovalidateP.alliaceatraditionaluseforcancer treatment(Chirinos,1992;Gupta,1995;CorreaandBernal,1998). Previously,P.alliaceaextracthasbeenproventoinduce apopto-sisandtodecreasecolonycellgrowthin4T1cells(Urue˜naetal., 2008).Inaddition,hereinweshowedthatthedryextractfromP. alliaceaiscytotoxicinadose-dependentmannertohumanbreast (IC5030g/ml)andcolon(IC5088g/ml)cancercelllines. How-ever,theplantextractislesscytotoxictomurinefibroblastsand non-tumorigenicepithelialbreastcellline,whenculturedathigh xenobioticconcentration(Fig.1).Thecytotoxicityshowninbreast butnotcoloncancercellsisassociatedwiththedecreasein gly-colyticrate,giventhatthelactateproductionisreduced(Fig.2A). Although,wedidnotfindchangesinglycolyticenzymes expres-sion,thefluctuationsinratewereacuteandreversible.
Suolinnaetal.(1975)havedemonstratedthatflavonoidshaving hydroxylgroupsat3′,4′,7either3or5positions,likefisetin, lute-olinorquercetindecreaseglycolyticrateinErhlichascitestumor cells. Such behaviorhasbeenexplained by thelossofNa+–K+ -ATPaseactivitythatlowersintracellularADPandPi,requiredfor glycolyticratemaintenance(Suolinnaetal.,1975).Previouslywe haveshown,thatP.alliaceaextractcontainsflavonoidsasleridol, petiveraland4-ethylpetiveralwhichhavehydroxylgroupsat5,7 and6positionsrespectively,althoughmethoxylsubstituentsmay bepresentat5or7positions(Urue˜naetal.,2008).Thisunique flavonoidcombinationcouldberesponsibleforthelactate secre-tiondecreaseinbreastcancercelllines.
Herein, we have demonstrated a decrease in -F1-ATPase expressionandmitochondrialrespiration(basalandafterOLIGO addition)inhumanand murinebreast cancercelllinesafter P. alliaceaextracttreatment(Figs.3and4).Also,changesin maxi-mumrespirationcanbeaccountedbytheeffectofP.alliaceaextract onrespiratorycomplexesexpression(Fig.3).Inmammaliancells -F1-ATPaseexpressionisprimarilyregulatedbymechanisms con-trolledattheleveloftranslation(WillersandCuezva,2011).Hence, wesuggestthatP.alliaceaextractmightpartiallyinhibittranslation of-F1-ATPasemRNA.
Moreover, flavonoids like quercetin, kaempferol and morin affectmitochondrial ATPaseactivity(ZhengandRamirez,2000). Specifically,quercetinbindstothehydrophobicpocketbetween ␥-andTP-subunitbymeansofvanderWaalsforcesandH-bonds preventingtherotationofF1catalyticdomain(Gledhilletal.,2007). Asdiscussedabove,P.alliaceaextractcontainsseveralflavonoids havingaplanarconformationlikequercetin.Wehypothesizedthat P.alliaceaextractflavonoids behavein aquercetin-like manner suchastheonereportedbyZhengandRamirez(2000),bybinding toF1hydrophobicpocketsanddecreasingtherespirationrate.
Celldeathisaddressedtonecrosisorapoptosisdependingupon ATPlevels.StrongATPdepletion(>50%)isacommitmentto necro-siswhilehigherATPlevelsfavorsapoptosis(Leistetal.,1997).Also, wehaveestablisheda2.5-folddecreaseinATPlevelsassociatedto adeclineinglycolyticfluxandOCRin4T1cells(Fig.5).Previously, ourgrouphasreportedthatP.alliaceaextractinducesapoptotic celldeath(Hernandezetal.,2014)whereATPdepletioncouldbe implied.
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314
Glucose
Glucose 6P
PEP
Pyruvate
P. alliacea
extract
ADP ATP
TCA
OxPhos ADP
Breast cancer cell
Intracellular ATP
cell proliferation
ATP
Basal respiration
O2 consumption
F1-ATP synthase Acetyl-CoA
Lactate Lactate
Fig.9. AmodelfortheproposedmetabolicmechanismofactionofthecytotoxicextractofPetiveriaalliacea.
