ContentslistsavailableatScienceDirect
Behavioural
Brain
Research
j o ur na l h o me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / b b r
Research
report
Rosmarinic
acid
prevents
against
memory
deficits
in
ischemic
mice
Analu
Aragão
Fonteles
a,c,
Carolina
Melo
de
Souza
b,
Julliana
Catharina
de
Sousa
Neves
a,
Ana
Paula
Fontenele
Menezes
b,
Marta
Regina
Santos
do
Carmo
a,
Francisco
Diego
Pinheiro
Fernandes
b,
Patrícia
Rodrigues
de
Araújo
b,
Geanne
Matos
de
Andrade
a,b,c,∗aPost-GraduateProgrammeinPharmacology,DepartmentofPhysiologyandPharmacology,Fortaleza,Brazil
bPost-GraduateProgrammeinMedicalSciences,DepartmentofMedicine,FacultyofMedicine,FederalUniversityofCeará,Fortaleza,Brazil cInstituteofBiomedicineofBrazilianSemi-Arid,Fortaleza,Brazil
h
i
g
h
l
i
g
h
t
s
•Rosmarinicacidpreventsmemorydeficitsinducedbypermanentfocalcerebralischemia.
•Rosmarinicacidinducedsynaptogenesisinischemicmice.
•IncreasedBDNFwereobservedinischemicmicetreatedwithrosmarinicacid.
•RosmarinicaciddiminishedMPOactivityandastrogliosisinischemicmice.
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received10August2015 Receivedinrevisedform 17September2015 Accepted23September2015 Availableonline9October2015
Keywords: Cerebralischemia Memorydeficits Polyphenols Rosmarinicacid
a
b
s
t
r
a
c
t
Polyphenolshaveneuroprotectiveeffectsafterbrainischemia.Ithasbeendemonstratedthatrosmarinic acid(RA),anaturalphenoliccompound,possessesantioxidantandanti-inflammatoryproperties.To evaluatetheeffectivenessofRAagainstmemorydeficitsinducedbypermanentmiddlecerebralartery occlusion(pMCAO)miceweretreatedwithRA(0.1,1,and20mg/kg/day,i.p.beforeischemiaandduring5 days).Animalswereevaluatedforlocomotoractivityandworkingmemory72hafterpMCAO,andspatial andrecognitionmemories96hafterpMCAO.Inaddition,inanothersetofexperimentsbraininfarction, neurologicaldeficitscoreandmyeloperoxidase(MPO)activitywereevaluates24hafterthepMCAO. Finally,immunohistochemistry,andwesternblot,andELISAassaywereusedtoanalyzeglialfibrillary acidicprotein(GFAP),andsynaptophysin(SYP)expression,andBDNFlevel,respectively.Theworking, spatial,andrecognitionmemorydeficitsweresignificantlyimprovedwithRAtreatment(20mg/kg).RA reducedinfarctsizeandneurologicaldeficitscausedbyacuteischemia.ThemechanismforRA neuro-protectioninvolved,neuronallosssuppression,andincreaseofsynaptophysinexpression,andincrease ofBDNF.Furthermore,theincreaseofMPOactivityandGFAPimmunireactivitywerepreventedinMCAO grouptreatedwithRA.TheseresultssuggestthatRAexertsmemoryprotectiveeffectsprobablydueto synaptogenicactivityandanti-inflammatoryaction.
©2015ElsevierB.V.Allrightsreserved.
1. Introduction
Strokeisthemajorcauseofdisabilityandthefourthleading causeofdeathworldwide.Approximately100,000deathsoccurs annuallyduetostroke[1,2,].Ischemicinjury isassociated with
∗Correspondingauthorat:Post-GraduateProgrammeinPharmacology, Depart-mentofPhysiologyandPharmacology,FacultyofMedicine,FederalUniversityof CearáRuaCel.NunesdeMelo1127,PorangabussuFortaleza60430-270,CE,Brazil. Fax:+558533668333.
E-mailaddress:gmatos@ufc.br(G.M.deAndrade).
vascularleakage, inflammation,tissue injury,andcelldeath[3]. Cellularchangesassociatedwithischemiaincludeimpairmentof metabolism,energy failure,free radicalproduction, excitotoxic-ity,alteredcalciumhomeostasis,andproteaseactivation;allthese eventsaffectbrainfunctionandcontributetolongterm disabili-ties[4].Ithasbeenconfirmedthatstrokecanresultincognitive impairment,andtheprevalenceofpost-strokecognitive impair-mentrangesfrom20% to80%[5]and morethana thirdof the patientshavecognitiveimpairmentaftertransientischemicattack [6].Amechanisminvolvedincognitivedeficitsafterstrokeis synap-ticproteinloss[7].Thedisappearanceofsynapticactivityisone
ofthefirstconsequencesofischemia[8].Abloodflowlessthan 20%leadstoalossofaxonsanddendritesinminutes[9,10]. More-over, inflammation plays a significantrole in the pathogenesis ofischemicstroke[11].Theinflammationresponsesafterbrain ischemiaincludeastrocyteandmicroglialactivation,followedby peripheralinflammatorycellinfiltrationinthecoreandpenumbra zoneofischemia[12,13].Ischemiaevokesstrongreactive astroglio-sisthatactivatesinducednitricoxidesynthase(iNOS)andNADPH oxidasetoproduceNOandsuperoxide,whichareinvolvedinlipid peroxidation,andthereleaseofcytokines,suchasTNF andIL-1 [12].Thesecytokines,dependingontheconcentrations,mayinhibit synaptictransmissionoractasneuromodulators[14].Afterbrain ischemia,astrogliosishasbothbeneficialanddeleteriouseffects andastrocyteactivationisanimportantproliferativecomponent duringsynaptogenesis[8,15].Studiesshowthatastrocytesrelease mediatorsthatinfluencefromthegenesisofsynapsesuntiltheir stabilization[16,17].Inadditiontoastrogliosis,neurotrophic fac-torssuchasBDNFplayapivotalroleinsynapticplasticity[18], neuronalsurvival, and growth[19].Studiesshowthat systemic administrationofBDNFpromotesrecoveryafterstroke[20].
Rosmarinic acid (RA) is a phenolic compound with potent antioxidantandanti-inflammatoryactivities[21].RAhas neuro-protectiveactioninanimalmodelsofneurodegenerativediseases suchasAlzheimer’sdisease[22–24]and,Parkinson’sdisease[25], aswellasinischemia/reperfusionmodels[26].However,currently, therearenoreportsregardingtheprotectiveeffectsofrosmarinic acidonmemorydeficitsfollowingfocalcerebralischemia.Thus,the aimofthepresentworkwastoinvestigatetheeffectofRAon mem-orydeficitsinducedbypermanentfocalcerebralischemia,thereby investigatingthemechanismofaction.
2. Methods
2.1. Subjects
MaleSwissmiceweighing25–30gobtainedfromtheCentral AnimalHouseofPhysiologyandPharmacologyDepartmentof Fed-eralUniversityofCearáwereused.Animalswerehousedundera
12-h-light,12-h-darkcycleand allowedfreeaccesstofoodand water.Allproceduresinthisstudywereinagreementwiththe GuidefortheCareandUseofLaboratoryAnimalsbytheInstitute forLaboratoryAnimalResearchoftheNationalResearchCouncil publishedbytheNationalAcademiesPress(Washington,District ofColumbia,USA)andwereapprovedbytheethicscommitteeon animalexperimentationoftheFederalUniversityofCeará.
2.2. Drugs
The following drugs were used: rosmarinic acid (SIGMA, USA);xylazine(2%,Kensol®
,König,Argentina)andketamine(5%, Vetanarcol®
,König,Argentina).Allotherreagentswereof analyti-calgrade.
2.3. Inductionofpermanentmediacerebralarteryocclusion (pMCAO)
Permanentmiddlecerebralarteryocclusion(pMCAO)was pro-ducedbyelectrocoagulationoftheleftmiddlecerebralarteryas reportedpreviously[27].Briefly,animalswereanaesthetizedwith xylazine (10mg/kg, intraperitoneally) and ketamine (90mg/kg, intraperitoneally), an incision was made on the left temporo-parietalregion,andthetemporalismusclewaspartiallyremoved.A burrholewasdrilledintotheskulloverthemiddlecerebralartery andthevesselwasoccludeddirectly,proximaltothelateral lenticu-lostriatebranches,usingelectrocoagulationwithamicro-unipolar coagulator.Thecompleteinterruptionofthebloodflowwas con-firmedbyvisualinspection.Bodytemperaturewaskeptcloseto 37◦C. Sham-operatedanimals(SOgroup)weresubjectedtothe
sameprocedure,withtheexceptionofcauterizationofthe mid-dlecerebralartery.Thirtyminutesbeforeand 1hafterpMCAO, theanimalsreceivedeitherNaCl0.9%(pMCAOgroup)orRA(0.1,1 and20mg/kg/day,intraperitoneally)for5consecutivedays.There weretwogroupsofsham-operatedanimals:onegroupreceived NaCl0.9%andtheothergroupwastreatedwithRA(20mg/kg/day). Inthefirstsetofexperiments,animalsweretestedfor neurologi-calevaluationandeuthanizedforischemicdamageevaluation24h
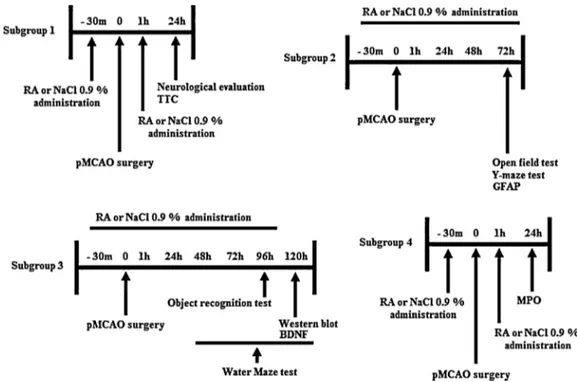
afterischemia(n=6/group).Inasecondset,animalswere evalu-atedforlocomotoractivityandworkingmemory(n=8/groups)at 72haftersurgery,andimmunohistochemistry(n=4/group)at96h aftersurgery.Inathirdset,animalswereevaluatedat4and5days for recognitionandspatial memories(n=8/group) and western blot(n=4/group).Immunohistochemistryandwesternblot anal-ysisandbehavioralinvestigationswereperformedattheindicated timepoints(Fig.1).
