w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Update
on
the
2012
Brazilian
Society
of
Rheumatology
Guidelines
for
the
treatment
of
rheumatoid
arthritis:
position
on
the
use
of
tofacitinib
Licia
Maria
Henrique
da
Mota
a,b,∗,
Bóris
Afonso
Cruz
c,
Cleandro
Pires
de
Albuquerque
d,
Deborah
Pereira
Gonc¸alves
e,f,g,
Ieda
Maria
Magalhães
Laurindo
h,i,
Ivanio
Alves
Pereira
j,k,
Jozélio
Freire
de
Carvalho
l,
Geraldo
da
Rocha
Castelar
Pinheiro
m,
Manoel
Barros
Bertolo
n,o,
Maria
Raquel
da
Costa
Pinto
p,q,
Paulo
Louzada-Junior
r,
Ricardo
Machado
Xavier
s,t,
Rina
Dalva
Neubarth
Giorgi
u,
Rodrigo
Aires
Corrêa
Lima
v aHospitalUniversitáriodeBrasília,UniversidadedeBrasília(UnB),Brasília,DF,BrazilbPostgraduatePrograminMedicalSciences,MedicineSchool,UniversidadedeBrasília(UnB),Brasília,DF,Brazil
cBiocorInstituto,NovaLima,MG,Brazil
dServiceofRheumatology,HospitalUniversitáriodeBrasília,UniversidadedeBrasília(UnB),Brasília,DF,Brazil
eServiceofRheumatology,HospitalUniversitárioWalterCantídio,Fortaleza,CE,Brazil
fServiceofRheumatology,HospitalGeralDr.CésarCals,Fortaleza,CE,Brazil
gProgramofMedicalResidenceinRheumatology,HospitalGeralDr.CésarCals,Fortaleza,CE,Brazil
hUniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil
iBiobadaBrasil,Brazil
jDisciplineofRheumatology,UniversidadedoSuldeSantaCatarina(UNISUL),Florianópolis,SC,Brazil
kNucleusofRheumatology,TeachingHospital,UniversidadeFederaldeSantaCatarina(UFSC),Florianópolis,SC,Brazil
lHospitalAlianc¸a,Salvador,BA,Brazil
mDisciplineofRheumatology,MedicalSciencesSchool,UniversidadedoEstadodoRiodeJaneiro(UERJ),RiodeJaneiro,RJ,Brazil
nDisciplinedeRheumatology,MedicalSciencesSchool(FCM),UniversidadeEstadualdeCampinas(UNICAMP),Campinas,SP,Brazil
oDepartmentofInternalMedicine,MedicalSciencesSchool(FCM),UniversidadeEstadualdeCampinas(UNICAMP),Campinas,SP,Brazil
pRheumatoidArthritisOutpatientClinic,HospitaldasClínicas,UniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,MG,
Brazil
qMedicineSchool,UniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,MG,Brazil
rFaculdadedeMedicinadeRibeirãoPreto,UniversidadedeSãoPaulo(USP),RibeirãoPreto,SP,Brazil
sMedicineSchool,UniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
tServiceofRheumatology,HospitaldeClínicasdePortoAlegre,PortoAlegre,RS,Brazil
uServiceofRheumatology,HospitaldoServidorPúblicoEstadual“FranciscoMoratodeOliveira”(HSPE-FMO),SãoPaulo,SP,Brazil
vServiceofRheumatology,HospitaldeBasedoDistritoFederal,Brasília,DF,Brazil
∗ Correspondingauthor.
E-mailaddresses:licia@unb.br,liciamhmota@gmail.com(L.M.H.daMota).
http://dx.doi.org/10.1016/j.rbre.2015.09.003
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received9August2015 Accepted11August2015 Availableonline21October2015
Keywords:
Rheumatoidarthritis Treatment
Brazil Tofacitinib
a
b
s
t
r
a
c
t
In 2014, tofacitinib, a target-specific, synthetic disease modifying anti rheumatic drug (DMARD)anda selectiveinhibitorofJanuskinase(JAK)wasapprovedforuseinBrazil. This position paper aimsto update the recommendationsof the BrazilianSociety of Rheumatology(SBR)onthetreatmentofrheumatoidarthritis(RA)inBrazil,specifically regarding theuseoftarget-specificsynthetic DMARDs.The methodofthis recommen-dationconsistedofaliteraturereviewofscientificpapersheldontheMedlinedatabase. Afterthisreview,atextwasproduced,answeringquestionsinPicostructure, consider-ing efficacyandsafetyissuesoftofacitinib useforRAtreatment indifferentscenarios (suchasfirst-linetreatmentafterfailurewithmethotrexate[MTX]orotherconventional syntheticDMARDsafterfailurewithbiologicaltherapy).Basedonexistingevidence,and consideringtheavailabledataonefficacy,safetyandcostofmedicationsavailabletotreat thediseaseinBrazil,theRACommissionofSBR,afteraprocessofdiscussionand vot-ingonproposals,establishedthefollowingpositionontheuseoftofacitinibfortreatment ofRAinBrazil:“Tofacitinib,aloneorincombinationwithMTX,isanalternativeforRA patients withmoderateor highactivityafterfailureofatleast twodifferentsynthetic DMARDsandonebiologicalDMARD.”Thelevelofagreementwiththisrecommendation was7.5.
Thispositionmaybereviewedinthecomingyears,inthefaceofagreaterexperience withtheuseofthismedication.
©2015ElsevierEditoraLtda.Allrightsreserved.
Posicionamento
sobre
o
uso
de
tofacitinibe
no
algoritmo
do
Consenso
2012
da
Sociedade
Brasileira
de
Reumatologia
para
o
tratamento
da
artrite
reumatoide
Palavras-chave:
Artritereumatoide Tratamento Brasil Tofacitinibe
r
e
s
u
m
o
Em2014,otofacitinibe,ummedicamentomodificadordocursoda doenc¸a(MMCD) sin-tético,alvo-específico,inibidorseletivodasJanusquinases(JAK),foiaprovadoparausono Brasil.Estedocumentodeposicionamentotemoobjetivodeatualizarasrecomendac¸ões daSociedadeBrasileiradeReumatologia(SBR)sobreotratamentodaartritereumatoide (AR)noBrasil,especificamentecomrelac¸ãoaousodeMMCDsintéticosalvo-específicos. O métododessa recomendac¸ãoincluiu revisão bibliográficade artigoscientíficos, feita na base de dados Medline. Após a revisão, foi produzido um texto, que responde a perguntas na estrutura Pico, e considera questões de eficácia e seguranc¸a do usodo tofacitinibeparatratamentodeARemdiferentessituac¸ões(comoprimeiralinhade trata-mento,apósfalhaaometotrexato[MTX]ououtrosMMCDsintéticosconvencionais,após falhadaterapiabiológica).Combasenasevidênciasexistentes,econsiderandoosdados disponíveissobreeficácia,seguranc¸aecustodasmedicac¸õesdisponíveisparatratamento da doenc¸ano Brasil,a Comissão deARda SBR,apósprocesso de discussãoevotac¸ão de propostas,estabeleceuoseguinteposicionamentosobreousode tofacitinibeparao tratamento da ARno Brasil:“Tofacitinibe, em monoterapiaouem associac¸ãoao MTX, é umaopc¸ãoparaospacientescomARematividademoderadaoualta,apósfalhade pelo menos doisesquemascomdiferentesMMCDsintéticos eum esquemade MMCD biológico”.Ograudeconcordânciacomessarecomendac¸ãofoi7,5.Esseposicionamento poderáserrevistonospróximosanos,comamaiorexperiênciaadquiridacomousodo medicamento.
