O R I G I N A L P A P E R
Involvement of monoaminergic systems in anxiolytic
and antidepressive activities of the standardized extract of
Cocos
nucifera
L.
Eliane Brito Cortez Lima1•Caren Na´dia Soares de Sousa1• Lucas Nascimento Meneses1• Yuri Freitas e Silva Pereira1•Nata´lia Castelo Branco Matos1•Rayanne Brito de Freitas2•
Nycole Brito Cortez Lima3•Manoel Cla´udio Azevedo Patrocı´nio3•Luzia Kalyne Almeida Moreira Leal2• Glauce Socorro Barros Viana1• Silvaˆnia Maria Mendes Vasconcelos1
Received: 20 May 2016 / Accepted: 5 October 2016 / Published online: 21 October 2016
ÓThe Japanese Society of Pharmacognosy and Springer Japan 2016
Abstract Extracts from the husk fiber ofCocos nucifera are used in folk medicine, but their actions on the central nervous system have not been studied. Here, the anxiolytic and antidepressant effects of the standardized hydroalco-holic extract of C. nucifera husk fiber (HECN) were evaluated. Male Swiss mice were treated with HECN (50, 100, or 200 mg/kg) 60 min before experiments involving the plus maze test, hole-board test, tail suspension test, and forced swimming test (FST). HECN was administered orally (p.o.) in acute and repeated-dose treatments. The forced swimming test was performed with dopaminergic and noradrenergic antagonists, as well as a serotonin release inhibitor. Administration of HECN in the FST after intraperitoneal (i.p.) pretreatment of mice with sulpiride (50 mg/kg), prazosin (1 mg/kg), orp-chlorophenylalanine (PCPA, 100 mg/kg) caused the actions of these three agents to be reversed. However, this effect was not observed after pretreating the animals with SCH23390 (15lg/kg, i.p.) or yohimbine (1 mg/kg, i.p.) The dose
chosen for HECN was 100 mg/kg, p.o., which increased the number of entries as well as the permanence in the open
arms of the maze after acute and repeated doses. In both the forced swimming and the tail suspension tests, the same dose decreased the time spent immobile but did not disturb locomotor activity in an open-field test. The anxiolytic effect of HECN appears to be related to the GABAergic system, while its antidepressant effect depends upon its interaction with the serotoninergic, noradrenergic (a1
receptors), and dopaminergic (D2 dopamine receptors) systems.
Keywords AnxiolyticAntidepressiveC. nuciferaL.
Hydroalcoholic extract ofC. nuciferahusk fiber
Abbreviations BUP Bupropion
CNS Central nervous system DZP Diazepam
EPM Elevated plus maze test FLUM Flumazenil
FLX Fluoxetine
FST Forced swimming test
HECN Hydroalcoholic extract ofC. nuciferahusk fiber HPLC High-performance liquid cromathography IMP Imipramine
NA Noradrenaline
NEOA Number of entries in open arms OFT Open-field test
PCPA p-Chlorophenylalanine PRA Prazosin
SLA Spontaneous locomotor activity test SUL Sulpiride
TPC Determination of total phenolic content TPOA Time of permanence in open arms TST Tail suspension test
YOH Yohimbine & Silvaˆnia Maria Mendes Vasconcelos
silvania_vasconcelos@yahoo.com.br; silvania@pq.cnpq.br
1 Neuropsychopharmacology Laboratory, Department of Physiology and Pharmacology, Universidade Federal do Ceara´, Cel. Nunes de Melo Street, 1127, Fortaleza, CE CEP: 60431-270, Brazil
2 Department of Pharmacy, Federal University of Ceara´,
Capita˜o Francisco Pedro Street, 1210, Fortaleza, CE CEP: 60430-170, Brazil
3 Pharmacology Laboratory, Department of Medicine,
Introduction
The species Cocos nucifera L. (Arecaceae), popularly known as the coconut tree, is one of the most naturally widely distributed fruit plants, as it can be found on most continents [1]. In Brazil, it is largely found on the north-eastern coast in its typical (giant) and nana (dwarf) vari-eties and as hybrids of these two [2].
