Behavioural pharmacology
Time course of the effects of lipopolysaccharide on prepulse inhibition
and brain nitrite content in mice
Charllyany Sabino Custódio
a,b, Bruna Stefânia Ferreira Mello
a,b,
Rafaela Carneiro Cordeiro
b, Fernanda Yvelize Ramos de Araújo
b, João Henrique Chaves
b,
Silvânia Maria Mendes Vasconcelos
b, Hélio Vitoriano Nobre Júnior
a,
Francisca Cléa Florenço de Sousa
b, Mariana Lima Vale
c, André Férrer Carvalho
d,
Danielle Silveira Macêdo
b,d,naPostgraduate Program in Medical Microbiology, Department of Pathology, Faculty of Medicine, Federal University of Ceará, Fortaleza, CE, Brazil bNeuropharmacology Laboratory, Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Fortaleza, CE, Brazil cLaboratory of inflammation and cancer pharmacology, Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Fortaleza, CE, Brazil
dPsychiatry Research Group, Faculty of Medicine, Federal University of Ceará, Fortaleza, CE, Brazil
a r t i c l e
i n f o
Article history:
Received 22 November 2012 Received in revised form 20 April 2013
Accepted 26 April 2013 Available online 9 May 2013
Keywords: Lipopolysaccharide Sickness behavior Depressive-like behavior Neuroinflammation Nitric oxide
a b s t r a c t
The systemic administration of lipopolysaccharide (LPS) induces time-dependent behavioral alterations, which are related to sickness behavior and depression. The time-course effects of LPS on prepulse inhibition (PPI) remain unknown. Furthermore, the time-dependent effects of LPS on central nitrite content had not been investigated. Therefore, we studied alterations induced by single LPS (0.5 mg/kg, i.p.) administration to mice on parameters, such as PPI, depressive- and anxiety-like behaviors, working memory, locomotor activity and motor coordination, 1.5 and 24 h post-LPS administration. IL-1β and TNFαin the blood and brain as well as brain nitrite levels were evaluated in the prefrontal cortex (PFC), hippocampus (HC) and striatum (ST). An overall hypolocomotion was observed 1.5 h post-LPS, along with depressive-like behaviors and deficits in working memory. Increments in IL-1βcontent in plasma and PFC, TNFαin plasma and decreases in nitrite levels in the ST and PFC were also verified. Twenty-four hours post-LPS treatment, depressive-like behaviors and working memory deficits persisted, while PPI levels significantly reduced along with increases in IL-1βcontent in the PFC and a decrease in nitrite levels in the HC, ST and PFC. Our data demonstrate that a delayed increase (i.e., 24 h post-LPS) in PPI levels ensue, which may be useful behavioral parameter for LPS-induced depression. A decrease in nitrergic neurotransmission was associated with these behavioralfindings.
&2013 Elsevier B.V. All rights reserved.
1. Introduction
Over-activation of the innate immune system and abnormal secretion of inflammatory mediators (e.g., cytokines) have been implicated in the physiopathology of depression (Dellagioia et al., 2012; Dunjic-Kostic et al., 2012; Dunn and Swiergiel, 2005;Dunn et al., 2005) and it has been proposed that treatment with lipopolysacharide (LPS) or interleukin-1 (IL-1) could induce time-dependent depressive-like behavioral in rodents (Dunn et al., 2005).
Whether depression is a form or consequence of sickness behavior has been highlighted as a question of considerable
translational importance (Maes et al., 2012a). In fact, there are considerable phenomenological similarities between sickness behavior and depression; behavioral inhibition, anorexia, weight loss, anhedonia, physio-somatic (fatigue, hyperalgesia, malaise) symptoms, anxiety and neurocognitive impairment can be char-acteristic of both conditions.
Some studies have identified time-related behavioral altera-tions induced by systemic LPS administration (Dantzer et al., 2008; Fu et al., 2010). It has been suggested that short-term behavioral alterations (i.e., during the peak secretion of cytokines) are related to sickness behavior, while long-term depressive behavior is pathophysiologically linked to depression (Dantzer et al., 2008).
Possibly the most compelling evidence implicating cytokines in depressive illness comes from the induction of depressive-like behavior following treatment with endotoxin or recombinant cytokines, such as interleukin-2 (IL-2) and interferon-α (IFN-α) (Capuron and Dantzer, 2003). This includes alterations in neuro-transmitter function and neuroendocrine output (Dunn, 2006) Contents lists available atSciVerse ScienceDirect
journal homepage:www.elsevier.com/locate/ejphar
European Journal of Pharmacology
0014-2999/$ - see front matter&2013 Elsevier B.V. All rights reserved. http://dx.doi.org/10.1016/j.ejphar.2013.04.040
n
Corresponding author at: Federal University of Ceará, Department of Physiology and Pharmacology, Rua Coronel Nunes de Melo 1127, 60431-270 Fortaleza, Ceara, Brazil. Tel.:+55 85 3366 8337; fax:+55 85 3366 8333.
similar to those observed in depressed patients (Anisman et al., 2002;Capuron and Dantzer, 2003). Moreover, healthy volunteers treated with LPS report a decrease in positive mood and increase in anxiety (Grigoleit et al., 2011; Kullmann et al., 2012). Taken together, these studies support the utility of the LPS-induced animal model of depression.
