1
Vol.60: e17160741, January-December 2017 http://dx.doi.org/10.1590/1678-4324-2017160741
ISSN 1678-4324 Online Edition
BRAZILIAN ARCHIVES OF BIOLOGY AND TECHNOLOGY
A N I N T E R N A T I O N A L J O U R N A L
Antioxidant Effects of Biochanin A in Streptozotocin
Induced Diabetic Rats
Hamideh Sadri
1, Mohammad Taghi Goodarzi
2, Zahra Salemi
1,3*, Morteza Seifi
4.
1Department of Biochemistry, Arak University of Medical Sciences, Arak, Iran; 2Research Center for Molecular
Medicine, Hamadan University of Medical Sciences, Hamadan, Iran; 3Molecular and Medicine Research Center, Arak University of Medical Sciences, Arak, Iran; 4Department of Medical Genetics, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada.
ABSTRACT
Bioflavonoid-containing diets have been reported to be beneficial in diabetes. In the current study, the effect of Biochanin A (BCA) on blood glucose, antioxidant enzyme activities and oxidative stress markers in diabetic rats were investigated. 30 male Wistar rats were divided into five groups. Two of them were selected as control; group1: control (receiving 0.5%DMSO), and group2: Control+BCA (receiving 10 mg/kg.bw BCA). Diabetes was induced in other rats with injection of (55 mg/kg.bw) streptozotocin; group3: diabetic control (receiving 0.5%DMSO), groups 4 and 5 were treated with 10 and 15 mg/kg.bw BCA respectively. After 6 weeks the following results were obtained. Fasting blood glucose (FBG), Triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C) and malondialdehyde (MDA) levels significantly increased and body weight, high density lipoprotein cholesterol (HDL-C), superoxide dismutase (SOD) and catalase (CAT) activity and total antioxidant status (TAS) significantly decreased in diabetic rats as compared to control rats. Oral administration of BCA in 10 and 15 mg/kg.bw, FBG, TG, TC, LDL-C, VLDL-C were decreased significantly in all treated rats. MDA was decreased in all treated rats but it was significant just in 15 mg/kg.bw BCA. HDL, CAT, SOD, and TAS were significantly increased in treated group with 15 mg/kg.bw. The obtained results indicated hypoglycemic and hypolipidemic effect of BCA. Also BCA reduced oxidative stress in diabetic rats.
Key Words: Biochanin A, Diabetic rats, Oxidative stress, STZ
*
Author for correspondence: Dr.zsalemi@arakmu.ac.ir
According to International Diabetes Federation (IDF), the total number of people with diabetes worldwide was predicted to be 552 million by 2030 (1). Diabetes is characterized by hyperglycemia, resulting from defects in insulin secretion, insulin action, or by both. Accumulation of glucose in the blood and sustained hyperglycemia, often leading to various macrovascular and microvascular complications (2). In diabetes, oxidative stress is caused by advanced glycation end products (AGEs) and free radical formation by autoxidation of unsaturated lipids in plasma and membrane proteins. It may be amplified and propagated by an autocatalytic cycle of metabolic stress, tissue damage, and cell death leading to a simultaneous increase in free radical production and compromised inhibitory and scavenger mechanisms, which further exacerbate the oxidative stress (3). The important goal of diabetes treatment is to keep blood glucose, lipid and lipoprotein levels close to normal and detoxify or scavenge free radicals. The first line of antioxidant defense are enzymes, like superoxide dismutase (SOD) and catalase (CAT) (4). The total antioxidant status (TAS) reflect the antioxidant level of antioxidant enzymes and nonenzymatic compounds including albumin, bilirubin and uric acid [endogenous antioxidant components] (5), as well as exogenous antioxidants such as carotenoids, vitamin E, ascorbic acid and flavonoids. The TAS has been developed to determine the synergistic role of all enzymatic as well as nonenzymatic antioxidants, rather than using the simple sum of individual antioxidants (6). Malondialdehyde (MDA) is the most commonly used marker of lipid peroxidation (7)
. It appears from several studies that oxidative stress and lipid peroxidation are increased in STZ induced diabetic rats (8).