2.4. QuantificationofinfarctareathroughTTCstaining
Six animals of each group were euthanizedby cervical dis-location 24h after ischemia. Brains were removed and 2-mm coronal sections were made from the prefrontal cortex to the midbrain. Slices were immersed in 1% solution of TTC (2,3,5-triphenyltetrazolium)innormalsalineat37±◦Cfor15min.This
saltacceptsaprotonfromsuccinatedehydrogenaseintheinner membraneofthemitochondriawhichreducesittoitsred, insol-ubleformknownasformazan[28].Thus,anareawithinactive enzymesandtheinfarctionisnotstainedandappearspale.The unstainedareasweremeasuredbytheOsirissoftware(University ofGeneva,Switzerland,Switzerland)andcalculatedasthepercent oftheentirecoronalsection.
2.5. Neurologicalevaluation
Neurologicalevaluationwasperformed24hafterischemiaby aninvestigatorwhowasblindedtotheexperimentalgroupdesign. Theneurologicfindingswerescoredusingascaledescribed previ-ouslybyGarciaetal.[29].Sixitemsweremeasuredandthetotal scorerangedfrom3to18;thehigherthescore,thebetterthemotor performance.Items1–4(spontaneousactivity,symmetryof move-ments,symmetryofforelimbs,andclimbingthewallofwirecage) measuredmotorperformance,items5–6(reactiontotouchonand responsetovibrissaetouch)measuredsensoryfunction(Table1).
2.6. Open-fieldtest
Theanimalsinthesubgroup2weretestedforlocomotor activ-ityusinganopen-fieldapparatus,whichconsistedofablackacrylic chamber(30×30cm)withthefloordividedintoninesquaresof equalareas[30].Seventy-twohoursaftersurgery,theanimalwas positionedinthecentreofthearenaandallowedtoexplorefreely. Thenumberofcrossings(horizontalexploration)andrearings (ver-ticalexploration)werescoredfor5min.Thearenawascleanedwith 20%ethylalcoholtoremoveanyodorsbeforethenexttest.
2.7. Y-mazetest
The third day after surgery, spatial working memory was assessed byrecordingspontaneousalternation (SA)behavior in theY-maze[31].Themazewasconstructedofwhitewoodwith threeidenticalarms(40×15×4.5cm)positionedatequalangles. Animalswereplacedattheendofonearmandallowedtomove freelythroughthemazeduring an8-minsession. Theseriesof armsentrieswasrecordedvisuallyandarmentrywasconsidered tobecompletewhenthehindpawsofthemicewerecompletely placedinthearm.Theabilitytoalternaterequiresthatthemice torememberwhicharmshavealreadybeenvisited.Each experi-mentwasscored,andthepercentageofspontaneousalternation wascalculatedusingthefollowingformula:
SAs(%)= Alternationbehavior
Maximumalternations×100
wherealternationbehaviorisdefinedasconsecutiveentriesinto eachofthethreearms,withoutrepetition,andmaximum alterna-tionsarethetotalnumberofarmentries,minustwo.
2.8. Objectrecognitiontest
Thefourthdayaftersurgery,recognitionmemorywasassessed by the object recognitiontest [32]. Theobject recognitiontest wasperformedin ablack acrylicchamberopen-fieldapparatus (30×30cm).Animalswereplaced intheexperimentalroomat least30minbeforetesting.Intheacquisitionsessionanimalswere allowedtofreelyexploretwoobjectsplacedinthebackleftand rightcornersoftheapparatusfloor-fixedfor10min.Theanimals were placed at the mid-point of the wallopposite thesample objects.Whenplaced,itsbodywasparalleltothesidewallsand itsnosepointingawayfromtheobjects.Aftertheplanned sample-objectexposuretime,theanimalwasremovedfromtheapparatus andreturnedtothecage.Theobjectswerecleanedbetweeneach trialusing20%ethylalcoholtoavoidodortrails.Inthetest ses-sion (1h after acquisition session), one of the familiar sample objectswasplaced inonebackcorneroftheapparatusand the novelobjectintheotherbackcorner.Theobjectsweredifferent in shapesandcolors. Recognitionmemorywasevaluatedusing arecognitionindex(RI)calculatedforeachanimalusingthe for-mula:(N−F/N+F)correspondingtothedifferencebetweenthe timeexploringthenovel(N)andthefamiliarobject(F),corrected fortotaltimeexploringbothobjects.
2.9. Watermazetest
Inthisversionofthewatermazetest,previouslydescribedby Morrisetal.[33],themicelearntoswim atashortestpossible
Table1
DefinitionoftheneurologicalscoreaccordingtoGarciaetal.[29].
Test Score
0 1 2 3
Spontaneousactivity(in cagefor5min)
Nomovement Barelymoves Movesbutdonotapproach atleastthreesidesofthe cage
Movesandapproachat leastthreesidesofthe cage
Symmetryofmovements (4limbs)
Contralateralside:no movement
Contralateralside: slighmovement
Contralateralside:moves slowly
Bothsides:move symmetrycally Symmetryofforelimbs
(outstretchingwhileheld bytail)
Contralateralside:no movement,no outreaching
Contralateralside: slighmovementto outreach
Contralateralside:moves andoutreacheslessthan therightside
Symmetricaloutreach
Climbingwallofwirecage ... Failstoclimb Contralateralsideisweak Normalclimbing Reactiontotouchoneither
sideoftrunk
... Noresponseon Contralateralside
Weakresponseonright side
Symmetricalresponse
Responsetovibrissaetouch ... Noresponseon contralateralside
Weakresponseon contralateralside
distancefromtheedgesofawatertankforahiddenplatformbelow thesurface.Theylearnguidedbycluesinthewallsorothervisual stimuliexternaltothetank.Thistaskspaceisdependentonthe hippocampus.
Theanimalwasplacedrandomlyinacircularpool(90cm diam-eter×60cmdeep)containingturbidwater(whitenontoxicpaint) at26◦C,dividedspatially intofourquadrants,andshouldfinda platform(7cmdiameter)submerged2cmbelowthewaterlevel. Theanimalhad60stofindtheplatform(whichremainedinthe sameplaceeverytrail)andremainedtherefor10s.Thistraining wasconductedfourtimesadayatintervalsof30sfortwodays (learning)and48hafterthelasttrainingsession,theanimalswere tested,nowwithouttheplatform,whichwasassessedmemory retention.On occasion,theanimalremainedinthepoolfor60s andwasrecordedatthetimethattheanimalremainsinthe quad-rantwheretheplatformshouldbe,thelatencytimetofindthe locationoftheplatformandthenumberoftimeshecrossedthe exactlocationtheplatform.
2.10. MyeloperoxidaseassayBradleyetal.[34]
Eightanimalsofeachgroupwereeuthanized24hafterpMCAO andcortex,hippocampusandstriatumwereremoved.Briefly,brain sampleswerehomogenized(50mg/mL)inasolutionofphosphate buffer(PBS)(50mM,pH6.0)containing0.5%of hexadecyltrimethy-lammoniumbromide(HTAB)(Sigma–Aldrich,USA).Homogenates werecentrifugedat 14,000g,4◦C, for2min,and then30L of
thesupernatant was mixed with 0.167mg/mL of o-dionisidine dihydrochloride(Sigma–Aldrich, USA)and 0.0005%ofhydrogen peroxide(CarloHerba, Italy)inafinal volumeof230L,plated ina96wellplate.Thentheabsorbancechangeat460nmwas mea-suredwithaplatespectrophotometerin0.1and3min.Oneunit ofactivitywasdefinedasthatdegrading1molofperoxide/min. ResultsarepresentedasMPOactivity(U/mgtissue).
