w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Polycarpol
in
Unonopsis
,
Bocageopsis
and
Onychopetalum
Amazonian
species:
chemosystematical
implications
and
antimicrobial
evaluation
Felipe
M.A.
da
Silva
a,∗,
Bruna
R.
de
Lima
a,
Elzalina
R.
Soares
a,
Richardson
A.
de
Almeida
a,
Francinaldo
A.
da
Silva
Filho
a,
Wallace
R.
Corrêa
b,
Marcos
J.
Salvador
b,
Antonia
Q.L.
de
Souza
c,
Hector
H.F.
Koolen
d,
Afonso
D.L.
de
Souza
a,
Maria
L.B.
Pinheiro
aaDepartamentodeQuímica,UniversidadeFederaldoAmazonas,69077-000Manaus,AM,Brazil bInstitutodeBiologia,UniversidadeEstadualdeCampinas,13083-970Campinas,SP,Brazil cFaculdadedeCiênciasAgrárias,UniversidadeFederaldoAmazonas,69077-000Manaus,AM,Brazil dInstitutodeQuímica,UniversidadeEstadualdeCampinas,13083-970Campinas,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received7December2014 Accepted8January2015 Availableonline11February2015
Keywords:
Antimicrobialactivity Bocageopsis
Chemotaxonomicmarker Onychopetalum Polycarpol Unonopsis
a
b
s
t
r
a
c
t
Polycarpol,arecurrentlanostane-typetriterpeneinAnnonaceaefamily,wasconfirmedbythinlayer chromatographyandmassspectrometryanalysisintheaerialparts(twigsandtrunkbarks)ofUnonopsis duckeiR.E.Fr.,U.floribundaDiels,U.rufescens(Baill.)R.E.Fr.,U.stipitataDiels,Onychopetalum amazon-icumR.E.Fr.andBocageopsispleiospermaMaas.Itschemotaxonomicsignificancewasdiscussedforthese threegenera,aswellfortheAnnonaceaefamily.Inaddition,theantimicrobialactivityagainstseveral strainsofmicroorganismswasevaluatedforthefirsttimeforthiscompound,beingobservedsignificant antibacterialactivityagainstStaphylococcusaureus(ATCC6538),Staphylococcusepidermidis(ATCC1228) andEscherichiacoli(ATCC10538andATCC10799)withminimalinhibitoryconcentrationvaluesbetween 25and50gml−1.
©2014SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
The Annonaceae family comprises about 2500 species dis-tributedin130genera.Thisfamilyconsistsoftrees,shrubsand climbers,witha predominantdistributioninlowlands of tropi-calandsubtropicalregions,beingconsideredapantropicalfamily (Richardsonetal.,2004).Thisfamilyischemicallycharacterized bythepresenceofalkaloids,particularlyisoquinoline-derivedand terpenoids,wheremonoterpenesandsesquiterpenesare predom-inantin thecomposition ofessential oils (Leboeuf et al.,1982; Fournieretal.,1999).Anotherremarkablefeatureisthepresenceof annonaceousacetogenins,aclassexclusivefromthisfamily,that hasattractedinterestduetothegrowinglistofnewlydescribed structuresandbiologicalactivities(Changetal.,1999;Cunhaetal., 2009).Acetogeninsalongwiththelanostane-typetriterpene poly-carpolhavebeensuggestedaspotentialchemotaxonomicmarkers forthisfamily(Leboeufetal.,1982;Goulartetal.,1986;Jungetal., 1990).
∗ Correspondingauthor.
E-mail:felipemas@ufam.edu.br(F.M.A.d.Silva).
Polycarpol displays a growing list of biological activities describedintheliterature,suchasantifilarial(Nyasseetal.,2006), antineoplastic(Matosetal.,2006),antitrypanosomal(Ngantchou etal.,2009)andmorerecentlyanti-inflammatory(Saadawietal., 2012).Althoughtriterpenespossessprovenantimicrobial activi-ties(Haraguchietal.,1999;Katerereetal.,2003;Djoukengetal., 2005;Angehetal.,2007)alackofstudiesdescribingthe antibacte-rialactivitiesofpolycarpolisobserved.Thecontinuoussearchfor newantimicrobialdrugsisstimulatedbytheincreasing appear-anceofantibiotic-resistantorganisms,suchasindividualsofthe Staphylococcus,Pseudomonas,Enterococcus,andPneumococcus gen-era(Pachecoetal.,2012).