In spite of the use of many oral hypoglycemic drugs, all of them are expensive and show limited efficacy and certain adverse effects. Comparatively very less side effects and low cost of phytochemicals from natural resources open new avenues for the treatment of various diseases including diabetes (9). Flavonoides are phenolic compounds with their hydroxyl groups, they possess strong antioxidative activity, they do not act as conventional hydrogen-donating antioxidants but may exert modulatory actions in cells through various signaling pathways (10). Biochanin A (BCA) is an O-methylated isoflavone; it is found in red clover, soy, alfalfa sprouts, peanuts and chickpea. A broad spectrum of biological benefits for BCA has been reported, including anti-inflammatory, antioxidative and antineoplastic effects (11). The aim of the present study was to evaluate the effect of BCA on fasting blood glucose levels, lipid peroxidation, activity of antioxidant enzymes and total antioxidant status in STZ-diabetic rats.
MATERIALS AND METHODS
Chemicals
Streptozotocin [STZ], biochanin A, and dimethyl sulfoxide (DMSO) were obtained from Sigma Aldrich. All other chemicals used in this study were of analytical grade obtained from E Merck.
Animals
30 Male Wistar rats (200-220 g) were purchased from the central animal house, Tehran University of Medical Sciences, and maintained in standard air conditioned
room (23±2 ˚C) and humidity (55±5%) with a 12 h light/12 h dark cycle. All the rats were fed with commercially available rat normal pellet diet and water ad libitum.
Biochanin A and diabetes
3
Arak University of Medical Sciences, Arak, Iran. All necessary efforts were made to minimize the number of animals and their suffering. Body weight was measured in the morning of the first and the last day of administration by Balance: Sartorius TE64 (Germany). All experiments were approved by the local Ethics Commission (Arak university of medical science) under project number 93-186-28 and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 80-23, revised 1996).
Animal Groups and Diabetes Induction
From thirty male Wistar rats, we selected two groups (n=6) randomly. Groups 1: Control (receiving 0.5% DMSO), and group 2: Control + BCA (receiving 10 mg/kg bw/day). Diabetes was induced in other rats by a single intraperitoneal injection of 55mg/kg b.w Streptozotocin. STZ was dissolved in citrate buffer, pH =4.5, while the respective control rats were given vehicle citrate buffer and normal saline. Hyperglycemia was confirmed by the elevated fasting blood glucose levels determined at 72h after injection. The rats with fasting blood glucose (FBG)> 250 mg/dl were considered diabetic and selected for further studies (12). The diabetic rats were divided randomly as follow: group3: Diabetic control (receiving 0.5% DMSO); group 4: Diabetic + BCA (receiving 10 mg/kg bw/day) and group5 Diabetic + BCA (receiving 15 mg/kg bw/day). The initial and final body weight and FBG of all groups were recorded after 42 days in fasting condition. The animals were anesthetized using IP ketamine (75 mg/kg) and xylazine (10 mg/kg) (13). Blood sample was collected by cardiac puncture using syringes for serum and EDTA-coated syringes for plasma. Samples were centrifuge at the 3000 rpm for 15 min at 4 °C. The plasma and serum samples were kept at -20°C until analysis.
Biochemical Analysis
Determination of serum glucose and lipid profile
Serum concentrations of FBG, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured using enzymatically commercial kits (Parsazmun company, Tehran, Iran) with the aid of a spectrophotometer (JENWAY 6505, Europe Union]. Low density lipoprotein cholesterol (LDL-C) was calculated by Friedewald formula: LDL-C = Total cholesterol – (HDL-C + [TG/5)) (14).
Estimation of malondialdehyde (MDA)
Lipid peroxidation is estimated indirectly by the measurement of a secondary product ie. malondialdehyde (MDA) (15). MDA level in the plasma samples was measured using the reaction with thiobarbituric acid. The standards of 0.1 to 20
μmol/L tetramethoxypropane (TMP) were used to obtain the calibration curve (16).
