49
microscopy
A tool for imaging,
manipulating
biomolecules,
and quantifying
single-molecule
interactions in
biological systems
Catarina S. Lopes,
Filomena A. Carvalho,
and Nuno C. Santos
Contents
Introduction . . . .50
Applications . . . . 51
Imaging . . . . 51
Atomic force microscopy-based force spectroscopy . . . . 52
Single-molecule interactions . . . . 54
Indentation . . . . 56
Single-cell adhesion studies . . . . 56
Combining with fluorescence studies . . . . 57
Scanning probe lithography . . . . 57
Limitations . . . . 59
Future . . . . 60
Acknowledgments . . . . 61
50
Introduction
Atomic force microscopy (AFM) is a technique that can be used for high-resolution real-time studies of properties of molecules at the nanoscale . It has been applied to structural and functional studies of dynamic pro-cesses in biological systems .1–4 AFM has contributed significantly to the
nanotechnology field, with developments in nanodiagnostic and thera-peutics, contributing to the improvement of the state of the art in health care,5 pathology,6,7 identification of biomolecules8 and cellular
micro-environments, physiology and functions of living cells and organisms,9
understanding of the structure–function relationship of DNA,10 cell and
molecular recognition, signaling, adhesion, and fusion .11 AFM is a tool
that allows imaging and force probing biological samples with nano-meter (nm)3 topographical and piconewton (pN) force resolution .12 This
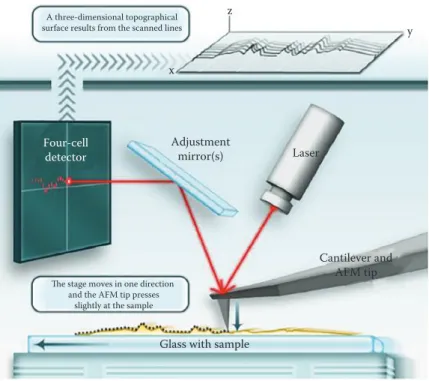
microscope central element is a small cantilever, which distance from the sample is controlled in the z-axis by a piezoelectric crystal . A sharp tip is usually mounted at the end of the microfabricated flexible cantile-ver (typically 20–200 μm long), used as a kind of spring to measure the force between the tip and the sample surface .2,13 AFM is a surface probe
technique, which uses the tip of this soft cantilever to image surfaces, or to measure or apply forces while interacting with them .14 The cantilever
behaves similarly to a Hookean spring when the AFM is used for bio-logical applications; it has spring constants on the order of 0 .1 N/m .15 On
bringing the tip in close proximity to the sample surface, attractive and repulsive forces cause the deflection of the cantilever .3,16 These
deflec-tions cause changes in the position of the reflection of the light beam pointed to the cantilever’s top surface . This information may be trans-lated into applied force through Hooke’s Law: F = −KΔx . The cantilever deflection versus scanner displacement curve data can be converted into a force–distance curve17 . Consequently, these means the force (F) needed
to extend the cantilever with spring constant (K) depends in a linear way on the cantilever deflection (∆x) .16,17 This deflection is detected and
pro-cessed as a function of the position on the (x, y) plane .3,5,17–22 The
bend-ing and torsion of the cantilever are measured based on the light beam reflection point of incidence on a four-quadrant photodetector .16,23–27 An
optical lever is created by this light beam, from a laser or light-emitting diode, reflected by the cantilever onto the position-sensitive photodiode .13
The tip movement relative to the sample can follow different operation modes, including contact mode (in which the tip is in continuous contact with the sample’s surface), noncontact mode (the cantilever vibrates and variations from its resonance frequency are used to generate images), and intermittent contact (the cantilever moves rapidly, with a large oscilla-tion, between repulsive and attractive forces) .3,16 When the tip contacts
the sample, the deflection of the cantilever provides information on the mechanical properties of the sample .27,28 AFM has other applications,
based on force spectroscopy, where attractive and repulsive forces are measured (Figure 4 .1) .24
51
Applications
AFM can be applied in various modalities to study different biologi-cal systems . In addition to imaging, one of the most promising areas of applications is the quantification of the forces resulting in the interac-tion between the tip (or something attached to it) and a sample, taking advantage of the pN sensitivity of the equipment . These approaches are generally termed force spectroscopy . By measuring the variations of the force exerted on the sample, AFM enables the detection of specific inter-action forces at the single-molecule level . The possibility of modifying the surface and manipulating individual molecules made AFM an ideal tool for biological and biomedical applications .5
Imaging
AFM has been applied on the characterization of nanometric features at the surfaces of the samples and also on the mapping of the spatial distribution of their physicochemical properties . These equipments have
Glass with sample
Cantilever and AFM tip Laser Adjustment mirror(s) Four-cell detector
The stage moves in one direction and the AFM tip presses
slightly at the sample A three-dimensional topographical surface results from the scanned lines
z
x
y
Figure 4.1 Schematic illustration of the imaging mode using a sample scanner AFM: A laser beam is reflected from the cantilever, the interaction forces of the tip with the piezo-positioned samples are deflected and the adjustment mirror directs the signaling for a segmented photodetector. A pseudo three-dimensional topographical image of the sample surface results from the scanned lines. (From Schön, P., Methods, 103, 25–33, 2016.)