Unonopsis,BocageopsisandOnychopetalumarebotanicallyclose generadistributedthroughneotropicalregions.The morphologi-calsimilaritiesamongthesegenerawereexpressedbyFries,when he placed them in the informal “Unonopsis-Gruppe”. Recently thiscloserelationshipwassupportedbyphylogeneticresearches (Maasetal.,2007).Unonopsisisthelargestgenusamong these three,comprising approximately50 species.Ontheotherhand,
Bocageopsisand Onychopetalumcomprise few species, fourand
two, respectively (B. mattogrossensis, B. canescens, B. multiflora andB.pleiosperma,O.amazonicumandO.periquino)(Maasetal., 2007).Unonopsisisalsothemostexploredfromthechemicaland
http://dx.doi.org/10.1016/j.bjp.2015.01.003
biologicalpointsof view(Siqueiraet al.,1998; Waechteretal., 1999; Silva et al., 2012b,c, 2014). The chemical information regardingtheBocageopsisandOnychopetalumgeneraisstilllimited, beingfocuseddirectedtothestudyoftheessentialoilsand alka-loidcompositions(Almeidaetal.,1976;Oliveiraetal.,2014;Soares etal.,2015).Inthiswork,thepresenceofthetriterpenepolycarpol (1)intheaerialparts(trunkbarksandtwigs)ofAmazonian
Boca-geopsis, Onychopetalum and Unonopsis species was investigated
bythinlayerchromatography(TLC)andmassspectrometry(MS) approaches.Thechemotaxonomicsignificanceofpolycarpolwas discussedforthesethreegeneraandfortheAnnonaceaefamily. Inaddition,polycarpolwassubmittedtoinvitroassaysto evalu-ateitsantimicrobialactivityagainstStaphylococcusaureus(ATCC 6538),Staphylococcusepidermidis(ATCC1228),Pseudomonas aeru-ginosa(ATCC27853),Enterobacterfaecalis(Ef),Bacillussubtilis(Bs),
Escherichiacoli(ATCC10538and10799),Candidaalbicans(ATCC
10231and1023),Candidaparapsilosis(ATCC22019),Candida tropi-calis(ATCC157andct),Candidaglabrata(ATCC30070)andCandida dubliniensis(ATCC778157).
OH
(1) HO
Materialsandmethods
Plantmaterial
Theaerialparts (twigsand trunkbarks)of Unonopsisduckei R.E.Fr.,(voucherno.3289), Onychopetalumamazonicum R.E.Fr. (voucherno.218341)andBocageopsispleiospermaMaas(voucher no.183125)werecollectedintheReservaFlorestalAdolphoDucke, atthemunicipalityofManaus,Brazil,fromindividualspreviously identifiedbyspecialists.Thevoucherspecimensaredepositedin theherbariumoftheInstitutoNacionaldePesquisasda Amazô-nia(INPA).The samepartsfor U.floribunda (voucherno.6701)
and U. rufescens (voucherno. 3767) were collectedin the
Dis-tritoAgropecuáriodaSUFRAMAinthesamecity,fromindividuals previously identified byspecialists. The voucher specimens are depositedinthebotanycollectionofPDBFF/INPA(ProjetoDinâmica BiológicadeFragmentosFlorestais).Theplantmaterial(twigsand trunkbarks)of U.stipitata(voucher no.8250) wascollectedin thecampusoftheUniversidadeFederaldoAmazonas(UFAM).A voucherspecimenwasdepositedintheHerbariumofthe institu-tion.ThespecimencollectedonthecampusofUFAMwasidentified byProf.AntonioCarlosWebberfromtheDepartamentode Biolo-giaoftheUFAM.TheSISBIOlicensenumberforthecollectionis 34677-1.
Extractionandconfirmationofthepolycarpol
TheextractionofpolycarpolfromU. duckei,U. floribunda, U. rufescens,U.stipitata,O.amazonicumandB.pleiospermawasmade accordingto theadapted methoddescribed for U. guatterioides (Silvaetal.,2012a),briefly:10gofpowderedmaterial(twigsand trunkbarks)weremaceratedforthreedayswithhexane(80ml). Theextractwasconcentrated atreduced pressure. The precipi-tateformedwaswashedwithhexaneandre-crystallizedinethyl
Table1
AntimicrobialactivityinvitroofpolycarpolwiththeirMICvalues(gml−1).