Animal models of depression, such as the separation-induced model, promote traditional depressive-like behavior together with a decrease in prepulse inhibition (PPI) levels (Martin and Brown, 2010). This decrease in PPI levels observed in preclinical models of depression may be related to stress-induced increases in corticotropin-releasing hormone levels (Tejeda et al., 2010), which are observed in depressed subjects (Bangasser and Valentino, 2012). There is also evidence to suggest cytokines may impair PPI (Mizuno et al., 2007). To our knowledge, changes in PPI levels 24 h post systemic LPS treatment have not been investigated.
Nitrergic signaling is involved in the neurobiology of stress-related psychiatric disorders such as anxiety and depressive disorders (Zhou et al., 2007). In the chronic mild stress depression model hippocampal production of nitric oxide (NO) by neuronal nitric oxide synthase isoform (nNOS) is altered (Palumbo et al., 2007; Zhou et al., 2007). However, an association between LPS-induced depression and central alterations in nitrite levels has yet to be reported.
Following previously reported LPS-induced time-dependent behavioral alterations (Dantzer et al., 2008), we investigate the time-course of behavioral changes (depressive/anxiety-like behaviors and alterations in PPI levels) induced by a single systemic adminis-tration of LPS to adult mice, as well as the associated changes in cytokine (IL-1βand TNFα) and nitrite content in brain areas puta-tively related to affective symptoms (Drevets et al., 2008); the prefrontal cortex (PFC), hippocampus (HC) and striatum (ST).
2. Materials and methods
2.1. Drugs
Lipopolysaccharide (LPS) from Escherichia coli, strain 055:B5 (Sigma-Aldrich Corp., St Louis, USA) and ketamine (Cristália Chemical and Pharmaceutical Products, Itapira, Brazil) were used. Drugs were freshly prepared. All other chemicals used were of analytical grade.
2.2. Animals
The experiments were performed in male Swiss mice (weighting 20–30 g) obtained from the Animal House of Federal University of Ceará. The animals were housed 10 per cage in standard poly-carbonate cages (4220.520 cm) and standard environmental conditions (22711C; humidity 6075%; reversed 12-h light/dark cycle with lights on at 19:00) with access to food (FRI-LAB Rat II, FRI-Ribe) and water ad libitum. All experimental procedures were conducted between 8:00 and 14:00 h and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH, 2011) and the Brazilian College of Animal Experimentation (COBEA). The research protocol was approved by the local ethical committee of Federal University of Ceará.
2.3. Experimental design
The dose of LPS was chosen based on previous studies evaluat-ing LPS-induced behavioral and neurochemical alterations in mice (Kessler et al., 2003;Lejuez et al., 2011). In the case of ketamine, the dose selection was based on studies using this drug as an animal model of schizophrenia (de Araujo et al., 2011). The animals were randomly divided into two experimental groups: LPS-treated group
(LPS 0.5 mg/kg, i.p., dissolved in 0.2 ml of volume,n¼32) and control group (n¼32, i.p., injected with 0.2 ml of vehicle - sterile endotoxin-free PBS). To avoid potential influence of behavioral testing on cytokine levels, cytokine assays and behavioral testing were per-formed on different animals (Bossu et al., 2012). Behavioral testing and cytokine analyses were performed in controls and after LPS challenge at two time-points (1.5 and 24 h). Different animals were used at each time-point. One additional group of mice (n¼8) received a single sub-anesthetic dose of ketamine (20 mg/kg, i.p.) and PPI levels were evaluated. The behavioral assessments were performed on four distinct different animal group; (1) openfield, plus maze and rota rod tests in that order; (2) forced swimming test; (3) the Y-maze and (4) PPI determinations. In all behavioral deter-minations, the rater was blind to the experimental treatment.
After each time-point of observation the animals used for cytokines and nitrite determinations were killed by cervical dislocation. Blood was immediately collected, centrifuged and the plasma isolated for posterior analyses. The brain areas dis-sected were: prefrontal cortex (PFC), hippocampus (HC) and striatum (ST). All biological material was immediately stored at −701C until assay.
2.4. Behavioral determinations
2.4.1. Openfield test (OFT)
To analyze the effects of LPS treatment on locomotor activity, animals were evaluated in an openfield test. The arena was made of acrylic (406050 cm) with thefloor divided into nine equal squares. The exploratory activity of the animal was registered during 5 min (Archer, 1973). The number of squares crossed by the animal and number of groomings (stereotyped behavior) and rearings (vertical exploratory activity) were observed. The experi-ments were conducted in a sound-attenuated room, under low-intensity red light.
2.4.2. Rota rod test (RRT)
The rota rod test was used to evaluate motor coordination. In this test, animals were placed with the four paws on a rotating swivel 25 cm above thefloor, turning at 12 rpm (Ugo Basile, Italy). For each animal, the number of falls (up to three falls) during 1 min was registered (Dunham and Miya, 1957).
2.4.3. Forced swimming test (FST)
The mice were individually placed into an acrylic cylinder (25 cm height, 10 cm diameter) containing 8 cm of water main-tained at 22–241C. After 1 min of habituation the time of immo-bility (s) of the animals was rated during 5 min in a total time of 6 min inside the cylinder. Immobility was defined as the absence of active, escape-oriented behaviors such as swimming, jumping, rearing, sniffing, or diving (Porsolt et al., 1978). Any mouse appearing to have difficulty keeping its head above water was removed from the cylinder and excluded from the study. The procedure has been validated in our laboratory by demonstrating that imipramine treatment (10 mg/Kg, i.p.) dramatically decreases immobility time (data not shown).
