online | memorias.ioc.fiocruz.br
RNA viruses in trypanosomatid parasites: a historical overview
Danyil Grybchuk1, Alexei Y Kostygov1, Diego H Macedo1, Claudia M d’Avila-Levy2, Vyacheslav Yurchenko1/+
1University of Ostrava, Faculty of Science, Life Science Research Centre, Ostrava, Czech Republic
2Fundação Oswaldo Cruz-Fiocruz, Instituto Oswaldo Cruz, Laboratório de Estudos Integrados em Protozoologia,
Coleção de Protozoários, Rio de Janeiro, RJ, Brasil
Viruses of trypanosomatids are now being extensively studied because of their diversity and the roles they play in flagellates’ biology. Among the most prominent examples are leishmaniaviruses implicated in pathogenesis of
Leishmania parasites. Here, we present a historical overview of this field, starting with early reports of virus-like particles on electron microphotographs, and culminating in detailed molecular descriptions of viruses obtained using modern next generation sequencing-based techniques. Because of their diversity, different life cycle strategies and host specificity, we believe that trypanosomatids are a fertile ground for further explorations to better understand viral evolution, routes of transitions, and molecular mechanisms of adaptation to different hosts.
Key words: trypanosomatidae - RNA viruses - leishmaniavirus
doi: 10.1590/0074-02760170487
Financial support: This work was supported by the Grant Agency of Czech Republic awards 16-18699S and 17-10656S to VY, CAPES, CNPq, FAPERJ, FIOCRUZ (to CMDL).
DG and DHM were funded by grants from the University of Ostrava. + Corresponding author: vyacheslav.yurchenko@osu.cz
Received 10 November 2017 Accepted 20 December 2017
Viruses are obligate intracellular parasites having no metabolism on their own. Instead, they employ resources from their hosts. Because of their high adaptability and diversity, viruses are the most abundant biological ob-jects on Earth (Forterre 2010). Indeed, they can parasitise all cellular types; cases of simultaneous co-infection by different viral species are widespread. This observation indicates a deep evolutionary connection between cells and viruses possibly dating back to the origin of life itself (Forterre & Prangishvili 2009, Moreira & López-García 2009). Understandably, the virus research is mostly fo-cused on the disease-causing agents of humans and live-stock. However, the rise of methods for massively paral-lel nucleic acid sequencing enabled broad-scale studies of viral ecology and diversity in those groups of hosts, which were previously neglected (Cook et al. 2013, Li et al. 2015, Shi et al. 2016, Aswad & Katzourakis 2017). In particular, this lead to the significant progress in the study of viruses in protists (La Scola et al. 2003, Blanc et al. 2015, Maumus & Blanc 2016, Schulz et al. 2017). One of the better studied groups in this respect are trypano-somatids, flagellate parasites of vertebrates, including humans and domestic animals, plants and invertebrates (Maslov et al. 2013, Lukeš et al. 2014).
Methods of RNA viruses’ detection in parasitic pro-tists - Early reports of virus-like particle (VLPs) in vari-ous protists were mostly based on transmission electron microscopy (TEM), which was a part of routine species description procedures since 1950s (Anderson 1959).
Unfortunately, for the most part, microscopic images were inconclusive and insufficient to prove the viral in-fection (Patterson 1990, Wang & Wang 1991). Moreover, nucleic acids sequencing methods were not well devel-oped at that time further complicating description of new viruses from protists. Standards of virology require enrichment of viral particles by ultracentrifugation with subsequent demonstration of their infectivity in the orig-inal host (Hamilton et al. 1981). This technique had lim-ited success with viruses of protists, since usually they cause only latent infections (Miller et al. 1988a, b). Not all of the early descriptions of putative protist-infecting viruses withstood further scrutiny. For example, nuclear filamentous and cytoplasmic polyhedral VLPs from
Entamoeba histolytica were extensively studied by TEM throughout 1970s (Diamond et al. 1972, Mattern et al. 1972, 1977). However, all attempts to purify VLPs failed and the nature of supposed viral agent has not been re-vealed thus far (Hruska et al. 1973, Banik et al. 2014).
b, Tarr et al. 1988). Also, virus particles were isolated form these cells using CsCl gradient ultracentrifugation, and association of dsRNA with these particles was con-firmed (Miller et al. 1988, Widmer et al. 1989, Khoshnan & Alderete 1993). A recently developed real time quan-titative polymerase chain reaction (RT-qPCR)/ultracen-trifugation-based protocol for viral purification couples molecular identification with TEM and has a potential to greatly facilitate viral detection (de Souza et al. 2014).
