T
h e f a m i l y A r a c e a e i s m o s t abundantly represented by the genus Homalomena which comprises approximately 120 species. Most of theHomalomena species are distributed in South and Central America, the Mexican lowlands, the Caribbean islands, southern Florida and the Asian tropical areas (Hay & Herscovitch, 2002; Bown, 2000). Most of the Homolomena species are found in tropical climates. However, many aroids, including Homalomena
species, are native to temperate climates (Mayo et al., 1997). Species of genus
Homalomena have been used for traditional medicinal purposes. H.
coerulescens was used to treat various kinds of skin diseases. H. grifflthii
was found to accelerate childbirth and relieve backache. H. purpurascens was used to relieve hoarseness while H. sagillifolia was reported to treat fever and abdominal flatulence. H. occulta
was commonly used to relieve pain and edema due to traumatic injury (Sulaiman & Mansor, 2002; Bensky & Gamble, 1993). The oil extracted from the rhizome of H. aromatica showed significant antifungal activity against Fusarium graminearum (Singh et al., 2000). The natives of Papua New Guinea eat the leaves of Homalomena
species combined with the leaves and bark of Galbulimima belgraviana as a narcotic (Schultes, 1970). A few species, such as H. philippinensis and H. rubescens are used as ornamental plants.
Homalomena pineodora, a dwarf and creeping aroid plant of about 25 cm in height, was found in Peninsular Malaysia and described as a new species by Sulaiman & Boyce (2005). Leaves of H. pineodora are dark green in color, heart shaped with smooth and glossy appearance and clustered towards the shoot tip. H. pineodora was recommended as an ornamental plant because of its impressive large
Malaysia. Horticultura Brasileira 30: 39-43.
Micropropagation of
Homalomena pineodora
Sulaiman & Boyce
(Araceae): a new species from Malaysia
Christine Stanly; Arvind Bhatt*; Baharuddin Sulaiman; Chan Lai Keng
School of Biological Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia; drbhatt@rediffmail.com; *Corresponding author
ABSTRACT
Homalomena pineodora (family Araceae) is a species found to have impressive foliage characteristics which remain evergreen throughout the year. Therefore, H. pineodora can be grown as an ornamental plant. Generally H.pineodora needs 3-5 years to propagate and multiply. However, the demand for new ornamental plants is increasing worldwide and the quality of planting material
is a basic need for boosting productivity. Therefore an efficient
micropropagation protocol for large-scale production of H.pineodora was developed. In vitro shoot cultures were initiated from the rhizomatous buds on MS basal medium. The best conditions for propagating H.pineodora was found to be MS medium supplemented with 3% sucrose and 0.5 mg L-1 BA (6-benzyladenine) under 24 h
of cool fluorescent light which produced an average of 3.8 shoot
per explant. Presence of an auxin was not necessary for plantlet production. Liquid MS medium supplemented with 0.5 mg L-1 BA, enhanced the shoot production of H.pineodora as compared to agar-gelled medium with same composition. All the in vitro plantlets of H. pineodora were successfully acclimatized with 100% survival
rate. Scanning electron microscopy confirmed the similarity of
leaf microstructures between the in vitro and mother plants of H. pineodora.
Keywords: Homalomena pineodora, acclimatization, scanning electron microscopy, cytokinin, in vitro culture, shoot proliferation.
RESUMO
Micropropagação de Homalomena pineodora Sulaiman & Boyce (Araceae): uma nova espécie da Malásia
Homalomena pineodora (família Araceae) é uma espécie que apresenta como característica a folhagem perene durante todo o ano. Portanto, H. pineodora pode ser cultivada como planta ornamental. Geralmente H. pineodora necessita 3-5 anos para sua propagação e multiplicação. No entanto, a demanda por novas plantas ornamentais vem aumentando no mundo e a qualidade do material de plantio é uma necessidade básica para aumentar a produtividade. Com essa
finalidade foi desenvolvido um eficiente método de micropropagação
para produção em larga escala de H. pineodora. Foram desenvolvidos cultivos in vitro de gemas dos rizomas em meio MS. As melhores condições para propagação de H. pineodora foram obtidas com o emprego do meio MS, acrescido de 3% de sacarose e 0,5 mg L-1 de BA (6-benziladenina) sob 24 h de luz fluorescente, sendo produzidas
uma média de 3,8 brotos por explante. O uso de auxina se mostrou dispensável na produção de plântulas. O uso do meio MS na forma líquida, suplementado com 0,5 mg BA L-1, foi mais eficiente na
produção de explantes de H. pineodora, em comparação com o
mesmo meio na forma de agar-gelificado. Todas as plântulas de H. pineodora foram aclimatizadas com successo, com 100% de taxa
de sobrevivência. A microscopia eletrônica de varredura confirmou
a semelhança da microestrutura de folhas das plântulas obtidas in vitro e das plantas mãe de H. pineodora.
