www.jped.com.br
ORIGINAL
ARTICLE
Effects
of
erythromycin
on
␥
-glutamyl
cysteine
synthetase
and
interleukin-1

in
hyperoxia-exposed
lung
tissue
of
premature
newborn
rats
夽
Cheng
Cai
a,∗,
Gang
Qiu
a,
Xiaohui
Gong
a,
Yihuan
Chen
a,
Huanhu
Zhao
baDepartmentofNeonatology,ShanghaiChildren’sHospital,ShanghaiJiaoTongUniversity,Shanghai,China
bChineseMinorityEthnicGroups’TraditionalMedicineResearchCenter,CentralUniversityforNationalities,Beijing,China
Received8November2013;accepted14January2014
Availableonline27May2014
KEYWORDS Erythromycin; Hyperoxia; Lunginjury; Glutathione; Interleukin-1-beta
Abstract
Objective: Toexploretheeffectoferythromycinonhyperoxia-inducedlunginjury.
Methods: One-day-oldpretermoffspringSprague-Dawley(SD)ratswererandomlydividedinto fourgroups:group1,air+sodiumchloride;group2,air+erythromycin;group3,hyperoxia+ sodiumchloride;andgroup4,hyperoxia+erythromycin.Atone,seven,and14daysof expo-sure,glutathione(GSH)andinterleukin-1beta(IL-1beta)weredetectedbydouble-antibody sandwichenzyme-linkedimmunosorbentassay(ELISA),andbicinchoninicacid(BCA)wasused todetectGSHprotein.␥-glutamine-cysteinesynthetase(␥-GCS)mRNAwasdetectedbyreverse transcription-polymerasechainreaction(RT-PCR).
Results: Comparedwithgroup1,expressionsofGSHand␥-GCSmRNAingroup3were signifi-cantlyincreasedatoneandsevendaysofexposure(p<0.05),butexpressionof␥-GCSmRNAwas significantlyreducedat14days;expressionofIL-1betaingroup3wassignificantlyincreased atsevendaysofexposure(p<0.05),andwassignificantlyreducedat14days.Comparedwith group3,expressionsofGSHand␥-GCSmRNAingroup4weresignificantlyincreasedatone, seven,and14daysofexposure(p<0.05),butexpressionsofGSHshowedadownwardtrendat 14days;expressionofIL-1betaingroup4wassignificantlyreducedatoneandsevendaysof exposure(p<0.05).
Conclusions: Changesinoxidant-mediatedIL-1betaandGSHareinvolvedinthedevelopment ofhyperoxia-inducedlunginjury.Erythromycinmayup-regulatetheactivityof␥-GCS, increas-ingtheexpressionofGSH,inhibitingthelevelsofoxidant-mediatedIL-1betaandalleviating hyperoxia-inducedlunginjuryviaanantioxidanteffect.
©2014SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
夽
Please citethisarticleas:CaiC,Qiu G, GongX, ChenY,Zhao H.Effects oferythromycin on␥-glutamylcysteine synthetase and interleukin-1inhyperoxia-exposedlungtissueofprematurenewbornrats.JPediatr(RioJ).2014;90:493---9.
∗Correspondingauthor.
E-mail:caicheng2004@163.com(C.Cai). http://dx.doi.org/10.1016/j.jped.2014.01.013
PALAVRAS-CHAVE Eritromicina; Hiperóxia; Lesãopulmonar; Glutationa; Interleucina-1beta
Efeitosdaeritromicinasobrea␥-glutamil-cisteína-sintetaseeainterleucina-1no tecidopulmonarexpostoàhiperóxiaderatosrecém-nascidosprematuros
Resumo
Objetivo: Exploraroefeitodaeritromicinasobrelesõespulmonaresinduzidasporhiperóxia.
Métodos: UmaprolederatosSprague-Dawley(SD)prematuroscomumdiadevidafoidividida aleatoriamente emquatro grupos:grupo1ar+cloretodesódio,grupo2ar+eritromicina, grupo3hiperóxia+cloretodesódioegrupo4hiperóxia+eritromicina.Comum,setee14 diasdeexposic¸ão,foramdetectadasGlutationa(GSH)eInterleucina-1beta(IL-1beta)pelo ensaioimunossorventeligadoàenzima(ELISA),eoácidobicinconinico(BCA)foiutilizadopara detectaraproteínaGSH.OmRNAda␥-glutamil-cisteina-sintetase (␥-GCS)foidetectadopor reac¸ãoemcadeiadapolimeraseviatranscriptasereversa(RT-PCR).