2.4.4. Elevated plus maze test (EPM)
and closed sections of the maze. These data were used to calculate % open entries (i.e., open entries/total entries100) and % time in open and closed arms. The procedure has been validated in our laboratory by demonstrating that diazepam treatment (1 mg/kg, i.p.) increases the proportion of open arm entries and total time spent in the open arms (data not shown).
2.4.5. Prepulse inhibition of startle (PPI)
The mice were placed in a stabilimeter, which consisted of a wire-mesh cage (844.5 cm) suspended within a PVC frame (2599 cm) attached to the response platform with four thumbnail-screws. The stabilimeter and platform were located inside a ventilated plywood sound attenuating chamber (646040 cm) (Insight, São Paulo, Brazil). Thefloor of the stabilimiter consisted of six stainless steel bars. The startle reaction of the animals generated a pressure on the response platform and analogue signals were amplified, digitized and analyzed by a software of the startle measure system (Insight, São Paulo, Brazil), which also controlled other parameters of the session (intensity of the acoustic stimulus, inter-stimulus interval, etc.). Two loudspeakers located 10 cm above the floor, on each lateral side of the acoustic isolation chamber, were used to deliver the prepulse stimulus, the acoustic startle stimulus and continuous background noise (65 dB). Calibration procedures were conducted before the experiments to ensure equivalent sensitivities of the response platforms over the protocol.
The test session began by placing a subject in the stabilimeter cage for a 5 min exposure to the background noise. After this acclimatization period, the subject was presented with a series of 10 stimuli (pulse alone—120 dB, 50 ms duration), with an inter-trial interval of 15 s. The purpose of this phase was to allow within-session habituation to the startle stimulus. Thereafter, the PPI modulation of the acoustic startle was tested in 74 trials pseudo-randomly divided into seven different categories pre-sented with an inter-trial interval of 15 s: 20 presentations of pulse alone (120 dB, 50 ms duration), 8 presentations of each prepulse intensity alone (70, 75 and 80 dB, 3000 Hz frequency, 20 ms duration) and 10 presentations of each prepulse intensity
+pulse (with 50 ms interval) (Blaszczyk et al., 2000; Gururajan et al., 2010). Mean amplitude of startle response to pulse-alone (P) and prepulse-pulse (PP+P) trials were calculated for each
subject. The PPI level of each mouse was determined by expressing the prepulse+pulse startle amplitude as a percentage decrease
from pulse-alone startle amplitude, according to the following formula: % PPI¼100−[100(PP/P)]. Using this formula, a 0% value denotes no difference between amplitude of startle response to pulse alone and to the prepulse+pulse and, consequently, no PPI.
2.4.6. Y-maze
Immediate working memory performance was assessed by recording spontaneous alternation behavior during a single ses-sion in a Y maze (Hughes, 2004). Each mouse, new to the maze (30 cm long by 6 cm wide by 20 cm high), was placed at the end of one arm and allowed to move freely through the maze during an 8 min session. The series of arm entries was recorded visually. Alternation was defined if mice entered different arms three times in succession from the results of consecutive arm entering. The percentage of correct alternation was calculated as follows: total of alternations/(total arm entries−2), as described elsewhere (Dall'Igna et al., 2007).
2.5. Neurochemical determinations
2.5.1. ELISA for IL1-βand TNFα
The brain areas (PFC, HC and ST) were homogenized in eight volumes of PBS buffer with protease (EMD Biosciences) and
phosphatase (Sigma-Aldrich) inhibitors and centrifuged (10,000 rpm, 5 min). Plasma was used without dilution. The concentration of the cytokines in 50ml samples was determined by ELISA (R&D systems, Minneapolis, MN, USA), following manufacturer's directions and expressed in pg/g tissue.
2.5.2. Nitrite assay
This determination was based on the method described byGreen et al. (1982). The assay was based on the Griess reaction to determine
C LPS 0.5 C LPS 0.5
0 20 40 60
*
1.5 h after LPS 24 h after LPS
C
rossi
ngs (
5
m
in)
C LPS 0.5 C LPS 0.5
0 2 4 6 8 10
*
1.5 h after LPS 24 h after LPS
R
e
ar
in
gs (
5
m
in)
C LPS 0.5 C LPS 0.5
0 2 4 6 8 10
*
&
1.5 h after LPS 24 h after LPS
G
room
in
gs (
5
m
in)
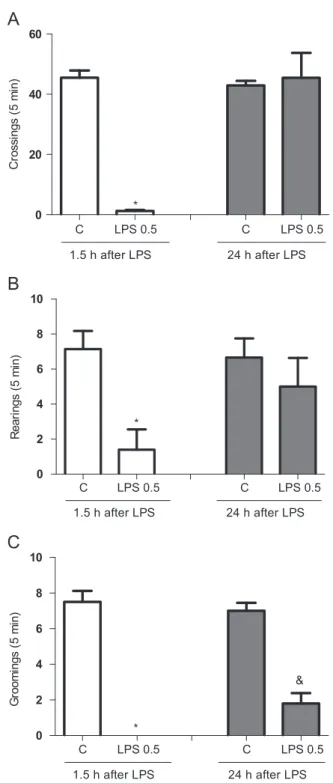
Fig. 1.Number of crossings (A), rearings (B) and groomings (C) 1.5 and 24 h after lipopolysaccharide (LPS) 0.5 mg/kg, i.p. administration (n¼8–10 animals for each
the production of NO. Briefly, 100 ml of the supernatant was incubated with 100 ml of the Griess reagent which consisted of equal parts (1:1:1:1) of 1% sulfanilamide dissolved in 1% H3PO4, 0.1% N-(1-naphthyl)-thylenediamine dihydrochloride and distilled water at room temperature for 10 min. The absorbance was measured at 560 nm in a microplate reader. Nitrite content was determined from a standard nitrite curve generated by using NaNO2(ranging from 0.75 to 100 mM) as standard and was expressed as nM/g tissue.