The rate of new RNA virus discovery accelerated with the advent of high-throughput sequencing analyses of environmental samples (Kristensen et al. 2010, Moki-li et al. 2012). The dawn of next-generation sequenc-ing era coincided with a gradual shift of attention from human-pathogenic viruses toward the broader study of associated commensal viruses from various biotopes (Dutilh et al. 2017). Although awe-inspiring, all these results must be taken with a caution because metatran-scriptomic studies have a number of fundamental limita-tions. The most significant one is that it is not possible to unambiguously identify a true host of the virus. When a metatranscriptome of a macroscopic organism is con-cerned, there is a chance that the actual viral host is a unicellular symbiont or a parasite. Another vulnerability of such an approach is its reliance on homology search algorithms to identify putative viral genes.
Exploration of viruses in Trypanosomatidae by elec-tron microscopy - During second half of the last century there were numerous reports of VLPs in dixenous (= two hosts in the life cycle) trypanosomatids of the genera
Leishmania, Trypanosoma, and Phytomonas. Historical-ly, the very first observation of VLPs in Trypanosomati-dae were made byMolyneux in Leishmania hertigi [now
Paraleishmania hertigi (Kostygov & Yurchenko 2017)] isolated from the prehensile-tailed porcupine (Molyneux 1974). Groups of well discernable rounded electron-dense objects 55-60 nm in diameter were documented in the cytoplasm near kinetoplast. VLPs had regular arrangement (paracrystalline array) and were reported to segregate between dividing cells. Further ultrastruc-tural studies demonstrated that VLPs had electron-dense outer ring and electron-translucent core and were some-times associated with tubular cisternae and vesicular bodies. It was proposed that these unusual cytoplasmic structures might be manifestations of viral infection and could be implicated in VLP morphogenesis. However, continuous three-year long observations of in vitro cul-tured parasites revealed that VLPs were stably present and did not affect the cells(Croft & Molyneux 1979). In the same study P. hertigi promastigotes were differenti-ated in the mouse peritoneal macrophage model. There were less VLPs in the resultant amastigotes compared to their almost ubiquitous presence in promastigotes. Thus, it was concluded that the putative virus might go through a cryptic stage during trypanosomatid differen-tiation. Numerous attempts to purify virus particles and their nucleic acid from P. hertigi cultures failed. These negative results, together with disappearance of VLPs in amastigotes, casted doubts on their viral nature (Eley et al. 1987). Similar arrays of 40-80 nm particles of
vari-ous shapes were also reported in four out of seven iso-lates of Endotrypanum spp. from sloths and Lutzomyia
flies in Central America (Croft et al. 1980). However, the pleomorphy of particles and apparent absence of cy-topathic effects pushed authors to assume their non-viral nature. The VLPs were also reported in Trypanosoma melophagium - a parasite of sheep transmitted by the ec-toparasitic sheep ked (Molyneux & Heywood 1984), but were never characterised by modern molecular methods. Most recently, VLPs were documented in T. cruzi, an etiological agent of Chagas disease. The study describes two types of 32 and 48 nm particles located in endo-plasmic reticulum, Golgi apparatus and cytoplasm of epimastigotes. The incidence of VLPs was only 2% and none were found in other life cycle stages. Apart from TEM, no additional analyses were performed to char-acterize a putative virus (Fernández-Presas et al. 2017).
The first monoxenous (= with one host) trypanosomatid, where VLPs were recorded, was the endosymbiont-bearing
Angomonas (formerly Crithidia) desouzai. Similarly to the case of P. hertigi, VLPs were located in hexagonally orga-nized groups near the nucleus and were observed in cells throughout 2 years of in vitro cultivation with no perceiv-able impact on the host (Motta et al. 1991). Further work showed that putative viral particles contained RNA, but not DNA (Soares et al. 1989, Motta et al. 2003), yet all attempts to isolate this RNA from Angomonas cultures failed. Vi-ral particles and RNA were also absent in the recently de-scribed relative of Angomonas, Kentomonas sorsogonicus
(Votýpka et al. 2014). Hence, presence of RNA virus in A. desouzai needs to be further validated.
Recently, VLPs were also documented in the monox-enous trypanosomatids Leptomonas moramango and
Crithidia pragensis. In L. moramango clusters of round shaped electron-dense objects of around 55-60 nm were observed in proximity of kinetoplast and basal body, whereas in C. pragensis,the 35-40 nm VLPs were found inside the mitochondrion lumen (Yurchenko et al. 2014).