Resultados: Comparadasao grupo1,asexpressões domRNAdaGSHeda␥-GCSnogrupo3 aumentaramsignificativamentecomumesetediasdeexposic¸ão(p<0,05),porémaexpressão demRNAda␥-GCSdiminuiusignificativamenteaos14dias;aexpressãodeIL-1betanogrupo 3aumentousignificativamenteaos7diasdeexposic¸ão(p<0,05)ediminuiusignificativamente aos14 dias. Comparadas ao grupo3, as expressões domRNAda GSHe da␥-GCS nogrupo 4aumentaramsignificativamentecomum,setee14diasdeexposic¸ão(p<0,05),porémas expressõesdeGSHmostraramumatendênciadequedaaos14dias;aexpressãodeIL-1beta nogrupo4foireduzidasignificativamentecomumesetediasdeexposic¸ão(p<0,05).
Conclusões: As variac¸ões de IL-1 beta e GSH mediadas por oxidantes estão envolvidas no desenvolvimento de lesão pulmonar induzida por hiperóxia. A eritromicina poderáregular positivamenteaatividadeda␥-GCS,aumentandoaexpressãodeGSH,inibindoosníveis de interleucina-1betamediadaporoxidanteealiviandoalesãopulmonarinduzidaporhiperóxia pormeiodeumefeitoantioxidante.
©2014SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
With the rapid development of maternal health technol-ogyandperinatology,thesurvivalrateofprematureinfants is increasing, especially in very low birth weight infants (VLBWI).1However,thelungsofprematureinfantsareoften
immatureandindirectcontactwithoxygen,andtheyare
oneofthemostsensitive organstooxygentoxicity.
More-over, premature infants need to receive various oxygen
therapies for a long time after birth. Unfortunately, this
undoubtedly aggravates oxidative stress in the immature
lungsof prematureinfants, which may lead toacuteand
chroniclunginjury.2
Hyperoxia-inducedlunginjuryisamajorcauseofchronic
respiratory disease from infancy to adulthood, and has
becomeoneofthemostdifficultproblemsintheneonatal
intensive care unit. However,itsetiology and
pathogene-sisarenotfully understood.3 Nowadays,most researchers
believe that immature lung tissue directly exposed to
the hyperoxic environment results in oxidative stress,
whichhasacrucialroleinthedevelopmentof
hyperoxia-induced lung injury.4,5 Oxidative stress can disturb the
oxidant/antioxidant balance, and is one of the primary
pathogenic factors.6 Glutathione (GSH) is an important
intracellular antioxidant and has a key role in
maintain-ingintegrity andpreventing oxidative damage in alveolar
epithelial cells.7 ␥-glutamine-cysteine synthetase (␥-GCS)
is therate-limiting enzyme of GSH protein synthesis,and
regulatesintracellularlevelsof GSH.8 IL-1betais present
intheearlyphaseof bronchopulmonarydysplasia(BPD)in
prematureinfants,andmayhave animportantroleinthe
development of BPD. However,the exact pathogenesis of
BPD remains unclear, and clinically effective treatments
remainlimited.
The non-antibacterial effectoferythromycin has
grad-ually attracted the attention of several researchers.9 It
exhibits many important physiological functions,
includ-ing: effective antibacterial activity, non-specific
anti-inflammatory effects in asthma, immune regulation,
induced chemical adhesion, promoted gastrointestinal
motility, and an anti-tumor effect.10 Erythromycin
effec-tivelytreatsmanynon-bacterial,infective chronic
inflam-matory diseases, some of which show imbalanced redox
reactions.11 However,it remains unclear howthe
expres-sion levels of GSH, ␥-GCS, and IL-1 beta are affected in
hyperoxia-exposed lung tissue. In the present study, the
authorsexploredtheeffectoferythromycinon
hyperoxia-induced lung injury in premature rats and examined the
expressionlevelsofGSH,␥-GCS,andIL-1betainpremature
ratpulmonarytissues.