2.6. Statistical analyses
Data from behavioral and neurochemical determinations are presented as mean7S.E.M. (standard errors of the mean) and were compared with controls using Student'st-test. For the analyses of the PPI produced by the three different prepulse intensities, repeated measures two-way ANOVA with“experimental groups”and“prepulse intensities” as factors was used, with Bonferroni test for post hoc comparisons. The significance level was set atp≤0.05. The statistical program used was GraphPad Prism 5.0 Version for Windows, GraphPad Software (San Diego, CA, USA).
3. Results
The analyses of OFT data, presented inFig. 1(A–C), showed that 1.5 h after LPS administration there was a significant decrease in all parameters evaluated, i.e., number of crossings (t¼8.976, df¼23, Po0.0001), number of rearings (t¼3.654, df¼10,P¼0.0044) and groomings (P¼0.035) when compared to control animals. Twenty four hours post-LPS treatment only the decrease in grooming behavior in relation to control animals was observed (t¼7.076, df¼8,Po0.0001). The evaluation of motor coordination by the RRT presented no significant alterations at any time of observation (data not shown).
Increments in the immobility time were observed in the FST 1.5 h (t¼2.322, df¼10, P¼0.0427) and 24 h (t¼4.102, df¼11, P¼0.018) after LPS administration (Fig. 2) when compared to control animals. In the EPM test a significant decrease in the number of open arms entries (t¼2.809, df¼24,P¼0.0097) (Fig. 3A), total number of arms entries (t¼2.351, df¼10,P¼0.0406) (Fig. 3B) and total time spent in
C LPS 0.5 C LPS 0.5
0 2 4 6 8
1.5 h after LPS 24 h after LPS
*
Open E
n
tr
ies
(
n
)
C LPS 0.5 C LPS 0.5
0 5 10 15
1.5 h after LPS 24 h after LPS
*
T
o
tal Ent
ries (
n
)
C LPS 0.5 C LPS 0.5
0 20 40 60
1.5 h after LPS 24 h after LPS
Op
en
/T
ota
l E
n
tries
(%
)
C LPS 0.5 C LPS 0.5
0 20 40 60
1.5 h after LPS 24 h after LPS
*
Ope
n D
uration/T
otal
D
u
rat
ion (%
)
Fig. 3.Behavioral determinations 1.5 and 24 h after LPS administration in EPM. The parameters evaluated were: (A) Number of entries in the open arms; (B) Number of total entries; (C) Number of entries in the open/total arms (open+close); (D) Time spent in the open/total arms (open+closed). Bars represent means7standard error of means (S.E.M.) of the immobility time in seconds;*Po0.05 vs. control (1.5 h) according to Student
'sttest.
C LPS 0.5 C LPS 0.5
0 50 100 150 200
1.5 h after LPS 24 h after LPS
&
Immobility time (s) in FST
Fig. 2.Immobility time in the forced swimming test (FST) 1.5 and 24 h after lipopolysaccharide (LPS) 0.5 mg/kg, i.p. administration (n¼8–10 animals for each
group). Bars represent means7standard error of means (S.E.M.) of the immobility time in seconds;n
open arms (t¼2.967, df¼11,P¼0.0128) (Fig. 3D) was registered 1.5 h after LPS treatment when compared to control animals. The relation between open/total entries did not change among experimental groups (Fig. 3C).
The analyses of PPI data by repeated measures two-way ANOVA revealed a significant main effect of“prepulse intensities”(df¼2, F¼13.2, Po0.0001) and “experimental groups” (df¼2, F¼16.85, Po0.0001). Bonferronipost hoctest showed a significant decrease in PPI levels 24 h post-LPS treatment using the prepulse intensities of 75 (Po0.05) and 80 dB (Po0.01) in relation to control animals. Ketamine treatment (20 mg/kg) administration caused a signifi -cant decrease in PPI values in all prepulse intensities evaluated. When compared to controls the decrease was significant in the prepulse intensities of 70, 75 and 80 dB (Po0.001), while when compared to data from animals evaluated 24 h after LPS challenge the significance occurred in the prepulse intensities of 75 and
80 dB (Po0.001) (Fig. 4). The spatial working memory was evaluated by the Y-maze spontaneous alternation task and animals treated with LPS presented a significant decrease on the percen-tage of correct alternations 1.5 h (t¼5.867, df¼12,Po0.0001) and 24 h (t¼5.363, df¼15, Po0.0001) after the endotoxin adminis-tration when compared to control animals (data not shown).
