Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor
Cell Transplants Improve Recovery after Cervical Spinal
Cord Injury
J
ASONS
HARP, J
ENNIFERF
RAME, M
ONICAS
IEGENTHALER, G
ABRIELN
ISTOR, H
ANSS. K
EIRSTEADReeve-Irvine Research Center, Sue and Bill Gross Stem Cell Research Center, Department of Anatomy &
Neurobiology, School of Medicine, 2111 Gillespie Neuroscience Research Facility, University of California
at Irvine, Irvine, California 92697-4292
Key Words. Spinal cord injury•Human embryonic stem cells•Forelimb•Pathogenesis•Neuroprotection
A
BSTRACTEvidence that cell transplants can improve recovery out-comes in spinal cord injury (SCI) models substantiates treatment strategies involving cell replacement for humans with SCI. Most pre-clinical studies of cell replacement in SCI examine thoracic injury models. However, as most human injuries occur at the cervical level, it is critical to assess potential treatments in cervical injury models and examine their effectiveness using at-level histological and functional measures. To directly address cervical SCI, we used a C5 midline contusion injury model and assessed the efficacy of a candidate therapeutic for thoracic SCI in this cervical model. The contusion generates reproducible, bilateral movement and histological deficits, although a number of injury parameters such as acute severity of injury, affected gray-to-white matter ratio, extent of
en-dogenous remyelination, and at-level locomotion deficits do not correspond with these parameters in thoracic SCI. On the basis of reported benefits in thoracic SCI, we transplanted human embryonic stem cell (hESC)-derived oligodendrocyte progenitor cells (OPCs) into this cervical model. hESC-derived OPC transplants attenuated lesion pathogenesis and improved recovery of forelimb function. Histological effects of transplantation included robust white and gray matter sparing at the injury epicenter and, in particular, preservation of motor neurons that corre-lated with movement recovery. These findings further our understanding of the histopathology and functional out-comes of cervical SCI, define potential therapeutic targets, and support the use of these cells as a treatment for cervi-cal SCI.STEMCELLS2010;28:152–163
Disclosure of potential conflicts of interest is found at the end of this article.
I
NTRODUCTIONThe pathogenesis of contusive spinal cord injury (SCI) involves a complex process that begins with cord compres-sion, immediate axon and cell damage, hemorrhage, and hy-poperfusion [1]. This trauma initiates a secondary degenera-tive cascade that exacerbates the loss of neurons, oligodendrocytes, and myelin, as well as axons [2, 3]. Con-comitant to these secondary processes and cell loss are inflammation and immune responses [4], the proliferation of progenitor cells [5–7], migration of astrocytes to the injury [8], and gliosis and cyst formation [9, 10]. Thus, treatment of this multifactorial injury will likely require a combination therapy capable of addressing disparate injury components.
More than half of all human spinal cord injuries occur at the cervical level, with C4-C6 injuries accounting for nearly 40% of all cases [11]. As in thoracic injuries, cervical injuries
interrupt axonal tract conduction between the cranial and cau-dal central nervous system. Notable differences in cervical relative to thoracic cord include enlarged neuronal pools, proximity of descending axons to their cell bodies, and at-level neuronal mediation of limb movement. In persons with SCI, injury level changes the priorities of recovery targets between the tetraplegic population and the paraplegic popula-tion, most notably, that recovery of hand and arm function is ranked as the priority of the tetraplegic population [12]. These differences highlight an increased clinical relevance for stud-ies of cervical SCI models that examine the direct relation of at-level histological effects and motor function. The C5-level is the most common level at which to induce bilateral [13– 15] and unilateral [16] cervical injuries. Anatomical evidence of cervical innervations indicates that, in general, C2-C5 level motor neurons project to muscles of the shoulder and proxi-mal forelimb and C6-C8 level motor neurons project to distal forelimb muscles [17]. Accordingly, injuries to C5 are
Author contributions: J.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.F.: collection and/or assembly of data, manuscript writing; M.S.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; G.N.: provision of study material or patients, collection and/or assembly of data; H.S.K.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Correspondence: Dr. Hans S. Keirstead, Ph.D., Reeve-Irvine Research Center, Sue and Bill Gross Stem Cell Research Center, Department of Anatomy and Neurobiology, 2111 Gillespie Neuroscience Research Facility, College of Medicine, University of
California at Irvine, Irvine, CA 92697-4292. Telephone: (949) 824-6213; Fax: (949) 824-5352; e-mail: hansk@uci.edu Received May
8, 2009; accepted for publication October 16, 2009; first published online inSTEMCELLSEXPRESSOctober 28, 2009.VC AlphaMed Press
1066-5099/2009/$30.00/0 doi: 10.1002/stem.245
demonstrated to affect both at-level and below-level outcomes such as locomotion and digit function, respectively. A broader understanding of the histopathology and functional outcomes of cervical SCI could hasten the identification of appropriate therapeutic targets for this injury and support the translation of potential therapeutics to the sizable cervical SCI population.
Cell replacement strategies are inherently combination therapies, as they confer phenotype-specific benefits as well as neurotrophic benefits to surrounding tissue. We have previ-ously shown that transplantation of human embryonic stem cell (hESC)-derived oligodendrocyte progenitor cells (OPCs) [18] to thoracic SCI resulted in pathotropism, cell survival and differentiation, enhanced remyelination, and improved locomotor outcomes [19] without harmful effects [20]. Other studies identified a number of neurotrophic factors and che-mokines expressed by hESC-derived OPCs and demonstrated that the secreted factors enhanced neuronal survival and neu-rite outgrowth [21, 22]. Thus, hESC-derived OPCs may con-fer benefit by pleiotropic effects such as myelinating demyeli-nated axons or providing neurotrophic support to surrounding tissue. In the present experiment, we explored the ability of hESC-derived OPCs to provide anatomical and functional benefit following transplantation into acute cervical SCIs.
M
ATERIALS ANDM
ETHODSSpinal Cord Injury
Cervical spinal cord contusion injuries were performed on female Sprague Dawley adult rats (200–220 g) as previously described [14]. A dorsal laminectomy was performed on the fifth cervical vertebra (C5) to expose the spinal cord, and the rat was sus-pended by clamps on the vertebrae cranial and caudal to the lami-nectomy. Contusion injury was induced using the Infinite Horizon Impactor (Precision Systems, Kentucky, IL, http://www.presysin.
com) with a force of 200 kDyn (n ¼ 46). After contusion, the
deep and superficial muscle layers were sutured, and the skin was closed with stainless-steel wound clips. Immediately after sur-gery, animals were given subcutaneous saline and Baytril (2.5 mg/kg/d, s.c.; Bayer, Shawnee Mission, KS, http://www.bayerus.-com) and maintained on an isothermic pad until alert and mobile. Animals received manual bladder expression twice daily and were inspected for weight loss, dehydration, and distress, with appropriate veterinary care as needed.