Palavras-chave: Homalomena pineodora, aclimatização, microscopia eletrônica de varredura, citocinina, cultivo in vitro, reprodução de explantes.
leaves. This plant was found to remain evergreen throughout the year. They also observed that H.pineodora did not propagate and multiply for up to 3-5 years. The demand for new ornamental plants is increasing worldwide and the quality of planting material is a basic need for boosting productivity. If H. pineodora is to be recommended as an ornamental plant, the growers will face the problem of getting sufficient plants due to its slow propagation rate. If this plant is to be harvested from its natural habitat without control, it will lead to eventual extinction of this species. Therefore, development of an efficient micropropagation protocol was proposed in this paper for the conservation and rapid propagation of this species.
MATERIAL AND METHODS
Plant materials and culture conditions - Plants of H. pineodora were grown under greenhouse conditions at the School of Biological Sciences, Universiti Sains Malaysia, Penang.
Rhizomatous stem explants (2 cm) were excised from the mother plants and washed thoroughly with detergent and kept under running tap water for one hour. The explants were immersed in 90% ethanol for 30 secondsand rinsed with sterile distilled water. They were then surface-sterilized with 100 mg L-1 (w/v) HgCl2 solution for 15 minutes, followed by rinsing three times with sterile distilled water. They were again surface-sterilized with 15% Clorox®
(containing 5.3 % sodium hypochlorite, NaClO) solution added with 2-3 drops of Tween-20 for another 10 minutes and again rinsed three times with sterile distilled water. The surface-sterilized rhizomatous stems were trimmed to remove the damaged and dead tissue and cut into small pieces, each piece containing one bud. The explants were inoculated into 350 mL glass jars containing Murashige & Skoog (MS) medium (1962), supplemented with 30 g L-1 sucrose and 0.8% agar (Algas, Chile) for four weeks. The pH of the medium was adjusted to 5.7-5.8 prior to autoclaving at 121°C at 1.06 kg cm-2
for 11 minutes. All the cultures were
maintained at 25±2°C in a culture room with continuous lighting provided with cool, white fluorescent tubes at 35 µmol m-2 s-1.
Effect of 6-benzylaminopurine
(BA) on shoot proliferation of H.
pineodora- In order to determine the effect of BA on shoot proliferation of
H. pineodora, the aseptic shoot explants were inoculated into 350 mL glass jars containing MS medium supplemented with different concentrations of BA (0.0-10 mg L-1) in a complete randomized
design. Six replicates were used for each concentration of BA and the experiment was repeated twice. Number of shoots produced from each explant was recorded after 4, 8 and 12 weeks of culture.
Since the result obtained indicated that the addition of 2 mg L-1 BA was
sufficient to induce multiple shoot formation from the bud explants, another experiment was carried out to effect the low concentration of BA on shoot proliferation. The shoot explants were inoculated in 350 mL glass jars containing MS medium supplemented with different concentrations of BA (0.0-2.0 mg L-1) in a complete randomized
design for 4 weeks.
Effect of combination of BA and 3-indole butyric acid (IBA) on shoot
growth and rooting - The multiple
shoots were separated into single shoots and were used as explants. The shoot explants were inoculated in 350 mL glass jars containing MS medium supplemented with 0.5 mg L-1 BA and different concentrations
of IBA (0.0-2 mg L-1) in a complete
randomized design. Six replicates were used for each combination treatment and the experiment was repeated twice. Parameters, such as average shoot height, number of shoots, leaves and roots produced from each explant, were recorded after 4 weeks of culture.