52
the capacity to visualize nanotopographical features and correlate them to surface-charge density and potential relationships between topography and local characteristics of biomaterials (e .g ., charge and conductivity) . The contact and oscillation modes are most commonly used to image reconstituted membrane proteins and native membranes . AFM imaging of cells generates structural information about them, enabling the iden-tification of functional components within such structures, despite the heterogeneity of the cell surface in terms of protein composition and dis-tribution .3,15,16,29,30 AFM imaging of living mammalian cells remains
lim-ited to resolutions in the 50–100 nm range, meaning that the individual components of the cell-surface machinery cannot be observed . Living cells are highly dynamic, and continuously respond to environmental changes .30 These methodologies can be applied to blood cells, such as
human erythrocytes and platelets from patients or healthy donors .2,5,8
They are also used for imaging of cancer cells, including lymphoma Raji cells31 and human lung adenocarcinoma cells32 and, recently, neuronal
cells from mice with chronic epilepsy .33 Other typical applications can be
found for biomolecules, such as proteins (e .g ., fibrinogen13), DNA,10 and
RNA (including its assemblies and aggregates12), and microorganisms,
such as viruses, their membrane and capsid,34 bacteria35,36 and
mycobac-teria .30 Membrane channels, such as potassium channels and
microfila-ments (polymerization and depolymerization),29 phospholipid bilayers,
and their perturbation by antibiotics,37 viral fusion inhibitors,38,39 singlet
oxygen production34 or antibodies,40 and antimicrobial peptides41–43 were
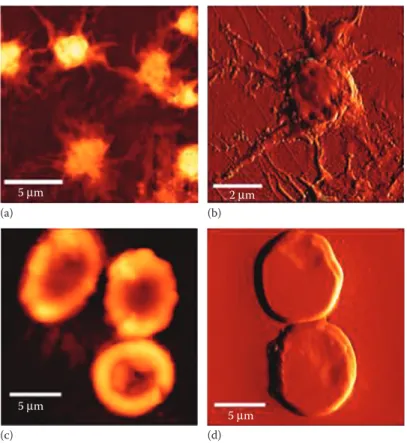
also studied by these approaches (Figure 4 .2) .
Atomic force microscopy-based force spectroscopy
AFM-based force spectroscopy allows the measurement of inter- and intramolecular interaction forces required to separate the tip from the sample . It is seldom used to quantify the interaction between the tip and a specific spot of the sample .5,13,17
The interaction force depends on the nature of the sample, the probe tip, and the distance between them . The force–distance curve depends on these characteristics and of the medium composition, and there are obvi-ous differences between curves obtained in air and in liquid medium . In force spectroscopy measurements, the cantilever moves in the vertical direction (z-axis) toward the surface and then in the opposite direction . During this procedure, the cantilever deflection as a function of the ver-tical displacement of the piezoscanner can be recorded . The result is a cantilever-deflection versus scanner-displacement curve, which can be converted into a force–distance curve after applying the Hooke’s law of elasticity .17 To calculate the quantitative parameters, an accurate
calibra-tion of the spring constant of the cantilevers used is necessary .13
This technique is a highly sensitive, rapid, and low operation cost nanotool for the diagnostic and unbiased functional evaluation of the
53 severity of hematological diseases arising from genetic mutations .
AFM-based force spectroscopy can be used to measure the binding force between fibrinogen and cell receptors, a strategy successfully used on the identification of the fibrinogen receptor on human erythrocytes .8
Different types of forces can be studied using AFM-based force spec-troscopy, both attractive and repulsive .24
The cycle begins with the tip away from the cell surface, and the forces measured by the cantilever deflection can change as it is moved toward or away from the sample on its neutral position, at 0 pN of force, from which it starts moving down toward the surface, reaching the contact point . On the approach curve, the van der Waals interactions are the main type of force present on the approaching of two hard surfaces in the absence of long-range interactions . This force is characterized by a small deflec-tion of the cantilever, at the approach curve, before the contact point . The jump to contact causes instability in the position of the cantilever because it occurs when the gradient of force between the tip and the sample exceeds the stiffness of the cantilever . If the approach curve has
(a) (b)
(c) (d)
5 μm 2 μm
5 μm
5 μm
Figure 4.2 AFM imaging in air of human platelets (a, b) and erythrocytes (c, d) from healthy donors. (a) and (c) are height images, whereas (b) and (d) are error signal images. (Adapted from Carvalho, F.A. et al.,
ACS Nano, 4, 4609–4620, 2010.)
54
a smooth and exponentially increasing repulsive force, it is expectable that electrostatic or polymer-brush forces are present . Viscoelastic prop-erties of some biological systems have been determined using AFM force curves . A waiting time should be kept before starting the retraction curve . Afterward, the tip and cantilever begin the upward movements away from the sample, in the opposite direction, reaching the contact/adhesion point . In a retraction curve where adhesion occurs, the force depends on the sample and appears as a deflection of the cantilever below the zero-deflection line . The central basis of adhesion forces is the development of a capillary bridge between the tip and the sample . This capillary force depends on whether the measurements are made in air or in liquid . In air, samples usually have several nanometers of water molecules adsorbed to their surface . In liquid conditions, the adhesion force not only depends on the interaction energies between the tip and the sample, but also on the solution used . If no binding occurs between the molecules attached to the tip and the cell surface, the tip continues its upward movements and reaches back the neutral position at a defined z-distance . If a bond is formed between the tip and the sample, as the cantilever moves upward, it bends down to negative values in force . Cantilever stiffness depends on the shape and on the material properties of the cantilever .16,24
AFM-based force spectroscopy can be used for diverse applications .