Derivation of OPCs from hESCs
The WA07 (H7) hESC line at passage 32 was obtained from Geron (Menlo Park, CA, http://www.geron.com). Cells were expanded in hESC growth media [23] and differentiated accord-ing to published protocols [18, 24, 25]. Briefly, dissociated colo-nies were placed into low attachment flasks in 50% hESC growth media and 50% glial restriction media (GRM) for 2 days. On day 1, the media was supplemented 4 ng/ml of basic fibroblast growth factor (FGF), 20 ng/ml of epidermal growth factor (EGF), and on
day 2, it was supplemented with EGF and 10 lM all-trans
-reti-noic acid (RA) (Sigma-Aldrich, St. Louis, http://www.sigmaal-drich.com). This media was then replaced with 100% GRM sup-plemented with EGF and RA for an additional 7 days. Cells were then exposed for 18 days to GRM/EGF without RA. At day 28, yellow spheres were plated in flasks coated with 1:30 Matrigel for 7 days. Cultures were then trypsinized, remnant spheres were excluded, and cells were replated on Matrigel and cultured for 7 days in GRM/EGF. The total time for the differentiation protocol was 42 days.
For immunocytochemistry, cells were plated on slides coated with poly-L-lysine and human laminin (Sigma-Aldrich). For
transplantation, cells were concentrated to 100,000 cells/ll.
Try-pan blue exclusion testing indicated that this preparation con-sisted of 87–98% viable cells at time of transplant.
Behavioral Testing
Forelimb movement scores were determined from videotape of animals crossing a clear Plexiglas walkway marked with 1 cm grid lines on the floor. Prior to testing, each animal was accli-mated to the apparatus and trained to cross for intermittent food reward. During test trials, animals were videotaped from under-neath (used for forelimb stride length) and from the side (used for proximal forelimb step range and passed-perpendicular step frequency) of the walkway using a Canon NTSC digital video camcorder (ZR10; Canon, Tokyo, Japan, http://www.canon.com). Videos were analyzed at the middle of a pass, frame by frame, using media player software. All behavioral tests and assessments
were conducted in a blinded manner (n¼13/group).
For forelimb stride length, animals were videotaped from below the walkway each week for weeks 1–3, and then every other week for weeks 5–9. The videos were scored for forelimb stride length as outlined for hindlimb analysis by Gonzalez et al. [26]. Briefly, forelimb stride length was defined as distance from the start of a step with the forepaw through to the end of that step with the same paw. Measurements were taken on each side for five steps and averaged for each animal.
Measures of proximal forelimb included proximal forelimb step range and passed-perpendicular step frequency. These meas-ures were selected to correspond with reported C5 motor neuron innervation of proximal forelimb by McKenna et al. [17]. These measures were recorded from videotape taken from the side view during week 9. For the step range parameter, the range of proxi-mal forelimb movement, expressed as an angle, was determined. The angle of proximal forelimb movement was defined as the inside angle of the proximal forelimb at the placement of a step to the inside angle at the lift off of that same step. Measurements were taken on the left and right sides for three steps and averaged for each animal. For the passed-perpendicular step frequency, the proximal forelimb positions at the lift-off of each of five steps were determined for the left and right sides. The position of the
forelimb at this point was compared to a line perpendicular (90)
to the floor and aligned at the shoulder of the animal. The num-ber of steps that the proximal forelimb crossed the line perpendic-ular to the floor was counted as passed-perpendicperpendic-ular. The occur-rence of steps passed-perpendicular relative to the total number of steps was averaged for each animal.
Transplantation
Both groups received cyclosporine A (20 mg/kg/d, s.c.; Bedford Laboratories, Bedford, OH, http://www.bedfordlabs.com) 1 day prior to transplantation/vehicle administration and then everyday for the duration of the study. Cell transplantation or vehicle (con-trol) administration occurred 7 days after contusion injury. Ani-mals were anesthetized as above, and the laminectomy site was re-exposed. After immobilization of the spinal process cranial to
the contusion site, a 10 ll Hamilton syringe (Hamilton, Reno,
NV, http://www.hamiltoncompany.com) was lowered into the spi-nal cord using a stereotactic manipulator arm. Cell suspensions were injected along the midline of the spinal cord at a depth of 1.2 mm into one site cranial and one site caudal to the lesion
epi-center, in a total volume of 7.5ll (1,500,000 cells) at a rate of 1
ll/minutes. Control animals received an equal volume of vehicle
only at the same injection rate. The needle was removed after 5 minutes.
hESC-Derived OPC Gene Expression Profile
Reverse transcription (RT) of mRNAs was performed using M-MLV Reverse Transcriptase (Ambion) with random hexamers and poly-dT as primers. cDNAs generated by RT were used for subsequent 30-cycle PCRs using a Mastercycler thermal cycler (Eppendorf, Westbury, NY, http://www.eppendorf.com), Platinum Taq DNA polymerase (Invitrogen), and specific primers for
TGFb1 (forward: 50-agagatacgcaggtgcaggt-30, reverse: 50-tca
gagttgcactccgaaga-30), PDGFRa (forward: 50
-tgtgtgggacattcattgct-30, reverse: 50-gggtactgccagctcacttc-30), Neuregulin1 (forward: 50
-caaagaaggcagaggcaaag-30, reverse: 50-aactggtttcacaccgaagg-30),
Neuregulin2 (forward: 50-ggagaccagagaccgcctac-30, reverse: 50
-taaaaacgcctttgccgtta-30), and Nkx2.2 (forward: 50-agccta
catttctgcgtgct-30, reverse: 50-gcctcacttggtcaattcgt-30) (Invitrogen).
PCR products were analyzed by gel electrophoresis.
Histology
Animals were killed 8 weeks after cell transplantation under pento-barbitone anesthesia by aortic perfusion with isotonic, heparinized saline followed with 4% paraformaldehyde (Fisher Scientific, Pitts-burgh, PA, http://www.fisherscientific.com) in 0.1 M phosphate buffer, pH 7.4. The spinal cord was divided into eight 1 mm blocks that extended 4 mm cranial to and 4 mm caudal to injury epicenter. Alternate blocks were processed to produce resin or cryostat sections. Resin sections were used to determine the num-ber of remyelinated axons, the gross pathology of the transplant environment, and morphometric measurements. Cryostat sections were used for immunodetection and used for immunodetection.