Estimation of superoxide dismutase [SOD]
Erythrocyte SOD activities was determined using Randox kits (Randox Laboratories). The hemoglobin content of the erythrocytes was determined using the cyanmethaemoglobin method (17). The determination of SOD activity was based on the production of O2- anions by the xanthine/xanthine oxidase system (18). O2
Catalase (EC 1.11.1.6) activity was determined by the method of Aebi. The principle of the assay is based on the determination of the rate constant k (dimension: s-1) of the hydrogen peroxide decomposition. By measuring the absorbance changes per minute, the rate constant of the enzyme was determined. Activities were expressed as U/g Hb (19).
Measurement of plasma total antioxidant status [TAS]
TAS was measured in plasma by means of a commercial kit (Randox Laboratories).
The assay was based on the incubation of
2,2'-azino-di-(3-ethylbenzthiazolinesulphonate) (ABTS) with a peroxidase (methmyoglobin) and hydrogenperoxide to produce the radical cation ABTS+, which has a relatively stable blue-green color, measured at 600 nm (20).The suppression of the color was compared with that of the Trolox. The assay results were expressed as Trolox equivalent (mmol/L) (21).
Statistical Analysis
All the data were expressed as mean±standard deviation (S.D) of three replicates for six rats in each group. Stata software, version 13 (Stata Corp, College Station, TX, USA) was used for all statistical analysis. Normality assumption was checked using Shapiro Wilk test. One-way ANOVA was applied for determining differences between mean of the variables in the studied groups. Post hoc Test (Tukey) was used to compare the data. Values of p < 0.05 were considered statistically significant.
RESULTS
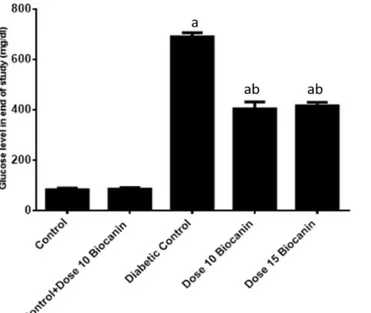
Figure 1 shows the effect of BCA on changes in FBG levels in diabetic and control rats. In the STZ induced diabetic rats serum glucose level significantly (p < 0.05) increased compared with the normal control rats. Oral administration of BCA at doses of 10 mg/kg.bw and 15 mg/kg.bw led to reduction in glucose levels significantly (p < 0.05) in the induced diabetic rats; although, there was no significant difference between the effect of two treatment doses.
Figure 1. Serum glucose levels in different studied groups. Each value is mean ± SD for 6 rats in each
group. ap< 0.05: in comparison with normal rats
b
Biochanin A and diabetes
5
Diabetic rats showed decrease in body weight significantly; administration of BCA at two different doses, improved body weight, however, the changes were not statistically significant.