2.11. Immunohistochemistry
Brainswerefixedwith4%paraformaldehydebytranscardiac perfusionandpost-fixedinthesamesolutionfor24hat4◦Cand
thenstoredincryoprotectantsolutionofsucrose30%.Free-floating sectionswerefirstrinsedinphosphatebufferedsalinefor5min and then three times for 5min with Tris buffered saline(TBS, 0.05MTrizmabasebuffercontaining150mMofNaCl,pH7.2)at roomtemperature.Slicesweresimultaneouslypermeabilizedand blockedwithTBScontaining0.2%TritonX-100and10%ofnormal goatserum,for1hatroomtemperature.Afterwards,sliceswere incubated,free-floating,withthe primaryantibodyprepared in theblockingsolution(anti-GFAP,1:500dilution,rabbitpolyclonal, Sigma–Aldrich,USA)overnightat4◦C.Sectionswerethenrinsed
3×10mineach,inTBSandsubsequentlyincubated2hatroom temperature,withgoatanti-rabbitsecondaryantibodyconjugated withafluorophore(AlexaFluor594,diluted1:200intheblocking solution,Invitrogen,Portugal).Afterrising3×for10mininTBS, thebrainsectionswereincubatedwithDAPI(VectorLaboratories, UK)andthenmountedongelatincoatedslidesusingFluoromount mountingmedium(Sigma–Aldrich,USA).Theslideswereallowed todry protected fromlight beforebeingstored at −20◦C until
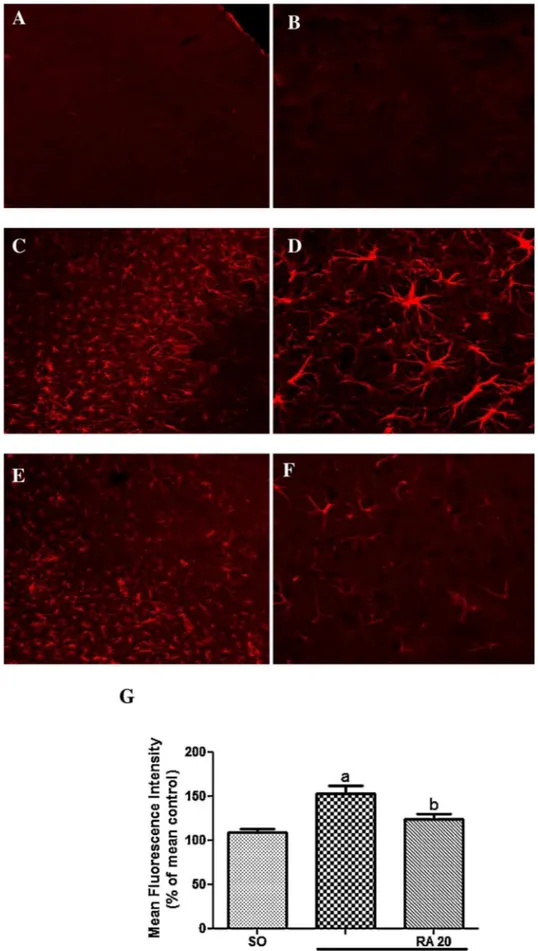
analysis.Sectionswerevisualizedunderafluorescencemicroscope (ZeissImagerZ2)orwithalaserscanningconfocalmicroscope(LSM 510Meta,Zeiss).Theimmunoreactivitywasquantifiedinaseries of8coronalsections(50mthickand300mapart) representa-tiveofthestriatumandcortexin4animalsfromeachexperimental group.Themeanfluorescenceintensity(MFI)wasusedasa mea-sureoftheimmunoreactivityandwasquantifiedusingtheprogram ImageJ.ThevaluesofMFIobtainedforthecontrolgroup(SO)were
averagedandalloftheMFIvalueswerecalculatedasapercentage ofthatmean.
2.12. Westernblot
Cortical tissue lysates were prepared fifthday after pMCAO andRAtreatment.Briefly,brainsampleswerehomogenizedin5 volumesofice-cold buffer(0.32Msucrose, 1mMEDTA, 10mM Hepes and 1% BSA, pH7.4) usinga ground-glass homogenizer. Homogenateswerecentrifugedat3000×gfor10min,thenthe supernatantwascollectedandcentrifugedat25,000×gfor1h.The pelletwasresuspendedin5%SDSwithproteaseinhibitorcocktail andproteinconcentrationwasdeterminedbytheLowrymethod. Samples were heated at 95◦C for 5min, and proteins (25g)
wereseparatedbySDS-PAGE(12%gels)andelectricallytransferred ontoa PVDFmembrane (BIORAD,USA).Theblots wereblocked with5%nonfatdrymilkinTris-bufferedsalinein0.1%Tween20 (TBS-T)at4◦Candincubatedwithrabbitanti-synaptophysin
anti-body(1:2,000;SantaCruzBiotechnology,CA)andmouseanti-␣
tubulinaantibody(1:4,000;Sigma,St.Louis,MO).Afterwashing withbufferTBS-Tthemembranewasincubatedwith horseradish-peroxidase-conjugated goatanti-mouse IgG (1:2,000; Millipore, Bedford, MA) or horseradish-peroxidase-conjugated goat anti-rabbit IgG (1:3,300; Bio-Rad; USA) for 1 hour. Immunoreactive bandswerevisualizedwith3,3′-diaminobenzidine.Thedensityof
synaptophysinimmunoreactivebandswasmeasuredusingImageJ software.
2.13. BDNFassay
Sixanimalsofeachgroupwereeuthanized120hafterpMCAO and hippocampus, and striatum were removed. Briefly, brain samples were homogenized (10% v/v) in a solution of phos-phatebuffer(PBS)(50mM,pH6.0)containingproteaseinhibitor (Sigma–Aldrich,USA).Homogenateswerecentrifugedat14.000g, 4◦C,for30min,andtheconcentrationsofBDNFinthesupernatans
weremeasuredwithELISAkit(Quantikine®ElisaR&DSYSTEMS),
accordingtothemanufacturesdirections.
2.14. Statisticalanalysis
DataareexpressedasmeanSEMorasmedian(interquartile range)and statistical differenceswereestimated usingeither a one-way analysis of variance, followed by a Newman-Keuls or Bonferronipost-hoc test,or Kruskal–WallisandMann–Whitney U-tests,withasignificancelevelof95%.
3. Results
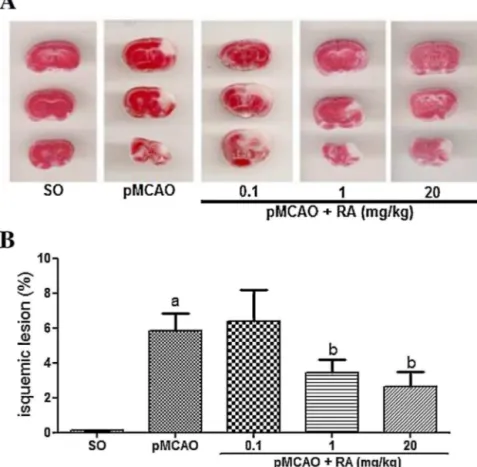
3.1. Rosmarinicacidtreatmentreducesinfarctarea24hafter pMCAO
Infarctvolumeswereevaluatedat24hafterpMCAO.The rep-resentative photomicrographs shown in Fig. 2A indicated that the infarctareas were located mainly in the ipsilateral cortex, andtoalesserextentinstriatumandhippocampus.Theanimals treated withrosmarinicacidat dosesof 1 and 20mg/kg had a significantlowerischemicneuronaldamagecomparedtopMCAO group(pMCAO+RA0.1:6.39±1.77%,pMCAO+RA1:3.43±0.7%5, pMCAO+RA20:2.65±0.85%)(Fig.2B).
3.2. RAtreatmentpreventedneurologicaldeficit24hafter pMCAO
com-Fig.2. Rosmarinicacid(RA)treatmentreducestheischemia-inducedinfarctedarea.SwissmalemicereceivedNaCl0.9%andRA(0.1,1or20mg/kg,i.p.).(A)Representative coronalsectionsstainedwith1%triphenyltetrazoliumchloride.Thepale-coloredregionshowstheischemicregionwhereasthedarkcoloredregionshowstheviabletissue. (B)Bargraphshowingthepercentageofinfarctarea24hafterpMCAO.avsSO,bvspMCAO,p<0.05.Dataareexpressedasmean
±S.E.M.,n=6pergroup,Kruskall–Wallis andMann–Whitneytest.
pared to sham-operated animals (SO: 18 (17–18); pMCAO:16 (14–17).ThepMCAOanimalspresentedreducedmotorabilityand alsodecreasedabilitytorespondtostimulionthesideofthebody contralateral toischemia.Animals treatedwithrosmarinic acid showedasignificantimprovementinneurologicaldeficitsinduced by pMCAO at doses of 1 and 20mg/kg (pMCAO+RA 0.1:15.5 (15–16),pMCAO+RA1:17.5(16–18),pMCAO+RA20:17(17–18), p<0.05)(Fig.3).
3.3. RAhasnoeffectinlocomotoractivityinpMCAOmice
Intheevaluationoflocomotoractivity72hafterischemiawere notobserved significantchanges inthe numberof crossingsor rearingsafterpMCAOand/orRAtreatment(Fig.4AandB).
3.4. RAreducesmemorydeficitsinducedbypMCAO
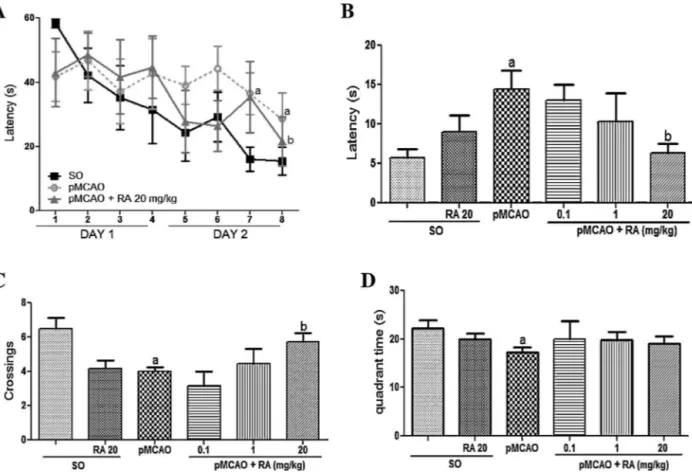
Theanimalssubjectedtoischemiashoweddeficitson work-ingmemory(SA-SO:70.2±2.5%;SO+RA20:69.2±6.0%;pMCAO: 60.9±2.6%)andontherecognitionmemory(recognition index-SO: 0.21±0.1; SO+RA 20: 0.39±0.13; pMCAO: −0.09±0.07) (Figs. 5 and 6). In assessing the retention of spatial memory, 120h after ischemia, pMCAO group had significant deficits in theparameterslatencytoreachtheplatform,number of cross-ings and time spent in the quadrant (latency: SO: 5.8±1.1s; SO+RA20:9.0±2.1s;pMCAO:14.4±2.5s, numberofcrossings: SO:6.5±0.6;SO+RA20:4.2±0.5;pMCAO:4.0±0.3,timespent inthequadrant:SO:22.3±1.6s;SO+RA20:20.0±1.2s;pMCAO: 17.2±1.0s)(Fig.7B–D).