For resin processing, blocks were postfixed for 24 h in 4% glutaraldehyde (Fisher Scientific) and embedded in resin (Elec-tron Microscopy Sciences, Hatfield, PA, http://www.emsdiasum.
com) according to standard protocols. Transverse semithin (1lm)
sections were cut from the cranial face, stained with alkaline tolu-idine blue, coverslipped, and examined by light microscopy on an Olympus AX-80 microscope and a 2 megapixel MagnaFire digital camera using Olympus MicroSuite B3SV software (Olympus America, Melville, NY, http://www.olympusamerica.com). For electron microscopy, blocks were trimmed and sections were cut at 100 nm, mounted on copper grids, uranyl acetate and lead ci-trate stained, and viewed under a Hitachi EM 600 electron micro-scope at 75 kV.
Immunodetection
Antibody detection was performed on fixed cultured cells or cryo-sectioned spinal cord using standard protocols, as previously described [19]. Antibodies used included Oct-4 (rabbit, 1:500), O4 (mouse, 1:50), NG2 (rabbit, 1:100), Olig1 (rabbit, 1:200),
A2B5 (mouse, 1:100), PDGFRa (rabbit, 1:200), RIP (mouse,
1:200) mouse anti-human nuclei (1:30) (all from Millipore,
Temecula, CA, http://www.millipore.com), class III b-tubulin
(mouse, Tuj1; 1:200), Pax6 (rabbit, 1:100) (all from Covance Research Products, Denver, PA, http://www.covance.com), cow GFAP (rabbit, 1:500; DakoCytomation, Glostrup, Denmark, http://www.dakocytomation.com), mouse anti-APC/CC1 (1:200; Calbiochem, San Diego, CA, http://www.emdbiosciences.com), mouse anti-human CXCR4 (1:200 Abcam, Cambridge, U.K., http://www.abcam.com), rabbit anti-rat/mouse CXCL12 (eBio-science, San Diego, CA, http://www.ebioscience.com), and stage-specific embryonic antigen four (SSEA4) supernatant (mouse, 1:5; gift from Geron Corporation).
Cell and sections were imaged using an Olympus AX-80 microscope and 2 megapixel MagnaFire digital camera with the Olympus MicroSuite B3SV software. For transplant cultures, the percentage of immunopositive cells was determined by dividing the total number of immunopositive cells by the total number of Hoechst-positive cells in each imaging chamber and then averag-ing the results from three chambers per marker. To determine dis-tribution of transplanted cells, the number of human nuclei
immu-nopositive cells were counted on three sections 100 lm apart
from each tissue block for each animal and averaged; the counts within corresponding blocks from animals within a group were
then used for statistical comparison. Double labeling of cells with anti-human nuclei plus cellular differentiation markers was con-firmed using confocal microscopy (MRC 1000; Bio-Rad, Hercu-les, CA, http://www.bio-rad.com; Zeiss, Thornwood, NY, http:// www.zeiss.com). Single confocal plane images of human nuclei and phenotype markers were collected and combined to produce three-dimensional reconstruction and determine label colocaliza-tion. Cells immunopositive for Olig1 or APC/CC1 were quanti-fied from three fields within the region(s) of interest per section. The Olig1 or APC/CC1 counts were averaged from three sections for each animal, and the averages were used for statistics. Motor neuron survival was determined from the average of motor
neu-ron counts taken from five transverse sections 100 lm apart and
centered on the injury epicenter. The motor neuron counts for each section were averaged for each animal. The averaged counts were used for statistical comparison of motor neuron preservation across treatment groups.
Myelin Pathology Counts
To quantify normally myelinated, demyelinated, and oligodendro-cyte- or Schwann cell (SC)-remyelinated axons, regions of
pa-thology were located on 1lm resin sections at 40x magnification
and traced using Olympus MicroSuite B3SV software to calculate perimeter and area as previously reported [14]. Normally myelin-ated, demyelinmyelin-ated, and oligodendrocyte- or SC-remyelinated axons were counted using the line-sampling technique detailed by Blight [27]. The average number of normally myelinated, demye-linated, and oligodendrocyte- or SC-remyelinated axons within
five 25lm225lm2areas along the radial line yielded an
esti-mate of the total number of axons within a region of pathology and is calculated as the number of axons per square millimeter. At least 10% of the area of pathology is used to determine total axons for each area of pathology. The number of oligodendro-cyte-remyelinated, SC-remyelinated, and demyelinated axons was used to determine the oligodendrocyte-remyelinated efficiency, calculated as the ratio of oligodendrocyte-remyelinated axons to SC-remyelinated and demyelinated axons.
Morphometry
Morphometric analysis was performed as previously described
[14]. Breifly, 1 lm transverse semithin spinal cord sections were
imaged at 40x using an Olympus AX-80 microscope and 2 mega-pixel MagnaFire digital camera, and measurements were traced on the image using the Olympus MicroSuite B3SV software to determine area and perimeter values. The accuracy of delineation was checked by viewing delineations at 200x and 1000x magnifi-cation. Spared white matter area was defined as white matter area(s) marked by less than 50% pathology that included aberrant hallmarks such as swelling, damaged axons, hypercellularity, and gross demyelination. Multiple areas of pathology within a section were individually traced and then summed. Spared gray matter area was defined as gray matter area(s) that were clearly identifia-ble from white matter and cavitation of transplant. The measured perimeters per section were used to derive the value of maximal area for each parameter as detailed by Schrimsher and Reier [15]. Maximal area was used for comparisons between animals to account for potential tissue processing artifacts. Maximal area is defined as the area of the circle calculated from the perimeter of the area measured. The maximal areas were averaged from ani-mals within each group.
Quantitative, Differential Gene Expression Profile in
Spinal Cord
Differential expression was assessed on epicenter segments of C5-injured, adult female rats at days 14 and 21 post-injury and
compared to uninjured rats (n ¼ 4/group). These time points
reflect the gene expression profile of untreated spinal cord at days
7 and 14 post-transplantation, respectively (n¼4/group).