Microorganism Polycarpol Positivecontrolsb
MICa MIC
Staphylococcusaureus(ATCC6538)c 25 25 Staphylococcusepidermidis(ATCC1228)c 50 50 Bacillussubtilis(Bs)d –e 50 Escherichiacoli(ATCC10538)c 50 50 Escherichiacoli(ATCC10799)c 50 50 Pseudomonasaeruginosa(ATCC27853)c – >500 Enterobacterfaecalis(Ef)d – 50 Candidaalbicans(ATCC10231)c 250 12.5 Candidaalbicans(ATCC1023)c 250 12.5 Candidaparapsilosis(ATCC22019)c – 12.5 Candidatropicalis(ATCC157)c – 12.5 Candidatropicalis(ct)d – 12.5 Candidaglabrata(ATCC30070)c – 12.5 Candidadubliniensis(ATCC778157)c 250 12.5
aMICminimuminhibitoryconcentrationingml−1.
bPositivecontrol:chloramphenicolforbacteriastrainsandketoconazoleforyeast strains.
c Standardstrain. d Fieldstrain.
e(–)withoutinhibitionofdevelopment.Compoundsevaluatedintherangeof10 and500gml−1.
acetate.Approximately1mgoftheeachsolidwasseparatedfor TLCandMSanalysis,beingpolycarpol(1)identifiedbycomparison withanauthenticstandard(Silvaetal.,2012a).
Biologicalassay
Thepurestandardofpolycarpolwasevaluatedforits antimi-crobial activity using the brothmicrodilution method(96-well microtiterplates),aspreviouslydescribed(Salvadoretal.,2002; Barrosetal.,2009; Costaet al.,2010;Bataglionet al.,2014)to giveconcentrations between 10 and 500gml−1. The minimal inhibitoryconcentration(MIC)wascalculatedasthelowest con-centrationshowingcompleteinhibitionofatestedstrain.Inthese tests, chloramphenicol and ketoconazole were used as experi-mentalpositivecontrol,whilethesolutionpropyleneglycol-sterile distilledwater(5:95,v/v)servedasthenegativecontrol.Each sensi-tivitytestwasperformedinduplicateforeachmicroorganismand repeatedthreetimes.Thestrainsutilizedintheassaysareshown inTable1.
Instrumentsandmaterials
200 300 400 500 600 700 800 900 1000
m/z 0
10 20 30 40 50 60 70 80 90
100 423.33
441.31 405.34
455.27
207.24 338.42
121.15 269.37
535.26 577.45 637.71 674.49 727.48 775.74 810.48 882.82 934.80 HO
HO HO
[M–2H2O+H]+ [M+H]+
[M–H2O+H]+
H
H
H H O
+
+ + +
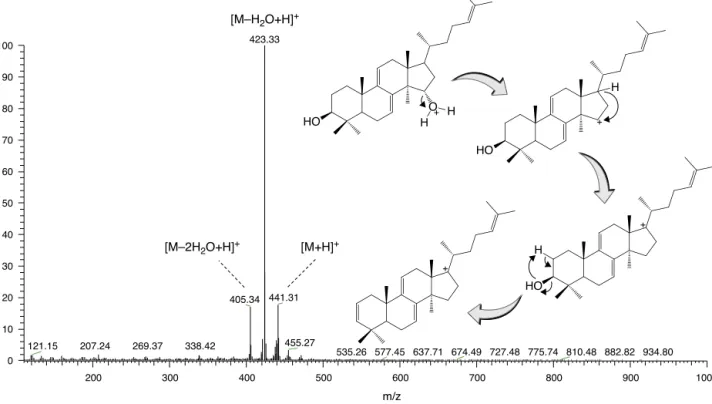
Fig.1. MassspectrumandinsourcethermaldissociationproposalforthesequentiallossesobservedforAPCIionizationofpolycarpol.