Derivatives of the fruit ofC. nuciferahave been used in the food, cosmetic, and craft industries [3]. In Brazil, the high consumption of coconut fruit by these indus-tries has led to a large amount of unwanted coconut barks, which correspond to 60 % of the volume of household waste [4].
The raw extract of the husk fiber of the coconut fruit is generally used in folk medicine in the northeastern region of Brazil to treat diarrhea and arthritis, while heating it generates an oil which is used to treat mycotic infections in Indian folk medicine [5].
Studies have shown that the husk fiber has various biological activities, including antibacterial, antiviral, antifungal, anthelmintic, analgesic, antitumor, antioxidant, and anti-inflammatory activities [5–11].
Research performed on several kinds ofC. nuciferahusk fiber extract has shown that they are rich in flavonoids, catechins, and condensed tannins [5,12,13]—compounds that have shown a potential antidepressant action in animal models [14–17]. In a recent study performed in our labo-ratory, we obtained the total polyphenols as well as two phenol compounds, catechin and chlorogenic acid, from hydroalcoholic extract ofC. nucifera husk fiber (HECN). In that study, the catechins in the HECN were linked to the antidepressant-like, antioxidant, and neurotrophic effects of HECN [18].
The aim of the study reported in the present paper was to analyze the effects produced by an acute dose and repeated doses (7 days) of HECN in mice. Behavioral tests such as the open-field test, plus maze test, hole-board test, tail suspension test, and forced swimming test were employed to evaluate the motor, anxiolytic, and antidepressant effects of HECN, as well as to elucidate its mechanism of action.
Materials and methods
Preparation of the hydroalcoholic extract ofCocos
nucifera(HECN)
The extract was prepared according to a protocol used by our laboratory previously [18].
Animals
Adult male Swiss mice (29–34 g) were housed (10 per cage) in standard polycarbonate cages (42920.59
20 cm) and under standard environmental conditions (22±1°C, humidity 60±5 % and 12 h light:12 h dark
cycle) with food and water ad libitum. The animals were obtained from the Central Animal Facility of the Federal University of Ceara´. The experimental procedures were performed from 8:00 am to 14:00 pm and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Brazilian College of Animal Experimentation (COBEA). The research protocol was approved by the Ethics Committee of the Federal Univer-sity of Ceara´ (no. 32/2011).
Drugs
Diazepam, a benzodiazepine anxiolytic (DZP; Unia˜o Quı´-mica, Brazil); imipramine, a tricyclic antidepressant (IMP; Geigy); bupropion, an atypical antidepressant (BUP; GlaxoSmithKline); fluoxetine, a serotonin release inhibitor (FLX; Eli-lilly) and flumazenil, specific antagonist for benzodiazepines (FLUM, Roche) were used as reference drugs. Methyl ester ofp-chlorophenylalanine (PCPA), (R )-(?
)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrah-ydro-1H-3-benzazepine (SCH23390), sulpiride (SUL), prazosin (PRA), and yohimbine (YOH) were used as neurotransmitter antagonists (Sigma–Aldrich, Brazil). HECN was administered orally (p.o.), while DZP, IMP, BUP, FLUM, PCPA, SUL, SCH23390, PRA, and YOH were administered intraperitoneally (i.p.). The doses used were given in a constant volume of 10 ml/kg weight. HECN and the other drugs were dissolved in 0.9 % saline. The control group received 0.9 % saline.
Experimental protocol
Animals received HECN at doses of 50, 100, or 200 mg/ kg, p.o., and were separated into two different groups based on treatment duration: an acute dose group, with mice treated 60 min before the behavioral tests; and a repeated dose group, where the mice were treated for seven con-secutive days and the behavioral tests were applied 60 min after the last dose. A control group received the same volume (10 ml/kg) orally as used in the treatment groups, but of the vehicle (0.9 % saline) only.
evaluate the anxiolytic and antidepressant actions of HECN, the plus maze, hole-board, tail suspension, and forced swimming tests were performed.