(Fisher Scientific). Total RNA was DNAse treated with Turbo DNAfree reagent (Ambion) and cleaned with an RNeasy kit (Qia-gen) according to manufacturers’ instructions. RT of mRNAs was performed using M-MLV Reverse Transcriptase (Ambion) with random hexamers and poly-dT as primers. cDNAs generated by RT were used for subsequent 40-cycle Real-Time PCRs using a Mastercycler Realplex thermal cycler (Eppendorf) and a Sensi-Mix NoRef kit (Quantance/Bioline, San Mateo, CA, http:// www.quantance.com). Mastermix included 200 nM concentration
of specific primers for GAPDH (forward: 50
-atgactctacccacgg-caag-30, reverse: 50-acgccagtagactccacgac-30), HGF (forward: 50
-tggctgtacaatccctgaaa-30, reverse: 50-gagctactcg taataaaccatctgc-30),
TGFb2 (forward: 50-atgaacctttcattgcccttg-30, reverse: 50
-gctcagttc-tataacggctcaca-30), Caspase 4 (forward: 50
-tctccaaactcatttcctgctt-30, reverse: 50-gccttttcaaatgattgttgc-30), GADD45 (forward: 50
-gcttcctccttcagtctcacc-30, reverse: 50-acgc cagtagactccacgac-30),
Pycard (forward: 50-caacacaggcaagcactcat-30, reverse: 50
-caggctg-gagcaaagctaaa-30), Fas (forward: 50-tgattg catctcgtttgtgg-30,
reverse: 50-tgcagcctgtaagtgatatttga-30), TNRFSF1A (forward: 50
-accaagtgccacaaaggaac-30, reverse: 50-ctggaaatgcgtctcactca-30),
TNFRSF1B (forward: 50-catgtcaacgtcacctgcat-30, reverse: 50
-ctgggactgagagggacact-30), CRP (forward: 50-ttcgtatttcccggagtgtc-30,
reverse: 50-tctcgttaaagctcgtcttgg-30), CD40 (forward: 50
-tctgagccctg-gaactgttt-30, reverse: 50-tattactgcggacccctgac-30), IL10 (forward: 50
-cctgctcttactggctggag-30, reverse: 50-tgtccagctggtccttcttt-30), IL10
(forward: 50-tggagcaaatctcccagttc-30, reverse: 50
-atagcagccat-catccttgg-30), and ChAT (forward: 50-gaggagcagttcaggaagagcc-30,
reverse: 50-agatgaggctggctgcaaacc-30), and 100 ng/ll concentration
of cDNA. Primers were designed using NCBI or Primer3 websites. Quantitative polymerase chain reaction (qPCR) data was confirmed
by melt curve plot and analyzed by the comparative CT method
[28].
Statistical Methods
Forelimb stride length scores were analyzed using two-way, repeated measures ANOVA with Tukey’s multiple comparison test at each time point. The treatment group (transplant versus control) was set as the between-groups factor, and the week inter-val was set as the within-group, repeated-measures factor. The
Statistical Package for the Social Sciences 11.5t-test was used to
determine differences between proximal forelimb range scores for
transplanted and control groups. The SPSS 11.5t-test was used to
determine differences between quantitative histological and qPCR values.
R
ESULTSDirected Differentiation of OPCs from hESCs
Undifferentiated hESCs expressed SSEA4 and Oct-4 (data not shown). At the end of the 42-day differentiation protocol, cells had a bipolar morphology characteristic of immature OPCs and a typical antigenic profile; they were immunoposi-tive for the OPC markers Olig1 (>80%), NG2 (>90%) (Fig.
1A), and PDGFRa (>70%) (Fig. 1B). A small number of
A2B5-positive cells (<5%), GFAP-positive astrocytes (<1%)
and Tuj1-positive neuronal cells (<5%) were also identified.
No cells were detected within the transplant population that were labeled with the more mature oligodendrocyte markers O4 and RIP (Fig. 1C). Also absent were cells that were la-beled with the hESC markers Oct-4 and SSEA4 or the neural progenitor marker Pax6 (Fig. 1C).
hESC-derived OPCs were additionally profiled by RT-PCR (Fig. 1D). Confirmation of PDGFRa mRNA expression supports the antibody detection of this receptor on OPCs (Fig. 1B). Selected cDNAs for the secreted proteins TGFb1, Neure-gulin 1, and NeureNeure-gulin 2 and for the transcription factor Nkx2.2 were present in detectible quantities.
Bilateral Cervical Contusion Pathology
Contusion produced bilateral forelimb impairment and did not result in respiratory distress or failure in any animals, indica-tive of an overall functional preservation of more cranial phrenic motor neuron pools. All animals regained bladder function and were capable of hindlimb locomotion and self-access to food and water within 7 days post-injury.
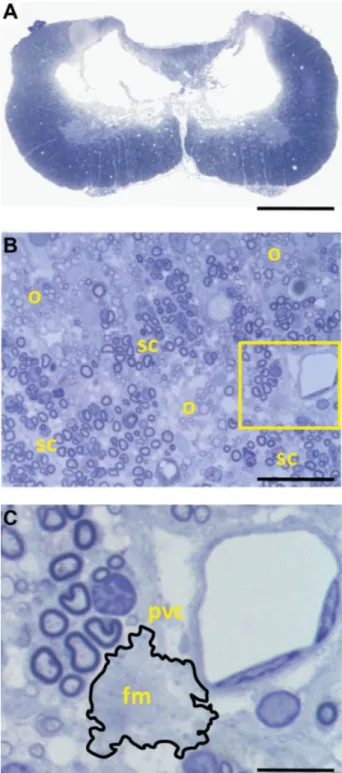
(Fig. 2A). Morphometric analysis at this site revealed that the maximal area of the cavity comprised approximately 18% of the total area of the spinal cord. The injured spinal cord showed marked contusion pathology that included gray matter loss, axonopathy, scattered demyelination, oligodendrocyte-remyelination, partitioned Schwann cell (SC)-remyelination (Fig. 2B), inflammatory infiltrates, and perivascular cuffing (Fig. 2C).
Transplanted OPCs Survive, Localize to Injury,
and Differentiate
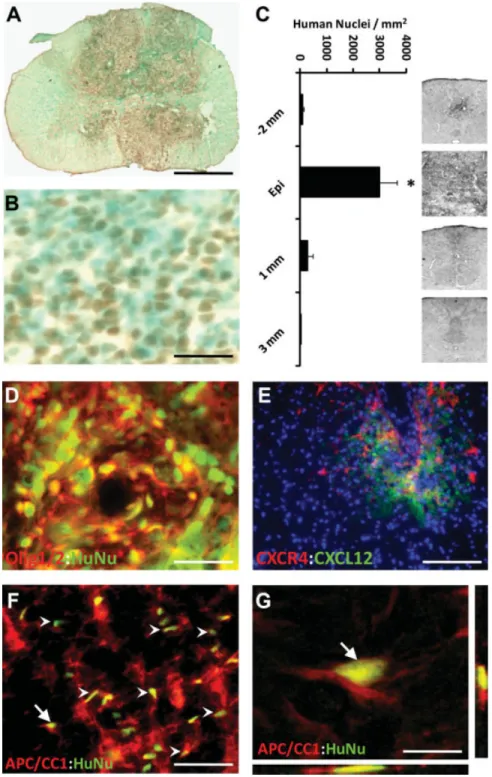
Transplanted hESC-derived OPCs survived and localized to the injury site during the nine-week study period (Fig. 3). OPCs were detected by anti-human nuclear staining and were present in all transplanted animals (Fig. 3A), confirming xeno-graft survival. Control animals did not exhibit such labeling. At the injury epicenter, anti-human positive cells were identi-fied throughout the transverse plane but were concentrated around the area of former cavitation (Fig. 3A). Rodent cells were also present within this area, as identified by methyl green-only staining (Fig. 3B). Quantitation of human nuclei revealed the highest number of cells at the injury epicenter and very few cells cranial and caudal to the epicenter (Fig. 3C).