Single-molecule interactions
Single-molecule force spectroscopy (SMFS) is an extremely powerful tool for detecting and localizing single-molecule recognition events, and for exploring the energy landscape of molecular interactions . Different mol-ecules, or their domains, can be attached to the tip, and each part can break contact separately or altogether . After attaching a molecule to the tip and/or to the substrate surface, the unfolding, stretching, or adhesion of single molecules can be studied . The tips are commonly functionalized with one or a small amount of probe molecules . These molecules can rec-ognize a specific target molecule on the sample surface . When analyzing the stretching of biomolecules, and in order to measure specific and strong interactions between tip and sample, it is necessary to specifically attach to the tip the biomolecule under study . The attachment should be firm enough to avoid reallocations, but it should maintain some autonomy of the molecule to change its conformation during or before the interaction . In force measurements, both the tip on the cantilever and the surface can be chemically modified to form specific and strong bonds .24 AFM has
been used as a nanodiagnostic tool for patient cells, such as the interac-tion between fibrinogen and erythrocytes in chronic heart failure patients, which affects blood microcirculatory flow conditions .44 With AFM-based
force spectroscopy, fibrinogen–erythrocyte interactions were assessed at the single-molecule level using the adhesion profiles obtained on each force curve to evaluate the increased cardiovascular risk .5,8,44,45
55 Another study by this methodology was the assessment of the cranberry
juice constituents that most strongly influence Escherichia coli adhesion forces and adhesive properties of an antibiotic-resistant clinical bacte-rial strain .46 Studies of folding and unfolding kinetics of proteins on the
membrane of the same bacteria were also successfully conducted .24,36 It
was possible to compare the barriers observed for unfolding from the N-terminus with those from the C-terminus . The barrier positions and heights found in bacteriorhodopsin when probed from both sides were located not only in or at the ends of stiff α-helical rods, but also in loops that are not well resolved by other structural biology methods .28 This
approach was also used for lipid bilayers of dioleoylphosphatidylcholine (DOPC), for therapeutic drugs or protein molecules that target specific receptors .11 It was also used for understanding the molecular
determi-nants and identifying the ligand for the dengue virus capsid protein on intracellular lipid droplets and plasma lipoproteins (Figure 4 .3) .17,47–50
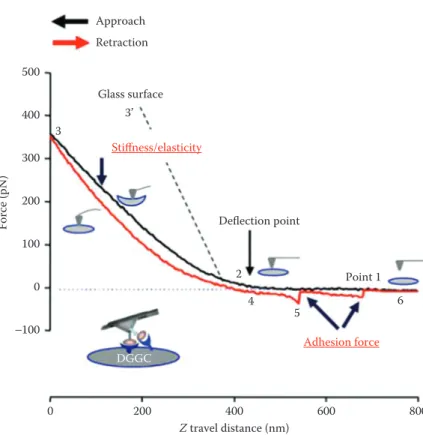
Glass surface Z travel distance (nm) Force (pN) Approach Retraction Stiffness/elasticity Deflection point Adhesion force 2 4 5 6 3’ 3 Point 1 DGGC 500 400 300 200 100 −100 0 600 800 400 200 0
Figure 4.3 Example of the force curves data from fibronectin (FN)-coated AFM probe on dentate gyrus granule cells (DGGC) approach/attaching (black trace) and retract/withdrawal (red trace) from single DGGC. The stages of attaching and withdrawal are shown on points 1–6. On the approach curve, it is possible to measure the stiffness or/and elasticity from the sample. On the retraction curve, it is possible to measure the adhesion force or interaction between cell and molecule under study. (Adapted from Wu, X. et al., Front. Aging Neurosci., 8, 1–12, 2016.)