Introduction
Rheumatoidarthritis(RA)isasystemic,autoimmune inflam-matorydiseasecharacterizedbyinvolvementofthesynovial membraneofperipheraljoints.Itisestimatedthatthe preva-lenceofRAis0.5–1%ofthepopulation,mainlyinwomenand inpeopleagedfrom30to50years.1
ThetreatmentofRAhasprogressedsubstantiallyinrecent decades,duetoamajorbreakthroughinunderstandingthe pathophysiologicalmechanismsofthedisease,development ofnewtherapeuticclassesandimplementationofdifferent strategiesoftreatmentandfollow-upofpatients,forinstance, intensivediseasecontrolandinterventionintheearlyphase ofsymptoms.1
In2012and 2013, the RACommissionofthe Sociedade BrasileiradeReumatologia(SBR)publishedaseriesofpapers aimedatproducingrecommendationsonthediagnosisand treatmentofRAinBrazil.Thepurposeofthesedocuments wastoestablish consensusguidelines forthe treatmentof RAinBrazilandtosupportBrazilianrheumatologists,using evidencefromscientificstudiesandtheexperienceofa com-mitteeofexpertsonthesubjectinordertostandardizethe therapeuticapproachofRAinthe Braziliansocioeconomic context,maintainingtheautonomyofthe physicianinthe indication/choiceoftreatmentoptionsavailable.2–5
Atthattime,thedocumentspredictedthat,duetotherapid advancesofknowledgeinthisfieldofscience,itwouldbe nec-essarytomakeperiodicupdatesofsuchrecommendations.
Sincethen,adrugbelongingtoadifferent classof pre-viouslyexistingdrugs,tofacitinib,asynthetic,target-specific disease-modifyinganti-rheumaticdrug(DMARD)and selec-tiveinhibitorofJanuskinase(JAK)hasbeenapprovedforusein Brazil.6TofacitinibwassubmittedforapprovaltotheNational HealthSurveillanceAgency(ANVISA) onApril18,2012and approvedonDecember8,2014.7
Thus,acontingentupdateoftheTreatmentGuideline pre-viouslyreportedbySBRwasinordertoestablishtheposition oftheRACommissionontheuseoftarget-specific,synthetic DMARDsinBrazil.
Objective
ThispositionpaperaimstoupdateSBRrecommendationson thetreatmentofRAinBrazil,specificallyregardingtheuseof target-specific,syntheticDMARDs.
Methods
Theliterature reviewof scientificarticles of this guideline wasconductedonMEDLINEdatabase.Thesearchforevidence camefrom actualclinicalsettings,usingthefollowing key-words(MeSH terms): Arthritis, Rheumatoid, Therapy, Efficacy,
Safety, Prognosis, Remission, Tofacitinib, Herpes zoster vaccine. StudiespublisheduptoMay2015wereevaluated.
Afteraliteraturereview,atextwasproduced,answering questionsinthePICOstructure(Population/patient, Interven-tion/indicator, Comparator/control, Outcome),8 taking into account efficacy and safety issues of tofacitinib use for
treatment of RA in different scenarios (such as first-line treatment after failure with methotrexate [MTX] or other conventionalsyntheticDMARDs,afterfailurewithbiological therapy).
Basedonthereviewconductedandonexpertopinion, pro-posals ofrecommendations on the use oftofacitinib were drawn up, subjectedto successiveroundsofvotingamong membersoftheCommitteegatheredinpersonforthis pur-pose, pending approval of positioning (recommendations). The degree of agreement with the text of the approved recommendationonanumericalscalefrom0(strongly dis-agree)to10(completelyagree)wasalsoestablished,withthe finaldegreeofagreementcalculatedbyaveragingthevalues assignedindividuallybyeachofthemembersofthe Commis-sion.Dependingontheapprovedrecommendation,anupdate oftheflowchartforthetreatmentofRAinBrazilwas devel-oped,consideringascenariooftofacitinibinsertion.
Tofacitinib–generalaspects
Tofacitinib is a preferential inhibitor of JAK1/JAK3 (mem-bersofthetyrosinekinasefamily,intracellularJanuskinase thattransducecytokine-mediatedsignalsthroughJAK-STAT signalingpathway).Thisagentisindicatedforpatientswith activeRAwhohaveexperiencedtreatmentfailurewith syn-thetic DMARDs, or with TNF inhibitors (TNFi). Tofacitinib can be usedin combination withsynthetic DMARDsor as monotherapy.6Theapproveddosagefortofacitinibis1tablet (5mg)twicedaily.7
Regarding its main adverse effects, the safety profileis similar tobiological immunomodulatorydrugs withhigher incidenceofseriousinfections,tuberculosisandherpeszoster compared to the controlgroup. Theobserved incidenceof herpeszosterhasbeen higherthanthat reportedforother immunobiological agents, mostly milder cases. Laboratory abnormalitiesobservedincludeincreasesintotalcholesterol, fractionsoflow-densitylipoprotein–LDL andhigh-density lipoprotein–HDL,allreversiblewithspecifictreatment; neu-tropeniaandlymphopenia,increasedliverenzymes,creatine phosphokinase(CPK)andaslightincreaseincreatinine(not associated with increased incidence of renal failure). The increasedincidenceofmalignancies,nonmelanomaskin can-cersandlymphoproliferativediseaseshasnotbeenobserved sofar,butonemustkeepstrictsurveillance,consideringthat this isapotentimmunosuppressiveagent. Briefly,patients with RA who will be treated with tofacitinib should be assessedpriortotreatmentwithrespecttopotentiallatent TB(tuberculinskintest,chestX-rayandpriorhistoryof con-tactwithpeopleinfectedwithtuberculosis)andperiodically monitoredwithcompletebloodcounts,assessmentofrenal function,liverenzymesandlipidogram.9–15
Tofacitinibisanexpensivedrug,generallysimilarto bio-logicalDMARDs.