The ability of transplanted OPCs to retain their phenotype and mature post-transplantation was examined by double im-munostaining for human-nuclei and the oligodendrocyte-spe-cific markers Olig1 or adenomatous polyposis coli tumor sup-pressor protein (APC/CC1). Cells that colabeled with human nuclei and Olig1 antibodies comprised 94%65% of the total number of human nuclei positive cells (Fig. 3D). Cells that colabeled with human nuclei and APC/CC1 comprised 9%6
4% of the total number of human nuclei positive cells. In sec-tions 2–3 mm away from the injection site and cranial to the injury epicenter, hESC-derived OPCs localized to the dorsal column. Human specific anti-CXCR4 labeled transplanted cells were found juxtaposed to mouse/rat specific CXCL12-positive loci in these same areas (Fig. 3E). In the white mat-ter, APC/CC1 cells constituted a much larger percentage of the human-nuclei positive cells, comprising 66% 6 10% of the total human nuclei positive cell population (Fig. 3F), indi-cating that the transplanted OPCs became oligodendrocytes in regions appropriate for myelinogenic differentiation. Cells double-labeled with human nuclei and APC/CC1 processes were confirmed with reconstruction of confocal thin-plane scans (Fig. 3G).
OPC Transplantation Improves Forelimb Motor
Function After Injury
To assess the effects of transplanted OPCs on recovery of forelimb function after injury, we examined forelimb stride length as a general gait parameter. Because C5 innervation impacts proximal forelimb function, we also analyzed proxi-mal forelimb step range and passed-perpendicular step frequency.
Assessment of forelimb locomotion on the forelimb stride length task was significant (p < .05) on two-way,
repeated measures analysis. The difference in the outcome measure between transplanted and nontransplanted groups became significant (p < .05) by 4 weeks post-transplant,
and the transplanted group showed significantly (p < .001)
longer stride length than controls for the duration of the study (Fig. 4A). In addition, stride length within the control group peaked at 1 week post-transplant, whereas stride length within the transplant group peaked at 6 weeks post-transplant. Thus, the transplanted group demonstrated more
extensive recovery before reaching a functional plateau. As a reference, the mean stride length of uninjured animals that were trained to the same apparatus for 9 weeks was 17.5 cm (dashed line).
Examination of the proximal forelimb range of motion exhibited during stepping for each group showed a significant (p < .01) increase in step range of the transplanted group as
compared to the nontransplanted group (Fig. 4B). The increased step range observed in the transplanted group occurred at the lift-off of the step and not at the placement, as determined by a significant (p < .01) difference of the
inside-angle between the forelimb and the floor at lift-off and
no significant (p > .05) difference in the inside-angle at
placement (Fig. 4C). Notably, the mean lift-off angle of the control group was about 90, or perpendicular to the floor.
Stepping function was also analyzed by determining the fre-quency of steps that passed the perpendicular plane. The occurrence of passed-perpendicular steps was significantly (p
< .001) more frequent in the transplanted group as compared
to the nontransplanted group (Fig. 4D). This measure is con-sistent with a classification of frequent (>50%) in transplants
versus occasional (50%) in controls. Together these findings suggest that forelimb locomotion and range of motion are improved by OPC transplants.
Figure 3. Transplantation of human em-bryonic stem cell (hESC)-derived oligoden-drocyte progenitor cells (OPCs) into acute cervical spinal cord injury resulted in cell survival, limited migration from the site of implantation, and differentiation to mature oligodendrocytes. (A): The distribution of hESC-derived OPCs was detected using anti-human-positive nuclei staining (brown). No human cells were detected in control animals. (B): Close image of trans-plant area reveals non-human cells (blue– green) among the anti-human nuclei-posi-tive, transplanted cells (brown). Within the transplant area, human nuclei positive cells were homogeneously distributed and were not located in discrete clusters or rosette-like formations in any of the transplanted animals as might suggest neuroblastoma/ glioma. (C): Distribution of total numbers of anti-human nuclei-positive cells within spinal cord transverse sections along the cranial-caudal axis. Cells were significantly localized to the injury epicenter (*, p <
OPC Transplantation Alters Lesion Pathogenesis
The injury epicenter within nontransplanted spinal cords exhibited widespread loss of white matter and gray matter (Fig. 5A). Cavity borders lacked cells and axons, consistent with glial scar formation (Fig. 5C). White matter pathology was most evident in the dorsal column and medial ventral white matter (Fig. 5A). Nontransplanted spinal cords con-tained extensive oligodendrocyte remyelination and parti-tioned SC-remyelination with scattered demyelination (Fig. 5E). In addition, perivascular cuffing and inflammatory infil-trates were present in the control animals, suggestive of dynamic, ongoing pathology within these focal lesions.
The injury epicenter within transplanted spinal cords was characterized by broad white matter and gray matter sparing (Fig. 5B). Most notably, the transplanted spinal cords lacked cavitation (Fig. 5B) and a distinct lesion border (Fig. 5D). The transplant area contained a high concentration of OPCs with a homogeneous distribution that suggests a lack of astro-cyte partitioning. As in control spinal cords, the white matter of transplanted cords contained oligodendrocyte-remyelinated axons and scattered demyelinated axons. In contrast to the nontransplanted spinal cords, transplanted spinal cords con-tained more normally-myelinated axons and fewer SC-remye-linated and demyeSC-remye-linated axons (Fig. 5F).
Quantification of normally myelinated, oligodendrocyte-remyelinated, SC-remyelinated, and demyelinated axons within the dorsal column exhibited significantly (p < .001)
more normally myelinated axons and significantly fewer SC-remyelinated (p<.001) and demyelinated (p<.05) axons in
the transplanted group compared to the nontransplanted group (Fig. 5G). Because the number of oligodendrocyte-remyeli-nated axons is limited by the number of SC-remyelioligodendrocyte-remyeli-nated and demyelinated axons, the ratio of oligodendrocyte-remyelinated to SC-remyelinated and demyelinated axons was determined in order to assess the efficiency of oligodendrocyte-remyelina-tion. The nontransplanted animals had an average oligoden-drocyte-remyelination efficiency of 1.13 6 0.31. The trans-plant group had a significantly (p < .001) higher
oligodendrocyte-remyelination efficiency of 7.646 0.75 (Fig. 5H). The transplant group, therefore, had an oligodendrocyte-remyelination efficiency that was approximately 680% that of the control group.