Exploration of viruses in Trypanosomatidae by mo-lecular methods - In the early 1990s dsRNA, viruses of the family Totiviridae were discovered in different Leish-mania spp. (Tarr et al. 1988, Widmer et al. 1989, Guil-bride et al. 1992). Leishmania RNA virus1 (LRV1) from
L. guyanensis M4147 was the first virus from kinetoplas-tids fully characterized in molecular terms. It was origi-nally discovered as an unusual electrophoretic band of to-tal nucleic acid extract. Further ultracentrifugation with subsequent negative-stain TEM revealed that RNA was associated with spherical proteinaceous particles 32 nm in diameter (Tarr et al. 1988). Partial sequence similari-ties between LRV1 and vesicular stomatitis virus (VSV) polymerase indicated that these viruses might be related. This view was supported by the fact that both VSV and
most similar to that of Saccharomyces cerevisiae L-A, a dsRNA virus of yeast. Similar viruses were described from other isolates of L. guyanensis, as well as from one isolate of L. braziliensis (Widmer et al. 1989, Guilbride et al. 1992). All these viruses from New World Leishmania
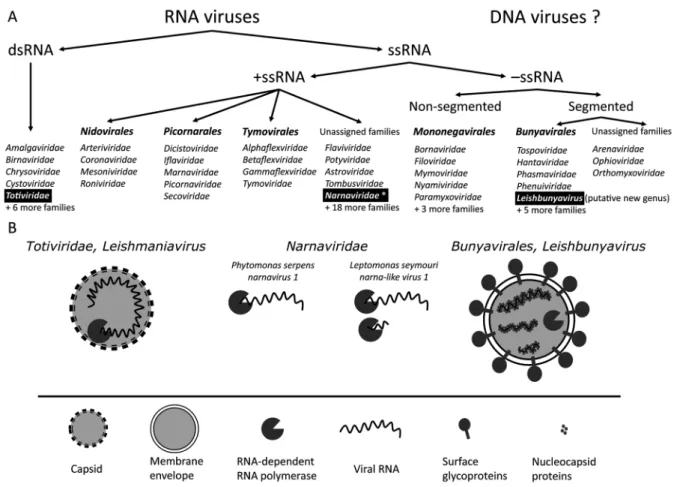
originating from Amazon basin were assigned to the ge-nus Leishmaniavirus within the family Totiviridae (Pat-terson & Larsen 1992) (Fig. 1). Totiviruses are known to infect a wide range of hosts, including protists (Hartley et al. 2012), fungi, yeast (Ghabrial et al. 2015), arthro-pods (Poulos et al. 2006), and vertebrates (Løvoll et al. 2010). All of them have at least two core proteins - capsid and RDRP (Fig. 2). In LRV1, the RDRP was identified as a product of ORF3 (Stuart et al. 1992). The upstream ORF2 encodes a capsid protein, as was demonstrated a few years later (Cadd & Patterson 1994a, b). These two ORFs overlap in all LRV1 representatives with no clear independent start codon for ORF3, leading to the conclu-sion that RDRP is translated only as a C-terminal exten-sion of the capsid (Fig. 2). The fuexten-sion protein is thought to be produced by +1 ribosomal frameshift facilitated by stem-loop structures and pseudoknots within the overlap region (Scheffter et al. 1994). In mid-1990s research on leishmaniaviruses continued with the emphasis on vari-ous aspects of its molecular biology, such as virus-host interactions (Armstrong et al. 1993, Ro et al. 1997), mu-tational analysis of the capsid protein (Cadd et al. 1994a,
b), and the mechanisms of translation and +1 ribosomal frameshift (Maga et al. 1995, Lee et al. 1996).
LRV1 is restricted to South America, yet its distribu-tion is segregated between Amazon (North) and South, where L. braziliensis strains from Minas Gerais (Mace-do et al. 2016) and Rio de Janeiro (Pereira et al. 2013) are not infected with the virus.
In 1995 the complete sequence of the new totivirus from the Old World L. major was reported (Scheffter et al. 1995). The virus was clearly related to LRV1, how-ever, it was quite divergent in terms of amino acid se-quences (only 38% and 47% identical amino acids in the capsid and RDRP proteins, respectively). Poor sequence conservation, probably, accounted for the failed detection by cross-reacting polyclonal antibodies raised against LRV1 capsid (Cadd et al. 1993). It also lacked the over-lap between ORF2 and ORF3, suggesting either different mechanism of fusion protein translation or independent initiation of RDRP synthesis (Scheffter et al. 1995). The virus from L. major was assigned to the genus Leisma-niavirus as LRV2 (Fig. 2, Supplementary data, Table).
Because of the differences in sequences and genome organizations of LRV1 and LRV2 (Fig. 2), it was pro-posed that these viruses diverged upon separation of Old and New World leishmanias. In support of this view, a study focused on virus-host co-evolution demonstrated similar topologies of phylogenetic trees inferred for
virus-infected Leishmania isolates (6 American and 1 Asian, Supplementary data, Table) and respective vi-ruses (Widmer & Dooley 1995). This model was in full agreement with the predominantly vertical transmission of totiviruses in yeasts (Fujimura & Esteban 2011) and asexual reproduction of Leishmania (Panton et al. 1991, Tibayrenc & Ayala 1991, Rougeron et al. 2017). Later, leishmaniaviruses were described in L. aethiopica and
L. infantum isolates (Fig. 2, Supplementary data, Table), allowing even broader phylogenetic analysis (Zangger et al. 2014, Hajjaran et al. 2016). Taken together these re-sults support the model of segregation of viruses to the Old and New World groups, further reinforcing the co-evolution hypothesis and posing a question on the role of virus in Leishmania infectivity or transmission.