56
Indentation
Nanoindentation experiments, for cell elasticity assessment, can be car-ried out on live cells . AFM-based detection of stiffness is highly depen-dent on the appropriate use of theoretical models . Cell elasticity can be measured by nanoindentation with the most common AFM cantile-vers, via the Hertzian theory . This theory of elastic contact is the most widely used approach to estimate the elastic properties of cells from force indentation curves, using the depth of indentation to assess elas-ticity in terms of the Young’s elastic modulus . AFM can measure the apparent stiffness of mammalian cells, which ranges between 1 to 10 kPa, and cells with cell wall (~100 to 1000 kPa) .9 The stiffness
measure-ments depend on cell type, on probe geometry, rate of force application, and force magnitude .51 Specifically, this method allows characterizing
the elasticity of biological structures, comparing different types of cells or even organelles, matching experimental conditions concerning the indenters’ shape, or the thickness of the sample .17
Indentation was used for the tensile testing and bulge testing to deter-mine the elastic modulus of the cornea and other eye components .52
Erythrocytes from patients with type 2 diabetes demonstrated signifi-cant aggregation of surface proteins, increased tip–cell adhesion, as well as increased stiffness in comparison with healthy cells .51 Erythrocytes
from chronic heart failure patients are also stiffer than those from healthy donors .44 This method can be applied in human lung
adenocarci-noma cells in different medium conditions for anticancer effect studies .32
Single-cell adhesion studies
AFM-based single-cell force spectroscopy (SCFS) is used to quantify the contribution of cell-adhesion molecules to the binding of cells to specific substrates at both the cell and single-molecule level .53 In
AFM-based SCFS, a single cell is attached to a cantilever, commonly facil-itated by an adhesive coating (e .g ., concanavalin A, poly-L-lysine, or CellTak) . The attached cell is lowered (in the approach) onto a substrate, which can be a protein-coated surface, another cell or a biomaterial, until a set force is reached . Then, the cell is kept stationary for a set time to allow the formation of adhesive interactions . During the subse-quent retraction of the of the cantilever, the force acting on the cell and the distance between cell and substrate are recorded in a force–distance curve . The force range that can be detected with AFM-based SCFS is typically from ~10 pN up to ~100 nN; thereby, SCFS allows both the overall cell adhesion and the contribution of single adhesion receptors to be quantified .53 During initial cantilever retraction, the upward force
acting on the cell increases until the force needed to initiate cell deadhe-sion is reached and unbinding events occur . At the cellular level, single cells can be ingeniously fixed onto the cantilever, becoming the probe
57 for the dynamic quantification of cell–substrate interactions . Parameters
such as maximum detachment force and work necessary for the entire cell detachment, as well as the number of detachment events of single cellular tethers have already been successfully quantified . The maxi-mum force is termed as the adhesion force and is a measure of how strong the cell adhered to the substrate . Single unbinding events can be characterized from individual rupture (jumps) or tether events depicted on the force–distance curve . The analysis of these unbinding events may be used to characterize the strength of single bonds and cell membrane properties .54,55
Cell adhesion is a fundamental aspect on both health and disease . In the past decade, single-cell adhesion studies have contributed to the under-standing of adhesion proteins and their regulation . AFM-based SCFS has been used to quantify adhesion of numerous cell types to a diverse set of substrates, including extracellular matrix (ECM) proteins, bio-materials, and cell–cell adhesion proteins .54 SCFS has been applied as
a tool to quantify cell adhesion between two cells to identify key pro-teins regulating the differential adhesive behavior of zebrafish mesen-dodermal progenitor cells to fibronectin, thus providing an insight into the germ layer formation and separation between gastrulation zebraf-ish cells .53,56 It has also contributed for the understanding of
differen-tial cell adhesion and cell-cortex tension in germ layer organization in Chinese hamster ovary (CHO) cells with integrin activators .53 AFM
allows recording of the actual adhesion force between a bacterium (Pseudomonas aeruginosa) and Candida albicans . Bacterial adhesion to hyphae was always accompanied by strong adhesion forces, but did not occur on yeast cells (Figure 4 .4) .57
Combining with fluorescence studies
AFM has been used simultaneously with optical-fluorescence imaging in cell biology . These techniques are usually combined by successive applications and led to the implementation of new modalities in AFM to allow easy image superposition . AFM indentation can be performed while recording a fluorescence signal . AFM combined with fluorescence microscopy has helped to determine the forces that the immune synapse exert between immune cells, such as photostimulated T-cells, gaining insight on their mechanical properties, when activating a small GTPase, Rac, in real time .14 Approaches combining AFM with fluorescence
microscopy were also used to characterize peptide-planar–lipid bilayer interactions39 and to study hepatocytes infected by the malaria parasite .58
Scanning probe lithography
AFM lithography is a method in which it is possible to draw a pattern on a solid surface, by scratching, oxidation, or reduction, using the AFM tip . In this method, selected molecules are adsorbed by capillarity onto the
58
AFM tip, which is successively used in contact mode to transfer predeter-mined molecular patterns to the surface, with a resolution of 30–100 nm .3
This technique has been optimized on atomically flat and contaminant-free surfaces (e .g ., gold and silicon) . It can now count on a dramatically improved patterning efficiency and flexibility in depositing different ink molecules .3 In contact mode lithography on Si surfaces, an area is oxidized
to SiO2 when a positive voltage is applied to the tip, and an oxidized
pat-tern can be drawn .17 This approach was applied to microarrays composed
by DNA-directed protein immobilization, with a precise pattern, used to investigate the recruitment of transmembrane receptors in living cells .3
Different AFM nanolithography modes, such as dip-pen nanolithography, electrochemical AFM nanolithography, thermal AFM nanolithography, nanografting, nanoshaving, and tapping-mode AFM nanolithography of alkanethiols can be done .17 On a related matter, the AFM tip has also
been successfully used to reconstitute nanometer-scale defined areas of supported phospholipid bilayers .17,59 However, its translation to medically
relevant materials and applications may still be far, mainly because of the nonatomic planarity of most biomaterials and the practical need to
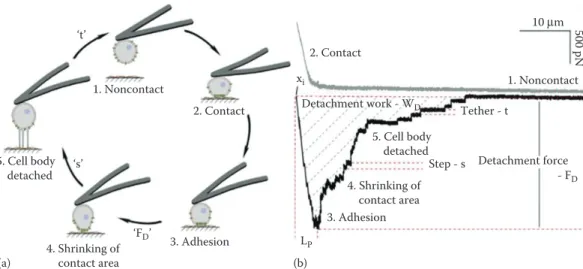
1. Noncontact 1. Noncontact - FD Detachment force 2. Contact ‘t’ Detachment work - WD 2. Contact Tether - t LP 10 μm 500 pN xi 3. Adhesion Step - s 5. Cell body detached 3. Adhesion 4. Shrinking of contact area (a) (b) 4. Shrinking of contact area 5. Cell body detached ‘s’ ‘FD’
Figure 4.4 Schematic illustration of a single-cell force spectroscopy experiment and of the adhesion events detected (a, b). In (b), the force–distance curve shows steps 1–5, corresponding to those outlined in (a). The AFM cantilever catches a single cell (1. noncontact [a, b]) and approaches to a substrate (1–2, a, b). In contact (2, gray line in b), cell adhesion molecules diffuse into the contact zone. The adhesive force between the cell and substrate increases. After a predefined contact time, the cell is retracted (black line in b) and the cantilever bends due to the adhesive force between cell and substrate (3). Once the force of the cantilever exceeds that of the interactions between cell and substrate, the cell starts to detach (3–4). The force at this point corresponds to the maximum detachment force (FD). On further retraction of the cantilever, the contact area between the cell and
substrate shrinks (4). The cell sequentially detaches from the substrate (5) and this force is generally followed by step-like events that correspond to the unbinding single-cell adhesion molecules from the substrate (step and tether events). The cell and substrate are completely separated again (1). (Adapted from Friedrichs, J. et al., Methods, 60, 169–178, 2013.)