Workingandrecognitionmemorydeficitswereprevented sig-nificantly byRA (SA:pMCAO+RA0.1: 62.9±4.3%; pMCAO+RA
Fig.3.Rosmarinicacid(RA)treatmentreducesischemia-inducedneuronaldeficits. SwissmalemicereceivedNaCl0.9%andRA(0.1,1or20mg/kg,i.p.).Bargraph showingthetotalscoresrangedfrom3to18ofsixitemsmeasured;thehigher thescore,thebetterthemotorperformance.avsSO,bvspMCAO,p<0.05.Data areexpressedasmedian(interquartilerange),n=6pergroup,Kruskall–Wallisand Mann–Whitneytest.
Fig.4. Rosmarinicacid(RA)treatmentpromotednoalterationsonmotoractivity. SwissmalemicereceivedNaCl0.9%andRA(0.1,1or20mg/kg,i.p.).(A)Bargraph showingthenumberofcrossings.(B)Bargraphshowingthenumberofrearings. Dataareexpressedasmean±S.E.M.
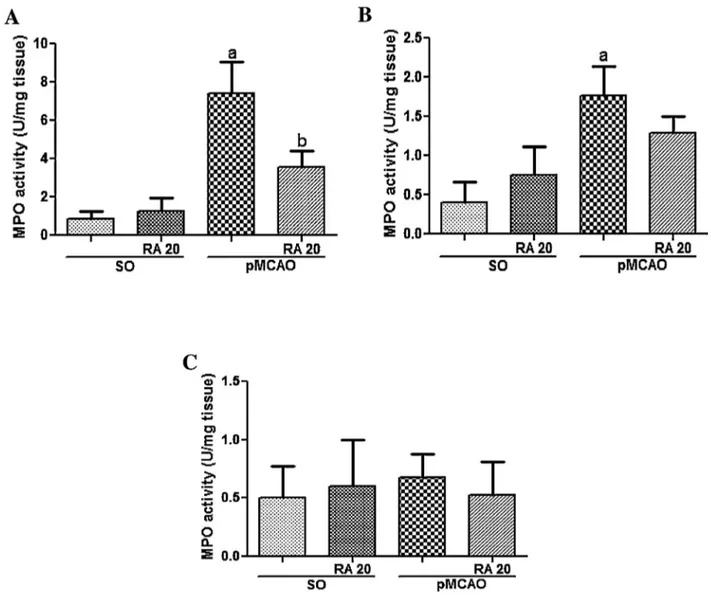
3.5. RAtreatmentpreventtheincreasedlevelsofMPOinducedby pMCAO
Ischemicmice showedincreased levelsof MPOin temporal cortex and striatum 24h after ischemia (Temporal cortex: SO: 0.85±0.42 U/mg tissue; SO+RA 20: 1.25±0.71U/mg tissue; pMCAO:7.4±1.67U/mgtissue,striatum:SO:0.39±0.27U/mg tis-sue;SO+RA20:0.75±0.36U/mgtissue;pMCAO:1.76±0.38U/mg tissue), but not in hipoccampus (SO: 0.50±0.27U/mg tissue; SO+RA 20: 0.60±0.39U/mg tissue; pMCAO: 0.68±0.20U/mg tissue).RA(20mg/kg/day)preventedsignificantlytheincreaseof MPOlevelsintemporalcortex(pMCAO+RA20:3.50±0.87U/mg tissue) but not in striatum (Striatum: pMCAO+RA 20: 1.29±0.23U/mgtissue)(Fig.8A–C).
Fig.5. Rosmarinicacid(RA)treatmentpreventsischemia-inducedworking mem-orydeficits.SwissmalemicereceivedNaCl0.9%andRA(0.1,1or20mg/kg,i.p.). Bargraphshowingthepercentageofspontaneousalterations72hafterpMCAO.a vsSO,bvspMCAO,p<0.05.Dataareexpressedasmean
±S.E.M.,n=8pergroup, Kruskall–WallisandMann–Whitneytest.
Fig.6.Rosmarinic acid(RA)treatment preventsischemia-inducedrecognition memorydeficits.SwissmalemicereceivedNaCl0.9%andRA(0.1,1or20mg/kg, i.p.).Bargraphshowingtherecognitionindex.avsSO,bvspMCAO,p<0.05.Data areexpressedasmedian(interquartilerange),n=6pergroup,Kruskall–Wallisand Mann–Whitneytest.
3.6. RAtreatmentpreventtheastrogliosis
GFAPexpressionwasfoundtobehigherinischemichemisphere ofpMCAOgroupcomparedtoSOgroup(SO:109.2±4.3%ofmean control;pMCAO:152.9±8.8%ofmeancontrol).Asignificant reduc-tioninGFAPexpressionwasobservedin ischemicmicetreated withRA(20mg)ascomparedtopMCAOgroup(pMCAO+RA20: 124.4±6.2%ofmeancontrol)(Fig.9A–G).
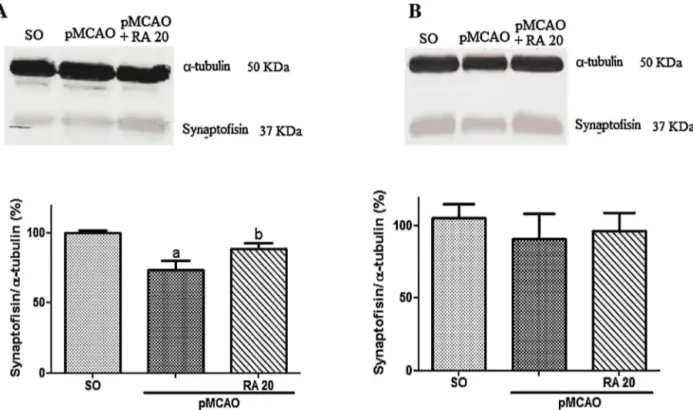
3.7. RAtreatmentpreventthedecreaseofSYPinducedbypMCAO
Ischemicanimalsshowedlowerexpressionofsynaptophysinin thestriatum5daysaftertheischemia,RAtreatmentinhibitedthe reductionofsynaptophysininischemicanimals(SO:100.0±1.9%; pMCAO:73.6±6.5%;pMCAO+RA20:88.6±4.0%)(Fig.AandC). No alterationwasfoundin thehipoccampus (SO: 105.3±9.5%; pMCAO:90.7±17.6%;pMCAO+RA20:96.2±13.1%)(Fig.10Aand B).
3.8. RAtreatmentincreasedBDNFlevelsinpMCAOmice
IschemicanimalsshowedincreasedlevelsofBDNFin hippocam-pusanddecreasedlevelsinstriatum,andRAtreatmentincreased BDNF levels in hippocampus of sham-operated and ischemic miceafter5 daysofpMCAOcomparedtopMCAOgroupandto SO group (Hippocampus: SO: 316.1±38.64pg/mL; SO+RA 20: 1148.0±92.0pg/mL; pMCAO: 516.8±49.51pg/mL; pMCAO+RA 20: 868.2±64.30pg/mL). RA treatment increased BDNF levels in striatumof ischemicmiceafter 5days of pMCAOcompared topMCAOgroup(Striatum:SO:511.2±65.8pg/mL;SO+RA20: 336.1±27.17pg/mL; pMCAO: 239.9±55.80pg/mL; pMCAO+RA 20:492.7±42.42pg/mL)(Fig.11AandB).
4. Discussion
cat-Fig.7.Rosmarinicacid(RA)treatmentpreventsischemia-inducedspatialmemorydeficits.SwissmalemicereceivedNaCl0.9%andRA(0.1,1or20mg/kg,i.p.).(A)Acquisition trailinthewatermaze,(B)latencytoreachthepreviouslocationoftheplatform,(C)numberofcrossings,(D)timespentinthequadrant.avsSO,bvspMCAO,p<0.05.Data arepresentedasmean±SEM,n=8pergroup.Kruskall–WallisandMann–Whitneytest.
echin, a polyphenol, in thedrinking water for 2 weeks. In the presentwork,infarctionareaandneurologicalevaluationshowed thatRAhadaneuroprotectiveeffectconsistentwiththeworkby Luanetal.[26]thatdemonstratedreducedcerebralinfarctvolume andneurologicaldeficitsinischemicdiabeticratsaftertreatment withRA(50mg/kg).Although,theliteraturehasdemonstratedthe neuroprotectiveeffectofRAonischemicdamage,nostudieshave demonstratedtheRAeffectonmemorydeficitinducedbycerebral ischemia.OurstudyfoundthatRAsignificantlyimprovedworking, recognitionandspatialmemoryincerebralischemicmice.
Y-mazeandobjectrecognitiontestsarerelatedtothe frontal-subcortical circuits rather than to thehippocampus [37]. Zhou et al. [38] demonstrated that transient focal cerebral ischemia lesionsofthestriatumandhippocampuscausedworkingmemory deficitsinratsassessedbytheY-mazetest.Recognitionmemory impairmentisrelatedtowhitemattersub-corticalstrokelesions inmice[39]andparietalcortexinC57B/6miceafterpMCAO[40]. Thesefindingsareconsistentwithourresultsthatshowedthatthe striatumandthecortexwerelargelyaffectedbyischemia,which inducedworkingandrecognitionmemorydeficits.Furthermore, poorperformanceintheMorriswater-mazetestwascorrelated withCA1regiondamage[41],althoughtheimpairmentinthe spa-tialmemoryinducedbypMCAOismorelikelyrelatedtocortical andstriatumdamage[42].Theneuralsubstratesmostfrequently implicatedinvariousformsofspatiallearningarethehippocampus andthedorsalstriatum,withtheformerbeinginvolvedin learn-ingwithreferencetoanarrayofdistalcues.Thisprocessisoften referredtoastheformationofa“cognitivemap”[43,33].Thedorsal striatumisinvolvedintheformationofanassociationbetweena proximalcueintheenvironmentandtheanimal’sownkinesthetic response,typicallyovermanyrepeatedtrials[44].Inourstudy,we observedthatmicewithpMCAOshowedspatialmemory
impair-ments,andRAsignificantlyattenuatedthesedeficits.RAprotects againstworkingandrecognitionmemoryimpairmentinducedby A(25–35)inmice[23]andenhancescognitiveperformancesinthe
Morriswater-mazetaskinrats[45].Previousstudieshaveshown that RAmetabolites, suchasCaffeic acid, have neuroprotective actions[46].Caffeicacidisreportedtoreducebraindamageand preventmemorydeficitsafterpermanentfocalcerebralischemia inmiceduetoanti-inflammatoryactions[47].