The apparent evolutionary cause of virus retention was discovered only 15 years later, when it was demonstrated that LRV1 [strain LRV1-LgyM4147 (Adams et al. 2014)] interferes with vertebrate host immune response against L. guyanensis (Ives et al. 2011). This is the most studied model for LRV1 function. Viral dsRNA stimulates the production
of pro-inflammatory interferon-β through the interaction
with endosomal Toll-like receptor 3. This, in turn, tips the balance toward T-helper 1 mediated immune response leading to chronic inflammation and increased metastatic potential of L. guyanensis (Hartley et al. 2012, 2014). This results in enhanced dissemination and parasite persistence, which ultimately increases the chances of both Leishmania
and its virus to be picked up by a sand fly and successfully complete their life-cycles (Márquez & Roossinck 2012). Thus, virus bearing presents a clear survival advantage in dixenous trypanosomatids. Another interesting question concerns RNA interference (RNAi). At first, it was thought that retention of dsRNA leishmaniaviruses is incompat-ible with RNAi, as the latter would counteract the selec-tive advantage presented by virus-bearing (Lye et al. 2010). However, it was demonstrated that RNAi can be used to eliminate virus from the infected New World Leishmania
by introducing small hairpin RNAs against viral genome. This implies that virus and RNAi are not mutually exclu-sive but instead are able to coexist in dynamical equilib-rium (Brettmann et al. 2016). In addition, recent broad
phylogenetic study of the distribution of RNAi components across Trypanosomatidae tree suggested at least seven in-dependent losses of RNAi machinery in different trypano-somatid lineages and apparent lack of selective forces driv-ing this process (Matveyev et al. 2017).
During the survey of trypanosomatid parasites iso-lates from lactiferous plants of the families Euphorbia-ceae and Apocynaceae, Phytomonas spp. were reported to bear 34 nm virus particles containing 4.7 kb linear dsRNA (Marche et al. 1993). The virus presence was documented only in flagellates infecting coconut and oil palms, but not in trypanosomatids from other lactifer-ous plants growing in the same locality. The dsRNA ge-nome, virion size and association with disease-causing unicellular parasite in many ways paralleled the situation with previously described totiviruses of Leishmania,
Trichomonas, and Giardia spp. Nevertheless, absence of sequence data did not allow establishing the true iden-tity of the virus at that time. Recently, a complete se-quence of the virus infecting P. serpens isolated from the tomato fruit sap has been reported (Akopyants et al. 2016a, b). This virus appears to be a typical narnavirus, consisting of bare RNA molecule encoding RDRP (Fig. 1). Its closest relative is a narnavirus from the oomycete plant pathogen Phytophthora infestans (PiRV-4). Narna-viruses do not have capsids and possess only one posi-tive sense single stranded genomic RNA almost entirely occupied by the RDRP gene. Much like totiviruses, they replicate in the cytoplasm and are transmitted either ver-tically or through cytoplasmic bridges between cells at the instance of mating (Ghabrial et al. 2015). Both Phy-tophthora and Phytomonas spp. are known to infect to-mato, thus, a possibility of virus exchange between the two cannot be formally excluded.
The dsRNAs of viral origin were reported in Lep-tomonas seymouri using nuclease digestion assays and anti-dsRNA antibodies (Kraeva et al. 2015). Soon after, the sequences of viruses infecting L. moramango and
L. seymouri were published (Akopyants et al. 2016b, Lye et al. 2016). The RDRP of L. seymouri virus had the highest sequence similarity to that of the narnavirus of
latter by having additional smaller RNA segment with two putative ORFs with no recognizable homologs in the NCBI database (Lye et al. 2016) The function and na-ture of these segments remain to be investigated further. A recent study also reported high prevalence of L. sey-mouri narna-like virus in clinical isolates from visceral leishmaniasis patients co-infected with L. donovani and
L. seymouri. Interestingly, the presence of virus and L.
seymouri always correlated, suggesting that L. donovani
cannot be the virus host (Sukla et al. 2017). Taken to-gether with the observation that L. seymouri contains high amount of viral dsRNA (Kraeva et al. 2015), it is plausible to suggest that this narnavirus may modulate the pathogenicity of L. donovani similarly to the way the LRV does.
The negative sense ssRNA virus from L. moramango
has a segmented genome consisting of large (L), medium (M), and small (S) fragments. Each segment was pre-dicted to encode a single ORF. Of these, the L segment-encoded RDRP and the S segment-segment-encoded nucleocap-sid protein displayed notable homology to Bunyaviridae
proteins. This virus was classified as a member of a new genus (tentatively, “Leishbunyavirus”) within the family
Bunyaviridae (Akopyants et al. 2016a, b).