Materials
and
methods
Experimentalanimalmodels12 andgrouping
UniversityforNationalities,Beijing,China.Thefirstdayof pregnancywasrecorded whenspermwasdetected in the vaginalsectionsoffemaleratsbymicroscopicexamination. Onday21ofgestation(term=22days)fetuseswere deliv-eredbyhysterectomy.Theone-day-oldpretermSDratswere randomlydividedintofourgroups(eightpupsineachgroup): group1receivedair(21%O2)+sodiumchloride;group2,air +erythromycin;group3,hyperoxia+sodiumchloride;and group4, hyperoxia+ erythromycin.Ratsin the airgroups wereexposedtoroomair,whereasthoseinthehyperoxia groupswereexposedtoO2concentrations>85%andCO2< 0.5%.Temperatureswerekeptat25-26◦Candhumidityat 60-70%,andtheoxygenandCO2levelsinthechamberwere monitored continuously with gas analyzers.12 The caudal
veinofthepretermratswasinjectedwithsodiumchloride
(0.15mL/kg)inthesodiumchloridegroups,and
erythromy-cin(50mg/kg) intheerythromycin groups.At one,seven,
and14daysofexposure,eightpupsfromeachgroupwere
anesthetizedand euthanized.Protein was extracted from
theleftlung, andtheright lungwasfrozen andstoredat
---70◦CinarefrigeratorforRT-PCR.
The study was approved by the experimental animal
welfaremanagementandethicscommitteeofShanghai
Chil-dren’s Hospital, Shanghai Jiao Tong University, Shanghai,
China.
DetectionofGSHandIL-1betainpulmonarytissue
homogenatesbyELISA
Lung tissues were collected, and total proteins were extracted using a protein extraction kit. Protein concen-tration wasmeasured usingtheBradford method(Bio-Rad - California,USA). GSH andIL-1 beta in pulmonary tissue homogenates were detected by ELISA kits obtained from NanjingJianchengBiologicalTechnologyCo.Ltd.,Nanjing, ChinaandWuhanHuameiCusabioBiologicalTechnologyCo. Ltd.,Wuhan,China,respectively.
All reagentswereallowedtoreachroom temperature. Therequired numberofstripswerearrangedandlabeled. 100-L of reagents were added to wells of polystyrene ELISA plates,and the wells werethoroughly washedwith phosphatebuffered saline(PBS)containing0.1% Tween-20 (PBS-Tween) (Bio-Rad Laboratories, CA, EUA) after each incubationstep.Allreagentswereprepared,including work-ingstandardsandsamples.100uLofstandards,controls,or samples wereadded tothe wellsand were incubatedfor twohoursat37◦C.Afterthewellswerewashed,100uLof goatanti-mouseGSH(orIL-1beta)polyclonalantibodywas addedtoeachwell(incubation,37◦C,30min).After exten-sivewash,100uLofrabbitanti-goatimmunoglobulinG(IgG) wasaddedtoeachwellforonehourat37◦C.Aftersubstrate solutionandstopsolutionincubation,theopticaldensityof each well wasread within30minutes,using amicroplate readersetto450nm.
DetectingofGSHproteinconcentrationsin
pulmonarytissuehomogenatesthrough
bicinchoninicacid(BCA)
Following the standard protocol for the Micro BCA Pro-tein AssayKit (Beijing BaitaikeBiological TechnologyCo.,
Beijing, China), the working solution consisted of 1 vol-umereagentCmixedwith25volumesofreagentB;then, 26 volumes of reagent A were added to the C/B mix-ture. The pH value of the working solution was 11.16 ± 0.06, measured with an Orion 310 (Thermo Scien-tific, MA, EUA) pH meter. Completely dissolved protein standard (5mg/ml), 10L diluted to 100L, so that the finalconcentrationwas0.5mg/ml,wouldbediluted stan-dards according to 0,1,2,4,8,12,16,20L respectively to 96-wellplate,andultra purewaterwouldall standardup to 20L, and 10L samples to 96-well plate, plus ultra pure water release liquid to 20L, the hole added with 200L BCA the working solution, gently tap the plate to ensurethoroughmixingwithasample addinggun,cooling thesamplestoroomtemperaturefrom37◦Cfor30-60min. Each measurement was performed in duplicate. All the absorbances were corrected by the corresponding blank replicate.Theabsorbance oftheblanksolutionwas0.048 ± 0.006. Absorbance at 562nm was measured by spec-trophotometerusingglasscuvetteswithopticalpathlength of0.1cm.