The characteristic injury-induced cavitation within non-transplanted spinal cords was absent within non-transplanted spi-nal cords. Morphometric aspi-nalyses at the injury epicenter showed no significant differences (p>.05) between the
trans-planted and nontranstrans-planted groups in the spinal cord maxi-mal area (Fig. 6A), but a significant (p < .05) reduction of
cavitation in the transplant group (Fig. 6B). This reduction in cavity was evident throughout the 2 mm extent of the lesion (data not shown). In addition, quantification of spared cord at the injury epicenter revealed significant increases in the total spared white matter area (p<.01) (Fig. 6C) and total spared
gray matter area (p< .001) (Fig. 6D) in the transplants
com-pared to controls. Further analysis of gray matter sparing Figure 4. Human embryonic stem cell (hESC)-derived oligodendrocyte progenitor cell (OPC)-transplants to cervical SCI significantly improved forelimb motor function. (A): Recovery of forelimb means stride length significantly improved in the transplanted group relative to the nontrans-planted group. The improvement was significant from at least 4 weeks after transplantation through the duration of the testing (*,p<.01). The
mean stride length of uninjured animals that were trained to the same apparatus for 9 weeks was 17.5 cm (dashed line). (B): The range of proxi-mal forelimb motion in degrees was significantly increased in the transplant group compared to the nontransplanted group (*,p<.01). (C): The increased range of proximal forelimb motion was resolved to the lift-off (rearmost) position of the step as opposed to the placement (foremost) by measurement of the inside angle between the forelimb and the floor at lift-off and placement (*,p<.01). (D): The occurrence of steps that
revealed a significant (p< .05) (Fig. 6E) increase in ventral
gray matter (VGM) sparing and a significant (p< .05) (Fig.
6F) increase in average spared motor neurons within the spared VGM.
To test for correlation between functional and histological outcomes, the test of proximal forelimb range of motion was
compared to several histological outcome measures. A signifi-cant correlation was found for proximal forelimb range of motion and mean motor neuron sparing (r¼ .7153,p<.01),
and ventral gray matter sparing (r¼.6612,p<.05),
suggest-ing that the reduction in motor neuron loss and gray matter loss may account for the improved locomotion in the Figure 5. Histopathogenesis in transplanted and nontransplanted animals. (A): Microthin sections from nontransplanted animals demonstrate myelin pathology concentrated around the dorsal and medial-ventral white matter and cavitation that eliminates a large portion of gray matter. (B): Transplanted animals lack cavitation, and myelin pathology is less extensive compared to nontransplanted animals. (C): The dorsal column along the cavitation (cav) contains mostly Schwann cell (SC)-remyelinated axons (sc) with an edge that is consistent with scar formation. (D): In contrast to nontransplanted animals (C), the dorsal column of transplanted animals contains mostly normally myelinated axons (n) and no delinea-tion between the transplanted (trans) and host cells. (E): Myelin pathology in non-transplanted animals shows thin oligodendrocyte-remyelination (o), partitioned SC-remyelination (sc), and few small-diameter axons. Inset illustrates the thin myelin sheath characteristic of oligodendrocyte-remyelination. (F): Transplanted animal spinal cords contained hypercellularity and extra-axonal swelling, spared myelination (n), as well as more small-diameter axons compared to controls. (G): Quantification of myelin pathology revealed a significant (***,p<.001) decrease in nor-mally myelinated axons and an increase in SC-remyelinated (***,p<.001) and demyelinated (*,p<.05) axons in the nontransplanted group relative to the transplanted group. These data indicate that normally myelinated and oligodendrocyte-remyelinated axons constitute about 95% of the total axon population within the area of dorsal column pathology in transplanted animals. (H): hESC-derived OPC transplants had signifi-cantly (***,p<.001) more oligodendrocyte-remyelinated axons relative to SC-remyelinated and demyelinated axons than controls. The trans-plants increased oligodendrocyte-remyelination efficiency to approximately 6.8-fold. Error bars represent6SEM. Scale bar: a,b, 1.25 mm; c,d, 60
transplant group. Together, these data suggest that hESC-derived OPC transplantation can attenuate tissue loss and thereby preserve spinal cord mediated function.
Transplantation of OPCs Affects Gene Expression
in Acute Phase of Spinal Cord Injury
To elucidate the acute events that precede the observed hESC-derived OPC transplantation-induced sparing, we examined the differential expression of select genes during the acute phase after SCI. The select genes of interest (GOIs), listed in the sup-plemental online Table 1, were detectible in injured spinal cord samples. Fold changes in expression at day 21 post-injury were detected as significantly (*, p < .05, **, p < .01, ***, p <
.001) different in select genes for neurotrophic factors, apopto-sis, inflammation, and a neuronal cell marker (Fig. 7A).
To assess the ability of hESC-derived OPC transplants to affect SCI-induced gene expression patterns, differential gene expression was examined in transplanted and nontransplanted animals at 21 days post-injury (14 days post-transplant). At this timepoint, expression of relevant, specific genes deviated from the injured pattern and tended toward an uninjured, or
spared, pattern. Specifically, fold difference expression of genes for HGF (p< .05), IL10 (p< .001), and ChAT (p <
.05) were significantly increased, whereas genes for Casp4 (p
< .001), GADD45 (p < .01), Pycard (p < .05), Fas (p <
.01), TNFR1a (p< .01), TNFR1b (p< .01), CRP (p<.01),
and CD40 (p < .05) were significantly decreased (Fig. 7B).
These results indicate that hESC-derived OPC transplantation can affect acute SCI-induced gene expression changes consist-ent with sparing and support the histological and functional findings at 8-weeks post-transplantation.
D
ISCUSSIONfunctional benefit following transplantation of a hESC-deri-vate to cervical SCI.
Cell-based therapeutics have proven successful in preclini-cal SCI models [30] at least in part due to their ability to address multiple features of SCI such as cell loss, demyelin-ation, or homeostatic loss. Several cell replacement strategies have emerged to treat SCI, including O-2A progenitors [31, 32], Schwann cells [29, 33, 34], or neural stem cells [35, 36]. Several recent studies indicate that myelinogenic transplants elicit histologic repair and functional recovery following SCI [36], validating demyelination as a therapeutic target for SCI [14, 37–39]. Nistor et al. [18] and subsequently Izreal et al. [40] described protocols to direct the differentiation of hESCs into high-purity OPC populations and demonstrated their myelination potential. The use of hESCs as a source for human transplant populations offers advantages over other cell types, including the inherently broad capacity for expan-sion and differentiation [41].