Conclusions and perspectives - The research on vi-ruses in trypanosomatids has started in early 1970s and the early investigations were primarily driven by TEM. Development of molecular biology techniques led to the new discoveries, notably of leishmaniaviruses, which are now being extensively studied due to the role they play in Leishmania pathogenesis. Today, next generation sequencing techniques are greatly accelerating the pace of studies into viral diversity. Trypanosomatids are a fertile ground for further explorations. Indeed, there are numerous groups of these flagellates parasitizing a wide range of hosts (from invertebrates to plants and verte-brates), which provides them with an opportunity to ac-quire viruses from different sources: hosts, food, and as-sociated microflora. These parasites can serve as models for the viral research in other protists helping to better understand viral evolution, ways of transitions between hosts, and molecular mechanisms governing adaptation of viruses to distantly related eukaryotes.
Note - While this manuscript was under review, a relevant paper has been published on this topic (Gryb-chuk et al. 2017, in press). The authors demonstrated that diversity of RNA viruses in trypanosomatids is substantially greater than it was envisioned before and revealed that representatives of tombus-like viruses, na-ranaviruses, leishbunyaviruses, and a novel supergroup (provisionally called “Ostravirus”) can be found in try-panosomatids. Importantly, no relatives of LRV1/2 were documented, keeping these viruses restricted to Leish-mania. Taken together, these data further justify the need of a broader sampling for trypanosomatid viruses.
REFERENCES
Adams MJ, Lefkowitz EJ, King AM, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Tax-onomy of Viruses. Arch Virol. 2014; 159(10): 2831-41.
Akopyants NS, Lye LF, Dobson DE, Lukeš J, Beverley SM. A Narna-virus in the trypanosomatid protist plant pathogen Phytomonas serpens. Genome Announc. 2016a; 4(4): e00711-6.
Akopyants NS, Lye LF, Dobson DE, Lukeš J, Beverley SM. A novel bunyavirus-like virus of trypanosomatid protist parasites. Ge-nome Announc. 2016b; 4(4): e00715-6.
Anderson E. The electron microscope: structure and ultra-structure. J Natl Med Assoc. 1959; 51(2): 87-96.
Armstrong TC, Keenan MC, Widmer G, Patterson JL. Successful transient introduction of Leishmania RNA virus into a virally in-fected and an uninin-fected strain of Leishmania. Proc Natl Acad Sci USA. 1993; 90(5): 1736-40.
Aswad A, Katzourakis A. A novel viral lineage distantly related to herpesviruses discovered within fish genome sequence data. Vi-rus Evol. 2017; 3(2): vex016.
Banik GR, Stark D, Rashid H, Ellis JT. Recent advances in molecular biology of parasitic viruses. Infect Disord Drug Targets. 2014; 14(3): 155-67.
Blanc G, Gallot-Lavallee L, Maumus F. Provirophages in the Big-elowiella genome bear testimony to past encounters with giant viruses. Proc Natl Acad Sci USA. 2015; 112(38): E5318-26.
Brettmann EA, Shaik JS, Zangger H, Lye LF, Kuhlmann FM, Akopyants NS, et al. Tilting the balance between RNA interference and replica-tion eradicates Leishmania RNA virus 1 and mitigates the inflamma-tory response. Proc Natl Acad Sci USA. 2016; 113(43): 11998-12005.
Cadd TL, Keenan MC, Patterson JL. Detection of Leishmania RNA virus 1 proteins. J Virol. 1993; 67(9): 5647-50.
Cadd TL, MacBeth K, Furlong D, Patterson JL. Mutational analysis of the capsid protein of Leishmania RNA virus LRV1-4. J Virol. 1994a; 68(12): 7738-45.
Cadd TL, Patterson JL. Synthesis of viruslike particles by expression of the putative capsid protein of Leishmania RNA virus in a recombi-nant baculovirus expression system. J Virol. 1994b; 68(1): 358-65.
Cai G, Myers K, Hillman BI, Fry WE. A novel virus of the late blight pathogen, Phytophthora infestans, with two RNA segments and a supergroup 1 RNA-dependent RNA polymerase. Virology. 2009; 392(1): 52-61.
Cook S, Chung BY, Bass D, Moureau G, Tang S, McAlister E, et al. Novel virus discovery and genome reconstruction from field RNA samples reveals highly divergent viruses in dipteran hosts. PLOS ONE. 2013; 8(11): e80720.
Croft SL, Chance ML, Gardener PJ. Ultrastructural and biochemi-cal characterization of stocks of Endotrypanum. Ann Trop Med Parasitol. 1980; 74(6): 585-9.