Becauseoxidativedamageandinflammationareimplicatedin theetiology of neurologicalcomplications incerebralischemia, floodingthesystemwithantioxidantsandanti-inflammatory com-poundsmaybeasensible step.Phenoliccompoundsareknown bytheirantioxidantandanti-inflammatoryeffects,whichinturn haveneuroprotectiveeffectsduringcerebralischemia[48]. Ros-marinicacidisaphenoliccompoundwithpotentantioxidantand anti-inflammatoryactionsandiscontainedinvariousLaminaceae herbsusedcommonlyasculinaryherbs,suchasRosmarinus offici-nalis[49,21].RAshowedpotentantioxidantactivitybymodulating H2O2-inducedcelldeathinhumandopaminergicneuronsthrough
reactiveoxygenspecies(ROS)scavenging[50].RAwasalsofoundto suppresslipidperoxidation[51].Additionally,ithasbeenreported thatRAhastheabilitytoinhibitcomplementfixationand lipoxy-genaseandcyclooxygenaseactivity[52–54].Furthermore,RAwas alsoabletoinhibitseasonalallergicrhinoconjunctivitisinhumans bysuppressingtheinflammatoryresponse[55].Inrats,RAisrapidly absorbedinthedigestivetract,andthemajormetabolitesfound inurinearecaffeicacid, ferulicacidandcoumaricacid[56].RA isalsoabsorbedinhumansafteroraladministrationandspecific metabolitescanbedetectedinurine[57].
Fig.8.Effectofrosmarinicacid(RA)20mg/kgonthemyeloperoxidase(MPO)activityinthecortex(A),inthestriatum(B)andinthehipoccampus(C)ofmicesubmittedto pMCAO.avsSO,bvspMCAO,p<0.05.Dataarepresentedasmean±S.E.M.,n=8pergroup.One-wayANOVAfollowedbyNewman–Keulsposthoc-test.
impairmentinducedbycerebralischemia.Ithasbeenshownthat RApossesses anti-inflammatoryactionsbydiminishingIL-1in anototoxicitymodelinducedbycisplatininmice[58],decreased COX-2positivecellsinamodelofcollageninducedarthritis[59], anddecreasedpro-inflammatorycytokinesIL-6,IL-1andTNF-␣
inaratmodelofsystemicinflammation[60].Furthermore,caffeic acid,amajorRAmetabolite,attenuateshippocampal inflamma-tionbydiminishing neutrophilinfiltrationin micesubjectedto pMCAO [47], RA diminished MPO activity induced by a renal ischemia/reperfusionmodelin rats[61],andotherpolyphenols, suchaspinocembrin, diminishedneutrophilinfiltrationina rat modelofglobalcerebralischemia/reperfusion[62].Forghaniand colleagues[63]demonstratedthatMPO-knockoutmicesubjected totransientMCAOshowedreducedlesionvolumeandimproved neurobehavioraleffects,suggestingthattheMPOactivityblockade is animportant neuroprotective strategy to attenuateischemic damage after 24h of stroke onset. In accordance with these findings,wealsofoundthatRA(20mg/kg)abolishedtheincrease MPOactivityafterischemicinjuryincerebralischemicmice.
BDNFis widelydistributed in nerve terminals and synapses throughout the brain, particularly in the hippocampus [64]. IncreasedBDNFlevelspromoteneuronalsurvivalandinfarct tol-erance[65].StudiesshowthatBDNFlevelsinischemicmiceare elevated,anditisprobablyduetothecleavageoftheexisting pro-BDNFinthecoreofthelesion[66,67].Inthelesionedhemisphere,
matureBDNFiselevated,althoughinthecontralateralhemisphere, bothpro-andmatureBDNFareelevated,indicatingthatinthe non-lesionedhemisphere,theoverexpressionofbothformscouldbe theresultofgeneinduction[67].TheoverexpressionofBDNFin ischemicconditionsmaydescribetheself-protectingpropertiesof neuronsafterischemicinjury[68].Inthepresentstudy,wefound anincreasinglevelofBDNFinthehippocampusofpMCAOmice 5daysafterstrokeonset.RAincreasesBDNFlevels,causing sig-nificantlyincreasedBDNFlevelsinthehippocampuscomparedto sham-operatedandischemicmice.Itoandcolleagues[69] demon-stratedthatRAinducedcellproliferationofnewborncellsinthe dentategyrusofthehippocampusofmice.Thesestudies corrobo-rateourresults,whichshowsthatRAhastheabilitytoself-induce cellproliferationthroughtheBDNFincreaseinthedentategyrus inthehippocampus;thismayexplaintheneuroprotectiveeffectof RAonmemorydeficits.Thestriatumisaregionofthebrainthat lacksBDNFmRNA[64],althoughtheBDNFproteincanbe trans-ferredbyananterogradetransport fromneuronalcellbodiesto theirterminals[70].BDNFwasfoundtobedecreasedinthecortex 7daysaftertransientMCAO,anareathatisthecoreofthesetypes ofischemiclesions[71].Thisfindingisconsistentwithourresults, whichshoweddecreasedBDNFlevelsinthestriatumofpMCAO mice5daysafterpMCAO.
Fig.10.Effectofrosmarinicacid(RA)20mg/kgontheexpressionofsynaptophysin(A)inthestriatumand(B)inthehipoccampusofmicesubmittedtopMCAO.Theresults arepresentedasmean±S.E.M.,n=4pergroup,avsSO,bvspMCAO,p<0.05.One-wayANOVAandNewman–Keulstest.
daysafterstrokeonset.Otherpolyphenols,suchasginsenosides, haveneuroprotectiveeffects, astheyincreasedneurogenesisby increasingBDNFlevelsinanoxygen-andglucose-deprivedmodel inPC12cells[72].OroxylinA, aflavonoidcompound,increased BDNFexpressioninthehippocampusandhasbeenshownto ame-liorate memory impairment and neuronal damage after global cerebralischemiainrats[68].Madinieretal.[67]demonstrated thatBDNFimmunoreactivity wascolocalizedinneuronsand in astrocytesafterpermanentfocalcerebralischemiaby photothrom-boticcorticalocclusioninrats.BDNFwasfoundtoreducecortical celldeathaftertransientMCAOinrats[73]andhasbeenshownto beessentialforsynaptogenesisandsynapticstructural composi-tionintheadultbrain[74,75].TherelationshipbetweenBDNFand synaptogenesisiswidelystudied.Chenetal.[76]haveshownthat statin,aBDNFupregulationinductor,isrelatedtoanincreaseof synaptophysinexpressionandtofunctionalrecoveryafterstroke. Pozzo-Milleretal.[77]haveshownthatBDNFknockoutmicehave areducedlevelofsynaptophysininhippocampalsynaptosomes.Jin etal.[78]havedemonstratedthatRA(10mg/kg)exertsa
neuropro-tectiveeffectbyincreasingastrocyticBDNFinthehippocampusvia modulationofERKphosphorylationinchronicunpredictablestress. Takentogether,synaptogenesisandneurogenesisaremechanisms involvedinlearningandmemoryrecoveryafterischemicevents [79,80].Thesemechanisms may,inpart,beresponsible forthe neuroprotectiveeffectofRAonmemorydeficitsinpMCAOinjured mice.
Synaptophysinhasarole inthebiogenesisofsecretory vesi-cles,stabilizationandmodificationofthefunctionofothersynaptic proteinsandprotectionduringischemicbraininjury.Itisusually overexpressed during neuronal remodeling [81]. Increased lev-elsof synaptophysinare interpretedtoindicate synaptogenesis [82].Ischemicpatientsshowmotorandcognitiverecoverysome time afterthestroke event[83],and themechanisms involved aresynaptogenesis,axongrowthandgenerationofnewcellsand bloodvessels[84,85].Studieshaveshownthatastrocytesplayan importantroleinsynaptogenesisthroughthereleaseofmediators thatmaintainthebrainmicroenvironmentevenunderconditions inwhichneuronslosetheirfunctionality,which occursin
bralischemia.Thewayinwhichtheactivationofastrocytesafter ischemiaoccursdiffersdependingonthetimeandthebrainarea thatisobserved[86,87].Inpathologicalconditions,astrocytes sup-portneuronswithantioxidantsandenergeticmetabolites,suchas lactateandATP[88,89].Theprotectivefunctionofreactive astro-cytes results from their ability of glutamate re-uptake [90,91], production of antioxidants suchas glutathione and superoxide dismutase [88], and stimulation of neurogenesis [92–94]. Dur-ingastrogliosisastrocytichypertrophy,GFAPup-regulation,gliotic scarformation,proliferation,andde-differentiation,whicharethe mostimportantfeaturesoftheastrocyticresponse,occur[95–97]. The mostcommonsignaling agents influencing these reactions includeneurotransmittersand gliotransmitters(ATP,glutamate, noradrenalin),reactiveoxygenspecies,cytokinesandinterleukins [88,98].Thus, thereactive astrogliosisrevealsboth detrimental andneuroprotectiverolesinthecourseofpathologicalprocesses. RA prevented the decrease in the synaptophysin levels in the striatumanddecreasedastrogliosis,whichmeansthatRAmight preventlearningandmemoryimpairmentbyinhibitingthelossof synapsesorenhancingsynaptogenesisbydecreasingastrogliosis andincreasingneurotrophicfactors,suchasBDNF,afterischemic injury.