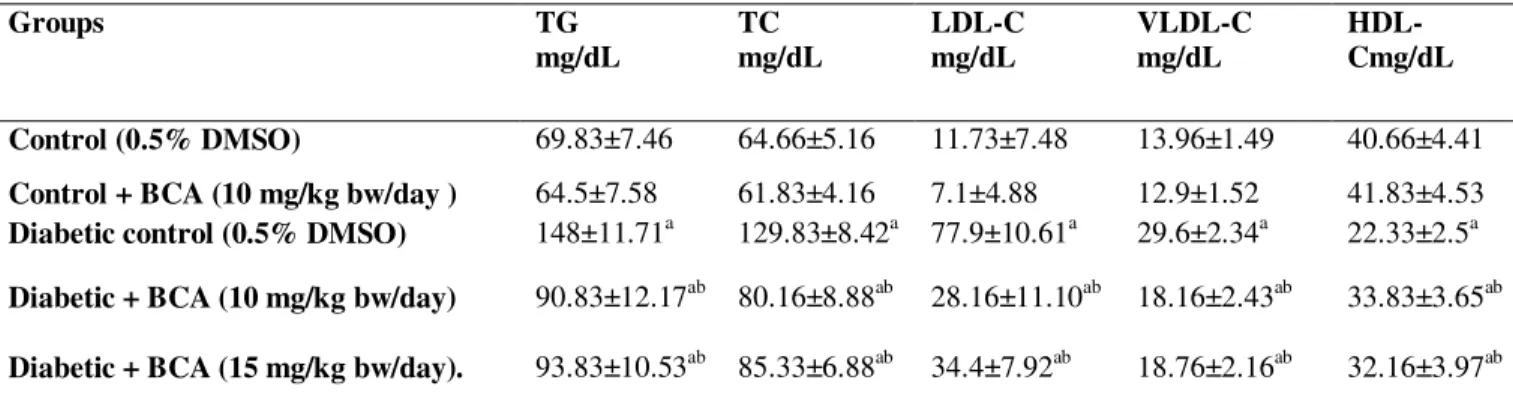
Effects of BCA administration on lipid profile is shown in Table 1. Serum TG , TC, LDL-C and VLDL-C were significantly increased and HDL-C was significantly decreased in diabetic rats comparing to normal rats. Oral administration of BCA 10 and 15 mg/kg.bw significantly decreased serum TG, TC, LDL-C and VLDL-C levels and increaesd HDL-C significantly compared to diabetic rats. BCA at 10 mg/kg.bw was more effective than 15 mg/kg.bw in lipid profile however, the difference was not statistically significant.
Table 1. Effect BCA on serum TG, TC, LDL-C, VLDL-C, and HDL-C of diabetic rats.
Groups TG
mg/dL
TC mg/dL
LDL-C mg/dL
VLDL-C mg/dL
HDL-Cmg/dL
Control (0.5% DMSO) 69.83±7.46 64.66±5.16 11.73±7.48 13.96±1.49 40.66±4.41
Control + BCA (10 mg/kg bw/day ) 64.5±7.58 61.83±4.16 7.1±4.88 12.9±1.52 41.83±4.53
Diabetic control (0.5% DMSO) 148±11.71a 129.83±8.42a 77.9±10.61a 29.6±2.34a 22.33±2.5a
Diabetic + BCA (10 mg/kg bw/day) 90.83±12.17ab 80.16±8.88ab 28.16±11.10ab 18.16±2.43ab 33.83±3.65ab
Diabetic + BCA (15 mg/kg bw/day). 93.83±10.53ab 85.33±6.88ab 34.4±7.92ab 18.76±2.16ab 32.16±3.97ab
Each value is mean ± SD of 6 rats in each group Abbreviations: TG, triglycerides; TC, total cholesterol, LDL-C, low-density lipoprotein cholesterol; VLDL-C, very-low-density lipoprotein cholesterol; and HDL-C, high density lipoprotein cholesterol. ap< 0.05 in comparison with normal rats bp< 0.05 in comparison with diabetic rats.
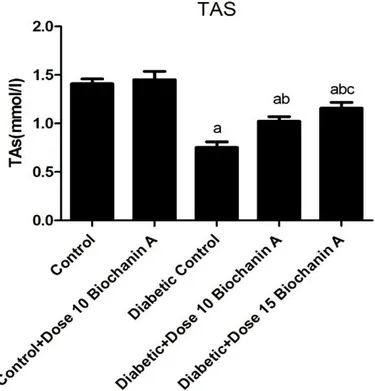
Table 2 demonstrates the activities of SOD, CAT, and levels of TAS and MDA in the controls and treatment groups. A significant (p < 0.05) reduction in the activities of SOD and CAT in diabetic rats was observed. Treatment with BCA increased the activities of enzymatic antioxidant; however, the difference was statistically significant in (p < 0.05) the group that received higher dose. TAS significantly decreased in the diabetic group compared with the control group. BCA administration increased this value significantly.
Table 2: The effect of BCA on the activities of SOD, CAT, and TAS in controls and treated groups.