Croft SL, Molyneux DH. Studies on the ultrastructure, virus-like particles and infectivity of Leishmania hertigi. Ann Trop Med Parasitol. 1979; 73(3): 213-26.
de Souza MM, Manzine LR, da Silva MV, Bettini J, Portugal RV, Cruz AK, et al. An improved purification procedure for Leish-mania RNA virus (LRV). Braz J Microbiol. 2014; 45(2): 695-8.
Diamond LS, Mattern CF, Bartgis IL. Viruses of Entamoeba histo-lytica. I. Identification of transmissible virus-like agents. J Virol. 1972; 9(2): 326-41.
Dutilh BE, Reyes A, Hall RJ, Whiteson KL. Editorial: virus dis-covery by metagenomics: the (im)possibilities. Front Microbiol. 2017; 8: 1710.
Fernández-Presas AM, Padilla-Noriega L, Becker I, Robert L, Jimenez JA, Solano S, et al. Enveloped and non-enveloped viral-like particles in Trypanosoma cruzi epimastigotes. Rev Inst Med Trop Sao Paulo. 2017; 59: e46.
Forterre P, Prangishvili D. The origin of viruses. Res Microbiol. 2009; 160(7): 466-72.
Forterre P. Defining life: the virus viewpoint. Orig Life Evol Biosph. 2010; 40(2): 151-60.
Fujimura T, Esteban R. Cap-snatching mechanism in yeast L-A dou-ble-stranded RNA virus. Proc Natl Acad Sci USA. 2011; 108(43): 17667-71.
Ghabrial SA, Castón JR, Jiang D, Nibert ML, Suzuki N. 50-plus years of fungal viruses. Virology. 2015; 479-480: 356-68.
Grybchuk D, Akopyants NS, Kostygov A, Konovalovas A, Lye L-F, Dobson DE, et al. Viral discovery and diversity in trypanosomatid protozoa with a focus on relatives of the human parasite Leishma-nia. Proc Natl Acad Sci USA. 2018; 115(3): E506-E15.
Guilbride L, Myler PJ, Stuart K. Distribution and sequence diver-gence of LRV1 viruses among different Leishmania species. Mol Biochem Parasitol. 1992; 54(1): 101-4.
Hajjaran H, Mahdi M, Mohebali M, Samimi-Rad K, Ataei-Pirkooh A, Kazemi-Rad E, et al. Detection and molecular identification of Leishmania RNA virus (LRV) in Iranian Leishmania species. Arch Virol. 2016; 161(12): 3385-90.
Hamilton RI, Edwardson JR, Francki RIB, Hsu HT, Hull R, Koenig R, et al. Guidelines for the identification and characterization of plant viruses. J Gen Virol. 1981; 54(2): 223-41.
Hartley MA, Drexler S, Ronet C, Beverley SM, Fasel N. The immu-nological, environmental, and phylogenetic perpetrators of meta-static leishmaniasis. Trends Parasitol. 2014; 30(8): 412-22.
Hartley MA, Ronet C, Zangger H, Beverley SM, Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Micro-biol. 2012; 2: 99.
Hruska JF, Mattern CF, Diamond LS, Keister DB. Viruses of Ent-amoeba histolytica. 3. Properties of the polyhedral virus of the HB-301 strain. J Virol. 1973; 11(1): 129-36.
Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Schutz F, et al. Leishmania RNA virus controls the severity of mucocuta-neous leishmaniasis. Science. 2011; 331(6018): 775-8.
Khoshnan A, Alderete JF. Multiple double-stranded RNA segments are associated with virus particles infecting Trichomonas vagi-nalis. J Virol. 1993; 67(12): 6950-5.
Kostygov AY, Yurchenko V. Revised classification of the subfamily Leishmaniinae (Trypanosomatidae). Folia Parasitol. 2017; 64: 020.
Kraeva N, Butenko A, Hlaváčová J, Kostygov A, Myškova J, Gryb -chuk D, et al. Leptomonas seymouri: adaptations to the dixenous life cycle analyzed by genome sequencing, transcriptome profil-ing and co-infection with Leishmania donovani PLOS Pathog. 2015; 11(8): e1005127.
Kristensen DM, Mushegian AR, Dolja VV, Koonin EV. New di-mensions of the virus world discovered through metagenomics. Trends Microbiol. 2010; 18(1): 11-9.
La Scola B, Audic S, Robert C, Jungang L, de Lamballerie X, Drancourt M, et al. A giant virus in amoebae. Science. 2003; 299(5615): 2033.
Lee SE, Suh JM, Scheffter S, Patterson JL, Chung IK. Identification of a ribosomal frameshift in Leishmania RNA virus 1-4. J Bio-chem. 1996; 120(1): 22-5.
Li CX, Shi M, Tian JH, Lin XD, Kang YJ, Chen LJ, et al. Unprec-edented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife. 2015; 4.