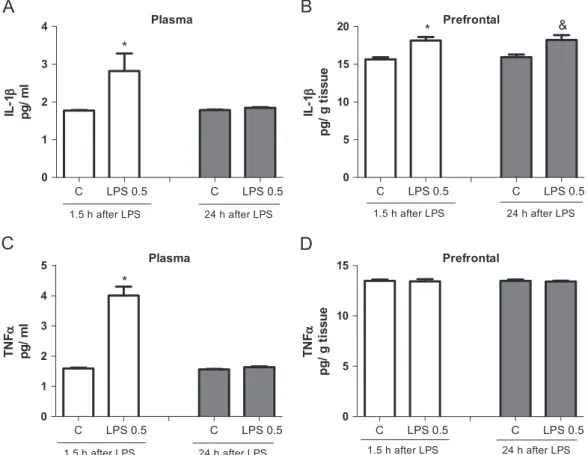
The levels of the cytokines IL-1βand TNFαin the plasma and PFC were tested at the two-time points following LPS treatment (Fig. 5). The content of IL-1βsignificantly increased only 1.5 h post-LPS challenge in plasma and 1.5 h and 24 h in the PFC (t¼4.158, df¼12,P¼0.013) in relation to controls (Fig. 5A and B). The levels of IL-1β in the HC and ST were unchanged when compared to control animals (data not shown). On the other hand, TNFαlevels significantly increased only 1.5 h after LPS administration in the plasma (Fig. 5C) when compared to control group (1.5 h:t¼8.661, df¼13,Po0.0001). No changes in TNFαcontent were observed in the PFC (Fig. 5D), as well as in the HC and ST (data not shown). Nitrite levels decreased in the HC only 24 h after LPS administra-tion (t¼3.535, df¼9, P¼0.0064), while in the ST and PFC this decrease occurred 1.5 h (ST: t¼4.746 df¼13, P¼0.0004; PFC: t¼13.60, df¼8, Po0.0001) and 24 h (ST: t¼2.859 df¼11, P¼0.0155; PFC:t¼8.909, df¼10,Po0.0001) following LPS admin-istration when compared to controls (Fig. 6).
4. Discussion
The results presented here corroborate previousfindings of time-dependent behavioral alterations induced by LPS (Dantzer, 2009; Dantzer et al., 2008) and identify a decrease in PPI levels and nitrite content in mood regulating brain areas of LPS-treated mice at 24 h post-treatment. Increased IL-1βlevels are also found in the PFC 24 h
70 dB 75 dB 80 dB
-40 -20 0 20 40 60
80 Control
LPS 0.5 (1.5 h) LPS 0.5 (24 h) ket 20 mg/kg
* *,& *
M
ean
P
P
I (
p
er
cen
t)
* *,&
Fig. 4.Percent of prepulse inhibition of startle (PPI) using three different prepulse intensities (70, 75 and 80 dB) 1.5 and 24 h after lipopolysaccharide (LPS) 0.5 mg/kg, i.p. administration (n¼8–10 animals for each group). Bars represent means7 stan-dard error of means (S.E.M.) of the percent of PPI;*Po0.05 vs. control and&Po0.05 vs. LPS 0.5 mg/kg for comparisons on each prepulse intensity according to Student's ttest.
C LPS 0.5 C LPS 0.5
0 1 2 3 4
*
Plasma
1.5 h after LPS 24 h after LPS
IL
-1β
pg
/ ml
Prefrontal
C LPS 0.5 C LPS 0.5
0 5 10 15
20
&
1.5 h after LPS 24 h after LPS
*
IL
-1β
pg
/ g t
is
s
u
e
Plasma
C LPS 0.5 C LPS 0.5
0 1 2 3 4 5
*
1.5 h after LPS 24 h after LPS
TN
F
α
pg
/ ml
Prefrontal
C LPS 0.5 C LPS 0.5
0 5 10 15
1.5 h after LPS 24 h after LPS
TN
Fα
pg
/ g t
is
s
u
e
Fig. 5.IL-1βlevels in plasma (A) and prefrontal cortex (B); TNFαlevels in plasma (C) and prefrontal cortex (D) 1.5 and 24 h after lipopolysaccharide (LPS) 0.5 mg/kg, i.p. administration (n¼8–10 animals for each group). Bars represent means7standard error of means (S.E.M.);n
post-treatment, which correlates with the appearance of depressive-like behavior (Ohgi et al., 2013;Park et al., 2011).
The development of depressive disorders is associated with a sustained activation of the innate immune system (Dantzer et al., 2008), while many conditions with an underlying chronic infl am-matory component, such as obesity, rheumatoid arthritis, athero-sclerosis and type II diabetes are associated with an increased prevalence of depressive disorders (Evans et al., 2005). Several studies have used LPS-induced behavioral effects to clarify the associations between neuroinflammation and depression (Dunn and Swiergiel, 2005;Fu et al., 2010;Park et al., 2011). LPS-induced behavioral effects have been shown to be time-dependent and evident 24 h post-treatment (Ohgi et al., 2013;Park et al., 2011).
Patients with major depressive disorder have lower PPI levels when compared to healthy subjects, although PPI levels are higher in depressed patients when compared to schizophrenic patients (Perry et al., 2004). Deficits in central inhibitory mechanisms, i.e., the organism's ability to inhibit or gate responses to sensory, motor, or cognitive information are manifested in several neurop-sychiatric disorders (Swerdlow and Geyer, 1993) and are evaluated using the PPI test (Swerdlow and Geyer, 1993). The association between PPI levels and LPS-induced depressive-like behavior was tested by comparing PPI values of LPS-treated animals (i.e., depressive-like behavior) to those of ketamine-treated mice (a drug known to disrupt PPI and widely employed as a pharma-cological model of schizophrenia) (Becker and Grecksch, 2004). Our results revealed significantly lower PPI levels only 24 h post-LPS treatment. Similar to previous clinical observations (Perry et al., 2004), this decrease in PPI levels in LPS-challenged animals was less severe than that observed in ketamine-treated mice. One possible explanation for the observed lower PPI levels 24 h post-LPS treatment may be the upregulation of indoleamine 2,3-dioxygenase (IDO) induced by pro-inflammatory cytokines, which is known to occur 24 h post-LPS treatment (Dantzer et al., 2008;Sublette and Postolache, 2012).