Expressionof␥-GCSmRNAdetectedbyRT-PCR
TotalRNA wasextractedusingtheRNAgentTotalRNA Iso-lation System (Promega Corporation, WI, EUA) according to the manufacturer’s instructions. The purity and yield of total RNA were determined spectrophotometrically by measuring the absorbance of an aliquot at 260nm and 280nm. RNA (4g) was reverse-transcribed into 50L of complementaryDNA(cDNA)usingtheM-MLVReverse Trans-criptase system (Jingmei Biotech Ltd, Shenzhen, China). The primer sequenceswere designed by Shanghai Biology EngineeringCo.,China,inaccordancewiththeliterature: ␥-GCS,forward:5′-TTGGCAGCCTTCCTGATTTC-3′,reverse: 5′-AACTTCTCCACAACCCTCTG-3′, product size 78bp; -actin, forward: 5′-AAC GCAGCTCAGTAACAGTC-3′, reverse: 5′-ATCCGT AAAAGCCTCTATGC -3′, productsize 280bp. ␥-GCSand -actinPCR reactionmixtures weresubjected to incubation for 5min at 94◦C, followed by 35 cycles of 94◦ C for 45 s, 50◦ C for one min, and 72◦ C for 30 s. A final extension was carried out at 72◦ C for ten min. PCR products were separated by electrophoresis on 2% agarosegels, stained withethidiumbromide(0.5g/mL), and observed using a UV transilluminator and evaluated usinga GDS-8000gel imagesystem (UVP Co., Cambridge, United Kingdom) by comparing the intensity of target product bands with that of -actin used as the internal standard.
StatisticalAnalysis
0 2 4 6 8 10 12 14
14d 7d
1d
day
Expression of GSH protein
(ng/ml)
Group 1
Group 2
Group 3
Group 4 a
a
b b
b
Figure1 ExpressionofGSHproteininprematureratlungsdetectedbyELISA.
GSH,glutathione;ELISA,enzyme-linkedimmunosorbentassay.
ap<0.05,comparedwithgroup1. bp<0.05,comparedwithgroup3.
Results
EffectoferythromycinonGSHin
hyperoxia-exposedlungtissue
Comparedwithgroup1,expression ofGSHin group3was significantlyincreased(p <0.05)atoneandsevendaysof exposure,butshowednosignificantreduction(p>0.05)at 14days.Comparedwithgroup3,expressionofGSHingroup 4wassignificantlyincreasedatone,seven,and14daysof exposure(p<0.05);thegeneraltendencydecreasedafter 14days(Figs.1and2).
EffectoferythromycinonIL-1betain
hyperoxia-exposedlungtissue
Comparedwithgroup1,expressionofIL-1betaingroup3 wassignificantlyincreased(p<0.05)atsevendaysof expo-sure;itsexpression wassignificantlyreduced(p<0.05)at 14daysofexposure.Comparedwithgroup3,expressionof IL-1betaingroup4becamesignificantlyreducedatoneand sevendaysofexposure(p<0.05)(Fig.3).
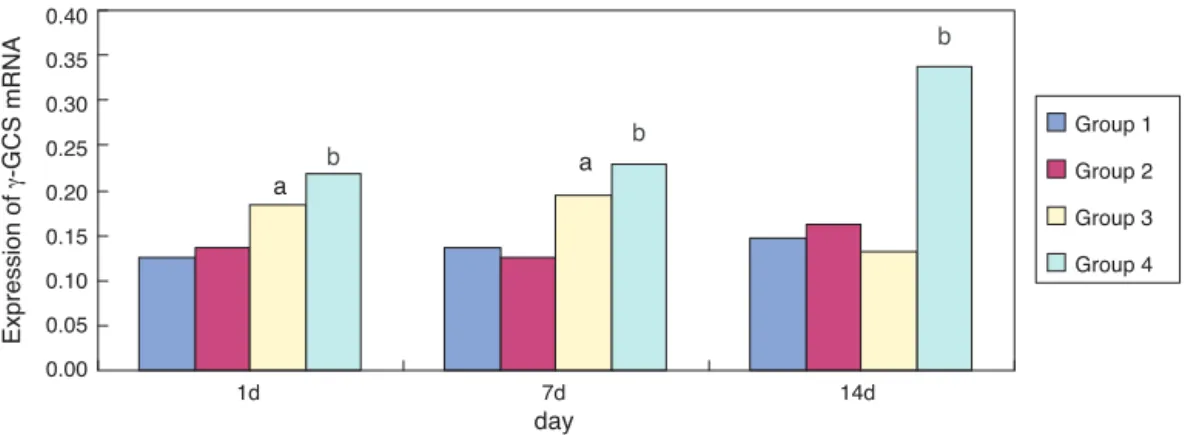
Effectoferythromycinon␥-GCSin
hyperoxia-exposedlungtissue
Comparedwithgroup1,expressionof␥-GCSmRNAingroup 3 wassignificantly increased (p < 0.05) at one and seven daysof exposure; itsexpression wassignificantly reduced (p < 0.05) at 14 days of exposure. Compared with group 3, expression of ␥-GCSmRNA in group 4was significantly increasedatone,seven,and14daysofexposure(p<0.05)
(Fig.4).Erythromycininterventionup-regulatedtheactivity
of␥-GCSmRNAparticularlyinthehyperoxia-exposedlung
tissues.