59 pattern areas whose dimensions lay beyond the range of this technique .
Nevertheless, the capacity of creating specific molecular patterns will undoubtedly benefit more fundamental studies in biomaterials surface science, focused, for example, on furthering the investigation of cellular response to specific chemical cues (e .g ., chemotaxis) and/or on the valida-tion of funcvalida-tional molecular nanostructures .3
Limitations
With the advance of this technique, AFM has been an ideal tool to study biological interactions, but every technique has its limitations .
Despite the development of high-speed atomic force microscopes, the time resolution of AFM applications is a limitation in several biological questions, as numerous biological interactions occur faster than the time required by the AFM to probe, in approximately 0 .001–1 s .60
The shape of the AFM tip can also have a drastic effect on the images that are acquired . In a real imaging situation, sample compression and deformation also need to be taken into consideration . The part of the tip that interacts with the sample is often a critical source of artifacts in AFM . Double tips may also occur on worn tips that have been dam-aged during scanning . When an interaction force is measured by the cantilever, the probe always also exerts some force on the sample . This can cause problems of distortion or damage to the sample, which may be deformed under this force, particularly since many biological samples are soft and delicate, and require particularly careful AFM imaging .16
Another limitation of the AFM application is a maximum scan speed in imaging rate . On increasing the imaging rate for capturing multiple frames per second, a major error associated to tip–sample interaction forces can be caused . Major limitations for fast scanning concern the mechanical proper-ties of the cantilever . Fast and accurate regulation of the cantilever deflec-tion is required to maintain a constant interacdeflec-tion between the tip and the sample . Additionally, this cantilever deflection regulation is needed to pre-vent force-induced conformational changes or damages of the sample . In liquid, to achieve a fast imaging for monitoring the dynamics of biomacro-molecules, high-resonance frequencies in stiff cantilevers are necessary .28
When the substrates are soft, the determination of the tip area contacting the substrate and spring-constant calculation are difficult .13 Furthermore,
the AFM tip can be easily contaminated by the adsorption of molecules coming from the sample surface .60
A problem with oscillation mode in liquid is the low quality factor of a cantilever . It is still debated whether the image quality is significantly improved compared to conventional feedback by tuning other scan parameters, or by scanning with small amplitudes and stiff cantilevers . The low quality factor in liquid also gives rise to unclean excitation
60
spectra when exciting the cantilever via its chip or the complete cantile-ver holder . As a result of the liquid coupling, system resonances of the AFM are superimposed to the cantilever spectrum .28
In cell adhesion studies, the limitations are in conducting n approach and retraction cycles between cells, which need to be repeated a sufficient number of times to obtain reproducible results, due to the complexity of living cells .6 Cells may also be in different states and thus show distinct
adhesive properties that may be difficult to compare .53
Future
AFM can be an important tool for nanotechnology and nanomedicine in biological systems . The microscope can be used alone or combined with other techniques (for example, optical microscopies) that provide complementary information, creating an even more powerful tool . The combination of nanophysics with cell biology establishes a mechanical assay that relates and is used for qualitatively and quantitatively char-acterizing specific interactions of surfaces from living cells, which can open an enormous variety of applications .
The evolution of AFM applications has been very important to the advance of science . This potential is based on the versatility of AFM force detection and mapping methods, as well as its toolbox, which allows func-tionalizing the AFM tip to tackle biological and biomedical questions that are difficult or impossible to address by other methods . AFM can be used to image living cells in aqueous solutions with nanometer resolution, so that the dynamic changes of cellular ultramicrostructures on single cells in response to a given molecule introduced on the volume of liquid sur-rounding the sample can be monitored by AFM . It can simultaneously obtain multiple physicochemical parameters, such as morphology, elastic-ity, adhesion, deformation, and energy dissipation of biological systems in a relatively short time (approximately several minutes) .