Resultsanddiscussion
ConfirmationofpolycarpolbyTLCandMSanalyses
Fromtheaerialparts ofU. duckei,U. floribunda,U. rufescens, U.stipitata,O.amazonicumand B.pleiospermawhitesolidswere obtainedafterextractionandre-crystallizationprocedures.Allthe samplesweredissolvedinethylacetateandanalyzedbyTLCin comparison withan authentic polycarpolstandard (Silvaet al., 2012a).Thesamplesandthestandardpresentedthesame reten-tion factor (Rf 0.6) when eluted with a hexane–ethyl acetate (7:3)mixtureandrevealedwithvanillin–sulphuricacidreagent (bluespot) andunderUV light(254nm).Theobservationofan intense blue spot in all samples is in agreement with results expected for triterpene compounds (Oleszek et al., 2008). The sampleswhensubjected toMSanalysisinfull scanmode, pre-sented three major ions at m/z441 [M+H]+, 423 (
−18Da) and
405 (−18Da) (Fig. 1). The ion at m/z 441 corresponds to the
protonatedmoleculeexpectedforpolycarpol,whilethem/z423 and 405are consistent with sequential neutral losses (−H2O). Hydroxilatedtriterpenespresentthisdissociativebehaviordueto theapplicationofhighdesolvationtemperaturesinAPCI exper-iments (Koolen et al., 2013). All samples presented the same threemajorionswhensubjectedtoMSanalysis.Theseevidence along withTLC observations assisted theconfirmation of poly-carpol (1) in aerial parts (twigs and trunk barks) of U. duckei, U. floribunda, U. rufescens, U. stipitata, O. amazonicum and B. pleiosperma,being this substance reportedfor the first time in
thisUnonopsisspeciesaswellinOnychopetalumandBocageopsis
genus.
Antibacterialactivity
Thepolycarpolwasevaluatedinvitroforantimicrobialactivity againstelevenstrainsofmicroorganism(Table1).Thissubstance presentedsignificantantimicrobialactivityagainstS.aureus(ATCC
6538),S.epidermidis(ATCC1228)andE.coli(ATCC10538andATCC 10799)withMICvaluesbetween25and50gml−1.Forthestrains ofB.subtilis,E.faecalisandP.aeruginosawerenotobserved inhi-bitionofthedevelopment.Theseobservationsareinagreement withliteraturedata,sincelannostanetriterpenesisolatedfromthe vegetalspeciesandfungiwhenassayedagainstgram-negativeand gram-positivebacteria,presentseveralactivestructures(Liuetal., 2010a,b;Mosaetal.,2014).Oleanane,ursane,lupane,friedelane, fernane and others miscellaneous-typetriterpenes alsopresent severalactivecompoundsagainstgram-negativebacteria,mainly P.aeruginosa,E.coli,Klebsiellapneumoniae,andSalmonellatyphi, andgram-positive,comprisingS.aureus,B.subtilis,Bacilluscereus, and S.faecalis.Thetentative understandingof therelationships betweenthechemicalstructuresoftriterpenesandthe antibac-terialactivitiesindicatesthat theactivitymayberelatedtothe presenceofanoxygenatedgroupatC-3(Pachecoetal.,2012).In polycarpolthispositionisoccupiedbyahydroxylgroup,however anothersubstituents,likecarbonyls,O-glycosides,esters(mainly acetylmoieties),orhydroxylaminescanbepresentinthisposition (Pachecoetal.,2012).
Thesignificantantibacterialactivitiesobservedforpolycarpol anddescribedinthisworkforthefirsttimeneedsfurther inves-tigations,which couldhelpin thesearchfor newantimicrobial drugs.
Chemotaxonomicsignificanceofpolycarpol
The chemotaxonomic importance of the polycarpol for the AnnonaceaefamilywasfirstlyrecognizedbyLeboeufetal.(1982)
duringa systematic researchwithseveral species belongingto neighboringfamilies,suchasLauraceae,Monimiaceaeand Menis-permaceae.Atthistime,polycarpolwasnotfound,beingsuggested for the first time in literature as chemotaxonomic marker for Annonaceae.Thisideawassupportedovertheyears(Goulartetal., 1986; Junget al., 1990).Polycarpol was recentlyisolated from Duguetiaglabriuscula (Pereira et al., 2003), Duguetiafurfuraceae (Silva et al., 2007), Artabotrys madagascariensis (Murphy et al., 2008),Piptostigmapreussi(Ngantchouetal.,2009)andArtabotrys spinosus(Sichaemetal.,2011),allspeciesfromAnnonaceae fam-ily,which enhancestheinitialpropositionof thiscompound as achemotaxonomicmarkerforthisfamily.Consideringitsbroad occurrence at genera level, is appropriate to restrict the term “chemotaxonomicmarker”atafamilylevel,beingsuggestedthe useoftheterm“chemicalmarker”togeneralevel.