Istofacitinibasafeandeffectivedrugforthetreatmentof RApatients,asfirstlinetherapy?
intherapeuticdoses,wererandomizedtotakeadoseof tofac-itinib5mgtwiceaday,tofacitinib10mgtwiceaday,orMTX.14
Effectiveness
Inthis study,tofacitinibwassuperiortoMTXincontrolling signsandsymptoms(ACR70responsesat6months:25.5%in thegroupreceivingtofacitinib5mgtwiceaday,37.7%inthe groupoftofacitinib10mgtwiceaday, and12% ofpatients receivingMTX,p<0.001.ACR70=animprovementof70%of theAmericanCollegeofRheumatologyscore–ACR).There wasalsoasignificantlygreaterreductionofstructural dam-age in patients receiving tofacitinib compared to patients receivingMTX(progressionofSharpindex[modified]insix months=0.2pointsinthegrouptreatedwithtofacitinib5mg and<0.1pointsinthe10-mggroup,comparedto0.8inthe groupreceivingMTX,p<0.001forbothcomparisons).14
Safety
Fivecasesofneoplasm,including3casesoflymphoma,have beenreportedinthegrouptreatedwithtofacitinib,compared to1caseinthe groupreceiving MTX.Infections,including herpeszoster,weremorecommoninpatientsreceiving tofac-itinib,whichalsowasassociatedwithincreasesofcreatinine, LDLandHDL.14
Thisstudysuggeststhattofacitinibiseffectivein control-lingsignsandsymptomsandinreducingstructuraldamageas afirst-linetreatmentinpatientswithRA.Theuseoftofacitinib incombinationwithotherDMARDshasnotbeenevaluatedin thispopulation.Thebenefitoftofacitinibshouldbeevaluated inthecontextoftheadverseeffectsdescribed.
Istofacitinibasafeandeffectivedrugforthetreatmentof RApatientsafterfailurewithMTXorothersynthetic DMARDs?
Effectiveness
Sixsystematicreviewswithameta-analysisevaluatedthe effi-cacyoftofacitinibversusplacebointhetreatmentofpatients withRA,followinganinadequateresponsetoaDMARD.16–21 Zhangetal.16evaluated10randomizedstudiestotaling4,929 patients. Tofacitinib was superior to placebo in the evalu-ation byACR20response(20%improvementin ACRscore), HAQ-DI(HealthAssessmentQuestionnaire–DisabilityIndex) score,andDAS28(28-jointDiseaseActivityScore)inweek12 (asmonotherapyorincombinationwithMTX)andinweek 24 (combined with MTX; monotherapy data not available). Song et al.17 included five randomized trials totaling 1590 patients.Tofacitinibatdosesof5mgand10mg(twicedaily) wassuperiortoplaceboonallevaluatedefficacyendpoints: ACR20 response, painful and swollen joint counts, visual scales ofpainand ofthe overall assessmentbythe physi-cianandpatient,HAQ-DIscoreandC-reactiveprotein(CRP) levels.
Berhan18conductedameta-analysisof8randomized tri-alstotaling4,283patientswithRA,followinganinadequate responseto a DMARDor a biological agent. Theodds ratio
for ACR20 response was 4 times greater with tofacitinib
versus placebo (OR=4.15;95% CI: 3.23–5.32). Theresults of tofacitinibcombined withMTX or asmonotherapydid not differsignificantlyfromeachother.Therewasadecreasein
HAQ-DIscoreinpatientstreatedwithtofacitinibversus con-trols (meanstandardizeddifference=−0.62,95%CI=−0.735
to −0.506). He et al.19 analyzed eightrandomized studies,
totaling 3,791 patients with RA, following an inadequate response to MTX. Higher ACR20 response rates at week 12 occurred with the use of tofacitinib 5mg (relative risk [RR]=2.20; 95% CI=1.58–3.07) and 10mg (RR=2.38; 95% CI: 1.81–3.14)versusplacebo.Theresponsesweremaintainedat week24.
Kawalecetal.20compiledeightrandomizedstudies com-paringtofacitinibversusplacebointhetreatmentofRA,after aninadequateresponsetoasyntheticorbiologicalDMARD. Tofacitinib was superior to placebo in ACR20, ACR50 and ACR70responseratesafter12weeksoftreatment(p<0.0001 forallcomparisons).Tofacitinibalsoresultedinan improve-ment in HAQ-DIversus placebo. Kaur et al.21 conducted a systematicreview(withoutmeta-analysis)of8phaseIIand IIIrandomizedtrials,comparingtofacitinibversusplaceboin patientswithRA,followinganinadequateresponsetoa syn-theticorbiologicalDMARD.Tofacitinibwassuperiortoplacebo after12weeksoftreatmentinACR20andACR50responses andindecreasesinHAQ-DIscore.Together,thesixsystematic reviewsincluded 12publications,related to11randomized clinicaltrials.9–13,22–28Thefindingscorroboratethe effective-nessoftofacitinibcombinedtoMTXorasmonotherapyinthe treatmentofRA.
Two randomized studies evaluated tofacitinib and adal-imumab in parallel groups versus placebo.10,27 No direct comparisonbetweentofacitinibandadalimumabwasheld.In thefirststudy(n=384),ACR20responseratesatweek12were 59.2%and 70.5%fortofacitinibatdoses of5mgand 10mg, respectively, and 22%in theplacebo group(p<0.0001). Dif-ferencesfavoringtofacitinibwerealsofoundinACR50(50% improvement in ACR score) and ACR70 responses, and in DAS28,HAQ-DIandSF-36(MedicalOutcomesStudy36-Item– Short-FormHealthSurvey)scores,andalsointheevaluationof fatiguebyFACIT-F(FunctionalAssessmentofChronicIllness Therapy–Fatigue).Inthisstudy,adalimumabdidnotdiffer significantlyfromplacebowithrespecttomostoutcomes.27In anotherstudy(n=717),ACR20responseratesafter6months oftreatmentwere51.5%and52.6%ingroupswithtofacitinib 5mg and 10mg respectively; 47.2% in groups treated with adalimumab;and28.3%intheplacebogroup(p<0.001forall comparisonsvs.placebo).Alsoahighernumberofsubjectsin remission(DAS28≤2.6)wereobservedafter6monthsinthe
activetreatmentgroups.Theresponsesweremaintaineduntil the12thmonthoffollow-up.10
vanderHeijdeet al.11conductedarandomized, double-blind, placebo-controlled trial to assess the prevention of structuraldamagebytofacitinibin797RApatients. After6 months of treatment (interimanalysis of data), tofacitinib 10mg(twicedaily)significantlyreducedtheprogressionofthe totalmodifiedSharp/vanderHeijdescoreversusplacebo.
Thedataavailablefrom phaseIIandIIIstudies indicate thattofacitinibiseffectiveinthetreatmentofRAafterfailure withaDMARD.