Invivo assay showedthat treatmentwithP. alliaceaextract twiceaweekviai.p.withadoseequivalenttotheLD50/6decreases the primary tumor growth and increases survival in the TS/A murinebreastcancermodel.TS/Aisahighlyheterogeneous mam-maryadenocarcinomaoriginatedspontaneouslyinaBALB/cmice thatdevelopsspontaneouslungmetastases andgenerates100% oftumordevelopmentafterinoculationof105cellsviaSC(Nanni etal.,1983).OurresultsshowedthatP.alliaceaextractincreases significantlythemediansurvivalofmicewithaninteresting50%of populationfreeoftumoruntil42days,suggestingthatATP deple-tioncausedbyP.alliaceatreatmentcouldarrestthetumorgrowth atthebeginningphase,astagecharacterizedbyahighlyglycolytic andmitochondrialactivity.Overallwehypothesizethatcontinuous treatmentof cancercells withP.alliaceaextract decrease -F1-ATPaseexpressionandglycolyticfluxtriggeringdiminishedATP levelsandfinallydecreasingcellproliferation.SoATPdepletionin breastcancercelllinescouldpartiallyexplainP.alliaceadryextract cytotoxicandantitumoralactivity.Aproposedmodeldisplaying themetabolismmechanismof actionoftheextract isshownin Fig.9.
Anticancerdrugsthatblockenergyproductionormimic low energyconditionrepresentanewclassofcancerdrugtherapy(Jose andRossignol,2013).Particularly,drugsaffectingmitochondrial complexes(I,IIandIV)likeVLX600anindol-1,2,4-triazinehave shownantitumoractivityincolontumorxenografts(Zhangetal., 2014).Similarly,acytotoxicaqueousextractfromScutellaria bar-batacontainingflavonoids,terpenesand alkaloidsreducesbasal respirationandglycolyticfluxinbreastcancercelllines(Chenetal., 2012).
Conclusions
HerewehavedemonstratedthatATPdepletionanddecreasein mitochondrialexpressionof-F1-ATPasecouldpartlyexplainthe P.alliaceadryextractcytotoxicactivity.Suchmechanismseems specifictoepithelialbreastcancercelllineshavingnoeffecton non-tumorigeniccounterparts.Currently,wearestudyingifATP depletionisonlyduetomitochondrialeffectsorNa+–K+-ATPase
changesareinvolvedinamechanismsimilartothedescribedby Suolinnaforhydroxylsubstitutedflavonoids.
Authorcontributions
SF,JMC, LFand JFH participate inthe studyconception and experimentsdesign.JFHandTASperformtheacquisitionofdata. JFH,CPU,MCCandTASparticipateindraftingofmanuscript.SF, MCCandJMCaccomplishthecriticalrevisionofmanuscript.All theauthorscontributetoanalysisandinterpretationofdata.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethattheyhave fol-lowed theprotocolsof theirwork centeronthe publicationof patientdata.
Right to privacy and informed consent.The authors have
obtainedthewritteninformedconsentofthepatientsorsubjects mentionedinthearticle.Thecorrespondingauthorisinpossession ofthisdocument.
Acknowledgments
J.F.Hernándezetal./RevistaBrasileiradeFarmacognosia27(2017)306–314
AdministrativodeCiencia,TecnologíaeInnovación“COLCIENCIAS” (120348925341)andPUJ(ID00004753)fortheirfinancialsupport. SomeexperimentsweresupportedbyMinisteriodeEconomíay Competitividad(SAF2013-41945-R),Spain.LFwasfinancially sup-portedbyAsociaciónEspa˜nolaContraelCáncer(AECC),Spain.PhD studentsJFHandTASwerefinanciallysupportedbyCOLCIENCIAS.
References
Acebo,P.,Giner,D.,Calvo,P.,Blanco-Rivero,A.,Ortega,Á.D.,Fernández,P.L., Ron-cador,G.,Fernández-Malavé, E., Chamorro,M., Cuezva,J.M., 2009. Cancer abolishesthetissuetype-specificdifferencesinthephenotypeofenergetic metabolism.Transl.Oncol.2,138–145.