Comparation of the efficiency of liquid and gelled medium for shoot
proliferation - The shoot explants were
inoculated in a 100 mL Erlenmeyer flask containing MS medium supplemented with 0.5 mg L-1 BA with or without
the addition of a gelling agent. Each flask contained 3 shoot explants and six vessels were used for each medium
type. The experiment was repeated twice. Average shoot height, number of shoots, leaves and roots produced from each explant were recorded after 4 weeks of culture. The obtained data were analyzed using student t-test at p≤0.05 with SPSS version 13.
Acclimatization - Four-week old
in vitro plantlets of H. pineodora with shoot height of 3-4 cm, 3-4 roots and at least 2-3 leaves were selected for the acclimatization process. The in vitro
plantlets (n=50) were removed from the culture vessels, washed thoroughly with tap water and transplanted into plastic pots (25 x 25 cm) containing a mixture of organic soil and sand (1:1). They were placed under greenhouse conditions with relative humidity of 80-90%, temperature of 28±2ºC during day and 24±2ºC during night time. The plantlets were watered twice a day (morning and evening) with tap water. Shoot height was measured from the base of the plant to the tip of the leaves and the percentage of surviving plants was recorded after four weeks of growing in the plant house.
Comparison of leaf morphology
of mother and in vitro clone - For
scanning electron microscopy (SEM), healthy and mature leaves of H. pineodora were collected from in vitro
as well as from mother plants of H. pineodora. Leaf segments were secured onto brass stubs with carbon conductive tape and the observations on leaf surface morphology were carried out using a Leo Supra 50 VP Field Emmision Scanning Electron Microscope (Carl-Zeiss SMT, Oberkochen, Germany) operated at 12 kV, with a working distance of 15 mm.
RESULTS AND DISCUSSION
Only 50% aseptic rhizomatous stems of H. pineodora was established using
HgCl2 and Clorox® surface-sterilization
technique. This method was found to be more effective for the establishment of aseptic explants of other plant species such as Zingiberaceae species (Chan & Thong, 2004), banana (Kulkarni et
al., 2006), Cymbopogon nardus (Chan
et al., 2005) and Maranta leuconeura
2000). After four weeks of culture on the MS basal medium, the axillary buds remained green in color while rhizomatous stem tissues turned brown and necrotic. The axillary bud explants were subcultured into fresh MS medium every four weeks for culture stabilization and uniform shoot production and subsequently used for further studies.
Preliminary results revealed that MS medium, supplemented with 2.0 -10.0 mg L-1 BA, did not show any significant
difference in the shoot multiplication rate of H. pineodora. MS medium supplemented with as low as 2.0 mg L-1 BA was sufficient to induce multiple
shoot formation and the number of shoots produced was not found to be significantly different from MS medium supplemented with 4–10 mg L-1 BA. An
average of 2.8-3.2 shoots was formed from each shoot explant on MS medium,
supplemented with 2.0 -10.0 mg L-1 BA
after four weeks of culture. Maintaining the shoots on the same culture media for 12 weeks did not show much increase in multiple shoots formation (Table 1). However the in vitro shoots of H. pineodora, cultured on MS medium supplemented with 10.0 mg L-1 BA,
turned pale green in color after 12 weeks of culture. This might indicate that high concentration of BA was detrimental to the shoot cultures of H. pineodora.
Subsequently, shoot explants of H. pineodora were cultured in MS medium, supplemented with lower concentration of BA (0.5-2.0 mg L-1). Shoot explants
that were cultured on MS medium, supplemented with 0.5 mg L-1 BA, produced significantly higher number of shoots (3.8 shoots per explant) as compared to other concentrations of BA (1.0-2.0 mg L-1) added into the culture
medium. MS medium, supplemented with low concentration of BA (0.5 mg L-1), also induced significantly higher
root length as compared to those shoots cultured on other concentrations of BA. However, shoot explants cultured on MS medium devoid of plant growth regulators, produced less shoots with the longest roots. There was no significant difference in the shoot height, number of roots and number of leaves produced by the shoots explants cultured on MS medium supplemented with different concentration of BA (Table 2).
When the shoot explants were cultured on MS medium supplemented with 0.5 mg L-1 BA in combination with
different concentrations of IBA (0.0-2.0 mg L-1), there was no significant
difference in shoot height, and the number of shoots, roots, and leaves produced from each shoot. The presence of higher concentration of IBA (1.0–2.0 mg L-1) had significantly reduced the
root length (Table 3). This indicated that IBA was not necessary and the addition of only low concentration of BA (0.5 mg L-1) was sufficient to promote shoot and
roots multiplication of H. pineodora. Thus MS medium, supplemented with 0.5 mg L-1 BA, was chosen as
the optimum proliferation medium for production of H. pineodora plantlets.