To investigate the possible involvement of the dopaminergic and noradrenergic systems in the antide-pressant action of the HECN, the mice were pretreated i.p. with 50 mg/kg sulpiride (D2 dopaminergic receptor antagonist), 15lg/kg SCH23390 (D1 dopaminergic
receptor antagonist); 1 mg/kg prazosin (a1-adrenergic
antagonist), or 1 mg/kg yohimbine (a2-adrenoceptor
antagonist), all administered 30 min before HECN (100 mg/kg), BUP (30 mg/kg), or vehicle only (0.9 % saline).
To discern the involvement of HECN in the serotonin system, the animals were pretreated once a day for four consecutive days withp-chlorophenylalanine methyl ester (PCPA, 100 mg/kg), an inhibitor of serotonin synthesis, or the vehicle only [5, 19, 20]. Then, 24 h after the last administration of PCPA, the mice were treated with HECN (100 mg/kg, p.o.), FLX (35 mg/kg), or vehicle only (0.9 % saline), and underwent the forced swimming test 1 h later. In all behavioral determinations, the animals were tested during the light period and observed in a closed room at constant temperature (25±1 °C) under poor illumination
with a 15-V red light, except in the forced swimming test, which was illuminated with normal light. All tests were performed on different days with distinct groups of animals.
Open-field test
The open-field area was made of acrylic (transparent walls and black floor, 30930915 cm) divided into nine
squares of equal area. This apparatus was used to evaluate the exploratory activity of each animal for 5 min [21]. The observed parameters were as follows: number of squares crossed (with the four paws) and number of rearings and groomings. Diazepam 2 mg/kg was used as the standard drug.
Elevated plus maze test
The plus maze test for mice [22] consisted of two per-pendicular open arms (3095 cm) and two perpendicular
closed arms (3095 925 cm). The open and closed arms were connected by a central platform (595 cm). The
platform and the lateral walls of the closed arms were made of transparent acrylic, and the floor of black acrylic. The maze was 45 cm above the floor.
Hole-board test
The hole-board test for exploratory behavior in mice was used as described previously by Clark et al. [23]. The
apparatus employed was a Ugo Basile (60930 cm) that
had 16 evenly spaced holes with built-in infrared sensors. In brief, the number of head dips into the holes in 5 min was counted for each animal. Diazepam (1 mg/kg) was used as the standard anxiolytic drug.
Tail suspension test
The tail suspension test for the evaluation of antidepressant activity has been described previously [24]. The animals were transported from the housing room to the testing area in their own cages and allowed to adapt to the new envi-ronment for 1 h before testing. For the test, the animals were suspended on the edge of a shelf, 58 cm above a table top, by an adhesive tape placed approximately 1 cm from the tip of the tail. The amount of time the animal stayed immobile was recorded for a period of 6 min.
Forced swimming test
Animals were subjected individually to an analysis of depressive-like behavior, based on a model adapted from Porsolt et al. [25]. In this task, the animal’s immobility during a 6-min period was registered; the greater the time spent immobile, the lower the animal’s motivation to escape, thus representing depressive-like behavior [26]. The animals were placed individually in an acrylic cylinder (25 cm high, 10 cm in diameter, and 8 cm deep) contain-ing water at 25°C, and were unable to escape from or to
touch the bottom of the cylinder. Any mouse that appeared to have difficulty in keeping its head above the water was removed from the cylinder and excluded from the study.