Chen,V.,Staub,R.E.,Fong,S.,Tagliaferri,M.,Cohen,I.,Shtivelman,E.,2012.Bezielle selectivelytargetsmitochondriaofcancercellstoinhibitglycolysisandOXPHOS. PLoSONE7,e30300.
Chirinos,D.N.,1992.Elmilagrodelosvegetales:Petiveriaalliacea,3rded.Bienes Lacónica,Caracas,Venezuela.
Ciavardelli,D.,Rossi,C.,Barcaroli,D.,Volpe,S.,Consalvo,A.,Zucchelli,M.,DeCola, A.,Scavo,E.,Carollo,R.,D’Agostino,D.,Forli,F.,D’Aguanno,S.,Todaro,M.,Stassi, G.,DiIlio,C.,DeLaurenzi,V.,Urbani,A.,2014.Breastcancerstemcellsrely onfermentativeglycolysisandaresensitiveto2-deoxyglucosetreatment.Cell DeathDis.5,e1336.
Cifuentes,C.,Casta˜neda,D.,Urue˜na,C.,Fiorentino,S.,2009.AfractionfromPetiveria alliaceainducesapoptosisviaamitochondriadependentpathwayandregulates Hsp70expression.UniversitasSci.14,125–134.
Correa,J.,Bernal,H.,1998.EspeciesVegetalesPromisoriasdePaisesdelconvenio AndrésBello,Bogotá,Colombia.
Cuezva,J.,Krajewska,M.,deHeredia,M.,Krajewski,S.,Santamaria,G.,Kim,H., Zapata,J.,Marusawa,H.,Chamorro,M.,Reed,J.,2002.Thebioenergeticsignature ofcancer:amarkeroftumorprogression.CancerRes.62,6674–6681.
Cuezva,J.M.,Ortega,A.,Willers,I.,Sanchez-Cenizo,L.,Aldea,M.,Sanchez-Arago, M.,2009.Thetumorsuppressorfunctionofmitochondria:translationintothe clinics.Biochim.Biophys.Acta1792,1145–1158.
Fantin,V.,St-Pierre,J.,Leder,P.,2006.AttenuationofLDH-Aexpressionuncovers alinkbetweenglycolysis,mitochondrialphysiology,andtumormaintenance. CancerCell9,425–434.
Formentini,L.,Martínez-Reyes,I.,Cuezva,J.,2010.Themitochondrialbioenergetic capacityofcarcinomas.IUBMBLife62,554–560.
Garcia-Barriga,H.,1974.FloraMedicinaldeColombia.InstitutodeCiencias Natu-rales.UniversidadNacional,Bogotá,Colombia.
Gledhill,J.,Montgomery,M.,Leslie, A.,Walker,J.,2007.Mechanismof inhibi-tionofbovineF1-ATPasebyresveratrolandrelatedpolyphenols.PNAS104, 13632–13637.
Gong,C.,Bauvy,C.,Tonelli,G.,Yue,W.,Delomenie,C.,Nicolas,V.,Zhu,Y.,Domergue, V.,Marin-Esteban,V.,Tharinger,H.,Delbos,L.,Gary-Gouy,H.,Morel,A.,Ghavami, S.,Song,E.,Codogno,P.,Mehrpour,M.,2013.Beclin1andautophagyarerequired forthetumorigenicityofbreastcancerstem-like/progenitorcells.Oncogene32, 2261–2272.
Govindarajan,B.,Sligh,J.,Vincent,B.,Li,M.,Canter,J.,Nickoloff,B.,Rodenburg,R., Smeitink,J.,Oberley,L.,Zhang,Y.,Slingerland,J.,Arnold,R.,Lambeth,J.,Cohen,C., Hilenski,L.,Griendling,K.,Martinez-Diez,M.,Cuezva,J.,Arbiser,J.,2007. Overex-pressionofAktconvertsradialgrowthmelanomatoverticalgrowthmelanoma. J.Clin.Invest.117,719–729.
Gupta,M.,1995.270PlantasmedicinalesIberoamericanas.ConvenioAndrésBello. ConvenioAndresBelloySubprogramaXdelCYTED,Bogotá,Colombia.
Hernandez,J.,Urue˜na,C.,Cifuentes,C.,Sandoval,T.,Pombo,L.,Castaneda,D.,Asea, A.,Fiorentino,S.,2014.APetiveriaalliaceastandardizedfractioninducesbreast adenocarcinomacelldeathbymodulatingglycolyticmetabolism.J. Ethnophar-macol.153,641–649.