Kynurenine metabolites are products of the IDO pathway, are involved in the pathophysiology of depression (Dantzer et al., 2008) and their levels have been linked to changes in PPI (Fletcher et al., 2001). Previous reports have found that the administration of LPS did not cause PPI alterations 60–75 min post-LPS treatment (Lockey et al., 2009). This is in agreement with ourfindings that PPI levels are unchanged 1.5 h post-LPS treatment. However, a novel later-onset (i.e., 24 h post-LPS treatment) decrease in PPI was identified.
Preclinical studies of LPS-induced behavioral alterations pro-vided evidence for a delayed depressive-like behavior (Fu et al., 2010) and changes in cytokine levels are known to persist in some areas of the brain (Dunn and Swiergiel, 2005;Dunn et al., 2005) or may not emerge until later (up to 24 h post-LPS treatment) (Bossu et al., 2012). In humans, administration of a bolus injection of bacterial LPS (0.4 ng/kg) induces a pronounced transient increase in the plasma levels of TNFα, IL-1ra, IL-6, IL-10 and cortisol, which is accompanied by a decrease in positive mood and an increase in anxiety (Grigoleit et al., 2011;Kullmann et al., 2012).
Although anxiety has been observed in human subjects follow-ing LPS treatment, it is difficult to translate these results to experimental animals because anxiogenic-like behavior is often observed during the early sickness-behavior period (Lacosta et al., 1999; Swiergiel and Dunn, 2007), together with decreased loco-motor activity (Swiergiel and Dunn, 2007) this may confound the interpretation of results. We find a similar pattern of behavior changes; a decrease in locomotor activity was observed 1.5 h post-LPS treatment together with anxiogenic-like behavior in EPM testing. Although neither of these parameters differed from con-trol mice at 24 h post-LPS treatment, we cannot rule out the possibility that the observed anxiogenic effects during EPM testing are influenced by a decrease in locomotor activity (Swiergiel and Dunn, 2007).
In the present study we identify a decrease in nitrite levels in all brain areas at 1.5 and 24 h post-LPS treatment. It has been reported that treatment with nitric oxide (NO) synthase inhibitor is able to increase the severity of LPS-induced sickness behavior in rats (Connor et al., 2002). Taken together, these results suggest that endogenous NO does not act as a mediator of LPS-induced sickness behavior but may have a protective role, acting in an inhibitory feedback loop to limit LPS-induced sickness. Indeed nitrite/nitrate levels are decreased in the cerebrospinal fluid of depressive patients (Gao et al., 2012) and there is evidence to suggest that aberrant NO neurotransmission may result in
C LPS 0.5 C LPS 0.5
0 100 200 300 400 500
1.5 h after LPS 24 h after LPS & Hippocampus
Nitrite Conten
t
nM
/g
tissu
e
C LPS 0.5 C LPS 0.5
0 100 200 300 400
1.5 h after LPS 24 h after LPS
*
& Striatum
Nitr
ite Conten
t
nM
/g
tissu
e
C LPS 0.5 C LPS 0.5
0 200 400 600 800
1.5 h after LPS 24 h after LPS
*
&Prefrontal
Nit
rit
e C
o
nt
en
t
n
M
/g
t
is
s
u
e
Fig.6.Nitrite levels in hippocampus, striatum and prefrontal cortex 1.5 and 24 h after lipopolysaccharide (LPS) 0.5 mg/kg, i.p. administration (n¼8–10 animals for each group). Bars represent means7standard error of means (S.E.M.);n
Po0.05 vs. control (1.5 h),&Po0.05 vs. control (24 h) according to Student
depressive-like behaviors via the cAMP response element binding protein (CREB) (Hu et al., 2012;Maes et al., 2009).
A role for endogenous NO in restraining over-activation of the hypothalamic-pituitary–adrenal (HPA) axis during periods of increased cytokine and/or neuropeptide secretion (e.g., during immune stimula-tion) (Jankord et al., 2009;Uribe et al., 1999), may account for the LPS-induced decrease in nitrite levels observed here. Indeed, gluco-corticoids play an important role in coping with unpredictable insult by maintaining allostasis (McEwen and Wingfield, 2003; Romero et al., 2009). Therefore, we can speculate that LPS-induced reductions in brain nitrite levels may promote an increase in corticosterone secretion and thus initially mitigate depressive-like behavior. Our findings suggest that NO is not only involved in the development of sickness behavior, but also may play a role in LPS-induced depressive-like behavior.
This study identifies increased IL-1β levels in the brain 1.5 and 24 h post-LPS treatment only in the PFC. The PFC plays an important role in cognitive control, such as planning, reasoning and problem solving (Miller and Cohen, 2001). A recent review article highlights that in translational models, IL-1βadministration elicits depressive-like behaviors, neuroinflammation and neuroprogression, while interleukin-1 receptor antagonists (e.g., IL-1R) were suggested to have antidepressant effects and possibly attenuate neuroprogression of severe mental disorders (Maes et al., 2012b).
In conclusion, we identify LPS-induced time-dependent altera-tions in PPI levels, brain nitrite content and prefrontal IL-1βlevels, supporting a specific role for NO pathways in LPS-induced beha-vioral alterations and suggest that drugs which modulate central NO signaling may have promise as novel neurotherapeutic targets for depression.
Acknowledgements
This study was supported by grants from FUNCAP—Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico, CAPES Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and CNPq—National Council for Scientific and Technological Development.
The authors would like to especially thank the team of American Manuscript Editors for the language and style editing of the manuscript.