AFM imaging will continue to be the simplest AFM methodology to use, as it is quite more straightforward to the user, while enabling a structural and morphological evaluation of the sample . Requiring additional train-ing and a thorough optimization for each new type of sample, AFM-based force spectroscopy has become an important tool on the study of biological systems . Single-molecule interaction studies have allowed, through the combination of nanophysics with cells, the establishment of a mechanical assay that relates qualitatively cooperative molecular processes during contact formation, or even quantitatively the expression of genes, to the function of its product in cell adhesion .53,54,61 This type
of force spectroscopy performed on live cells is directly applicable to a variety of different cell-adhesion systems .9,61 An extensive field of
appli-cation for this cell-based molecular assay is expectable, for instance, when investigating mutated cell-adhesion proteins or the coupling of
61 cell-adhesion molecules to the cytoskeleton and also when evaluating
adhesion-blocking drugs .31,61
Still regarding cell adhesion, the additional molecular insight offered by single-cell techniques, together with the continuing development and improvement of quantitative single-cell methods, is providing stimulating original insight into the dynamic regulation of cell–cell adhesion dur-ing the formation of multicellular organisms .62 Since the dysregulation
of adhesion receptors and their signaling function also can lead to an extensive variety of pathological defects, these tools can also improve our understanding of how changes in cell adhesion can contribute to differ-ent diseases, namely oncologic processes .9,32 Furthermore, the extended
effective pulling range of the instrument leads to the ability to detect the (un)binding forces associated to interactions going from short-range, unspecific interactions, up to specific tether-associated interactions . Most importantly, this method can be operated with an experimental microen-vironment as close to truly physiologic conditions in organisms as pos-sible . Most of the studies can be conducted at controlled conditions of temperature, pH, ionic strength, and other physiological parameters . In future studies, it is important to understand how cells are stimulated, how these cells interact between them, and how their properties can change under various conditions . AFM is expected to be used for these purposes in more and more routine studies, namely due to its special adequacy to understand the biochemical and biophysical mechanisms underlying different biological processes .
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia— Ministério da Ciência, Tecnologia e Ensino Superior (FCT– MCTES, Portugal) grants PTDC/BBB-BMD/6307/2014 and PTDC/ BBB-BQB/3494/2014 .
References
1 . Kim, H ., Arakawa, H ., Osada, T . and Ikai, A . Quantification of cell adhesion force with AFM: Distribution of vitronectin receptors on a living MC3T3-E1 cell . Ultramicroscopy 97, 359–363 (2003) .
2 . Karagkiozaki, V ., Logothetidis, S ., Laskarakis, A ., Giannoglou, G . and Lousinian, S . AFM study of the thrombogenicity of carbon-based coatings for cardiovascular applications . Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 152, 16–21 (2008) .
3 . Variola, F . Atomic force microscopy in biomaterials surface science . Phys. Chem. Chem. Phys. 17, 2950–2959 (2015) .
4 . Santos, N . C . and Castanho, M . A . An overview of the biophysical applica-tions of atomic force microscopy . Biophys. Chem. 107, 133–149 (2004) . 5 . Carvalho, F . A ., Freitas, T . and Santos, N . C . Taking nanomedicine
teach-ing into practice with atomic force microscopy and force spectroscopy . Adv. Physiol. Educ. 39, 360–366 (2015) .
62
6 . Simon, A . and Durrieu, M .-C . Strategies and results of atomic force micros-copy in the study of cellular adhesion . Micron 37, 1–13 (2006) .
7 . Dong, C ., Hu, X . and Dinu, C . Z . Current status and perspectives in atomic force microscopy-based identification of cellular transformation . Int. J. Nanomedicine 11, 2107–2118 (2016) .
8 . Carvalho, F . A . et al . Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes . ACS Nano 4, 4609–4620 (2010) .
9 . Dufrêne, Y . F . and Pelling, A . E . Force nanoscopy of cell mechanics and cell adhesion . Nanoscale 5, 4094–4104 (2013) .
10 . Lyubchenko, Y . L . and Shlyakhtenko, L . S . Imaging of DNA and protein-DNA complexes with atomic force microscopy . Crit. Rev. Eukaryot. Gene Expr. 26, 63–96 (2016) .
11 . Attwood, S . J ., Choi, Y . and Leonenko, Z . Preparation of DOPC and DPPC supported planar lipid bilayers for atomic force microscopy and atomic force spectroscopy . Int. J. Mol. Sci. 14, 3514–3539 (2013) .
12 . Schön, P . Imaging and force probing RNA by atomic force microscopy . Methods 103, 25–33 (2016) .
13 . Averett, L . E . and Schoenfisch, M . H . Atomic force microscope studies of fibrinogen adsorption . Analyst 135, 1201–1209 (2010) .
14 . Cazaux, S . et al . Synchronizing atomic force microscopy force mode and fluo-rescence microscopy in real time for immune cell stimulation and activation studies . Ultramicroscopy 160, 168–181 (2016) .
15 . Fotiadis, D . Atomic force microscopy for the study of membrane proteins . Curr. Opin. Biotechnol. 23, 510–515 (2012) .
16 . JPK Instruments AG . NanoWizard® AFM Handbook . pp . 1–55 (2012) .