Safety
patientsfromphaseI,29,30II,22,23,25,27andIII9–13,28,31 random-izedclinicaltrials.Overall,discontinuationoftreatmentwas observed in 842 patients (20.8%), and 437 patients (10.7%) werediscontinuedduetoadverseevents.Themainadverse eventsassociatedwithdiscontinuationwere infectionsand laboratoryabnormalities(anemia,neutropenia,lymphopenia, changes inliverenzymes, and serum cholesterol,LDL and creatinineincreases).32
Themostcommoninfectionswerenasopharyngitis,upper respiratorytractinfectionandurinarytractinfection. Infec-tionsofparticularinterestasherpeszosterandtuberculosis weremoreoftenobservedinAsianpatients, forwhomthe riskofdevelopingherpeszosterwashigher(6.7 eventsper 100patient-years)comparedtoCaucasianpatients(3.7events per 100 patient-years).32 The risk of developing tubercu-losis was evaluated in a model by Maiga et al.33 These authors showed that tofacitinib can induce reactivation of latenttuberculosisinfection(LTBI),becausethedruginduces increasesinmycobacteria replication. Long-termextension studies reported the occurrence of 10 cases of tuberculo-sisin 4,102 patients, reinforcing the recommendation of a LTBIsurveybeforestartingtreatmentwithtofacitinib.32The mostcommongastrointestinalmanifestationswerediarrhea (4.4%),nausea(3.3%)and gastritis(2.5%). Despitereportsof gastrointestinalperforation,theoccurrenceoftheseadverse eventsdidnotdifferfromthoseoccurringwithbiologicaland non-biologicalDMARDusers(0.05–0.17eventsper100 patient-years).34,35
Regarding laboratory changes, these effects were char-acterized as mild to moderate, not being, in most cases, required a permanent discontinuation of treatment with tofacitinib.32 Astoanemia,ahemoglobindropbelow7g/dL or a decrease greater than 3g/dL was observed in 1% of patients, with the majority of patients presenting anemia haddecreasesinhemoglobinvaluesintheorderof1–2g/dL (12.7%). The incidence of neutropenia was 4.9%, and no patientshadneutrophilcountsunder500cells/mm3;3.9%had neutrophilcountsof1500–1999cells/mm3.Liverenzyme ele-vationsoccurredin35%ofpatients,andthemostcommon findingwasaonefoldincreaseofnormalvalue(29.7%).These changesweretransientanddidnotresultintreatment discon-tinuation.Athreefoldincreaseofthenormalvalueoccurred in1.2%ofpatients,whose valuesreturned tonormalafter discontinuation oftofacitinib. An increase of creatinewas observedin3.3%ofpatients;thisfindingwasreversibleand transient,anddoesnotseemtoberelatedtoacuterenal fail-ureorprogressiveworseningofrenalfunction.32,36 Theuse oftofacitinibincreasesserumcholesterol,LDLandHDL,with ameanelevationofcholesterolof13mg/dL,avaluesimilar tothatobservedwithHDLelevation.Itwasnotyetproperly establishedtherealmeaningofthesemoderateelevationsin lipidprofile.37
Regardingtheoccurrenceofmalignancies,Curtisetal. ana-lyzed5,671tofacitinibusersin6phaseIIstudies,6phaseIII studiesandtwolong-termextensionstudies.Theyobserved theoccurrenceof107malignancies(excludingnon-melanoma skincancer).Themostcommonneoplasmwaslungcancer (n=24),followedbybreastcancer(n=19),lymphoma(n=10) andgastriccancer(n=5).Themalignancyratebysix-month intervalsofexposuretotofacitinibremainedstableduringthe
periodoftime analyzed.Theincidenceratesofthese neo-plasms wereasexpectedforRApatients withmoderate or severeactivity.38
IstofacitinibasafeandeffectivedrugfortreatmentofRA patientsafterfailurewithbiologicaldrugs?
Effectiveness
AphaseIII, double-blind,placebo-controlled,parallelgroup trialwithsixmonths’durationwasconductedinpatientswith moderatetosevereRAwhohadnotrespondedorwere intol-eranttooneormoreTNFis.12Allpatientsinvolvedinthestudy wereusing,andremainedinuse,ofMTX.399patientswere included(133intofacitinib5mgtwicedailygroup,134in tofac-itinib10mgtwicedailygroup,and132intheplacebogroup). Afterthreemonths,patientsintheplacebogroupwere dis-tributed bytofacitinib 5mgor10mggroups. Mostpatients were female(80.3%),white (84.8%)andwithameanageof 54.4–55.4yearsandameandiseasedurationof11.3–13years. ThemeannumberofpriortreatmentswithTNFiwas1.4–1.5 (64%hadbeenpreviouslytreatedwithaTNFi:26%withtwo and8%withthreeormoretreatments).TNFihadbeen dis-continued forlack ofefficacy in 65.2%,for lackof efficacy andintolerance in19.8%,andforintoleranceonlyin13.8%. BaselinemeanvaluesofHAQ-DIandDAS28-ESR(erythrocyte sedimentationrate)rangedfrom1.5–1.6and6.4–6.5, respec-tively.Theprimaryobjectivesofthestudyweretoevaluatethe ACR20responserate,meanHAQ-DIchangefromtheonsetof treatment,anddiseaseactivityindex(DAS28-VHS)rates<2.6 attheendofthreemonths.12
Afterthreemonths,theACR20responseoftofacitinib 5-mggroupwas41.7%(55of132;95%CI:6.06–28.41;p=0.0024)
versusplacebo24.4%(32of131).Alsoafterthreemonths,the ACR50responsetotofacitinib 5-mggroupwas 26.5%(35of 132;[9.21–27.02];p<0.0001)versusplacebo(8.4%;11of131)and theACR70responsetotofacitinib5-mggroupwas13.6%(18of 132;[5.89–18.32];p<0.0001)versusplacebo,1.5%(2of131).Still, afterthreemonths,themean variationofleastmeanleast squares(LMS)versusbaselineforHAQ-DIwas−0.43([95%CI: −0.36to−0.15]; p<0.0001) fortofacitinib 5-mggroupversus
placebo(−0.18).ImprovementinHAQ-DI≥0.22wasobserved
in54.2%(71of131;[1.76–25.71];p=0.0245) fortofacitinib 5-mggroupversusplacebo,40.5%(53of131).Improvementin HAQ-DI≥0.5wasobservedin35.9%(47of131;[4.52–26.01];