Result indicated that liquid MS medium, supplemented with 0.5 mg L-1 BA, induced a significantly higher
number of shoots as compared to the agar-gelled MS medium with same composition. The shoots also produced longer roots in liquid medium, as compared to the solid medium. However, there was no significant difference in Table 1. Effect of Murashige & Skoog medium (MS) supplemented with BA (0.0-10.0
mg L-1) on the shoot proliferation of H. pineodora explants over a duration of 12 weeks of
culture (efeito do meio Murashige e Skoog (MS) suplementado com BA (0,0-10,0 mg L-1) na
produção de explantes de H. pineodora durante 12 semanas de cultivo). Malaysia, USM, 2010.
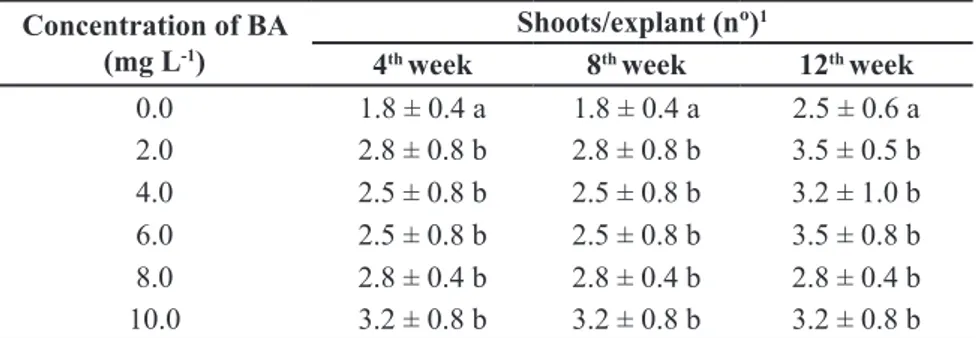
Concentration of BA (mg L-1)
Shoots/explant (nº)1
4th week 8th week 12th week
0.0 1.8 ± 0.4 a 1.8 ± 0.4 a 2.5 ± 0.6 a
2.0 2.8 ± 0.8 b 2.8 ± 0.8 b 3.5 ± 0.5 b
4.0 2.5 ± 0.8 b 2.5 ± 0.8 b 3.2 ± 1.0 b
6.0 2.5 ± 0.8 b 2.5 ± 0.8 b 3.5 ± 0.8 b
8.0 2.8 ± 0.4 b 2.8 ± 0.4 b 2.8 ± 0.4 b
10.0 3.2 ± 0.8 b 3.2 ± 0.8 b 3.2 ± 0.8 b
Mean values ± SD (standard deviation) within the same column followed by same letter are
not significantly different (Tukey test, p≤0.05) (valores médios ±SD (desvio padrão) dentro da mesma coluna seguidos de mesma letra não são significativamente diferentes (Tukey, p≤0,05)).
Table 2. Effect of Murashige & Skoog medium (MS) supplemented with BA (0.0-2.0 mg L-1) on shoot proliferation, shoot growth and root
production of H. pineodora after four weeks of culture (efeito do meio Murashige & Skoog (MS) suplementado com BA (0,0-2,0 mg L-1)
sobre a proliferação e crescimento de ramos e raizes de H. pineodora após quatro semanas de cultura). Malaysia, USM, 2010.