The ability of non-myelinating cells such as bone marrow stromal cells to improve the outcome of SCI exemplifies ther-apeutic benefits of a non-myelinogenic transplant [42, 43]. In an analogous manner, hESC-derived OPCs might contribute to at-level histological and functional outcomes via a non-myelin mechanism. A recent report identified 49 neurotrophic factors expressed by hESC-derived OPCs [22], including the neurotrophic factors insulin-like growth factor 1, brain-derived neurotrophic factor, NT-3, nerve growth factor, and trans-forming growth factor-b1. Co-cultures of hESC-derived OPCs with cortical neurons enhanced neurite outgrowth from the cortical neurons, indicating that the secreted factors can affect surrounding cells [21, 22]. These results suggest that hESC-derived OPCs might produce beneficial effects in SCI aside from remyelination, such as neuroprotection, suppression of inflammation, promotion of axonal regeneration, and/or homeostatic maintenance.
Our methods for cervical contusion produced cavitation and pathological features consistent with moderate to severe bilateral injuries reported by Schrimsher and Reier [15] and Pearse et al. [13]. Here, the lesion emanated a distance of less than 2 mm from the epicenter, and quantitative analysis revealed extensive remyelination. This is in contrast to tho-racic injuries of the same force, which resulted in lesions extending 6–12 mm either side of the lesion epicenter [14] with substantial demyelination and remyelination. This dif-ference in remyelination is consistent with the results of Franklin et al. [44], who found that the migratory potential of endogenous myelinogenic cells is restricted to approxi-mately 2 mm either side of a region of demyelination. The robust endogenous remyelination in this cervical model might also reflect enhanced axonal survival as a result of the axotomies being relatively closer to the cell bodies of origin [45]. Such potential asymmetries between cervical and thoracic injuries underscore the need to test the effec-tiveness of potential treatments for SCI in both injury models.
Most of the transplanted hESC-derived OPCs localized to the lesion epicenter. However, some transplanted cells were found up to 2 mm away from the site of injection. The expression of CXCR4 by hESC-derived OPCs and the detec-tion of human-CXCR4 positive cells within areas of rat-CXCL12 reactivity outside of the injury epicenter suggest that hESC-derived OPCs can follow migratory cues. These find-ings cannot rule out the possibility that dispersion during the transplant injection can also contribute to the cell distribution. Quantification of Olig1 and APC/CC1 positive cells in regions of high concentrations of human nuclei-positive cells within injury epicenter suggests that a lack of niche prevents matura-tion of a high percentage of the transplanted cells. This fea-ture is supported by evidence that, within the injured white matter niche, the percentage of APC/CC1 and human nuclei co-labeled cells is increased. The persistent detection of Olig1 or APC/CC1 on human cells localized to the injury epicenter suggests that transplanted hESC-derived OPCs retained an oli-godendroglial lineage despite the derivation method and diverse molecular milieu. These data, together with the ab-sence of human cells expressing astrocyte, neuronal, or em-bryonic markers, suggest that the transplant population did not trans-differentiate or become pluripotent.
Although transplanted hESC-derived OPCs differentiated into mature oligodendrocytes within white matter tracts, no significant difference in remyelination was detected between the transplanted and nontransplanted groups. This result is not unexpected given the extensive endogenous remyelination evi-denced in the control group. Importantly, the treatment group had significantly more normally myelinated axons and fewer Figure 7. Differential expression of select genes of interest (GOIs)
Schwann cell and demyelinated axons relative to control, sug-gesting that fewer axons were demyelinated in the treatment group. This would potentially present less substrate for remyelination and thereby reduce the number of remyelinated axons in the treatment group. Comparison of oligodendrocyte remyelination efficiency supports this interpretation, as the treatment group showed an increased efficiency relative to control.
The forelimb outcome measures used in this study to determine locomotor recovery were based on the results of anatomical tracing of C5 afferents to forelimb musculature by McKenna et al. [17]. In this cervical contusion model, ani-mals achieved a high level of recovery of the affected limbs at an earlier timepoint, as compared to that reported for tho-racic contusion animals [19]. The transplant group had an increase of stride length, proximal forelimb range of motion, specifically in the step lift-off, and a relative increase in fre-quency of passed-perpendicular steps. Thus, the improvement in forelimb function in the transplant group was observed across multiple forelimb gait parameters relevant to the level of injury.
Morphometry and cell quantification analyses revealed a number of significantly improved histological outcomes in the transplants compared to controls. Correlation of the different histological outcomes with a measure of proximal forelimb function in both transplants and nontransplants indicated a statistically significant correlation of forelimb function with spared motor neurons and gray matter sparing, underscoring the importance of preserving at-level motor neuron function in cervical SCI. Interestingly, no significant correlations were present when forelimb functional outcomes were compared to maximal cavitation or maximal cord areas, indicating that the reduction in cavitation alone was insufficient to account for the improved locomotion in the transplant group. Although this is consistent with hESC-derived OPC-mediated neuropro-tection of cultured neurons [21, 22], the differential gene expression results demonstrate that transplantation of OPCs reduces markers of SCI-induced apoptosis and inflammation and supports neuron survival in vivo during the acute phase after injury. Having identified significant gene expression changes at 21 days post-injury, we expect it will be possible to further investigate these pathways to form a better under-standing of how hESC-derived OPCs interact or interfere with
inflammation and cell death mechanism, such as TNFa– induced excitotoxicity [46] or microglial activation [47], to promote tissue sparing.
CONCLUSION
These findings demonstrate that transplantation of human OPCs into acute cervical SCI improves histological outcomes that correlate with improved recovery. Importantly, our data indicate that cervical SCI presents a distinct lesion pathogene-sis and that the mechanism and outcome of treatment can dif-fer from that in the thoracic spinal cord. These findings under-score the importance of using cervical injury models in addition to thoracic models for the preclinical development of therapeutics for SCI, in order to better address the human SCI population.
A
CKNOWLEDGMENTSThis work was supported by the Geron Corporation, the Univer-sity of California Discovery Grant, the Roman Reed Spinal Cord Injury Research Fund of California, Research for Cure, and indi-vidual donations to the Reeve-Irvine Research Center. J.S. and M.S. were supported by the Bill and Joan Jackson Scholarship. We thank Jane Lebkowski, Catherine Priest, Scott Thies, and Edward Wirth for discussion and advice. We thank Sharyn Rossi, Saba Motakef, Adrian Tripp, and Audrey Keebaugh for assistance with animal care, Sarah Park and Stephen Marley for data collection, and Adriana Gutierrez and David Ferguson for assistance with cell culture. H.S.K. is chairman of the scientific advisory board of California Stem Cells, Inc. G.N. is a member of the scientific advisory board of California Stem Cells, Inc.