Løvoll M, Wiik-Nielsen J, Grove S, Wiik-Nielsen CR, Kristoffersen AB, Faller R, et al. A novel totivirus and piscine reovirus (PRV) in Atlantic salmon (Salmo salar) with cardiomyopathy syndrome (CMS). Virol J. 2010; 7: 309.
Lukeš J, Skalický T, Týč J, Votýpka J, Yurchenko V. Evolution of
parasitism in kinetoplastid flagellates. Mol Biochem Parasitol. 2014; 195(2): 115-22.
Lye LF, Akopyants NS, Dobson DE, Beverley SM. A Narnavirus-like element from the trypanosomatid protozoan parasite Leptomonas seymouri. Genome Announc. 2016; 4(4): e00713-6.
Lye LF, Owens K, Shi H, Murta SM, Vieira AC, Turco SJ, et al. Reten-tion and loss of RNA interference pathways in trypanosomatid protozoans. PLoS Pathog. 2010; 6(10): e1001161.
Macedo DH, Menezes-Neto A, Rugani JM, Rocha AC, Silva SO, Melo MN, et al. Low frequency of LRV1 in Leishmania braziliensis
strains isolated from typical and atypical lesions in the state of Minas Gerais, Brazil. Mol Biochem Parasitol. 2016; 210(1-2): 50-4.
Maga JA, Widmer G, LeBowitz JH. Leishmania RNA virus 1-mediated cap-independent translation. Mol Cell Biol. 1995; 15(9): 4884-9.
Marche S, Roth C, Manohar SK, Dollet M, Baltz T. RNA virus-like particles in pathogenic plant trypanosomatids. Mol Biochem Par-asitol. 1993; 57(2): 261-7.
Márquez LM, Roossinck MJ. Do persistent RNA viruses fit the trade-off hypothesis of virulence evolution? Curr Opin Virol. 2012; 2(5): 556-60.
Maslov DA, Votýpka J, Yurchenko V, Lukeš J. Diversity and phylog-eny of insect trypanosomatids: all that is hidden shall be revealed. Trends Parasitol. 2013; 29(1): 43-52.
Mattern CF, Diamond LS, Daniel WA. Viruses of Entamoeba his-tolytica. II. Morphogenesis of the polyhedral particle (ABRM 2 leads to HK-9) leads to HB-301 and the filamentous agent (ABRM) 2 leads to HK-9. J Virol. 1972; 9(2): 342-58.
Mattern CF, Keister DB, Daniel WA, Diamond LS, Kontonis AD. Vi-ruses of Entamoeba histolytica. VII. Novel beaded virus. J Virol. 1977; 23(3): 685-91.
Matveyev AV, Alves JM, Serrano MG, Lee V, Lara AM, Barton WA, et al. The evolutionary loss of RNAi key determinants in kineto-plastids as a multiple sporadic phenomenon. J Mol Evol. 2017; 84(2-3): 104-15.
Maumus F, Blanc G. Study of gene trafficking between Acanthamoe-ba and giant viruses suggests an undiscovered family of amoeba-infecting viruses. Genome Biol Evol. 2016; 8(11): 3351-63.
Miller RL, Wang AL, Wang CC. Identification of Giardia lamblia
isolates susceptible and resistant to infection by the double-stranded RNA virus. Exp Parasitol. 1988a; 66(1): 118-23.
Miller RL, Wang AL, Wang CC. Purification and characterization of the Giardia lamblia double-stranded RNA virus. Mol Biochem Parasitol. 1988b; 28(3): 189-95.
Mokili JL, Rohwer F, Dutilh BE. Metagenomics and future perspec-tives in virus discovery. Curr Opin Virol. 2012; 2(1): 63-77.
Molyneux DH, Heywood P. Evidence for the incorporation of virus-like particles into Trypanosoma. Z Parasitenk. 1984; 70(4): 553-6.
Moreira D, López-García P. Ten reasons to exclude viruses from the tree of life. Nat Rev Microbiol. 2009; 7(4): 306-11.
Motta MC, de Souza W, Thiry M. Immunocytochemical detection of DNA and RNA in endosymbiont-bearing trypanosomatids. FEMS Microbiol Lett. 2003; 221(1): 17-23.
Motta MCM, Cava AMS, Silva PMF, Fiorini JE, Soares MJ, Desouza W. Morphological and biochemical characterization of the try-panosomatids Crithidia desouzai and Herpetomonas anglusteri. Can J Zool. 1991; 69(3): 571-7.
Panton LJ, Tesh RB, Nadeau KC, Beverley SM. A test for genetic exchange in mixed infections of Leishmania major in the sand fly
Phlebotomus papatasi. J Protozool. 1991; 38(3): 224-8.
Patterson DJ, Larsen J. A perspective on protistan nomenclature. J Protozool. 1992; 39: 125-31.