Concentration of
BA (mg L-1)
Shoots/explant (nº)
Shoot height (cm)
Leaves/explant (nº)
Roots/explant (nº)
Length of roots (cm)
0.0 1.8 ± 0.4 a 4.6 ± 0.5 a 2.3 ± 0.5 a 5.6 ± 1.0 a 1.5 ± 0.6 b
0.5 3.8 ± 0.5 c 4.8 ± 0.3 a 2.5 ± 0.5 a 5.0 ± 1.3 a 1.2 ± 0.3 b
1.0 2.5 ± 0.3 b 4.8 ± 0.5 a 2.3 ± 0.5 a 4.8 ± 1.7 a 0.8 ± 0.2 a
1.5 2.8 ± 0.6 b 4.9 ± 0.5 a 2.3 ± 0.5 a 5.2 ± 1.2 a 0.8 ± 0.1 a
2.0 2.8 ± 0.5 b 4.7 ± 0.2 a 2.3 ± 0.5 a 4.7 ± 1.2 a 0.8 ± 0.2 a
the shoot length, number of roots and the number of leaves produced by H. pineodora produced in solid as well as liquid proliferation medium (Table 4). The in vitro plantlets, produced either in the solid and liquid media were normal and healthy. More shoots produced in liquid medium than agar-gelled medium were also observed in other plant species such as zingiberaceae species
(Chan & Thong, 2004), Centaurium erythraea (Piatczak et al., 2005) and
Eucalyptus hybrid (Whitehouse et al., 2002). They explained that the high rate of proliferation of shoots in liquid medium was due to the fact that the shoots were completely submerged in the liquid medium, presenting a large surface area for the uptake of nutrients and plant growth regulators.
After 4 weeks of acclimatization in the greenhouse, all the in vitro
plantlets (100%) survived. The tissue cultured plantlets of H. pineodora were morphologically similar to their mother plants. The four week old acclimatized plantlets were found to be fairly uniform with an average height of 6.7±0.5 cm.
There was no variation in leaf morphology of the in vitro plantlets Table 3. Effect of Murashige & Skoog medium (MS) supplemented with BA (0.5 mg L-1) and IBA (0.0-2.0 mg L-1) on the shoot proliferation,
shoot growth and root production of H. pineodora after four weeks of culture (efeito do meio Murashige & Skoog (MS) suplementado com BA (0,5 mg L-1) e IBA (0,0-2,0 mg L-1) sobre a proliferação e crescimento de ramos e raizes de H. pineodora após quatro semanas de
cultura). Malaysia, USM, 2010.
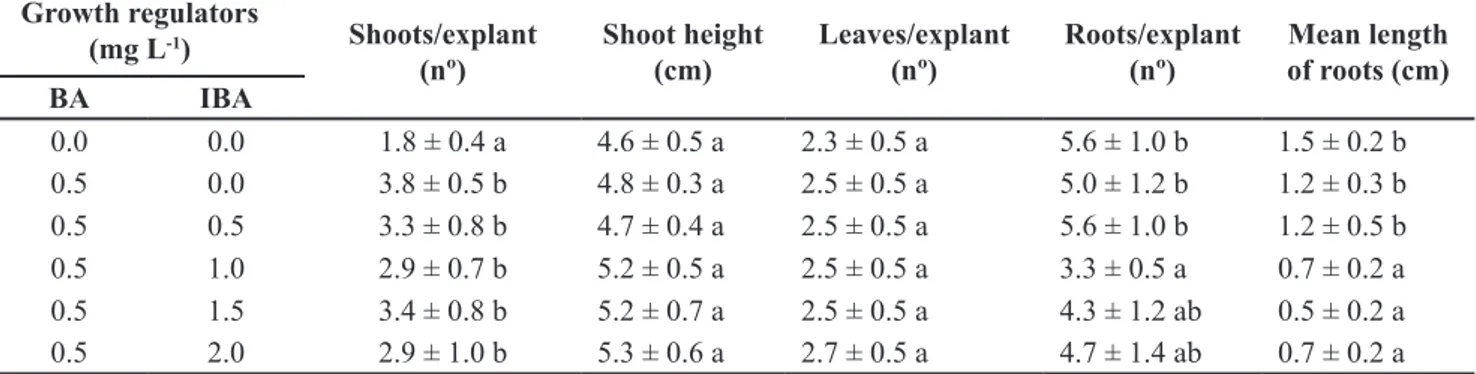
Growth regulators
(mg L-1) Shoots/explant
(nº)
Shoot height (cm)
Leaves/explant (nº)
Roots/explant (nº)
Mean length of roots (cm)
BA IBA
0.0 0.0 1.8 ± 0.4 a 4.6 ± 0.5 a 2.3 ± 0.5 a 5.6 ± 1.0 b 1.5 ± 0.2 b
0.5 0.0 3.8 ± 0.5 b 4.8 ± 0.3 a 2.5 ± 0.5 a 5.0 ± 1.2 b 1.2 ± 0.3 b
0.5 0.5 3.3 ± 0.8 b 4.7 ± 0.4 a 2.5 ± 0.5 a 5.6 ± 1.0 b 1.2 ± 0.5 b
0.5 1.0 2.9 ± 0.7 b 5.2 ± 0.5 a 2.5 ± 0.5 a 3.3 ± 0.5 a 0.7 ± 0.2 a
0.5 1.5 3.4 ± 0.8 b 5.2 ± 0.7 a 2.5 ± 0.5 a 4.3 ± 1.2 ab 0.5 ± 0.2 a
0.5 2.0 2.9 ± 1.0 b 5.3 ± 0.6 a 2.7 ± 0.5 a 4.7 ± 1.4 ab 0.7 ± 0.2 a
Mean values ± SD (standard deviation) within the same column followed by same letter are not significantly different (Tukey test, p≤0.05) (valores médios ±SD (desvio padrão) dentro da mesma coluna seguidos de mesma letra não são significativamente diferentes (Tukey, p≤0,05)).