InUnonopsisgenus,polycarpolhasbeenreportedin
Unonop-sisglaucopetala(Jayasuriyaet al.,2005), Unonopsisguatterioides (Touché et al., 1981; Silva et al., 2012a), Unonopsis spectabilis (Laprévoteetal.,1987)andUnonopsispacifica(Arangoetal.,1988). There-currentobservationofpolycarpolinallthesespecies sub-jectedtophytochemicalapproaches,aswellasinO.amazonicum andB.pleiospermaisanimportantchemicalevidencenotonlyto confirmthebotanicalproximityofthesegenera(Maasetal.,2007), butalsotoreinforcepolycarpolasa chemical markerforthese genera.Theinsufficientphytochemicalknowledgeregarding Ony-chopetalumandBocageopsisgenerastillnotdoesallowsaprofound evaluationofthechemicalsimilaritiesbetweenthemand Unonop-sis.Inthissense,phytochemicalinvestigationsdirectedatbetter understandingofthechemical relationshipsamongthese three close-relatedgeneraareindispensableinthefuture.
Conflictofinterest
Theauthorsdeclarenoconflictsofinterest.
Authorscontributions
FMAS,HHFK(PhDstudents),BRL,ERS(MScstudents),RAM(PhD student)andFASF (CIstudent)contributedequallyincollecting plantsamples,confirmationofthecompoundstructureandwritten ofthemanuscript.WRCandMJScontributedtoinvitrobiological assays.ADLS,MLBPandAQLSsupervisedthelaboratoryworkand contributedtocriticalreadingofthemanuscript.Alltheauthors havereadthefinalmanuscriptandapprovedthesubmission.
Acknowledgment
TheauthorsaregratefultoCAPES,CNPq,FINEP,FAPEAMand FAPESPforfinancialsupport.WethankPDBFFforproviding logis-ticalandfieldsupport.Thisispublicationnumber663inthePDBFF TechnicalSeries.
References
Angeh,J.E.,Huang,X.,Sattler,I.,Swan,G.E.,Dahse,H.,Hartl,A.,Eloff,J.N.,2007. Antimicrobialandanti-inflammatoryactivityoffour knownandonenew triterpenoidfromCombretumimberbe(Combretaceae).J.Ethnopharmacol.110, 56–60.
Almeida,M.E.L.,Braz-Filho,R.,Bülow,V.,Gottlieb,O.R.,Maia,J.G.S.,1976.Onychine, analkaloidfromOnychopetalumamazonicum.Phytochemistry15,1186–1187. Arango,G.J.,Cortes,D.,Cavé,A.,D’Ocon,P.M.,1988.7-7′-Bisdeshidroaporfnsde
Unonopsispacifica.An.Quim.C-Org.Bioq.84,124–127.
Barros,L.F.,Barison,A.,Salvador,M.J.,Mellosilva,R.,Cabral,E.C.,Eberlin,M.N., Ste-fanello,M.E.A.,2009.ConstituentsfromtheleavesofMagnoliaovata.J.Nat.Prod. 72,1529–1539.
Bataglion,G.A.,Silva,F.M.A.,Santos,J.M.,Santos,F.N.,Barcia,M.T.,Lourenc¸o,C.C., Salvador,M.J.,Godoy,H.T.,Eberlin,M.N.,Koolen,H.H.F.,2014.Comprehensive characterizationoflipidsfromAmazonianvegetableoilsbymassspectrometry techniques.FoodRes.Int.64,472–481.
Chang,F.R.,Chen,J.L.,Lin,C.Y.,Chiu,H.F.,Wu,M.J.,Wu,Y.C.,1999.Bioactive aceto-geninsfromtheseedsofAnnonaatemoya.Phytochemistry51,883–889. Costa, E.V., Pinheiro, M.L., Barison, A., Campos, F., Salvador, M.J., Maia, B.H.,
Cabral,E.,Eberlin,M.N.,2010.AlkaloidsfromthebarkofGuatteriahispida andtheirevaluationasantioxidantandantimicrobialagents.J.Nat.Prod.73, 1180–1183.