Discussion
Basedonthehistologicalfeatures,ratfetallung develop-mentcanbedividedintofourperiods:theembryonicperiod (zeroto13days),glandperiod(14to18days),canalicular stage(19to20days),andsaccularperiod(21to22days). Thesaccularperiodduringthedevelopmentofhumanlung correspondsto28to34weeksofgestationalage,theage ofbirth ofmostpretermneonates. Thepostnatal ratlung development is divided into threeperiods: the expansion
0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40
14d 7d
1d
day
Expression of GSH protein (mg/ml)
Group 1
Group 2
Group 3
Group 4
b b
b
a a
Figure2 ExpressionofGSHproteininprematureratlungsdetectedbyBCA.
GSH,glutathione;BCA,bicinchoninicacid.
0 5 10 15 20 25 30
14d 7d
1d
day
Expression of IL-1 beta
protein (ng/ml)
Group 1
Group 2
Group 3
Group 4 b
b a
a a
Figure3 ExpressionofIL-1betaproteininprematureratlungsdetectedbyELISA.
IL-1beta,interleukin-1beta;ELISA,enzyme-linkedimmunosorbentassay.
ap<0.05,comparedwithgroup1. bp<0.05,comparedwithgroup3.
period(onetofourdaysafterbirth),alveolarperiod(four to13daysafterbirth),andbalancedgrowthperiod(14to 21daysafterbirth).Thus,thedifferenttimepoints respec-tivelyrepresentthedifferentstagesoflungdevelopmentin preterminfantsafterbirth.
The pathogenesis and prevention of BPD in preterm infants hasmade significantbreakthroughs recently; how-ever, the exact pathogenesis of BPD remains unclear, and effective treatment is still significantly restricted.13
Macrolide antibiotics (MAs) contain the 12-22 carbon
chemical structure and belong to the lactone ring
car-bon antibiotics. Erythromycin A can inhibit the secretion
of pro-inflammatory cytokines such as tumor necrosis
factor-␣andIL-1beta.14Moreover,itisanextremely
broad-spectrumantibiotic, andhasantibacterialactivity against
Gram-positivebacteriaand someGram-negative bacteria,
anaerobic bacteria, Legionella, Chlamydia, Mycoplasma,
andRickettsia.15 Long-term clinical practiceand in-depth
pharmacological studies have showed that MAs not only
have antibacterial effects, but also possess non-specific
anti-inflammatory, anti-allergic, and immune regulation
properties.16 The main role ofantibiotics in somechronic
pulmonaryinflammatory diseasesmayberelatedto
inhib-itingthe oxidativeburstof neutrophilsandthereleaseof
inflammatorymediators. In addition,MAs areeffective in
preventingandtreating somerespiratorydiseases,
includ-ingasthma,pulmonaryfibrosis,diffusepanbronchiolitis,and
somenon-infectious inflammatory diseases, such asblood
diseases, skin diseases, and cancer; these functions have
nothingtodowiththeantibacterialactivities.17
Glutathione is a tripeptide-containing sulfonium
compound, composed of glycine, glutamic acid, and
cys-tine. ␥-GCS is the rate-limiting enzyme of GSH synthesis
thatregulates intracellularGSH levels.18 GSH isactivated
bytheinvivooxidation/reductionsystem,andprovidesthe
reductant for cystine, inhibiting the body’s production of
varioussubstancesin the processof oxidation of reactive
oxygen species (ROS), inactivating activity of membrane
peroxidase and inhibiting ROS, thus reducing ROS. Most
researchers have recognized that ROS caused by
oxida-tive stress has an important role in the development of
hyperoxia-inducedlunginjury.19Severalstudieswithinvivo
and in vitro experiments have demonstrated that, as an
important antioxidant, GSH played an important role in
maintainingtheairwayepithelialcellintegrityandresisting
lunginjuryandinflammation.20
Inthepresentstudy,comparedwiththeair+sodium
chlo-ridegroup,GSHexpressioninlungtissuesofprematurerats
0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40
14d 7d
1d
day
Expression of
γ
-GCS mRNA
Group 1
Group 2
Group 3
Group 4 b
b b
a
a
Figure4 Expressionof␥-GCSmRNAinprematureratlungsdetectedbyRT-PCR.