Table 4. Effect of culture medium type on the shoot proliferation, shoot growth and root production of H. pineodora after 4 weeks of culture (efeito do tipo de meio de cultura na proliferação de ramos, crescimento de brotos e produção de raizes de H. pineodora após 4 semanas de cultura). Malaysia, USM, 2010.
Type of medium
Shoots/explant (nº)
Shoot height (cm)
Leaves/explant (nº)
Roots/explant (nº)
Length of roots (cm)
Solid 3.3 ± 1.0 a 4.8 ± 0.3a 2.5 ± 0.5 a 5.0 ± 1.3 a 1.2 ± 0.2 a
Liquid 5.9 ± 0.2 b 4.7 ± 0.3a 2.7 ± 0.5 a 5.2 ± 0.7 a 1.7 ± 0.1 b
Mean values ± SD (standard deviation) within the same column followed by same letter are not significantly different (Tukey test, p≤0.05) (valores médios ±SD (desvio padrão) dentro da mesma coluna seguidos de mesma letra não são significativamente diferentes (Tukey, p≤0,05)).
Figure 1. Micropropagation of H. pineodora (A) in vitro plant of H. pineodora in MS medium supplemented with 0.5 mg/L BA after four weeks of culture. (B) multiple shoots of H. pineodorain vitro plantlet and (C) mother plant (micropropagação de H. pineodora (A) in vitro das plantas de H. pineodora em meio MS acrescido de 0,5 mg/L de BA após quatro semanas de cultura. (B) de brotações múltiplas de H. pineodora in vitro das plântulas e (C) planta-mãe). Malaysia, USM, 2010.
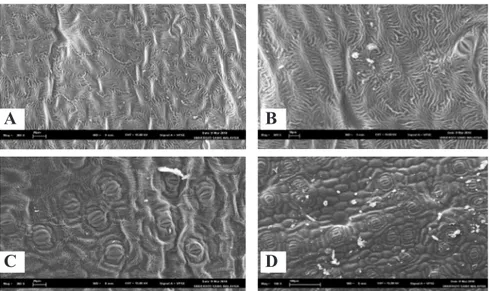
as well as the mother plants of H. pineodora when observed under the scanning electron microscope. The adaxial leaf surface has a distinctive patterned or ornamented cuticle in the form of striae (Figure 1A). Also, the striae are in the form of deep ridges sometimes forming a rosette pattern in certain areas of the leaf (Figure 1B). These ridges could probably allow the run off of water droplets which increased water repellency and reduced the adhesion of the contaminating particles on the leaf surface. This phenomenon was called the ‘lotus’ effect (Barthlott & Neinhuis, 1997). Stomata were in level with the epidermisand occurred on both leaf surfaces. It appeared that the density of stomata was lower on adaxial leaf surface as compared to the abaxial leaf surface. Both leaf surfaces showed the presence of stomata with well developed guard cells (Figures 1C, 1D). The stomata regulate the gases exchange and water vapor between the outside air and the interior of the leaf. Typically, the stomata are more numerous over the abaxial (lower) epidermis of the leaf than the (adaxial) upper epidermis. The similar trends of stomata distribution was reported in
Persea americana (Blanke & Lovatt, 1993). The lack of abundant stomata on the adaxial surface could be correlated with low gas-exchange rates on abaxial surfaces. The dissimilarity in the adaxial and abaxial leaf surface morphology could be an interesting feature and can also be used as a marker for the identification of H. pineodora.