Jose,C.,Rossignol,R.,2013.Rationaleformitochondria-targetingstrategiesincancer bioenergetictherapies.Int.J.Biochem.CellBiol.45,123–129.
Koukourakis,M.,Kontomanolis,E.,Giatromanolaki,A.,Sivridis,E.,Liberis,V.,2008.
SerumandtissueLDHlevelsinpatientswithbreast/gynaecologicalcancerand benigndiseases.Gynecol.Obstet.Invest.67,162–168.
Leist,M., Single, B.,Castoldi, A.S., Kuhnle,S., Nicotera, P., 1997. Intracellular adenosinetriphosphate(ATP)concentration:aswitchinthedecisionbetween apoptosisandnecrosis.J.Exp.Med.185,1481–1486.
Morales,C.,Gomez-Serranillos,M.,Iglesias,I.,Villar,A.,Caceres,A.,2001.Preliminary screeningoffiveethnomedicinalplantsofGuatemala.Farmaco56,523–526.
Nanni,P.,deGiovanni,C.,Lollini,P.,Nicoletti,G.,Prodi,G.,1983.TS/A:anew metas-tasizingcelllinefromaBALB/cspontaneousmammaryadenocarcinoma.Clin. Exp.Metastasis1,373–380.
Pampaloni,F.,Reynaud,E.,Stelzer,E.,2007.Thethirddimensionbridgesthegap betweencellcultureandlivetissue.Nat.Rev.Mol.CellBiol.8,839–845.
Papandreou, I., Goliasova, T., Denko, N., 2011. Anticancer drugs that tar-getmetabolism: isdichloroacetate thenewparadigm? Int.J. Cancer 128, 1001–1008.
Rossi,V.,1990.AntiproliferativeeffectsofPetiveriaalliaceaonseveraltumorallines. Pharmacol.Res.22,434.
Rossi,V.,Marini,S.,Jovicevic,L.,D’Atri,S.,Turri,M.,Giardina,B.,1993.Effects ofPetiveriaalliaceaL.oncellimmunity.Pharmacol.Res.27(Supplement1), 111–112.
Stock,D.,Leslie,A.,Walker,J.,1999.Moleculararchitectureoftherotarymotorin ATPsynthase.Science286,1700–1705.
Suolinna,E.,Buchsbaum,R.,Racker,E.,1975.Theeffectofflavonoidsonaerobic glycolysisandgrowthoftumorcells.CancerRes.35,1865–1872.
Urue˜na,C.,Cifuentes,C.,Castaneda,D.,Arango,A.,Kaur,P.,Asea,A.,Fiorentino, S., 2008. Petiveria alliacea extracts uses multiple mechanisms to inhibit growthofhumanandmousetumoralcells.BMCComplement.Altern.Med.8,
http://dx.doi.org/10.1186/1472-6882-8-60.
Urue˜na, C., Mancipe, J., Hernandez, J., Castaneda, D., Pombo, L., Gomez, Asea,A., Fiorentino,S., 2013. Gallotannin-richCaesalpinia spinosa fraction decreases the primary tumor and factors associated with poor progno-sisin amurinebreast cancermodel. BMC Complement.Altern.Med.13,
http://dx.doi.org/10.1186/1472-6882-13-74.
Willers,I.,Cuezva,J.,2011.Post-transcriptionalregulationofthemitochondrial H+ATPsynthase:akeyregulatorofthemetabolicphenotypeincancer.Biochim. Biophys.Acta1807,543–551.
Zhang,X.,Fryknäs,M.,Hernlund,E.,Fayad,W.,DeMilito,A.,Olofsson,M.,Gogvadze, V.,Dang,L.,Påhlman,S.,Schughart,L.,2014.Inductionofmitochondrial dys-functionasastrategyfortargetingtumourcellsinmetabolicallycompromised microenvironments.Nat.Commun.5,http://dx.doi.org/10.1038/ncomms4295. Zheng, J., Ramirez, V., 2000. Inhibition of mitochondrial proton F0F1-ATPase/ATPsynthasebypolyphenolicphytochemicals.Br.J.Pharmacol.130, 1115–1123.