References
Anisman, H., Hayley, S., Turrin, N., Merali, Z., 2002. Cytokines as a stressor: implications for depressive illness. Int. J. Neuropsychopharmacolog. 5, 357–373. Archer, J., 1973. Tests for emotionality in rats and mice: a review. Anim. Behav. 21,
205–235.
Bangasser, D.A., Valentino, R.J., 2012. Sex differences in molecular and cellular substrates of stress. Cell. Mol. Neurobiol. 32, 709–723.
Becker, A., Grecksch, G., 2004. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Prog. Neuropsychopharmacol. Biol. Psych. 28, 1267–1277.
Blaszczyk, J.W., Tajchert, K., Lapo, I., Sadowski, B., 2000. Acoustic startle and
open-field behavior in mice bred for magnitude of swim analgesia. Physiol. Behav. 70, 471–476.
Bossu, P., Cutuli, D., Palladino, I., Caporali, P., Angelucci, F., Laricchiuta, D., Gelfo, F., De Bartolo, P., Caltagirone, C., Petrosini, L., 2012. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-alpha and IL-18. J. Neuroinflamm. 9, 101. Capuron, L., Dantzer, R., 2003. Cytokines and depression: the need for a new
paradigm. Brain Behav. Immun. 17, S119–124.
Connor, T.J., O'Sullivan, J., Nolan, Y., Kelly, J.P., 2002. Inhibition of constitutive nitric oxide production increases the severity of lipopolysaccharide-induced sickness behaviour: a role for TNF-alpha. Neuroimmunomodulation 10, 367–378. Dall'Igna, O.P., Fett, P., Gomes, M.W., Souza, D.O., Cunha, R.A., Lara, D.R., 2007.
Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp. Neurol. 203, 241–245.
Dantzer, R., 2009. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 29, 247–264.
Dantzer, R., O'Connor, J.C., Freund, G.G., Johnson, R.W., Kelley, K.W., 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56.
de Araujo, F.Y., de Oliveira, G.V., Gomes, P.X., Soares, M.A., Silva, M.I., Carvalho, A.F., de Moraes, M.O., de Moraes, M.E., Vasconcelos, S.M., Viana, G.S., de Sousa, F.C., Macedo, D.S., 2011. Inhibition of ketamine-induced hyperlocomotion in mice by the essential oil of Alpinia zerumbet: possible involvement of an antioxidant effect. J. Pharm. Pharmacol. 63, 1103–1110.
Dellagioia, N., Devine, L., Pittman, B., Hannestad, J., 2012. Bupropion pre-treatment of endotoxin-induced depressive symptoms. Brain Behav. Immun. 12, 00470–00479.
Drevets, W.C., Price, J.L., Furey, M.L., 2008. Brain structural and functional abnorm-alities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 213, 93–118.
Dunham, N., Miya, T., 1957. A note on a simple apparatus for detecting neurological deficits in rats and mice. J. Am. Pharm. Assoc. 46, 208–209.
Dunjic-Kostic, B., Ivkovic, M., Radonjic, N.V., Petronijevic, N.D., Pantovic, M., Damjanovic, A., Poznanovic, S.T., Jovanovic, A., Nikolic, T., Jasovic-Gasic, M., 2012. Melancholic and atypical major depression—connection between cyto-kines, psychopathology and treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 1–6.
Dunn, A.J., 2006. Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 6, 52–68.
Dunn, A.J., Swiergiel, A.H., 2005. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol. Biochem. Behav. 81, 688–693.
Dunn, A.J., Swiergiel, A.H., de Beaurepaire, R., 2005. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci. Biobehav. Rev. 29, 891–909.
Evans, D.L., Charney, D.S., Lewis, L., Golden, R.N., Gorman, J.M., Krishnan, K.R., Nemeroff, C.B., Bremner, J.D., Carney, R.M., Coyne, J.C., Delong, M.R., Frasure-Smith, N., Glassman, A.H., Gold, P.W., Grant, I., Gwyther, L., Ironson, G., Johnson, R.L., Kanner, A.M., Katon, W.J., Kaufmann, P.G., Keefe, F.J., Ketter, T., Laughren, T.P., Leserman, J., Lyketsos, C.G., McDonald, W.M., McEwen, B.S., Miller, A.H., Musselman, D., O'Connor, C., Petitto, J.M., Pollock, B.G., Robinson, R.G., Roose, S.P., Rowland, J., Sheline, Y., Sheps, D.S., Simon, G., Spiegel, D., Stunkard, A., Sunderland, T., Tibbits Jr., P., Valvo, W.J., 2005. Mood disorders in the medically ill: scientific review and recommendations. Biol. Psychiatry 58, 175–189.
Fletcher, P.J., Selhi, Z.F., Azampanah, A., Sills, T.L., 2001. Reduced brain serotonin activity disrupts prepulse inhibition of the acoustic startle reflex. Effects of 5,7-dihydroxytryptamine and p-chlorophenylalanine. Neuropsychopharmacology 24, 399–409.
Fu, X., Zunich, S.M., O'Connor, J.C., Kavelaars, A., Dantzer, R., Kelley, K.W., 2010. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J. Neuroinflamm. 7, 43.
Gao, S.F., Qi, X.R., Zhao, J., Balesar, R., Bao, A.M., Swaab, D.F., 2012. Decreased NOS1 expression in the anterior cingulate cortex in depression. Cerebral Cortex 17, 17. Green, L.C., Wagner, D.A., Glogowski, J., Skipper, P.L., Wishnok, J.S., Tannenbaum, S.R., 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126, 131–138.