Cunha,M.M.,Nascimento,F.C.,Santos-Pimenta,L.P.,Boaventura,M.A.D.,Salas,C.E., Lopes,M.T.P.,2009.Screeningofcytotoxicactivityinhexanicandethanolic extractsofRollinialaurifolia.Lat.Am.J.Pharm.28,234–240.
Djoukeng,J.D.,Abou-Mansour,E.,Tabacchi,R.,Tapondjou,A.L.,Boudab,H.,Lontsi, D.,2005.Antibacterial triterpenesfrom Syzygiumguineense(Myrtaceae).J. Ethnopharmacol.101,283–286.
Fournier,G.,Leboeuf,M.,Cavé,A.,1999.Annonaceaeessentialoils:areview.J.Essent. OilRes.11,131–142.
Goulart,M.O.F.,Santana,A.E.G.,Oliveira,A.B.,Oliveira,G.G.,Maia,J.G.S.,1986. Azaflu-orenonesandazaanthraquinonefromGuatteriadielsiana.Phytochemistry25, 1691–1695.
Haraguchi,H.,Kataoka,S.,Okamoto,S.,Hanafi,M.,Shibata,K.,1999.Antimicrobial triterpenesfromIlexintegraandthemechanismofantifungalaction.Phytother. Res.13,151–156.
Hosoe,T.,Okada,H.,Itabashi,T.,Nozawa,K.,Okada,K.,Takaki,G.M.,Fukushima,K., Miyaji,M.,Kawai,K.I.,2000.Anewpentanorlanostanederivative,cladosporide A,asacharacteristicantifungalagentagainstAspergillusfumigatus,isolatedfrom Cladosporiumsp.Chem.Pharm.Bull.48,1422–1426.
Jayasuriya,H.,Herath,K.B.,Ondeyka,J.G.,Guan,Z.,Borris,R.P.,Tiwari,S.,Jong,W., Chavez,F.,Moss,J.,Stevenson,D.W.,Beck,H.T.,Slattery,M.,Zamora,N., Schul-man,M.,Ali,A.,Sharma,N.,Macnaul,K.,Hayes,N.,Menke,J.G.,Singh,S.B., 2005.Diterpenoid,steroid,andtriterpenoidagonistsofliverxreceptorsfrom diversifiedterrestrialplantsandmarinesources.J.Nat.Prod.68,1247–1258. Jung,J.H.,Pummangura,S.,Chaichantipyuth,P.,Patarapanich,C.,Mclaughlin,J.L.,
1990. Bioactiveconstituents of Melodorumfruticosum. Phytochemistry 29, 1667–1670.
Katerere,D.R.,Grev,A.I.,Nash,R.J.,Waigh,R.D.,2003.Antimicrobialactivityof pen-tacyclictriterpenesisolatedfromAfricanCombretaceae.Phytochemistry63, 81–88.
Kitagawa,I.,Kobayashi,M.,Inamoto,T.,Yasuzawa,T.,Kyogoku,Y.,1981.The struc-turesofsixantifungaloligoglycosides,stichlorosidesA1,A2,B1,B2,C1,andC2, fromtheseacucumberStichopuschloronotus(Brandt).Chem.Pharm.Bull.29, 2387–2391.
Kitagawa,I.,Kobayashi,M.,Inamoto,T.,Fuchida,M.,Kyogoku,Y.,1985.Marine naturalproducts.XIV.StructuresofechinosidesAandB,antifungal lanostane-oligosidesfromtheseacucumberActinopygaechinites(Jaeger).Chem.Pharm. Bull.33,5214–5224.
Kitagawa,I.,Kobayashi,M.,Son,B.W.,Suzuki,S.,Kyogoku,Y.,1989.Marinenatural products.XIX.PervicosidesA,B,andC,lanostane-typetriterpene-oligoglycoside sulfatesfromtheseacucumberHolothuriapervicax.Chem.Pharm.Bull.37, 1230–1234.
Koolen,H.H.F.,Soares,E.R.,Silva,F.M.A.,Oliveira,A.A.,Souza,A.Q.L.,Medeiros,L.S., Rodrigues-Filho,E.,Cavalcanti,B.C.,Pessoa,C.O.,Morais,M.O.,Salvador,M.J., Souza,A.D.L.,2013.Mauriticacid:anewdammaranetriterpenefromtheroots ofMauritiaflexuosaL.f.(Arecaceae).Nat.Prod.Res.27,2118–2125.