p=0.0053)fortofacitinib5-mggroupversusplacebo,20.6%(27 of131).TheproportionofpatientswithDAS28<2.6afterthree monthswas6.7%(8of119;[0–10.10];p=0.0496)fortofacitinib 5-mggroupversusplacebo(1.7%,2of120).Aftersixmonths, thisfigureincreasedto8.2%(10of122)withtofacitinib5mg
versusplacebo,5.0%(6of120).Inthethirdandsixthmonths, remission(definedbyBooleancriteria)wasreachedby6.1%(8 of132;[1.99–10.13];p=0.0035)fortofacitinib5-mggroupversus
0%intheplacebogroup.12
Regardingtheoutcomesreportedbypatients(PRO–Patient ReportedOutcomes)afterthreemonths,the proportionsof patients with greater than or equal answers to minimum clinicallyimportantdifference(MCDI)were:1.Overall assess-mentofdiseaseactivity–41.88%intheplacebogroupversus
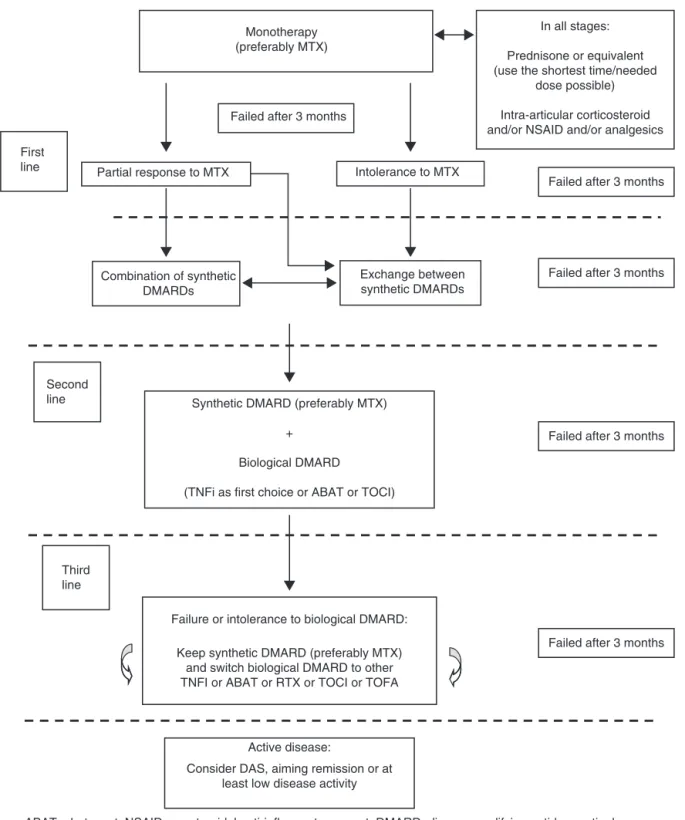
Monotherapy (preferably MTX)
First line
Failed after 3 months
Partial response to MTX Intolerance to MTX
In all stages:
Prednisone or equivalent (use the shortest time/needed
dose possible)
Intra-articular corticosteroid and/or NSAID and/or analgesics
Exchange between synthetic DMARDs
Synthetic DMARD (preferably MTX)
+
Biological DMARD
(TNFi as first choice or ABAT or TOCI)
Active disease:
Consider DAS, aiming remission or at least low disease activity Failure or intolerance to biological DMARD:
Keep synthetic DMARD (preferably MTX) and switch biological DMARD to other TNFI or ABAT or RTX or TOCI or TOFA
Failed after 3 months
Failed after 3 months
Failed after 3 months
Failed after 3 months Second
line
Third line
ABAT, abatacept; NSAID, nonsteroidal anti-inflammatory agent; DMARD, disease modifying antirheumatic drug; MTX, methotrexate; RTX, rituximab; TNFi, tumor necrosis factor inhibitors; TOFA, tofacitinib; TOCI, tocilizumab.
Combination of synthetic DMARDs
(p<0.0001);3.Fatigue(FACIT)–38.60%inthe placebogroup
versus61.54%intofacitinib5-mggroup(p<0.0001);4.SF-36v2 –physicalcomponent–49.14%intheplacebogroupversus
67.80%intofacitinib5-mg group(p<0.05), and 5.SF-36v2– mentalcomponent–37.07%intheplacebogroupversus54.24% intofacitinib5-mggroup(p<0.05).39
InanotherphaseIII,double-blind,placebo-controlledtrial, 611patientswithactiveRArefractoryorintoleranttoatleast onesyntheticorbiologicalDMARDwererandomizedingroups of monotherapy with tofacitinib 5mg twice daily (n=243), tofacitinib10mgtwicedaily(n=245)andplacebo(n=122).13A sub-analysiswithpatientswhohadalreadymadeuseofTNFi orotherbiologicalagentrevealedthat18of46(42.9%)patients inthetofacitinib5-mggroupand6of34(17.7%)patientsin theplacebogroupachievedACR20responsecriteriaatthree months.
Safety
Inthefirst threemonthsofthephaseIIItrial, 145adverse eventswere reported in71 of133 patientsintofacitinib 5-mg group (53.4%) versus 167 adverse events in 75 of 132 patients in the placebo group (56.8%). During this period, themostcommon adverseeventswerediarrhea(13of267; 4.9%),nasopharyngitis(11of267;4.1%),headache(11of267; 4.1%) and urinary tract infection (8 of267; 3.0%) in tofaci-tinib(5mgand10mg)groups;andnausea(9of132;6.8%)in the placebogroup. Seriousadverse events occurred in4of 267patients(1.5%)treatedwithtofacitinib(2in5-mggroup and 2in10-mggroup), and 6in132 patients(4.5%) inthe placebogroup.Noseriousinfectiouseventwasreported.Eight patients(6.0%)discontinuedtheprotocolbecauseofadverse eventsintofacitinib5-mggroupversus7(5.3%)intheplacebo group.12,13
Isthereevidenceforindicationoftheuseofherpeszoster vaccineintofacitinibusers?
Thepreventionofherpeszoster(HZ)isofgreatinteresttoRA patients,sincetheyareingreaterriskofinfectioncompared tothegeneralpopulation.40,41
InBrazil,the vaccineforHZprevention(andits compli-cations)contains liveattenuatedvirus,with14 timesmore antigensthanthevaricellavaccinecanhavefromthesame manufacturer.HZvaccineislicensed foruseinpeopleover 50yearsold.AccordingtotheImmunizationGuideprepared in2014bytheBrazilianSocietyofImmunizations(BSIm)and theBrazilianSocietyofRheumatology(SBR),its useshould beavoidedinpatientswithseveredrugimmunosuppression orwithimmunosuppressioncausedbydisease;however,this vaccinemaybeindicatedinpatientswithmild immunosup-pression.Patients withchronicdiseasescan bevaccinated, exceptincaseofsevereimmunosuppressionandifin treat-mentwithbiologicalagents.42
Atthemoment,however,thereislimitedinformationon thesafetyandefficacyofthisvaccineinRApatients.There are few studies evaluating its value inpatients being sub-mittedtodifferentimmunosuppressiveregimens,including tofacitinib.40
Takingintoaccountthatvaccinationisapreventive mea-surewiththegreatestimpactonreducing theincidenceof
infectioninanyagegroup,theSBRConsensusof2012on vac-cinationinRApatientsrecommendsareviewandupdatingof theimmunizationcardbeforetheuseofsyntheticor biolog-icalDMARDs,includingthevaccineagainstherpeszosterin patientsover50yearsold.43
Likewise, ACR recommends vaccination prior to the onset of treatment with synthetic or biological DMARDs in RA patients over 50 years old. During the use of syn-thetic DMARDs, the vaccine may also be applied, but this is not recommended during the use of biological DMARDs.1,3,4Theuseoftofacitinibwasnotaddressedinthese recommendations.40,44,45
Importantly, the vaccine is not available on the public HealthServiceandisnotcoveredbymosthealthplansand healthinsurancecompanies.