In conclusion, the present study is the first report that RA preventsmemorydeficitsinducedbycerebralischemia,probably byanti-inflammatoryandsynaptogenicmechanismsthroughthe BDNFpathway,althoughothermechanisms,suchasantioxidant andantiapoptoticactions,cannotbeexcluded.
Conflictofinterest
Therearenoconflictsofinterest.
Acknowledgements
TheauthorsthanktheBrazilianNationalResarchand Develop-mentCouncil(CNPq),INCT-IBISAB-Capes,andtheResearchSupport FoundationofCeará(FUNCAP)for financialsupportin theform ofgrantsandfellowshipawards.TheauthorsalsothankJuliana FernandesPereirafortechnicalassistance.
References
[1]H.E.López-Valdés,A.N.Clarkson,Y.Ao,A.C.Charles,S.T.Carmichael,M.V. Sofroniew,K.C.Brennan,Memantineenhancesrecoveryfromstroke,Stroke 45(7)(2014)2093–2100,http://dx.doi.org/10.1161/STROKEAHA.113.004476. [2]S.Kinlay,Changesinstrokeepidemiology,preventionandtreatmente,
Circulation124(2011)e494–e496.
[3]P.Lipton,Ischemiccelldeathinbrainneurons,Physiol.Rev.79(4)(1999) 1431–1568,http://dx.doi.org/10.1016/j.shpsa.2008.02.001.
[4]R.Brouns,P.P.DeDeyn,Thecomplexityofneurobiologicalprocessesinacute ischemicstroke,Clin.Neurol.Neurosurg.111(6)(2009)483–495,http://dx. doi.org/10.1016/j.clineuro.2009.04.001.
[5]J.-H.Sun,L.Tan,J.-T.Yu,Post-strokecognitiveimpairment:epidemiology, mechanismsandmanagement,Ann.Trans.Med.2(8)(2014),http://dx.doi. org/10.3978/j.issn.2305-5839.2014.08.05.
[6]F.G.VanRooij,P.Schaapsmeerders,N.A.M.Maaijwee,D.A.H.J.vanDuijnhoven, F.-E.deLeeuw,R.P.C.Kessels,E.J.vanDijk,Persistentcognitiveimpairment aftertransientischemicattack,StrokeJ.Cereb.Circ.45(8)(2014)2270–2274 http://doi.org/10.1161/STROKEAHA.114.005205.
[7]L.I.Sinclair,H.M.Tayler,S.Love,Synapticproteinlevelsalteredinvascular dementia,Neuropathol.Appl.Neurobiol.41(4)(2015)533–543,http://dx.doi. org/10.1111/nan.12215.
[8]A.G.Nikonenko,L.Radenovic,P.R.Andjus,G.G.Skibo,Structuralfeaturesof ischemicdamageinthehippocampus,Anat.Rec.292(12)(2009)1914–1921, http://dx.doi.org/10.1002/ar.20969.
[9]J.Hofmeijer,M.J.A.M.VanPutten,Ischemiccerebraldamage:anappraisalof synapticfailure,Stroke43(2)(2012)607–615,http://dx.doi.org/10.1161/ STROKEAHA.111.632943.
[10]T.H.Murphy,D.Corbett,Plasticityduringstrokerecovery:fromsynapseto behaviour,Nat.Rev.Neurosci.10(12)(2009)861–872,http://dx.doi.org/10. 1038/nrn2735.
[11]J.Pei,X.You,Q.Fu,Inflammationinthepathogenesisofischemicstroke, Front.Biosci.1(20)(2015)772–783.
[12]Y.Gu,J.Chen,J.Shen,Herbalmedicinesforischemicstroke:combating inflammationastherapeutictargets,J.NeuroimmunePharmacol.9(3)(2014) 313–339,http://dx.doi.org/10.1007/s11481-014-9525-5.
[13]T.Shichita,M.Ito,A.Yoshimura,Post-ischemicinflammationregulatesneural damageandprotection,Front.Cell.Neurosci.8(2014),http://dx.doi.org/10. 3389/fncel.2014.00319.
[14]M.Liguz-Lecznar,M.Kossut,Influenceofinflammationonpoststroke plasticity,NeuralPlast.(2013)258582,http://dx.doi.org/10.1155/2013/ 258582.
[15]A.Steliga,M.Waskow,Z.Karwacki,S.Wojcik,G.Lietzau,I.Klejbor,P. Kowianski,TranscriptionfactorPax6isexpressedbyastrogliaaftertransient brainischemiaintheratmodel,FoliaNeuropathol.3(2013)203–213,http:// dx.doi.org/10.5114/fn.2013.37704.
[16]L.-J.Cao,J.Wang,P.-P.Hao,C.-L.Sun,Y.-G.Chen,Effectsofulinastatin,a urinarytrypsininhibitor,onsynapticplasticityandspatialmemoryinarat modelofcerebralischemia/reperfusioninjury,Chin.J.Physiol.54(6)(2011) 435–442,http://dx.doi.org/10.4077/CJP.2011.BAA058.
[17]T.Fellin,Communicationbetweenneuronsandastrocytes:relevancetothe modulationofsynapticandnetworkactivity,J.Neurochem.108(3)(2009) 533–544,http://dx.doi.org/10.1111/j1471-4159.2008.05830.x.
[18]A.F.Schinder,M.M.Poo,Theneurotrophinhypothesisforsynapticplasticity, TrendsNeurosci.23(2000)639–645.
[19]R.M.Lindsay,S.J.Wiegand,C.A.Altar,P.S.DiStefano,Neurotrophicfactors, TrendsNeurosci.17(1994)182–1903.
[20]W.R.Schabitz,T.Steigleder,C.M.Cooper-Kuhn,S.Schwab,C.Sommer,A. Schneider,H.G.Kuhn,Intravenousbrain-derivedneurotrophicfactor enhancespoststrokesensorimotorrecoveryandstimulatesneurogenesis, Stroke38(2007)2165–2172.
[21]M.Petersen,M.S.J.Simmonds,Rosmarinicacid,Phytochemistry62(2)(2003) 121–125http://doi.org/10.1016/S0031-9422(02)00513-7.
[22]T.Iuvone,D.Filippis,G.DeEsposito,A.D.Amico,A.A.Izzo,A.D’Amico,The spicesageanditsactiveingredientrosmarinicacidprotectPC12cellsfrom amyloid-peptide-inducedneurotoxicity,J.Pharmacol.Exp.Ther.317(3) (2006)1143–1149http://doi.org/10.1124/jpet.105.099317.
[23]T.Alkam,A.Nitta,H.Mizoguchi,A.Itoh,T.Nabeshima,Anaturalscavengerof peroxynitrites,rosmarinicacid,protectsagainstimpairmentofmemory inducedbyA??25–35,Behav.BrainRes.180(2)(2007)139–145http://doi. org/10.1016/j.bbr.2007.03.001.
[24]T.Hamaguchi,K.Ono,A.Murase,M.Yamada,Phenoliccompoundsprevent Alzheimer’spathologythroughdifferenteffectsontheamyloid-beta aggregationpathway,Am.J.Pathol.175(6)(2009)2557–2565http://doi.org/ 10.2353/ajpath.2009.090417.
[25]J.Wang,H.Xu,H.Jiang,X.Du,P.Sun,J.Xie,Neurorescueeffectofrosmarinic acidon6-hydroxydopamine-lesionednigraldopamineneuronsinratmodel ofParkinson’sdisease,J.Mol.Neurosci.47(1)(2012)113–119http://doi.org/ 10.1007/s12031-011-9693-1.
[26]H.Luan,Z.Kan,Y.Xu,C.Lv,W.Jiang,Rosmarinicacidprotectsagainst experimentaldiabeteswithcerebralischemia:relationtoinflammation response,J.Neuroinflammation10(1)(2013)28http://doi.org/10.1186/ 1742-2094-10-28.
[27]M.Bernaudin,H.H.Marti,S.Roussel,D.Divoux,A.Nouvelot,E.T.MacKenzie, etal.,Apotentialroleforerythropoietininfocalpermanentcerebralischemia inmice,J.Cereb.BloodFlowMetab.19(1999)643–651.
[28]J.B.Bederson,L.H.Pitts,S.M.Germano,M.C.Nishimura,R.L.Davis,H.M. Bartkowski,Evaluationof2,3,5-triphenyltetrazoliumchlorideasastainfor detectionandquantificationofexperimentalcerebralinfarctioninrats, StrokeJ.Cereb.Circ.17(6)(1986)1304–1308http://doi.org/10.1161/01STR. 17.6.1304.
[29]J.H.Garcia,S.Wagner,K.F.Liu,X.J.Hu,Neurologicaldeficitandextentof neuronalnecrosisattributabletomiddlecerebralarteryocclusioninrats. Statisticalvalidation,StrokeJ.Cereb.Circ.26(4)(1995)627–634(Discussion 635)http://doi.org/10.1161/01STR.26.4.627.
[30]R.N.Walsh,R.A.Cummins,Theopen-fieldtest:acriticalreview,Psychol.Bull. 83(1976)482–504.
[31]M.Sarter,G.Bodewitz,D.N.Stephens,Attenuationofscopolamine-induced impairmentofspontaneousalterationbehaviorbyantagonistbutnotinverse agonistandagonistbeta-carbolines,Psychopharmacology(Berlin)94(1988) 491–495.