17 . Carvalho, F . A . and Santos, N . C . Atomic force microscopy-based force spec-troscopy–Biological and biomedical applications . IUBMB Life 64, 465–472 (2012) .
18 . Rappaz, B . et al . Comparative study of human erythrocytes by digital holo-graphic microscopy, confocal microscopy, and impedance volume analyzer . Cytometry. A 73, 895–903 (2008) .
19 . Wang, Y ., Wang, H ., Bi, S . and Guo, B . Nano-Wilhelmy investigation of dynamic wetting properties of AFM tips through tip-nanobubble interaction . Sci. Rep. 6, 1–14 (2016) .
20 . Schmitt, L ., Ludwig, M ., Gaub, H . E . and Tampé, R . A metal-chelating microscopy tip as a new toolbox for single-molecule experiments by atomic force microscopy . Biophys. J. 78, 3275–3285 (2000) .
21 . De Oliveira, R . R . L . Albuquerque, D . A . C ., Cruz, T . G . S, Yamaji, F .M . and Leite, F . L . Measurement of the nanoscale roughness by atomic force micros-copy: Basic principles and applications . At. Force Microsc.–Imaging, Meas. Manip. Surfaces At. Scale 147–174 (2012) . doi:10 .5772/37583
22 . Zeidan, A . and Yelin, D . Reflectance confocal microscopy of red blood cells: Simulation and experiment . Biomed. Opt. Express 6, 4335–4343 (2015) . 23 . Torre, B ., Braga, P .C . and Ricci, D . How the Atomic Force Microscope
Works? - Atomic Force Microscopy in Biomedical Research–Methods and Protocols, Humana Press–Springer Science 736, 3–18 (2011) . doi: 10 .1007/978-1-62703-239-1_1
24 . Carvalho, F . A ., Martins, I . C . and Santos, N . C . Atomic force microscopy and force spectroscopy on the assessment of protein folding and functionality . Arch. Biochem. Biophys. 531, 116–127 (2013) .
25 . Behary, N . and Perwuelz, A . Atomic force microscopy–for investigating surface treatment of textile fibers . Atomic Force Microscopy–Imaging, Measuring and Manipulating Surfaces at the Atomic Scale 231–256 (2012) . doi: 10 .5772/35656
63 26 . Morris, V . J . Atomic force microscopy (AFM) and related tools for the
imag-ing of foods and beverages on the nanoscale . Nanotechnology in the Food, Beverage and Nutraceutical Industries (Woodhead Publishing Limited, 2012) . doi:10 .1533/9780857095657 .1 .99
27 . Ramachandran, S ., Teran Arce, F . and Lal, R . Potential role of atomic force microscopy in systems biology . Wiley Interdiscip. Rev. Syst. Biol. Med. 3, 702–716 (2011) .
28 . Frederix, P . L . T . M ., Bosshart, P . D . and Engel, A . Atomic force microscopy of biological membranes . Biophys. J. 96, 329–338 (2009) .
29 . Jung, S .-H ., Park, D ., Park, J . H ., Kim, Y .-M . and Ha, K .-S . Molecular imag-ing of membrane proteins and microfilaments usimag-ing atomic force microscopy . Exp. Mol. Med. 42, 597–605 (2010) .
30 . Müller, D . J . and Dufrêne, Y . F . Atomic force microscopy: A nanoscopic win-dow on the cell surface . Trends Cell Biol. 21, 461–469 (2011) .
31 . Li, M ., Liu, L ., Xi, N . and Wang, Y . Nanoscale monitoring of drug actions on cell membrane using atomic force microscopy . Acta Pharmacol. Sin. 36, 1–14 (2015) . doi:10 .1038/aps .2015 .28 .
32 . Bernardes, N . et al . Modulation of membrane properties of lung cancer cells by azurin enhances the sensitivity to EGFR-targeted therapy and decreased β1 integrin-mediated adhesion . Cell Cycle 15, 1415–1424 (2016) .
33 . Wu, X ., Muthuchamy, M . and Reddy, D . S . Atomic force microscopy proto-col for measurement of membrane plasticity and extracellular interactions in single neurons in epilepsy . Front. Aging Neurosci. 8, 1–12 (2016) .
34 . Hollmann, A . et al . Effects of singlet oxygen generated by a broad-spectrum viral fusion inhibitor on membrane nanoarchitecture . Nanomed. 11, 1163– 1167 (2015) .
35 . Fang, H . H ., Chan, K . Y . and Xu, L . C . Quantification of bacterial adhesion forces using atomic force microscopy (AFM) . J. Microbiol. Methods 40, 89–97 (2000) .
36 . Domingues, M . M . et al . Antimicrobial protein rBPI21-induced surface changes on gram-negative and gram-positive bacteria . Nanomedicine 10, 543–551 (2014) .
37 . Santos, N . C ., Ter-Ovanesyan, E ., Zasadzinski, J . A ., Prieto, M . and Castanho, M . A . R . B . Filipin-induced lesions in planar phospholipid bilayers imaged by atomic force microscopy . Biophys. J. 75, 1869–1873 (1998) .