RACommissionofSBRpositionontheuseoftofacitinib forthetreatmentofRAinBrazil
Basedonpreviousevidenceandconsideringtheavailabledata onefficacy,safetyandcostofmedicationsavailabletotreatthe diseaseinBrazil,theRACommissionofSBR,afteraprocessof discussionandvotingonproposals,establishedthefollowing positionontheuseoftofacitinibfortreatmentofRAinBrazil: “Tofacitinib,aloneorincombinationwithMTX,isan alterna-tiveforRApatientswithmoderateorhighactivityafterfailure ofatleasttwodifferentsyntheticDMARDsandonebiological DMARD.”Thelevelofagreementwiththisrecommendation was7.5.
TheRACommissionconsidersitnecessarytoestablisha timelyandobjectiverecommendation,whichwouldhelpthe rheumatologistinhis/herdecisionmakingabouttheuseof thisnewmedicationinRAtreatmentflowchart.Butwealso considered–andthiswasquiteclearonthebasisofafairly debatedvotinganddiscussionprocess–that,undercertain conditionsandinveryspecificclinicalscenarios,theearlier useoftofacitinibcouldbeindicated,atthephysician’s discre-tion,since,asshown,thereisevidenceofefficacyofthisdrug atdifferenttimesinthetreatmentofRA.
ThedecisionofthisCommissionofexperts,toindicatethe useoftofacitinibafterfailureofatleasttwodifferentsynthetic DMARDsandonebiologicalDMARD,tookintoaccountmainly the stillrestrictedperiodofpost-marketingexperience.We believe that this aspect limits the informationrelevant on safety,comparedtoothermedicationsalreadyinuseforRA treatment.Thispositionmaybereviewedincomingyears,in thefaceoftheacquisitionofagreaterexperiencewiththeuse ofthisdrug.
RAtreatmentflowchartupdateinBrazil
Fig.1summarizestheupdatedflowchartofdrugtreatmentfor RAinBrazil,asproposedbytheRACommitteeofSBR.
Conclusion
issuggestedthatitsuseisconsideredinascenariooffailure inatleasttwosyntheticDMARDandatleastonebiological DMARD,thankstoalesserlong-termclinicalexperiencewith thisdruginclinicalpractice.
Thispositioningmayberevisedovertime,inthefaceofthe acquisitionofagreaterexperiencewiththeuseofthisdrug.
Conflicts
of
interest
Licia Maria Henrique da Mota is a consultant/speaker for Abbvie, Bristol-Myers Squib, Janssen, Hospira, Lilly, Pfizer, RocheandUCB.CleandroPiresdeAlbuquerquereportsgrants, personalfeesandnon-financialsupportfromPfizer,grants, personalfeesandnon-financialsupportfromAbbvie,grants, personalfeesand non-financialsupportfrom AstraZeneca, grantsandnon-financialsupportfromJanssen,non-financial support from Bristol-Myers-Squibb, outside the submitted work.RodrigoAiresCorrêaLimaworksasspeakerforAbvvie, PfizerJanssenandUCB.GeraldodaRochaCastelarPinheiro worksasconsultantforAstra-Zeneca,BristolMyersSquibb, Janssen,Novartis,Pfizer,Roche,RuiYiandSanofi.IedaMaria MagalhãesLaurindo works asa speaker and/orconsultant forAbbvie, Astra-Zenica, Bristol, GSK, Lilly, Janssen, Pfizer, RocheandUCB.ManoelBarrosBertololecturesforPfizer, Abb-vie,Roche,JanssenandAventis.Theauthorconductsclinical researchforMSD,Bristol,AventisandRoche.PauloLouzada JúniorheldservicesforUCBandRoche.RinaDalvaNeubarth Giorgi is a honorary member in clinical research for UCB, HGS(GSK),Sanofi-AventisandRoche;andworksas consult-ant and lecturesfor BMS,Pfizer, Janssen and Lilly.Ricardo Machado Xavier works as a speaker and consultant; and reportsresearchgrantsornon-financialsupportfor partici-pationinscientificeventsforAbbvie,Hospira,Janssen,Lilly, Pfizer,Roche.Theotherauthorsdeclarenoconflictsof inter-est.
r
e
f
e
r
e
n
c
e
s
1. SmolenJS,LandewéR,BreedveldFC,BuchM,BurmesterG,
DougadosM,etal.EULARrecommendationsforthe
managementofrheumatoidarthritiswithsyntheticand
biologicaldisease-modifyingantirheumaticdrugs:2013
update.AnnRheumDis.2014;73:492–509.
2. daMotaLM,CruzBA,BrenolCV,PereiraIA,FronzaLS,Bertolo
MB,etal.2011ConsensusoftheBrazilianSocietyof
Rheumatologyfordiagnosisandearlyassessmentof
rheumatoidarthritis.RevBrasReumatol.2011;51:
199–219.
3. daMotaLM,CruzBA,BrenolCV,PereiraIA,Rezende-Fronza
LS,BertoloMB,etal.2012BrazilianSocietyofRheumatology
Consensusforthetreatmentofrheumatoidarthritis.Rev
BrasReumatol.2012;52:152–74.
4. PereiraIA,MotaLM,CruzBA,BrenolCV,FronzaLS,Bertolo
MB,etal.2012BrazilianSocietyofRheumatologyConsensus
onthemanagementofcomorbiditiesinpatientswith
rheumatoidarthritis.RevBrasReumatol.2012;52:
474–95.
5. MotaLM,CruzBA,BrenolCV,PereiraIA,Rezende-FronzaLS,
BertoloMB,etal.Guidelinesforthedrugtreatmentof
rheumatoidarthritis.RevBrasReumatol.2013;53:158–83.
6.O’SheaJJ,KontziasA,YamaokaK,TanakaY,LaurenceA.
Januskinaseinhibitorsinautoimmunediseases.AnnRheum
Dis.2013;72Suppl.2:ii111–5.
7.DiárioOficialdaUnião-SuplementoAnvisa–DOU1–Edic¸ão nr237de08/12/2014Pag.13.Availableat:http://pesquisa.in. gov.br/imprensa/jsp/visualiza/index.jsp?jornal=1010&pagina
=13&data=08/12/2014[accessed19.06.15].