Patterson JL. Viruses of protozoan parasites. Exp Parasitol. 1990; 70(1): 111-3.
Pereira LOR, Maretti-Mira AC, Rodrigues KM, Lima RB, de Oliveira-Neto MP, Cupolillo E, et al. Severity of tegumentary leishmaniasis is not exclusively associated with Leishmania RNA virus 1 infec-tion in Brazil. Mem Inst Oswaldo Cruz. 2013; 108(5): 665-7.
Potgieter AC, Page NA, Liebenberg J, Wright IM, Landt O, van Dijk AA. Improved strategies for sequence-independent amplification and sequencing of viral double-stranded RNA genomes. J Gen Virol. 2009; 90(Pt 6): 1423-32.
Poulos BT, Tang KF, Pantoja CR, Bonami JR, Lightner DV. Purifi-cation and characterization of infectious myonecrosis virus of penaeid shrimp. J Gen Virol. 2006; 87(Pt 4): 987-96.
Ro YT, Scheffter SM, Patterson JL. Specific in vitro cleavage of a Leish-mania virus capsid-RNA-dependent RNA polymerase polyprotein by a host cysteine-like protease. J Virol. 1997; 71(12): 8983-90.
Rougeron V, de Meeus T, Banuls AL. Reproduction in Leishmania: a focus on genetic exchange. Infect Genet Evol. 2017; 50: 128-32.
Scheffter S, Widmer G, Patterson JL. Complete sequence of Leish-mania RNA virus 1-4 and identification of conserved sequences. Virology. 1994; 199(2): 479-83.
Scheffter SM, Ro YT, Chung IK, Patterson JL. The complete se-quence of Leishmania RNA virus LRV2-1, a virus of an Old World parasite strain. Virology. 1995; 212(1): 84-90.
Schulz F, Yutin N, Ivanova NN, Ortega DR, Lee TK, Vierheilig J, et al. Giant viruses with an expanded complement of translation system components. Science. 2017; 356(6333): 82-5.
Shi M, Lin XD, Tian JH, Chen LJ, Chen X, Li CX, et al. Redefining the invertebrate RNA virosphere. Nature. 2016; 540(7634): 539-43.
Soares MJ, Motta MC, de Souza W. Bacterium-like endosymbiont and virus-like particles in the trypanosomatid Crithidia desou-zai. Microbios Lett. 1989; 41: 137-41.
Stuart KD, Weeks R, Guilbride L, Myler PJ. Molecular organiza-tion of Leishmania RNA virus 1. Proc Natl Acad Sci USA. 1992; 89(18): 8596-8600.
Sukla S, Roy S, Sundar S, Biswas S. Leptomonas seymouri narna-like virus 1 and not leishmaniaviruses detected in kala-azar samples from India. Arch Virol. 2017; 162(12): 3827-35.
Tarr PI, Aline Jr RF, Smiley BL, Scholler J, Keithly J, Stuart K. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci USA. 1988; 85(24): 9572-5.
Tibayrenc M, Ayala FJ. Towards a population genetics of microor-ganisms: the clonal theory of parasitic protozoa. Parasitol Today. 1991; 7(9): 228-32.
Vogt VM. Purification and further properties of single-strand-specif-ic nuclease from Aspergillus oryzae. Eur J Biochem. 1973; 33(1): 192-200.
Votýpka J, Kostygov AY, Kraeva N, Grybchuk-Ieremenko A,
Tesařová M, Grybchuk D, et al. Kentomonas gen. n., a new genus of endosymbiont-containing trypanosomatids of Strigomonadi-nae subfam. n. Protist. 2014; 165(6): 825-38.
Wang AL, Wang CC. Discovery of a specific double-stranded RNA vi-rus in Giardia lamblia. Mol Biochem Parasitol. 1986a; 21(3): 269-76.
Wang AL, Wang CC. The double-stranded RNA in Trichomonas vag-inalis may originate from virus-like particles. Proc Natl Acad Sci USA. 1986b; 83(20): 7956-60.
Wang AL, Wang CC. Viruses of the protozoa. Annu Rev Microbiol. 1991; 45: 251-63.
Widmer G, Comeau AM, Furlong DB, Wirth DF, Patterson JL. Char-acterization of a RNA virus from the parasite Leishmania. Proc Natl Acad Sci USA. 1989; 86(15): 5979-82.
Widmer G, Dooley S. Phylogenetic analysis of Leishmania RNA vi-rus and Leishmania suggests ancient virus-parasite association. Nucleic Acids Res. 1995; 23(12): 2300-04.
Yurchenko V, Votýpka J, Tesařová M, Klepetková H, Kraeva N, Jirků M, et al. Ultrastructure and molecular phylogeny of four
new species of monoxenous trypanosomatids from flies (Dip-tera: Brachycera) with redefinition of the genus Wallaceina. Folia Parasitol. 2014; 61(2): 97-112.