Group SOD,
U/mg Hb
CAT, U/g Hb
MDA, nmol/ml
Control(0.5% DMSO) 1747.8±19.5 289.83±18.56 1.45±0.12
Control + BCA (10 mg/kg bw/day) 1765.6±20.3 277.12±33.28 1.48±0.11 Diabetic control (0.5% DMSO) 1589.8±21.7a 227.5±23.29a 2.6±0.17a
Diabetic + BCA (10 mg/kg bw/day) 1683.2±28.9 248.87±41.21 2.35±0.24a
Diabetic + BCA (15 mg/kg bw/day) 1736.1±26.7b 281.16±38.17b 2.13±0.24ab
Each value is mean ± SD for six rats in each group ap< 0.05 compared to Control control bp< 0.05 compared to diabetic control rats cp< 0.05 compared to diabetic + BCA(10 mg/kg bw) rats
decreased the MDA level, however the difference was significant (p < 0.05) only at 15 mg/kg bw dose. Our result showed in all measured parameters there was not any significant difference between control and control + BCA (receiving 10 mg/kg bw/day), therefore it appeared BCA had not any side effects.
Figure 2. TAS levels in different studied groups. Each value is mean ± SD for 6 rats in each group. ap< 0.05 compared to Control control bp< 0.05 compared to diabetic control rats cp< 0.05 compared to diabetic + BCA (10 mg/kg bw) rats
DISCUSSION
Our results showed that oral administration of BCA significantly decreased fasting blood glucose levels and oxidative stress and improved lipid profile in STZ-induced diabetic rats. STZ intrapritoneal injection at doses of 50-60 mg/kg into rats damages the pancreas and insulin levels fall to 10-30% of normal levels leading to hyperglycemia. However, diabetic rats can survive for some months without insulin replacement (22). STZ induced diabetes mellitus increases oxidative stress and the level of lipids usually rises in diabetes and such an elevation represents a risk factor for coronary heart disease. The abnormal high concentration lipids in diabetes are mainly due to increase in adipose tissue lipolysis in absence of insulin and mobilization of free fatty acids from the peripheral depots, since insulin inhibits the hormone sensitive lipase (23).
Hyperglycemia in diabetes causes oxidative stress by inducing free radical formation via glucose autoxidation, polyol pathway, and non-enzymatic glycation of proteins (24)
Biochanin A and diabetes
7
by utilization of glucose. BCA administration also controlled glucose metabolism by restoring activities of glucose 6- phosphatase and fructose 1,6-bisphosphatase to near normal. Although the activities of these enzymes increased significantly in the liver and kidney of diabetic rats (27). The streptozocin-induced diabetic rats significantly lost weight compared to control rats (28). Sever weight loss of diabetic rats is related to defect in glucose metabolism and excessive breakdown of tissue proteins. Administration of BCA increased body weight, possibly due to glycemic control (29). After 42 days treatment with BCA, a significant reduction in serum TG, TC, LDL-C, VLDL-C and a significant increase in the HDL-C level were observed. The possible mechanism of the hypolipidemic effects of BCA as a soy protein, including the improvement of insulin/glucagon ratio, which involves in lower fatty acid biosynthesis in liver through reducing the gene expression of sterol regulatory element binding protein (SREBP)-1. Moreover, soy protein isoflavones can increase serum cholesterol clearance through stimulating the transcription factor SREBP-2 (30)
enhancing the formation of glycated proteins (42).