8.SchardtC,AdamsMB,OwensT,KeitzS,FonteloP.Utilization
ofthePICOframeworktoimprovesearchingPubMedfor
clinicalquestions.BMCMedInformDecisMak.2007;7:16.
9.KremerJ,LiZG,HallS,FleischmannR,GenoveseM,
Martin-MolaE,etal.Tofacitinibincombinationwith
nonbiologicdisease-modifyingantirheumaticdrugsin
patientswithactiverheumatoidarthritis:arandomizedtrial.
AnnInternMed.2013;159:253–61.
10.vanVollenhovenRF,FleischmannR,CohenS,LeeEB,Garcia
MeijideJA,WagnerS,etal.Tofacitiniboradalimumabversus
placeboinrheumatoidarthritis.NEnglJMed.
2012;367:508–19.
11.vanderHeijdeD,TanakaY,FleischmannR,KeystoneE,
KremerJ,ZerbiniC,etal.Tofacitinib(CP-690,550)inpatients
withrheumatoidarthritisreceivingmethotrexate:
twelve-monthdatafromatwenty-four-monthphaseIII
randomizedradiographicstudy.ArthritisRheum.
2013;65:559–70.
12.BurmesterGR,BlancoR,Charles-SchoemanC,WollenhauptJ,
ZerbiniC,BendaB,etal.Tofacitinib(CP-690,550)in
combinationwithmethotrexateinpatientswithactive
rheumatoidarthritiswithaninadequateresponsetotumour
necrosisfactorinhibitors:arandomisedphase3trial.Lancet.
2013;381:451–60.
13.FleischmannR,KremerJ,CushJ,Schulze-KoopsH,Connell
CA,BradleyJD,etal.Placebo-controlledtrialoftofacitinib
monotherapyinrheumatoidarthritis.NEnglJMed.
2012;367:495–507.
14.LeeEB,FleischmannR,HallS,WilkinsonB,BradleyJD,
GrubenD,etal.Tofacitinibversusmethotrexatein
rheumatoidarthritis.NEnglJMed.2014;370:2377–86.
15.BulaXELJANZ,CitratodeTofacitinibe;2014.
16.ZhangX,LiangF,YinX,XiaoX,ShiP,WeiD,etal.Tofacitinib foracuterheumatoidarthritispatientswhohavehadan inadequateresponsetodisease-modifyingantirheumatic drug(MMCD):asystematicreviewandmeta-analysis.Clin Rheumatol.2014;33:165–73.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/24389749[accessed
10.06.15;cited25.04.15].
17.SongGG,BaeS-C,LeeYH.Efficacyandsafetyoftofacitinibfor activerheumatoidarthritiswithaninadequateresponseto methotrexateordisease-modifyingantirheumaticdrugs:a meta-analysisofrandomizedcontrolledtrials.KoreanJ InternMed.2014;29:656–63.Availablefrom:http://www. pubmedcentral.nih.gov/articlerender.fcgi?artid=4164730
&tool=pmcentrez&rendertype=abstract[accessed08.06.15;
cited31.03.15].
18.BerhanA.Efficacy,safetyandtolerabilityoftofacitinibin patientswithaninadequateresponsetodiseasemodifying anti-rheumaticdrugs:ameta-analysisofrandomized double-blindcontrolledstudies.BMCMusculoskeletDisord. 2013;14:332.Availablefrom:http://www.pubmedcentral. nih.gov/articlerender.fcgi?artid=4222887&tool=pmcentrez
&rendertype=abstract[accessed12.06.2015;cited29.03.15].
19.HeY,WongAYS,ChanEW,LauWCY,ManKKC,ChuiCSL, etal.Efficacyandsafetyoftofacitinibinthetreatmentof rheumatoidarthritis:asystematicreviewandmeta-analysis. BMCMusculoskeletDisord.2013;14:298.Availablefrom:
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=
3819708&tool=pmcentrez&rendertype=abstract[accessed
20.KawalecP,MikrutA,Wi´sniewskaN,PilcA.Theeffectiveness oftofacitinib,anovelJanuskinaseinhibitor,inthetreatment ofrheumatoidarthritis:asystematicreviewand
meta-analysis.ClinRheumatol.2013;32:1415–24.Available from:http://www.pubmedcentral.nih.gov/articlerender.fcgi? artid=3778229&tool=pmcentrez&rendertype=abstract
[accessed10.06.15;cited03.03.15].
21.KaurK,KalraS,KaushalS.Systematicreviewoftofacitinib:a newdrugforthemanagementofrheumatoidarthritis.Clin Ther.2014;36:1074–86.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/25047498[accessed
16.06.15;cited23.04.15].
22.KremerJM,BloomBJ,BreedveldFC,CoombsJH,FletcherMP, GrubenD,etal.ThesafetyandefficacyofaJAKinhibitorin patientswithactiverheumatoidarthritis:resultsofa double-blind,placebo-controlledphaseIIatrialofthree dosagelevelsofCP-690,550versusplacebo.ArthritisRheum. 2009;60:1895–905.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/19565475[accessed
30.05.15;cited28.02.15].
23.KremerJM,CohenS,WilkinsonBE,ConnellCA,FrenchJL, Gomez-ReinoJ,etal.AphaseIIbdose-rangingstudyofthe oralJAKinhibitortofacitinib(CP-690,550)versusplaceboin combinationwithbackgroundmethotrexateinpatientswith activerheumatoidarthritisandaninadequateresponseto methotrexatealone.ArthritisRheum.2012;64:970–81. Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/22006202[accessed
11.06.15;cited19.04.15].
24.TanakaY,TakeuchiT,YamanakaH.Tofacitinib(CP-690,550),
anoralJanuskinaseinhibitor,asmonotherapyinJapanese
patientswithactiverheumatoidarthritis:a12-weekphase2b
study[abstract].ArthritisRheum.2011;63Suppl.10:2192
[accessed09.06.15].
25.TanakaY,SuzukiM,NakamuraH,ToyoizumiS,ZwillichSH. PhaseIIstudyoftofacitinib(CP-690,550)combinedwith methotrexateinpatientswithrheumatoidarthritisandan inadequateresponsetomethotrexate.ArthritisCareRes (Hoboken).2011;63:1150–8.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/21584942[accessed
02.06.15;cited01.04.15].
26.TanakaY,TakeuchiT,YamanakaH,NakamuraH,Toyoizumi S,ZwillichS.Efficacyandsafetyoftofacitinibas
monotherapyinJapanesepatientswithactiverheumatoid arthritis:a12-week,randomized,phase2study.Mod Rheumatol.2014:1–25.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/25496464[accessed
05.06.15;cited03.05.15].
27.FleischmannR,CutoloM,GenoveseMC,LeeEB,KanikKS, SadisS,etal.PhaseIIbdose-rangingstudyoftheoralJAK inhibitortofacitinib(CP-690,550)oradalimumab
monotherapyversusplaceboinpatientswithactive rheumatoidarthritiswithaninadequateresponseto disease-modifyingantirheumaticdrugs.ArthritisRheum. 2012;64:617–29.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/21952978[accessed
05.06.15;cited19.04.15].