␥-GCS,␥-glutaminecysteinesynthetase;RT-PCR,reversetranscription-polymerasechainreaction.
wassignificantlyenhancedaftererythromycinintervention onday one, seven, and 14 in the erythromycin + sodium chloride group (p < 0.05); itsexpression wassignificantly enhancedondayoneandsevenafterexposuretohyperoxia in the hyperoxia + sodiumchloride group, and decreased significantlyonday14.GSH expressionin thehyperoxia+ erythromycingroups wassignificantlyenhancedunderthe exposuretohyperoxiaanderythromycininterventiononday one,sevenand14,butshowedasignificantdownwardtrend onday14.GSHexpressiondetectingbyBCAconfirmedthe ELISAresults.Afterexposuretohyperoxiaondayoneand seven,GSHexpressionwassignificantlyenhanced.Thebody mayhavesomemechanismforself-protectionandcanresist hyperoxicinjury.Asintracellular ROSincreases,thesulfur groups of cysteine in GSH have a strong affinity activity, andcanbeusedaselectrophilictargetsthatcombinewith ROS.TheyalsohavearoleineliminatingROSandlipid per-oxidation, thus avoiding alveolar cell membrane damage. However,exposuretohyperoxiacausedGSHproteinin alve-olar epithelial cells to be severely damaged by oxidative stressonday14,andGSHexpressionshowednosignificant reduction.
␥-glutamine-cysteine synthetase is the rate-limiting enzymeofGSHproteinsynthesis,whichregulates intracel-lularlevelsofGSH.21Thepresentstudydemonstratedthat
theinterventionoferythromycincaninhibitup-regulationof
GCSproteinlevelsinlungtissuesbyhyperoxiaexposureon
dayoneandseven(p<0.05);theinterventionof
erythromy-cinhadnoobviousinfluenceonhyperoxiaexposureonday
14,but␥-GCSmRNAexpressionwassignificantlyenhanced
ondaysevenand14(p<0.05),whichmayberelatedto
rel-evantregulatory proteins after ␥-GCS mRNAtranscription
becauseofhyperoxiaexposuredamage,resultingin
eryth-romycininhibiting theup-regulation ofGCSprotein levels
byhyperoxiaexposure.
Infection and inflammatory reactions are key factors
in thepathogenesis of BPD in preterm infants, which has
beenconfirmedbyanimalandclinicalstudies.22Ithasbeen
reportedthatIL-1beta asaproinfammatory cytokinehas
a central positionin the pathogenesisof BPD and has an
importantpathogenicroleinacuteandchroniclunginjury
inpreterminfants.23Inthepresentstudy,itwasfoundfound
that,comparedtotheairgroups,theexpressionofIL-1beta
inthelungtissueofprematureratsofhyperoxiagroupswas
significantly increased on day seven and reduced on day
14.Moreover,compared withthesodiumchloride groups,
theexpressionofIL-1betawassignificantlyreducedinthe
erythromycingroupsondayoneandseven.Bycontrast,the
expression of GSH in thelung tissueswas enhancedafter
theinterventionoferythromycinondayone,seven,and14.
Theseresultsdemonstratedthattheprimaryroleof
eryth-romycin may be related to inhibiting the oxidative burst
ofneutrophilsandthereleaseofinflammatorymediators.
Thus,oneofthemain mechanismsofMAsin treatingBPD
inthepreterminfantsmaybetheinhibition ofneutrophil
oxidativeoutbreakandthereleaseofinflammatory
media-tors.
In summary, erythromycin can inhibit the oxidative
outbreak of neutral granulocytes in lung tissue, improve
the antioxidant role of GSH, inhibit the release of the
inflammatory cytokine IL-1 beta, and thus has an
impor-tant rolein reducing oxidative stress in the development
of hyperoxia-induced lung injury, which may provide
a new theoretical basis for the clinical treatment of
hyperoxia-inducedlunginjury.