Krohn,K.,Ludewig,K.,Jones,P.G.,Doering,D.,Aust,H.J.,Draeger,S.,Schulz,B., 1992.Biologicallyactivemetabolitesfromfungi,21Anantifungalandherbicidal lanostanelactonefromSporormiellaaustralis.Nat.Prod.Lett.1,29–32. Laprévote,O.,Roblot,F.,Hocquemiller,R.,Cavé,A.,1987.Alcaloïdesdesannonacées,
84.bisaporphinoïdesdel’Unonopsisspectabilis.J.Nat.Prod.50,984–988. Leboeuf,M.,Cavé,A.,Bhaumik,P.K.,Mukherjee,B.,Mukherjee,R.,1982.The
phyto-chemistryoftheAnnonaceae.Phytochemistry21,2783–2813.
Liu, X.T.,Winkler,A.L.,Schwan, W.R.,Volk,T.J.,Rott,M.A.,Monte, A., 2010a. AntibacterialcompoundsfrommushroomsI:ALanostane-typetriterpeneand prenylphenolderivativesfromJahnoporushirtusandAlbatrellusflettiiandtheir activities againstBacilluscereusand Enterococcus faecalis. PlantaMed.76, 182–185.
Liu,X.T.,Winkler,A.L.,Schwan,W.R.,Volk,T.J.,Rott,M.A.,Monte,A.,2010b. Antibac-terialcompoundsfrommushrooms.II:Lanostanetriterpenoidsandanergostane steroidwithactivityagainstBacilluscereusisolatedfromfomitopsispinicola. PlantaMed.76,464–466.
Maas,P.J.M.,Westra,L.Y.T.,Vermeer,M.,2007.Revisionoftheneotropical gen-era Bocageopsis, Onychopetalum, andUnonopsis (Annonaceae).BLUMEA52, 413–554.
Matos,M.F.C.,Leite,L.I.S.P.,Brustolim,D.,Siqueira,J.M.,Carollo,C.A.,Hellmann,A.R., Pereira,N.F.G.,Silva,D.B.,2006.Antineoplasticactivityofselectedconstituents ofDuguetiaglabriuscula.Fitoterapia77,227–229.
Murphy,B.T.,Cao,S.,Brodie,P.J.,Miller,J.S.,Ratovoson,F.,Birkinshaw,C.,Rakotobe, E.,Rasamison,V.E.,Tendyke,K.,Suh,E.M.,Kingston,D.G.I.,2008. Antiprolifera-tivecompoundsofArtabotrysmadagascariensisfromtheMadagascarrainforest. Nat.Prod.Res.22,1169–1175.
Ngantchou,I.,Nkwengoua,E.,Nganso,Y.,Nyasse,B.,Denier,C.,Hannaert,V., Schnei-der,B.,2009.AntitrypanosomalactivityofpolycarpolfromPiptostigmapreussi (Annonaceae).Fitoterapia80,188–191.
Nyasse,B.,Ngantchou,I.,Nono,J.J.,Schneider,B.,2006.Antifilarialactivityinvitroof polycarpoland3-O-acetylaleuritolicacidfromcameroonianmedicinalplants againstOnchocercagutturosa.Nat.Prod.Res20,391–397.
Oliveira,E.S.C.,Amaral,A.C.F.,Lima,E.S.,Silva,J.R.A.,2014.Chemicalcomposition andbiologicalactivitiesofBocageopsismultifloraessentialoil.J.Essent.OilRes. 26,161–165.
Oleszek,W.,Kapusta,I.,Stochmal,A.,2008.TLCoftriterpenes(includingsaponins). In:Waksmundzka-Hajnos,M.(Ed.),ThinLayerChromatographyin Phytochem-istry.CRCPress,Taylor&FrancisGroup,LLC,NewYork,pp.519–537. Pereira,N.F.G.,Carollo,C.A.,Garcez,W.S.,Siqueira,J.M., 2003.Novelsantalane
sesquiterpenoidsfromthestembarkofDuguetiaglabriuscula–Annonaceae. Quim.Nova26,512–516.
Pacheco,A.G.,Alcântara,A.F.C.,Abreu,V.G.C.,Corrêa,G.M.,2012.Relationships betweenchemicalstructureandactivityoftriterpenesagainstgram-positive andgram-negativebacteria.In:Bobbarala,V.(Ed.),ASearchforAntibacterials Agents.InTech,Rijeka,pp.1–24.