[32]A.Ennaceur,One-trialobjectrecognitioninratsandmice:methodological andtheoreticalissues,Behav.BrainRes.215(2)(2010)244–254http://doi. org/10.1016/j.bbr.2009.12.036.
[33]R.Morris,Developmentsofawater-mazeprocedureforstudyingspatial learningintherat,J.Neurosci.Methods11(1)(1984)47–60http://doi.org/10. 1016/0165-0270(84)90007-4.
[34]P.P.Bradley,R.D.Christensen,G.Rothstein,Cellularandextracellular myeloperoxidaseinpyogenicinflammation,Blood60(1982) 618–622.
[35]A.Simonyi,Q.Wang,R.L.Miller,M.Yusof,P.B.Shelat,A.Y.Sun,G.Y.Sun, Polyphenolsincerebralischemia:noveltargetsforneuroprotection,Mol. Neurobiol.31(2005)135–147.
[36]O.Inanami,Y.Watanabe,B.Syuto,M.Nakano,M.Tsuji,M.Kuwabara,Oral administrationof(−)catechinprotectsagainstischemia-reperfusion-induced neuronaldeathinthegerbil,FreeRadic.Res.29(1998)359–365.
[38]Y.Zhou,J.Huang,W.He,W.Fan,W.Fang,G.He,Y.Li,N2amelioratesneural injuryduringexperimentalischemicstrokeviatheregulationof
thromboxaneA2production,Pharmacol.Biochem.Behav.124(2014) 458–465http://doi.org/10.1016/j.pbb.2014.06.009.
[39]F.Blasi,Y.Wei,M.Balkaya,S.Tikka,J.B.Mandeville,C.Waeber,M.A. Moskowitz,Recognitionmemoryimpairmentsaftersubcorticalwhitematter strokeinmice,Stroke45(5)(2014)1468–1473http://doi.org/10.1161/ STROKEAHA.114.005324.
[40]R.D.Ronca,A.M.Myers,D.Ganea,R.F.Tuma,E.A.Walker,S.J.Ward,Aselective cannabinoidCB2agonistattenuatesdamageandimprovesmemoryretention followingstrokeinmice,LifeSci.(2015)http://doi.org/10.1016/j.lfs.2015.05. 005.
[41]D.H.Kim,S.J.Jeon,K.H.Son,J.W.Jung,S.Lee,B.H.Yoon,J.H.Ryu,Effectofthe flavonoid,oroxylinA,ontransientcerebralhypoperfusion-inducedmemory impairmentinmice,Pharmacol.Biochem.Behav.85(3)(2006)658–668 http://doi.org/10.1016/j.pbb.2006.10.025.
[42]J.A.Nunn,E.LePeillet,C.A.Netto,H.Hodges,J.A.Gray,B.S.Meldrum,Global ischemia:hippocampalpathologyandspatialdeficitsinthewatermaze, Behav.BrainRes.62(1994).
[43]J.O’Keefe,Acomputationaltheoryofthehippocampalcognitivemap,Prog. BrainRes.83(1990)301–312.
[44]M.G.Packard,J.L.McGaugh,Inactivationofhippocampusorcaudatenucleus withlidocainedifferentiallyaffectsexpressionofplaceandresponselearning, Neurobiol.Learn.Mem.65(1)(1996)65–72.
[45]D.H.Park,S.J.Park,J.M.Kim,W.Y.Jung,J.H.Ryu,Subchronicadministrationof rosmarinicacid,anaturalprolyloligopeptidaseinhibitor,enhancescognitive performances,Fitoterapia81(6)(2010)644–648http://doi.org/10.1016/j. fitote.2010.03.010.
[46]T.Nakazawa,K.Ohsawa,Metabolismofrosmarinicacidinrats,J.Nat.Prod.61 (8)(1998)993–996http://doi.org/10.1021/np980072s.
[47]F.D.P.Fernandes,A.P.F.Menezes,J.C.S.Neves,A.A.Fonteles,A.T.A.Silva,P.A. Rodrigues,M.R.S.Carmo,C.M.Souza,G.M.Andrade,Behav.Pharmacol.25 (2014)637–647.
[48]T.Yunoki,K.Deguchi,Y.Omote,N.Liu,W.Liu,N.Hishikawa,K.Abe, Anti-oxidativenutrientrichdietprotectsagainstacuteischemicbrain damageinrats,BrainRes.1587(2014)33–39http://doi.org/10.1016/j. brainres.2014.08.056.
[49]M.R.Al-Sereiti,K.M.Abu-Amer,P.Sen,Pharmacologyofrosemary
(RosmarinusofficinalisLinn.)anditstherapeuticpotentials,IndianJ.Exp.Biol. 37(2)(1999)124–130.
[50]H.J.Lee,H.-S.Cho,E.Park,S.Kim,S.-Y.Lee,C.-S.Kim,H.S.Chun,Rosmarinic acidprotectshumandopaminergicneuronalcellsagainsthydrogen peroxide-inducedapoptosis,Toxicology250(2–3)(2008)109–115http://doi. org/10.1016/j.tox.2008.06.010.
[51]O.Fadel,K.ElKirat,S.Morandat,Thenaturalantioxidantrosmarinicacid spontaneouslypenetratesmembranestoinhibitlipidperoxidationinsitu, Biochim.Biophys.Acta—Biomembr.1808(12)(2011)2973–2980http://doi. org/10.1016/j.bbamem.2011.08.011.
[52]Y.Kimura,H.Okuda,T.Okuda,Studiesontheactivitiesoftanninsandrelated compounds:X:effectsofcaffeetanninsandrelatedcompoundson arachidonatemetabolisminhumanpolymorphonuclearleukocytes,J.Nat. Prod.50(1987)392–399.
[53]A.Sahu,N.Rawal,M.K.Pangburn,Inhibitionofcomplementbycovalent attachmentofrosmarinicacidtoactivatedC3b,Biochem.Pharmacol.57 (1999)1439–1446.
[54]M.A.Kelm,M.G.Nair,G.M.Strasburg,Antioxidantandcyclooxygenase inhibitoryphenoliccompoundsfromOcimumsanctumLinn.antioxidant, Phytomedicine7(2000)7–13.
[55]H.Takano,N.Osakabe,C.Sanbongi,R.Yanagisawa,T.Yoshikawa,Extractof
Perillafrutescensenrichedforrosmarinicacid,apolyphenolicphytochemical, inhibitsseasonalallergicrhino-conjunctivitisinhumans,Exp.Biol.Med.229 (2004)247–254.
[56]S.Baba,N.Osakabe,M.Natsume,J.Terao,Orallyadministeredrosmarinicacid ispresentastheconjugatedand/ormethylatedformsinplasma,andis degradedandmetabolizedtoconjugatedformsofcaffeicacid,ferulicacidand m-coumaricacid,LifeSci.75(2)(2004)165–178http://doi.org/10.1016/j.lfs. 2003.11.028.
[57]S.Baba,N.Osakabe,M.Natsume,A.Yasuda,Y.Muto,K.Hiyoshi,J.Terao, Absorption,metabolism,degradationandurinaryexcretionofrosmarinicacid afterintakeofPerillafrutescensextractinhumans,Eur.J.Nutr.44(1)(2005) 1–9http://doi.org/10.1007/s00394-004-0482-2.
[58]H.J.Jeong,Y.Choi,M.H.Kim,I.C.Kang,J.H.Lee,C.Park,H.M.Kim,Rosmarinic acid,activecomponentofdansam-eumattenuatesototoxicityofcochlearhair cellsthroughblockageofcaspase-1activity,PLoSOne6(4)(2011)http://doi. org/10.1371/journal.pone.0018815.
[59]J.Youn,K.-H.Lee,J.Won,S.-J.Huh,H.-S.Yun,W.-G.Cho,D.-J.Paik,Beneficial effectsofrosmarinicacidonsuppressionofcollageninducedarthritis,J. Rheumatol.30(6)(2003)1203–1207.
[60]J.Rocha,M.Eduardo-Figueira,A.Barateiro,A.Fernandes,D.Brites,R.Bronze, B.Sepodes,Anti-inflammatoryeffectofrosmarinicacidandanextractof rosmarinusofficinalisineatmodelsoflocalandsystemicinflammation,Basic Clin.Pharmacol.Toxicol.(2014)http://doi.org/10.1111/bcpt.12335. [61]H.Ozturk,H.Ozturk,E.H.Terzi,U.Ozgen,A.Duran,I.Uygun,Protectiveeffects
ofrosmarinicacidagainstrenalischaemia/reperfusioninjuryinrats,J.Pak. Med.Assoc.64(3)(2014)260–265.
[62]M.A.Saad,R.M.AbdelSalam,S.A.Kenawy,A.S.Attia,Pinocembrinattenuates hippocampalinflammation,oxidativeperturbationsandapoptosisinarat modelofglobalcerebralischemiareperfusion,Pharmacol.Rep.67(1)(2015) 115–122http://doi.org/10.1016/j.pharep.2014.08.014.
[63]R.Forghani,H.J.Kim,G.R.Wojtkiewicz,L.Bure,Y.Wu,J.W.Chen,
Myeloperoxidasepropagatesdamageandisapotentialtherapeutictargetfor subacutestroke,J.Cereb.BloodFlowMetab.35(2015)485–493http://doi. org/10.1038/jcbfm.2014.222.
[64]C.A.Altar,N.Cai,T.Bliven,M.Juhasz,J.M.Conner,A.L.Acheson,R.M.Lindsay, S.J.Wiegand,Anterogradetransportofbrain-derivedneurotrophicfactorand itsroleinthebrain,Nature389(1997)856–860.
[65]A.Ghosh,J.Carnahan,M.E.Greenberg,RequirementforBDNFin activity-dependentsurvivalofcorticalneurons,Science263(1994) 1618–1623.