Funding
This work was supported by funding from the Shanghai Science and Technology Committee (Project Number: 134119a0500).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.JobeAJ.ThenewBPD:anarrestoflungdevelopment.Pediatr Res.1999;46:641---3.
2.JobeAH.Thenewbronchopulmonarydysplasia.CurrOpin Pedi-atr.2011;23:167---72.
3.FarstadT,BratlidD,MedbøS,MarkestadT,NorwegianExtreme PrematurityStudyGroup.Bronchopulmonarydysplasia- preva-lence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr. 2011;100: 53---8.
4.SaugstadOD.Bronchopulmonarydysplasia-oxidativestressand antioxidants.SeminNeonatol.2003;8:39---49.
5.FernandesLV,GoulartAL, SantosAM,BarrosMC,GuerraCC, KopelmanBI.Neurodevelopmentalassessmentofverylowbirth weightpreterminfantsatcorrectedageof18-24 monthsby BayleyIIIscales.JPediatr(RioJ).2012;88:471---8.
6.DundarozR, Erenberk U, TurelO, DemirAD, OzkayaE, Erel O. Oxidativeand antioxidative statusof childrenwithacute bronchiolitis.JPediatr(RioJ).2013;89:407---11.
7.Hassanin KM, Abd El-Kawi SH, Hashem KS. The prospective protectiveeffectofseleniumnanoparticlesagainst chromium-induced oxidative and cellular damage in ratthyroid. Int J Nanomedicine.2013;8:1713---20.
8.Kim BY, Kwak SY, Yang JS, Han YH. Phosphorylation and stabilizationofc-MycbyNEMOrenders cellsresistantto ion-izing radiation through up-regulation of ␥-GCS. Oncol Rep. 2011;26:1587---93.
9.Swords WE,Rubin BK.Macrolide antibiotics,bacterial popu-lations and inflammatory airway disease. Neth J Med. 2003;61:242---8.
10.Amado-RodríguezL,González-LópezA,López-AlonsoI,Aguirre A,AstudilloA,Batalla-SolísE,etal.Anti-inflammatoryeffects ofclarithromycininventilator-inducedlunginjury.RespirRes. 2013;14:52.
11.Nair PM, Park SY, Chung JW, Choi J. Transcriptional regu-lationof glutathione biosynthesisgenes. ␥-glutamyl-cysteine ligaseandglutathionesynthetaseinresponsetocadmiumand nonylphenolinChironomusriparius.EnvironToxicolPharmacol. 2013;36:265---73.
12.YamJ,FrankL,RobertsRJ.Oxygentoxicity:comparisonoflung biochemicalresponsesinneonatalandadultrats.PediatrRes. 1978;12:115---9.
13.Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 8 days) postnatalcorticosteroidsfor preventing chronic lungdisease in preterm infants. Cochrane Database Syst Rev. 2010;(1): CD001146.
antibiotic from a marine-derived Micromonospora sp. Mar Drugs.2013;11:1152---61.
15.SatoK, SugaM,Akaike T, FujiiS, Muranaka H,Doi T, etal. Therapeuticeffectoferythromycinoninfluenzavirus-induced lung injury in mice. Am J Respir Crit Care Med. 1998;157: 853---7.
16.GotfriedMH.Macrolidesforthetreatmentofchronicsinusitis, asthma,andCOPD.Chest.2004;125:52S---60S,quiz60S-61S.
17.TamaokiJ.The effects ofmacrolides oninflammatory cells. Chest.2004;125:41S---50S,quiz51S.
18.RahmanI,vanSchadewijkAA,HiemstraPS,StolkJ,vanKrieken JH,MacNeeW,etal.Localizationofgamma-glutamylcysteine synthetasemessengerrnaexpressioninlungsofsmokersand patientswithchronicobstructivepulmonarydisease.FreeRadic BiolMed.2000;28:920---5.
19.Northway Jr WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonarydysplasia.NEnglJMed.1967;276:357---68.
20.Rahman I,MacNee W.Oxidativestress andregulationof glu-tathioneinlunginflammation.EurRespirJ.2000;16:534---54.
21.ChenHH,KuoMT.Roleofglutathioneintheregulationof Cis-platin resistance in cancer chemotherapy. MetBased Drugs. 2010:2010,pii:430939.
22.RochaG.Chorioamnionitisandlunginjuryinpretermnewborns. CritCareResPract.2013;2013:890987.