ACKNOWLEDGEMENT
The authors are thankful to Mr. PC Boyce for providing the plant material and School of Biological Sciences, Universiti Sains Malaysia for providing the facilities and support.
Christine Stanly would like to thank Universiti Sains Malaysia for providing USM Fellowship Scheme for the present study.
REFERENCES
BARTHLOTT W; NIENHUIS C. 1997. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202: 1-8. BENSKY D; GAMBLE A. 1993. Chinese herbal
medicine: Materia Medica (Revised edition). Eastland Press, Seatle, Washington. BLANKE MM; LOVATT CJ. 1993. Anatomy and
transpiration of the avocado inflorescence.
Annals of Botany 71: 543-547.
BOWN D. 2000. Aroids: Plants of the Arum Family [Illustrated]. Timber Press.
CHAN LK; DEWI PR, BOEY PL. 2005. Effect of plant growth regulators and regeneration of plantlets from bud cultures of Cymbopogon nardus L. and the detection of essential oils from the in vitro plantlets. Journal of Plant Biology 48: 142-146.
CHAN LK; THONG WH. 2004. In vitro propagation of Zingiberaceae species with medicinal properties. Journal of Plant Biotechnology 6: 181-188.
EBRAHIM MHK. IBRAHIM IA. 2000. Influence
of medium solidification and pH value on
in vitro propagation of Maranta leuconeura cv. Kerchoviana. Scientia Horticulturae 86: 211-221.
HAY A; HERSCOVITCH C. 2002. Two
remarkable new West Malesian Homalomena
(Araceae) Species. Garden’s Bulletin
Singapore 54: 171-178.
KULKARNI VM; SUPRASANNA P; BAPAT VA. 2006. Plant regeneration through multiple shoot formation and somatic embryogenesis in a commercially important and endangered Indian banana cv. Rajeli. Current Science 90: 842- 846.
MAYO SJ; BOGNER J; BOYCE PC. 1997. The genera of Araceae. Royal Botanic Gardens, Kew.
MURASHIGE T; SKOOG F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue culture. Plant Physiology 15: 473-497.
PIATCZAK E; WIELANEK M; WYSOKINSKA H. 2005. Liquid culture system for shoot multiplication and secoiridoid production in micropropagated plants of Centaurium erythraea Rafn. Plant Science 168: 431-437. SCHULTES RE. 1970. The botanical and
chemical distribution of hallucinogens. Annual Review of Plant Physiology 21: 571-598.
SINGH G; KAPOOR IPS; SINGH O; RAO GP; PRASAD YR; LECLERCQ PA; KLINKBY N. 2000. Studies on essential oils. Part. 28: chemical composition, antifungal and insecticidal activities of rhizome volatile oil of Homalomena aromatica (Schott.). Flavour and Fragrance Journal 15: 278-280. SULAIMAN B; BOYCE PC. 2005. A remarkable
new species of Homolomena (Araceae: homolomeneae) from Peninsular Malaysia. Garden’s Bulletin Singapre. 57: 7-11.
SULAIMAN B; MANSOR M. 2002. Medicinal
Aroids conservation: A case study of floral
garden, School of Biological Sciences, Universiti Sains Malaysia. Proceedings of the 4th IMT-GT UNINET Conference.
WHITEHOUSE AB; MARKS TR; EDWARDS GA. 2002. Control of hyperhydricity in Eucalyptus axillary shoot cultures in liquid medium. Plant Cell Tissue and Organ Culture 71: 245–252.
A B
C D
Figure 2. Scanning Electron Microscopy (SEM) micrographs showing leaf surface morphology of H. pineodora. The adaxial leaf surface showing distinctive patterned or ornamented cuticle in the form of striae (A); The striae in the form of deep ridges forming a rosette pattern (B). Distribution of stomata with well developed guard cells on adaxial leaf surface (C) abaxial leaf surface (D) (microscopia eletrônica de varredura (SEM) micrografias mostrando a morfologia da superfície foliar de H. pineodora. A face inferior das folhas mostra cutícula ou modelado ornamentado distinto, sob a forma de estrias (A); As estrias sob a forma de riscas profundas que formam um padrão de roseta (B). Distribuição de estômatos com células guarda bem desenvolvidas na face inferior das folhas (C) face abaxial (D)). Malaysia, USM, 2010.