Richardson,J.E.,Chatrou,L.W.,Mols,J.B.,Erkens,R.H.J.,Pirie,M.D.,2004.Historical biogeographyoftwocosmopolitanfamiliesoffloweringplants:Annonaceae andRhamnaceae.Phil.Trans.R.Soc.Lond.B359,1495–1508.
Saadawi,S.,Jalil,J.,Jasamai,M.,Jantan,I.,2012.Inhibitoryeffectsof acetylmelodor-inol,chrysinand polycarpolfrom Mitrellakentiionprostaglandin E2 and thromboxaneB2productionandplateletactivatingfactorreceptorbinding. Molecules17,4824–4835.
Salvador,M.J.,Ferreira,E.O.,Pral,E.M.F.,Alfieri,S.C.,Albuquerque,S.,Ito,I.Y.,Dias, D.A.,2002.BioactivityofcrudeextractsandsomeconstituentsofBlutaparon portulacoides(Amaranthaceae).Phytomedicine9,566–571.
Sichaem,J.,Ruksilp,T.,Worawalai,W.,Siripong,P.,Khumkratok,S.,Tip-pyang,S., 2011.AnewdimericaporphinefromtherootsofArtabotrysspinosus.Fitoterapia 82,422–425.
Silva,D.B.,Tulli,E.C.O.,Garcez,W.S.,Nascimento,E.A.,Siqueira,J.M.,2007.Chemical constituentsoftheundergroundstembarkofDuguetiafurfuracea(Annonaceae). J.Braz.Chem.Soc.18,1560–1565.
Silva,F.M.A.,Koolen,H.H.F.,Barisson,A., Souza,A.D.L.,Pinheiro,M.L.B.,2012a. Steroids and triterpenefrom the barkofUnonopsis guatterioides R. E. Fr. (Annonaceae).Int.J.Pharm.Pharm.Sci.4,522–523.
Silva,F.M.A.,Koolen,H.H.F.,Almeida,R.A.,Souza,A.D.L.,Pinheiro,M.L.B.,Costa,E.V., 2012b.Desreplicac¸ãodealcaloidesaporfínicoseoxoaporfínicosdeUnonopsis guatterioidesporESI-IT-MS.Quim.Nova35,944–947.
Silva,F.M.A., Koolen,H.H.F., Lima,J.P.S.,Santos,D.M.F.,Silva-Jardim,I.,Souza, A.D.L.,Pinheiro,M.L.B.,2012c.Leishmanicidalactivityoffractionsrichin apor-phinealkaloidsfromAmazonianUnonopsisspecies.Rev.Bras.Farmacogn.22, 1368–1371.
Silva,F.M.A.,Souza,A.D.L.,Koolen,H.H.F.,Barison,A.,Vendramin,M.,Costa,E.V., Ferreira,A.G.,Pinheiro,M.L.B.,2014.Phytochemicalstudyofthealkaloidal frac-tionsofUnonopsisduckeiR.E.Fr.guidedbyESI-IT-MSn.Phytochem.Anal.25, 45–49.
Siqueira,J.M.,Bomm,M.D.,Pereira,F.G.,Garcez,W.S.,Boaventura,M.A.D.,1998. EstudofitoquímicodeUnonopsislindmanii–Annonaceae,biomonitoradopelo ensaiodetoxicidadesobreaArtemiasalinaleach.Quim.Nova21,557–559. Soares, E.R., Silva, F.M.A., Almeida, R.A.,Lima, B.R., Koolen, H.H.F., Lourenco,
C.C.,Salvador,M.J., Flach,A.,Costa,L.A.M.A.,Souza,A.Q.L.,Pinheiro,M.L.B., Souza, A.D.L., 2015. Chemical composition and antimicrobial evaluation of the essential oils of Bocageopsis pleiosperma Maas. Nat. Prod. Res., http://dx.doi.org/10.1080/14786419.2014.996148(inpress).
Touché,A.,Desconclois,J.F.,Jacquemin,H.,Lelièvre,Y.,Forgacs,P.,1981. Constitu-antsdequelquesannonacéesguyanaisesanalysequalitativeetquantitativedes acidesaminésbasiqueslibres.Présenced’untriterpène,Lepolycarpol.Plant. Med.Phytother.15,4–9.