D
ISCLOSURE OFP
OTENTIALC
ONFLICTS OFI
NTERESTThe authors indicate no potential conflicts of interest.
R
EFERENCES1 Hagg T, Oudega M. Degenerative and spontaneous regenerative proc-esses after spinal cord injury. J. Neurotrauma 2006;23:264–280. 2 Crowe MJ, Bresnahan JC, Shuman SL et al. Apoptosis and delayed
degeneration after spinal cord injury in rats and monkeys. Nature Medicine 1997;3:73–76.
3 Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med 1999;22: 119–124.
4 Ankeny DP, Popovich PG. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 2009;158:1112–1121.
5 Horky LL, Galimi F, Gage FH et al. Fate of endogenous stem/progen-itor cells following spinal cord injury. J Comp Neurol 2006;498: 525–538.
6 Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 2005;131:177–187. 7 Yamamoto S, Yamamoto N, Kitamura T et al. Proliferation of
paren-chymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol 2001;172:115–127.
8 Frise´n J, Johansson CB, Torok C et al. Rapid, widespread, and long-lasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol 1995;131:453–464.
9 Faulkner JR, Herrmann JE, Woo MJ et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004; 24:2143–2155.
10 Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev 2004;5:146–156.
11 NSCISC. Facts and Figures at a Glance. Available at: http://www. spinalcord.uab.edu/show.asp?durki¼21446.
12 Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma 2004;21:1371–1383.
13 Pearse DD, Lo TP, Jr., Cho KS et al. Histopathological and behav-ioral characterization of a novel cervical spinal cord displacement con-tusion injury in the rat. J Neurotrauma 2005;22:680–702.
14 Siegenthaler MM, Tu MK, Keirstead HS. The extent of myelin pathol-ogy differs following contusion and transection spinal cord injury. J Neurotrauma 2007;24:1631–1646.
15 Schrimsher GW, Reier PJ. Forelimb motor performance following cervical spinal cord contusion injury in the rat. Exp Neurol 1992;117:287–298. 16 Gensel JC, Tovar CA, Hamers FP et al. Behavioral and histological
characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma 2006;23:36–54.
17 McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topogra-phy reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol 2000;419:286–296.
19 Keirstead HS, Nistor G, Bernal G et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005;25: 4694–4705.
20 Cloutier F, Siegenthaler MM, Nistor G et al. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen Med 2006;1:469–479. 21 Faulkner J, Keirstead HS. Human embryonic stem cell-derived
oligo-dendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol 2005;15:131–142.
22 Zhang YW, Denham J, Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic fac-tors. Stem Cells 2006;15:943–952.
23 Carpenter MK, Inokuma MS, Denham J et al. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol 2001;172:383–397.
24 Hatch MN, Nistor GI, Keirstead HS. Oligodendrocyte Differentiation from Human Embryonic Stem Cells. In: Loring J, Wesselschmidt RL, Schwartz PH, eds. Human Stem Cell Manual: A Laboratory Guide, 1st ed. New York: Elsevier; 2007:210–226.
25 Hatch MN, Nistor G, Keirstead HS. Derivation of high-purity oligo-dendroglial progenitors. Methods Mol Biol 2009;549:59–75. 26 Gonzalez R, Glaser J, Liu MT et al. Reducing inflammation decreases
secondary degeneration and functional deficit after spinal cord injury. Exp Neurol 2003;184:456–463.
27 Blight AR. Cellular morphology of chronic spinal cord injury in the cat: Analysis of myelinated axons by line-sampling. Neuroscience 1983;10:521–543.
28 Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the com-parative C(T) method. Nat Protoc 2008;3:1101–1108.
29 Schaal SM, Kitay BM, Cho KS et al. Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion. Cell Transplant 2007;16:207–228. 30 Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status
of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus 2008;24:E19.
31 Groves AK, Barnett SC, Franklin RJ et al. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature 1993;362:453–455.
32 Rosenbluth J, Schiff R, Liang WL et al. Xenotransplantation of trans-genic oligodendrocyte-lineage cells into spinal cord-injured adult rats. Experimental Neurology 1997;147:172–182.
33 Takami T, Oudega M, Bates ML et al. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance
in the moderately contused adult rat thoracic spinal cord. J Neurosci 2002;22:6670–6681.
34 Martin D, Robe P, Franzen R et al. Effects of Schwann cell transplan-tation in a contusion model of rat spinal cord injury. J Neurosci Res 1996;45:588–597.
35 Cummings BJ, Uchida N, Tamaki SJ et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A 2005;102:14069–14074.
36 Karimi-Abdolrezaee S, Eftekharpour E, Wang J et al. Delayed trans-plantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 2006;26:3377–3389.
37 Guest J, Herrera LP, Qian T. Rapid recovery of segmental neurologi-cal function in a tetraplegic patient following transplantation of fetal olfactory bulb-derived cells. Spinal Cord 2006;44:135–142.
38 Klussmann S, Martin-Villalba A. Molecular targets in spinal cord injury. J Mol Med 2005;83:657–671.
39 Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurology 2005;486:373–383. 40 Izrael M, Zhang P, Kaufman R et al. Human oligodendrocytes derived
from embryonic stem cells: Effect of noggin on phenotypic differen-tiation in vitro and on myelination in vivo. Mol Cell Neurosci 2007; 34:310–323.
41 Sharp J, Keirstead HS. Therapeutic applications of oligodendrocyte precursors derived from human embryonic stem cells. Curr Opin Bio-technol 2007;18:434–440.
42 Sheth RN, Manzano G, Li X et al. Transplantation of human bone marrow-derived stromal cells into the contused spinal cord of nude rats. J Neurosurg Spine 2008;8:153–162.
43 Hofstetter CP, Schwarz EJ, Hess D et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A 2002;99:2199–2204.
44 Franklin RJ, Gilson JM, Blakemore WF. Local recruitment of remyeli-nating cells in the repair of demyelination in the central nervous sys-tem. J Neurosci Res 1997;50:337–344.
45 Fernandes KJ, Fan DP, Tsui BJ et al. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: Differential regulation of GAP-43, tubulins, and neurofilament-M. J Comp Neurol 1999;414:495–510.
46 Hermann GE, Rogers RC, Bresnahan JC et al. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis 2001;8:590–599.
47 Nicholas R, Stevens S, Wing M et al. Oligodendroglial-derived stress signals recruit microglia in vitro. Neuroreport 2003;14:1001–1005.