In the present study, we observed the lipid peroxidation product (MDA) level was significantly increased by diabetes. The serum level of MDA in the treated diabetic groups decreased after BCA administration. This effect might be due to the direct scavenging activity of the genistein phenolic ring, activation of antioxidant defense gene transcription, and modulation of ROS producing enzyme expression (43). Therefore, we suggest that effect of BCA can be the same as genistein in ROS elimination. Total antioxidant status (TAS) indicates the total summation of the individual enzymatic and non-enzymatic antioxidants present in a sample. Thus, TAS may alter during oxidative stress. The reduced TAS in the diabetic rats might imply that there was an imbalance between free radical formation and antioxidant protection (44). BCA administration increased TAS significantly. This indicates that the administration of BCA has been able to prevent oxidative stress through elevation of the antioxidant enzymes and maintaining the plasma total antioxidant level. Andrea Lugasi et.al investigated the in vitro antioxidant properties of nine selected flavonoid aglycons, including BCA. They reported antioxidant effectiveness of a compound is significantly dependent on its chemical structure (45). From isoflavonoids, BCA and genistein (as a metabolite of BCA) were more effective than daidzein and formononetin. It is clear that both hydroxyl groups in the 4´ and 5 positions are needed for antioxidant activity of the molecule as in genistein. BCA has similar activity, OCH3 group at 4´ position do not modify considerably the antioxidant property. According to TAS result genistein is stronger than apigenin but the two other parameters as H-donor ability and reducing power show opposite property (45).
CONCLUSION
The obtained results indicated hypoglycemic and hypolipidemic effect of BCA. Also BCA played an important role in reducing oxidative stress in diabetic rats and it appeared BCA had not any side effects.
ACKNOWLEDGMENT
This study was supported by grants from Arak University of Medical Sciences.
REFERENCES
1.Federation I. IDF diabetes atlas update 2012. IDF diabetes atlas 5th ed Brussels, Belgium: International Diabetes Federation. 2011.
2.Control D, Trial C. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England journal of medicine. 2005;353(25):2643.
3.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405-12.
4.Jacob RA. The integrated antioxidant system. Nutrition research. 1995;15(5):755-66. 5.Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radical Biology and Medicine. 2000;29(11):1106-14.
6.Bartosz G. Non-enzymatic antioxidant capacity assays: limitations of use in biomedicine. Free radical research. 2010;44(7):711-20.
Biochanin A and diabetes
9
8.Ohkuwa T, Sato Y, Naoi M. Hydroxyl radical formation in diabetic rats induced by streptozotocin. Life sciences. 1995;56(21):1789-98.
9.Schlyer S, Horuk R. I want a new drug: G-protein-coupled receptors in drug development. Drug discovery today. 2006;11(11):481-93.
10. Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacological reviews. 2000;52(4):673-751.
11. Mishra P, Kale R, Kar A. Chemoprevention of mammary tumorigenesis and chemomodulation of the antioxidative enzymes and peroxidative damage in prepubertal Sprague Dawley rats by Biochanin A. Molecular and cellular biochemistry. 2008;312(1-2):1-9.
12. Thiraphatthanavong P, Wattanathorn J, Muchimapura S, Thukham-mee W, Lertrat K, Suriharn B. The combined extract of purple waxy corn and ginger prevents cataractogenesis and retinopathy in streptozotocin-diabetic rats. Oxidative medicine and cellular longevity. 2014;2014.
13. Kohn DF, Wixson SK, White WJ, Benson GJ. Anesthesia and analgesia in laboratory animals: Academic Press; 1997.
14. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389(6651):610-4. 15. Anoopkumar‐ Dukie S, Walker RB, Daya S. A sensitive and reliable method for the detection of lipid peroxidation in biological tissues. Journal of pharmacy and pharmacology. 2001;53(2):263-6.
16. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry. 1979;95(2):351-8.
17. Drabkin D. THE MOLECULAR WEIGHT OF HAEMOGLOBIN, ITS IRON AND NITROGEN CONTENT AND OPTICAL PROPERTIES--THEIR RELEVANCE IN THE PROBLEM OF A REFERENCE STANDARD FOR HAEMOGLOBIN MEASUREMENT. Bibliotheca haematologica. 1965;21:33.
18. Fairbanks V, Klee G. Biochemistry aspects of haematology. Tietz Fundamental of Clinical Chemistry 4th ed, Philadelphia, WB Saunders Co. 1996:704-30.
19. Aebi H. [13] Catalase in vitro. Methods in enzymology. 1984;105:121-6.
20. Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical science (London, England: 1979). 1993;84(4):407-12.