38 . Franquelim, H . G ., Veiga, A . S ., Weissmüller, G ., Santos, N . C . and Castanho, M . A . Unravelling the molecular basis of the selectivity of the HIV-1 fusion inhibitor sifuvirtide towards phosphatidylcholine-rich rigid membranes . Biochim. Biophys. Acta 1798, 1234–1243 (2010) .
39 . Franquelim, H . G ., Gaspar, D ., Veiga, A . S ., Santos, N . C . and Castanho, M . A . Decoding distinct membrane interactions of HIV-1 fusion inhibitors using a combined atomic force and fluorescence microscopy approach . Biochim. Biophys. Acta 1828, 1777–1785 (2013) .
40 . Franquelim, H . G . et al . Anti-HIV-1 antibodies 2F5 and 4E10 interact differ-ently with lipids to bind their epitopes . AIDS 25, 419–428 (2011) .
41 . Migliolo, L . et al . Structural and functional evaluation of the palindromic alanine-rich antimicrobial peptide Pa-MAP2 . Biochim. Biophys. Acta 1858, 1488–1498 (2016) .
42 . Bravo-Ferrada, B . M . et al . Study of surface damage on cell envelope assessed by AFM and flow cytometry of Lactobacillus plantarum exposed to ethanol and dehydration . J. Appl. Microbiol. 118, 1409–1417 (2015) .
43 . Cardoso, M . H . et al . A polyalanine peptide derived from polar fish with anti-infectious activities . Sci. Rep. 6, 21385 (2016) .
44 . Guedes, A . F . et al . Atomic force microscopy as a tool to evaluate the risk of cardiovascular diseases in patients . Nat. Nanotechnol. 11, 687–692 (2016) .
64
45 . Carvalho, F . A ., de Oliveira, S ., Freitas, T ., Gonçalves, S . and Santos, N . C . Variations on fibrinogen-erythrocyte interactions during cell aging . PLoS One 6, e18167 (2011) .
46 . Gupta, P ., Song, B ., Neto, C . and Camesano, T . A . Atomic force microscopy-guided fractionation reveals the influence of cranberry phytochemicals on adhesion of Escherichia coli . Food Funct. 7, 2655–2666 (2016) .
47 . Carvalho, F . A . et al . Dengue virus capsid protein binding to hepatic lipid droplets (LD) is potassium ion dependent and is mediated by LD surface pro-teins . J. Virol. 86, 2096–2108 (2012) .
48 . Faustino, A . F . et al . Understanding dengue virus capsid protein interaction with key biological targets . Sci. Rep. 5, 10592 (2015) .
49 . Faustino, A . F . et al . Understanding dengue virus capsid protein disordered N-terminus and pep14-23-based inhibition . ACS Chem. Biol. 10, 517–526 (2015) .
50 . Faustino, A . F . et al . Dengue virus capsid protein interacts specifically with very low-density lipoproteins . Nanomedicine 10, 247–255 (2014) .
51 . Haase, K . and Pelling, A . E . Investigating cell mechanics with atomic force microscopy . J. R. Soc. Interface 12, 1–16 (2015) .
52 . Last, J . A ., Russell, P ., Nealey, P . F . and Murphy, C . J . The applications of atomic force microscopy to vision science . Investig. Ophthalmol. Vis. Sci. 51, 6083–6094 (2010) .
53 . Friedrichs, J . et al . A practical guide to quantify cell adhesion using single-cell force spectroscopy . Methods 60, 169–178 (2013) .
54 . Yu, M ., Strohmeyer, N ., Wang, J ., Müller, D . J . and Helenius, J . Increasing throughput of AFM-based single cell adhesion measurements through multi-substrate surfaces . Beilstein J. Nanotechnol. 6, 157–166 (2015) .
55 . Bowman, K ., Saffell, J . Measuring the cell-cell adhesion force exerted by a cell adhesion molecule . JPK Instruments AG - Application Note, 1–4 (2012) . 56 . Puech, P .-H . et al . Measuring cell adhesion forces of primary gastrulating
cells from zebrafish using atomic force microscopy . J. Cell Sci. 118, 4199– 4206 (2005) .
57 . Ovchinnikova, E . S ., Krom, B . P ., Busscher, H . J . and van der Mei, H . C . Evaluation of adhesion forces of Staphylococcus aureus along the length of Candida albicans hyphae . BMC Microbiol. 12, 281 (2012) .
58 . Eaton, P ., Zuzarte-Luis, V ., Mota, M . M ., Santos, N . C . and Prudêncio, M . Infection by Plasmodium changes shape and stiffness of hepatic cells . Nanomedicine 8, 17–19 (2012) .
59 . Santos, N . C ., Ter-Ovanesyan, E ., Zasadzinski, J . A and Castanho, M . A . Reconstitution of phospholipid bilayer by an atomic force microscope tip . Biophys. J. 75, 2119–2120 (1998) .
60 . Muller, D . J ., Helenius, J ., Alsteens, D . and Dufrene, Y . F . Force probing sur-faces of living cells to molecular resolution . Nat Chem Biol 5, 383–390 (2009) . 61 . Benoit, M . and Gaub, H . E . Measuring cell adhesion forces with the atomic
force microscope at the molecular level . Cells Tissues Organs 172, 174–189 (2002) .
62 . Kashef, J . and Franz, C . M . Quantitative methods for analyzing cell–cell adhesion in development . Dev. Biol. 401, 165–174 (2015) .