28.CoombsJH,BloomBJ,BreedveldFC,FletcherMP,GrubenD, KremerJM,etal.Improvedpain,physicalfunctioningand healthstatusinpatientswithrheumatoidarthritistreated withCP-690,550,anorallyactiveJanuskinase(JAK)inhibitor: resultsfromarandomised,double-blind,placebo-controlled trial.AnnRheumDis.2010;69:413–6.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/19587388[accessed
12.06.15;cited19.04.15].
29.KrishnaswamiS,KudlaczE,WangR,ChanG.A supratherapeuticdoseoftheJanuskinaseinhibitor tasocitinib(CP-690,550)doesnotprolongQTcintervalin
healthyparticipants.JClinPharmacol.2011;51:1256–63. Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/21148043[accessed
04.06.15;cited05.05.15].
30.CohenS,ZwillichSH,ChowV,LabadieRR,WilkinsonB. Co-administrationoftheJAKinhibitorCP-690,550and methotrexateiswelltoleratedinpatientswithrheumatoid arthritiswithoutneedfordoseadjustment.BrJClin Pharmacol.2010;69:143–51.Availablefrom:http://www. pubmedcentral.nih.gov/articlerender.fcgi?artid=2824475
&tool=pmcentrez&rendertype=abstract[accessed25.05.15;
cited03.05.15].
31.LeeEB,FleishmannRM,HallS,vanVollenhovenRF,BradleyJ,
GrubenD,etal.Radiographic,clinicalandfunctional
comparisonoftofacitinibmonotherapyversusmethotrexate
inmethotrexate-naïvepatientswithrheumatoidarthritis
[abstract].ArthritisRheum.2012;64Suppl.:S1049[accessed
05.06.15].
32.WollenhauptJ,SilverfieldJ,LeeEB,CurtisJR,WoodSP,Soma K,etal.Safetyandefficacyoftofacitinib,anoralJanuskinase inhibitor,forthetreatmentofrheumatoidarthritisin open-label,longtermextensionstudies.JRheumatol. 2014;41:837–52.Availablefrom:http://www.ncbi.nlm.nih.gov/
pubmed/24692527[accessed17.06.15;cited03.05.15].
33.MaigaM,LunS,GuoH,WingleeK,AmmermanNC,Bishai WR.Riskoftuberculosisreactivationwithtofacitinib (CP-690550).JInfectDis.2012;205:1705–8.Availablefrom:
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=
3415851&tool=pmcentrez&rendertype=abstract[accessed
02.06.15;cited05.05.15].
34.CurtisJR,XieF,ChenL,SpettellC,McMahanRM,FernandesJ, etal.Theincidenceofgastrointestinalperforationsamong rheumatoidarthritispatients.ArthritisRheum.
2011;63:346–51.Availablefrom:http://www.pubmedcentral. nih.gov/articlerender.fcgi?artid=3031757&tool=pmcentrez
&rendertype=abstract[accessed03.06.15;cited05.05.15].
35.CurtisJR,LanasA,JohnA,JohnsonDA,SchulmanKL.Factors associatedwithgastrointestinalperforationinacohortof patientswithrheumatoidarthritis.ArthritisCareRes (Hoboken).2012;64:1819–28.Availablefrom:http://www. pubmedcentral.nih.gov/articlerender.fcgi?artid=3508293&tool
=pmcentrez&rendertype=abstract[accessed06.06.15;cited
05.05.15].
36.LundquistLM,ColeSW,SikesML.Efficacyandsafetyof tofacitinibfortreatmentofrheumatoidarthritis.WorldJ Orthop.2014;5:504–11.Availablefrom:http://www.
pubmedcentral.nih.gov/articlerender.fcgi?artid=4133456&tool
=pmcentrez&rendertype=abstract[accessed28.05.15;cited
29.03.15].
37.SoutoA,SalgadoE,ManeiroJR,MeraA,CarmonaL,
Gómez-ReinoJJ.Lipidprofilechangesinpatientswithchronic inflammatoryarthritistreatedwithbiologicagentsand tofacitinibinrandomizedclinicaltrials:asystematicreview andmeta-analysis.ArthritisRheumatol(HobokenNJ). 2015;67:117–27.Availablefrom:
http://www.ncbi.nlm.nih.gov/pubmed/25303044[accessed
29.05.15;cited25.04.15].
38.CurtisJR,LeeEB,KaplanIV,KwokK,GeierJ,BendaB,etal. Tofacitinib,anoralJanuskinaseinhibitor:analysisof malignanciesacrosstherheumatoidarthritisclinical developmentprogramme.AnnRheumDis.2015,
http://dx.doi.org/10.1136/annrheumdis-2014-205847,
pii:annrheumdis-2014-205847[accessed05.06.15].
39.StrandV,BurmesterGR,ZerbiniCAF,MebusCA,ZwillichSH,
GrubenD.Tofacitinibwithmethotrexateinthird-line
treatmentofpatientswithactiverheumatoidarthritis:
patient-reportedoutcomesfromaphaseIIItrial.Arthritis
40.WinthropKL,YamanakaH,ValdezH,MortensenE,ChewR,
KrishnaswamiS,etal.Herpeszosterandtofacitinibtherapy
inpatientswithrheumatoidarthritis.ArthritisRheumatol.
2014;66:2675–84.
41.SmittenAL,ChoiHK,HochbergMC,SuissaS,SimonTA,Testa
MA,etal.Theriskofherpeszosterinpatientswith
rheumatoidarthritisintheUnitedStatesandtheUnited
Kingdom.ArthritisRheum.2007;57:1431–8.
42.PileggiGS,BallalaiI,BrenolC,SaadCGS,TittonD,KlumbEM,
etal.GuiadeImunizac¸ãoSBIm/SBR.Reumatologia.2014/15.
43.BrenolCV,daMotaLM,CruzBA,PileggiGS,PereiraIA,
RezendeLS,etal.2012BrazilianSocietyofRheumatology
Consensusonvaccinationofpatientswithrheumatoid
arthritis.RevBrasReumatol.2013;53:4–23.
44.SinghJA,FurstDE,BharatA,CurtisJR,KavanaughAF,Kremer
JM,etal.2012updateofthe2008AmericanCollegeof
Rheumatologyrecommendationsfortheuseof
disease-modifyingantirheumaticdrugsandbiologicagents
inthetreatmentofrheumatoidarthritis.ArthritisCareRes
(Hoboken).2012;64:625–39.
45.HarpazR,Ortega-SanchezIR,SewardJF.Preventionofherpes
zoster:recommendationsoftheAdvisoryCommitteeon
ImmunizationPractices(ACIP).MMWRRecommRep.