21. Ghaffari T, Nouri M, Irannejad E, Rashidi M-R. Effect of vitamin E and selenium supplement on paraoxonase-1 activity, oxidized low density lipoprotein and antioxidant defense in diabetic rats. BioImpacts. 2011;1(2):121-8.
22. Oztürk Y, Altan VM, Yildizoğlu-Ari N. Effects of experimental diabetes and insulin on
smooth muscle functions. Pharmacological Reviews. 1996;48(1):69-112.
23. Marsh TG, Straub RK, Villalobos F, Hong MY. Soy protein supports cardiovascular health by downregulating hydroxymethylglutaryl–coenzyme A reductase and sterol regulatory element-binding protein–2 and increasing antioxidant enzyme activity in rats with dextran sodium sulfate–induced mild systemic inflammation. Nutrition Research. 2011;31(12):922-8.
24. Obrosova IG, Van Huysen C, Fathallah L, Cao X, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. The FASEB Journal. 2002;16(1):123-5.
25. Maritim A, Sanders a, Watkins rJ. Diabetes, oxidative stress, and antioxidants: a review. Journal of biochemical and molecular toxicology. 2003;17(1):24-38.
26. Gupta B, Ansari M, Singh J, Baquer N. Effect of insulin and thyroxine on catalase, glutathione-s-transferase, GSH and GSSG in alloxan diabetic rat red cells. Biochemistry international. 1992;27(5):793-802.
hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC complementary and alternative medicine. 2013;13(1):1.
29. Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life sciences. 2005;76(11):1223-38.
30. Torres N, Torre-Villalvazo I, Tovar AR. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. The Journal of nutritional biochemistry. 2006;17(6):365-73.
31. Jafarnejad A, Bathaie S, Nakhjavani M, Hassan M. Effect of spermine on lipid profile and HDL functionality in the streptozotocin-induced diabetic rat model. Life sciences. 2008;82(5):301-7.
32. Kumthekar MM, Katyare SS. Altered kinetic attributes of Na (+)+ K (+)-ATPase activity in kidney, brain and erythrocyte membranes in alloxan-diabetic rats. Indian journal of experimental biology. 1992;30(1):26-32.
33. Arivazhagan P, Thilakavathy T, Panneerselvam C. Antioxidant lipoate and tissue antioxidants in aged rats. The Journal of nutritional biochemistry. 2000;11(3):122-7.
34. Murugan P, Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin– nicotinamide induced diabetic rats. Life sciences. 2006;79(18):1720-8.
35. Sözmen EY, Sözmen B, Delen Y, Onat T. Catalase/superoxide dismutase (SOD) and catalase/paraoxonase (PON) ratios may implicate poor glycemic control. Archives of medical research. 2001;32(4):283-7.
36. Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radical Biology and Medicine. 2004;36(7):838-49.
37. Bandy B, Bechara EJ. Bioflavonoid rescue of ascorbate at a membrane interface. Journal of bioenergetics and biomembranes. 2001;33(4):269-77.
38. Moon YJ, Sagawa K, Frederick K, Zhang S, Morris ME. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. The AAPS journal. 2006;8(3):E433-E42.
39. Lee J-S. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life sciences. 2006;79(16):1578-84.
40. Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44(6):659-73. 41. Cameron NE, Cotter MA. The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes/metabolism reviews. 1994;10(3):189-224.
42. Kumar KM, Bobby Z, Selvaraj N, Das AK, Koner BC, Sen S, et al. Possible link between glycated hemoglobin and lipid peroxidation in hyperthyroidism. Clinica chimica acta. 2004;342(1):187-92.
43. Yoon G, Park S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutrition research and practice. 2014;8(6):618-24. 44. Erejuwa OO, Sulaiman SA, Wahab MSA, Salam SKN, Salleh MSM, Gurtu S. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. International journal of molecular sciences. 2011;12(1):829-43.
45. Lugasi A, Hóvári J, Sági KV, Bíró L. The role of antioxidant phytonutrients in the prevention of diseases. Acta Biologica Szegediensis. 2003;47(1-4):119-25.