[66]Y.Nakajo,D.Yang,J.C.Takahashi,Q.Zhao,H.Kataoka,H.Yanamoto,ERV enhancesspatiallearningandpreventsthedevelopmentofinfarcts, accompaniedbyupregulatedBDNFinthecortex,BrainRes.1610(2015) 110–123http://doi.org/10.1016/j.brainres.2015.03.042.
[67]A.Madinier,N.Bertrand,M.Rodier,A.Quirié,C.Mossiat,A.Prigent-Tessier,P. Garnier,Ipsilateralversuscontralateralspontaneouspost-strokeneuroplastic changes:involvementofBDNF?Neuroscience231(2013)169–181http://doi. org/10.1016/j.neuroscience.2012.11.054.
[68]D.H.Kim,S.J.Jeon,K.H.Son,J.W.Jung,S.Lee,B.H.Yoon,J.H.Ryu,Effectofthe flavonoid,oroxylinA,ontransientcerebralhypoperfusion-inducedmemory impairmentinmice,Pharmacol.Biochem.Behav.85(3)(2006)658–668 http://doi.org/10.1016/j.pbb.2006.10.025.
[69]N.Ito,T.Yabe,Y.Gamo,T.Nagai,T.Oikawa,H.Yamada,T.Hanawa, RosmarinicacidfromPerillaeherbaproducesanantidepressant-likeeffectin micethroughcellproliferationinthehippocampus,Biol.Pharm.Bull.31(7) (2008)1376–1380http://doi.org/10.1248/bpb.31.1376.
[70]Y.Kovalchuk,E.Hanse,K.W.Kafitz,A.Konnerth,Postsynapticinductionof BDNF-mediatedlong-termpotentiation,Science295(2002)1729–1734. [71]R.-Q.Yao,D.-S.Qi,H.-L.Yu,J.Liu,L.-H.Yang,X.-X.Wu,Quercetinattenuates
cellapoptosisinfocalcerebralischemiaratbrainviaactivationof
BDNF-TrkB-PI3K/Aktsignalingpathway,Neurochem.Res.(2012)2777–2786 http://doi.org/10.1007/s11064-012-0871-5.
[72]X.Liu,X.Zhou,J.Hou,H.Zhu,Z.Wang,J.Liu,Y.Zheng,GinsenosideRd promotesneurogenesisinratbrainaftertransientfocalcerebralischemiavia activationofPI3K/Aktpathway,ActaPharmacol.Sin.36(4)(2015)421–428 http://doi.org/10.1038/aps.2014.156.
[73]I.Ferrer,J.Krupinski,E.Goutan,E.Martí,S.Ambrosio,E.Arenas,Brain-derived neurotrophicfactorreducescorticalcelldeathbyischemiaaftermiddle cerebralarteryocclusionintherat,ActaNeuropathol.101(3)(2001)229–238 http://doi.org/10.1007/s004010000268.
[74]M.P.Mattson,Glutamateandneurotrophicfactorsinneuronalplasticityand disease,Ann.N.Y.Acad.Sci.1144(2008)97–112.
[75]E.G.Waterhouse,B.Xu,Newinsightsintotheroleofbrain-derived neurotrophicfactorinsynapticplasticity,Mol.Cell.Neurosci.42(2)(2009) 81–89http://doi.org/10.1016/j.mcn.2009.06.009.
[76]J.Chen,C.Zhang,H.Jiang,Y.Li,L.Zhang,A.Robin,M.Katakowski,M.Lu,M. Chopp,AtorvastatininductionofVEGFandBDNFpromotesbrainplasticity afterstrokeinmice,J.Cereb.BloodFlowMetab.25(2005)281–290http://doi. org/doi:10.1038/sj.jcbfm.9600034.
[77]L.D.Pozzo-Miller,W.Gottschalk,L.Zhang,K.McDermott,J.Du,R. Gopalakrishnan,C.Oho,Z.H.Sheng,B.Lu,Impairmentsinhigh-frequency transmission,synapticvesicledocking,andsynapticproteindistributionin thehippocampusofBDNFknockoutmice,J.Neurosci.19(1999)
4972–4983.
[78]X.Jin,P.Liu,F.Yang,Y.H.Zhang,D.Miao,Rosmarinicacidameliorates depressive-likebehaviorsinaratmodelofCUSandup-regulatesBDNFlevels inthehippocampusandhippocampal-derivedastrocytes,Neurochem.Res.38 (9)(2013)1828–1837http://doi.org/10.1007/s11064-013-1088-y.
[79]M.Gooney,K.Shaw,A.Kelly,S.M.O’Mara,M.A.Lynch,Long-termpotentiation andspatiallearningareassociatedwithincreasedphosphorylationofTrkB andERKindentategyrus:evidenceforaroleforBDNF,Behav.Neurosci.116 (2002)455–463.
[80]S.L.Patterson,Immunedysregulationandcognitivevulnerabilityintheaging brain:interactionsofmicroglia,IL-1,BDNFandsynapticplasticity, Neuropharmacology96(2014)11–18http://doi.org/10.1016/j.neuropharm. 2014.12.020.
[81]T.Fellin,Communicationbetweenneuronsandastrocytes:relevancetothe modulationofsynapticandnetworkactivity,J.Neurochem.108(3)(2009) 533–544http://doi.org/10.1111/j1471-4159.2008.05830.x.
[82]R.P.Stroemer,T.A.Kent,C.E.Hulsebosch,Enhancedneocorticalneural sprouting,synaptogenesis,andbehavioralrecoverywithd-amphetamine therapyafterneocorticalinfarctioninrats,StrokeJ.Cereb.Circ.29(11)(1998) 2381–2393,Discussion2393–2395http://doi.org/10.1161/01STR.29.11.2381. [83]J.J.Maller,Neuroplasticityinnormalandbraininjuredpatients:potential
relevanceofearwigglinglocusofcontrolandcorticalprojections,Med. Hypotheses83(6)(2014)838–843http://doi.org/10.1016/j.mehy.2014.11. 003.
[84]S.T.Carmichael,I.Archibeque,L.Luke,T.Nolan,J.Momiy,S.Li, Growth-associatedgeneexpressionafterstroke:evidencefora
[85]R.J.Felling,H.Song,Epigeneticmechanismsofneuroplasticityandthe implicationsforstrokerecovery,Exp.Neurol.268(2015)37–45http://doi. org/10.1016/j.expneurol.2014.09.017.
[86]H.K.Kimelberg,NIHpublicaccess,Neurothraputics7(4)(2010)338–353 http://doi.org/10.1016/j.nurt.2010.07.006.
[87]A.Steliga,M.Waskow,Z.Karwacki,S.Wojcik,G.Lietzau,I.Klejbor,P. Kowianski,TranscriptionfactorPax6isexpressedbyastrogliaaftertransient brainischemiaintheratmodel,FoliaNeuropathol.3(2013)203–213http:// doi.org/10.5114/fn.2013.37704.
[88]G.E.Barreto,J.Gonzalez,Y.Torres,L.Morales,Astrocytic-neuronalcrosstalk: implicationsforneuroprotectionfrombraininjury,Neurosci.Res.71(2011) 107–113.
[89]C.R.Figley,P.W.Stroman,Therolesofastrocytesandastrocyteactivityin neurometabolism,neurovascularcoupling,andtheproductionoffunctional neuroimagingsignals,Eur.J.Neurosci.33(2011)577–588.
[90]Y.David,L.P.Cacheaux,S.Ivens,E.Lapilover,U.Heinemann,D.Kaufer,A. Friedman,Astrocyticdysfunctioninepileptogenesis:consequenceofaltered potassiumandglutamatehomeostasis?J.Neurosci.29(2009)10588–10599. [91]L.Héja,P.Barabás,G.Nyitrai,K.A.Kékesi,B.Lasztóczi,O.Toke,G.Tárkányi,K.
Madsen,J.Kardos,Glutamateuptaketriggerstransporter-mediatedGABA releasefromastrocytes,PLoSOne4(2009)7153.
[92]B.Berninger,M.R.Costa,U.Koch,T.Schroeder,B.Sutor,B.Grothe,M.Götz, Functionalpropertiesofneuronsderivedfrominvitroreprogrammed postnatalastroglia,J.Neurosci.27(2007)8654–8664.
[93]K.Jin,Y.Sun,L.Xie,A.Peel,X.O.Mao,S.Batteur,D.A.Greenberg,Directed migrationofneuronalprecursorsintotheischemiccerebralcortexand striatum,Mol.CellNeurosci.24(2003)171–189.
[94]J.M.Parent,Z.S.Vexler,C.Gong,N.Derugin,D.M.Ferriero,Ratforebrain neurogenesisandstriatalneuronreplacementafterfocalstroke,Ann.Neurol. 52(2002)802–813.
[95]A.Buffo,I.Rite,P.Tripathi,A.Lepier,D.Colak,A.P.Horn,T.Mori,M.Götz, Originandprogenyofreactivegliosis:asourceofmultipotentcellsinthe injuredbrain,Proc.Natl.Acad.Sci.U.S.A.105(2008)3581–3586.
[96]K.A.Burns,B.Murphy,S.C.Danzer,C.Y.Kuan,Developmentalandpost-injury corticalgliogenesis:ageneticfate-mappingstudywithNestin-CreERmice, Glia57(2009)1115–1129.
[97]J.W.Zhao,R.Raha-Chowdhury,J.W.Fawcett,C.Watts,Astrocytesand oligodendrocytescanbegeneratedfromNG2+progenitorsafteracutebrain injury:intracellularlocalizationofoligodendrocytetranscriptionfactor2is associatedwiththeirfatechoice,Eur.J.Neurosci.29(2009)1853–1869. [98]M.V.Sofroniew,Moleculardissectionofreactiveastrogliosisandglialscar