Grigoleit, J.S., Kullmann, J.S., Wolf, O.T., Hammes, F., Wegner, A., Jablonowski, S., Engler, H., Gizewski, E., Oberbeck, R., Schedlowski, M., 2011. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6, 2. Gururajan, A., Taylor, D.A., Malone, D.T., 2010. Effect of testing conditions on the
propsychotic action of MK-801 on prepulse inhibition, social behaviour and locomotor activity. Physiol. Behav. 99, 131–138.
Hu, Y., Wu, D.L., Luo, C.X., Zhu, L.J., Zhang, J., Wu, H.Y., Zhu, D.Y., 2012. Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc. Nat. Acad. Sci. U.S.A. 109, 14224–14229.
Hughes, R.N., 2004. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 28, 497–505.
Jankord, R., McAllister, R.M., Ganjam, V.K., Laughlin, M.H., 2009. Chronic inhibition of nitric oxide synthase augments the ACTH response to exercise. Am. J. Physiol. Reg. I 296, R728–R734.
Kessler, R.C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K.R., Rush, A.J., Walters, E.E., Wang, P.S., 2003. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105.
Kullmann, J.S., Grigoleit, J.S., Lichte, P., Kobbe, P., Rosenberger, C., Banner, C., Wolf, O.T., Engler, H., Oberbeck, R., Elsenbruch, S., Bingel, U., Forsting, M., Gizewski, E.R., Schedlowski, M., 2012. Neural response to emotional stimuli during experimental human endotoxemia. Hum. Brain Mapp. 28, 22063.
Lacosta, S., Merali, Z., Anisman, H., 1999. Behavioral and neurochemical conse-quences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 818, 291–303.
Lejuez, C.W., Hopko, D.R., Acierno, R., Daughters, S.B., Pagoto, S.L., 2011. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav. Modif. 35, 111–161.
Lockey, A.J., Kavaliers, M., Ossenkopp, K.P., 2009. Lipopolysaccharide produces dose-dependent reductions of the acoustic startle response without impairing prepulse inhibition in male rats. Brain Behav. Immun. 23, 101–107.
Maes, M., Song, C., Yirmiya, R., 2012b. Targeting IL-1 in depression. Expert Opin. Ther. Targets 27, 27.
Maes, M., Yirmyia, R., Noraberg, J., Brene, S., Hibbeln, J., Perini, G., Kubera, M., Bob, P., Lerer, B., Maj, M., 2009. The inflammatory & neurodegenerative (I&ND) hypoth-esis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 24, 27–53.
Martin, A.L., Brown, R.E., 2010. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav. Brain Res. 207, 196–207. McEwen, B.S., Wingfield, J.C., 2003. The concept of allostasis in biology and
biomedicine. Horm. Behav. 43, 2–15.
Miller, E.K., Cohen, J.D., 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202.
Mizuno, M., Sotoyama, H., Narita, E., Kawamura, H., Namba, H., Zheng, Y., Eda, T., Nawa, H., 2007. A cyclooxygenase-2 inhibitor ameliorates behavioral impair-ments induced by striatal administration of epidermal growth factor. J. Neurosci. 27, 10116–10127.
NIH, 2011. Guide for the Care and Use of Laboratory Animals—National Research Council. The National Academies Press, Washington, DC..
Ohgi, Y., Futamura, T., Kikuchi, T., Hashimoto, K., 2013. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 103, 853–859. Palumbo, M.L., Fosser, N.S., Rios, H., Zorrilla Zubilete, M.A., Guelman, L.R.,
Cremaschi, G.A., Genaro, A.M., 2007. Loss of hippocampal neuronal nitric oxide synthase contributes to the stress-related deficit in learning and memory. J. Neurochem. 102, 261–274.
Park, S.E., Dantzer, R., Kelley, K.W., McCusker, R.H., 2011. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflamm. 8, 1742–2094.
Perry, W., Minassian, A., Feifel, D., 2004. Prepulse inhibition in patients with non-psychotic major depressive disorder. J. Affect Disord. 81, 179–184.
Porsolt, R.D., Bertin, A., Jalfre, M., 1978.“Behavioural despair”in rats and mice: strain differences and the effects of imipramine. Eur. J. Pharmacol. 51, 291–294. Romero, L.M., Dickens, M.J., Cyr, N.E., 2009. The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm. Behav. 55, 375–389.
Sublette, M.E., Postolache, T.T., 2012. Neuroinflammation and depression: the role of indoleamine 2,3-dioxygenase (IDO) as a molecular pathway. Psychosom. Med. 74, 668–672.
Swerdlow, N.R., Geyer, M.A., 1993. Prepulse inhibition of acoustic startle in rats after lesions of the pedunculopontine tegmental nucleus. Behav. Neurosci. 107, 104–117.
Swiergiel, A.H., Dunn, A.J., 2007. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and openfield tests. Pharmacol. Biochem. Behav. 86, 651–659.
Tejeda, H.A., Chefer, V.I., Zapata, A., Shippenberg, T.S., 2010. The effects of kappa-opioid receptor ligands on prepulse inhibition and CRF-induced prepulse inhibition deficits in the rat. Psychopharmacology 210, 231–240.
Uribe, R.M., Lee, S., Rivier, C., 1999. Endotoxin stimulates nitric oxide production in the paraventricular nucleus of the hypothalamus through nitric oxide synthase I: correlation with hypothalamic-pituitary–adrenal axis activation. Endocrinology 140, 5971–5981.