Statistical analyses
All results are presented as the mean±SEM. The data
were analyzed by ANOVA followed by Tukey’s post hoc multiple comparison test. The results were considered significant at P\0.05. The statistical program used was Prism 5.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Behavioral tests Open-field test
Repeated administration
control
HECN 50mg HECN 100mg HECN 200mg DZP2
0 10 20 30 40 50
Number of squares crossed Number of squares crossed a
control
HECN 200mg/kg HECN 100mg/kg
HECN 50mg/kg
DZP2 0
10 20 30 40 50
a
Acute treatment
Control HECN50
HECN100 HECN200 DZP2
Control HECN50
HECN100 HECN200 DZP2 0
5 10 15
a
Number of rearing
0 5 10 15 20 25
a
a a
a
Number of rearing
Acute treatment
Control
HECN50 HECN100 HECN200
DZP2 0
1 2 3 4
Number of grooming
Con trol
HECN50
HECN100 HECN200
DZP2 0
2 4 6
a
a
a
a
Number of grooming
Repeated administration Acute treatment
Repeated administration
(A) (B)
(C) (D)
(E) (F)
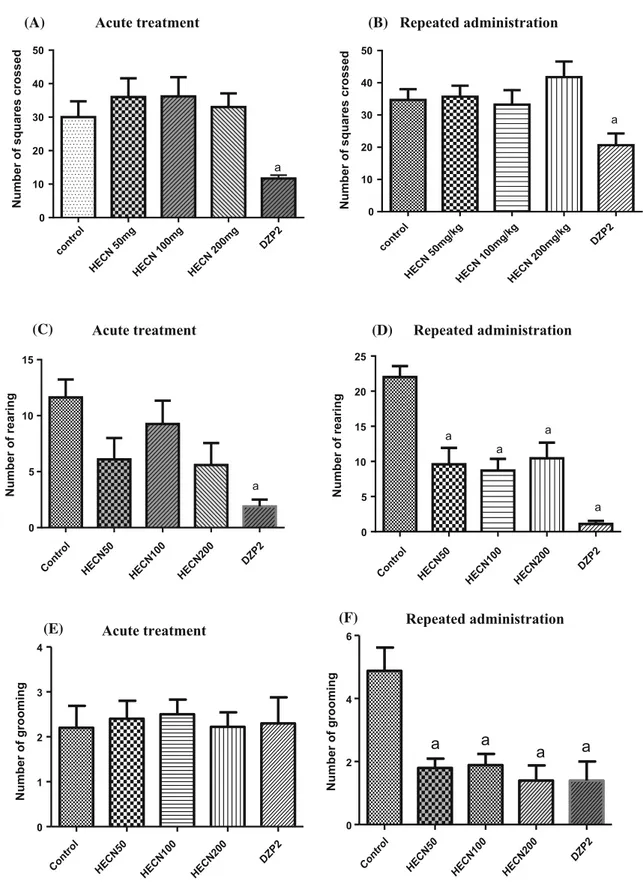
Fig. 1 Number of crossings (a,b), rearings (c,d), and groomings (e, f) in the open-field test of groups of mice that received either the vehicle only (control), HECN (50, 100, or 200 mg/kg, p.o.) in the acute and repeated treatments, or diazepam (DZP 2 mg/kg). The
parameters analyzed were the number of squares crossed, rearing, and grooming. The results are presented as the mean±SEM.aP\0.05
significantly decreased all of these parameters when com-pared to the control group (acute treatment: F(4,25)=
4.966, P=0.0044; repeated administration: F(4,40)=
4.711, P =0.0033). Fewer rearings and groomings were
also observed after repeated administration of HECN.
Plus maze and hole-board tests
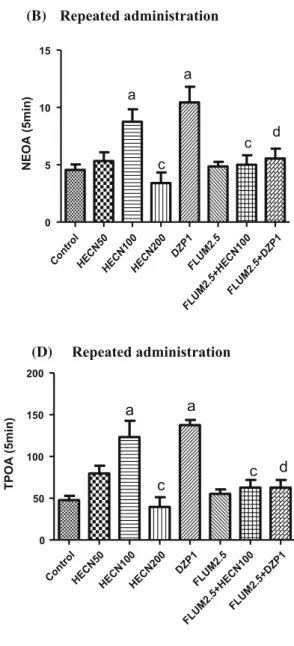
In the plus maze test (Fig.2a–d), using the 100 mg/kg dose of HECN significantly increased all of the parameters observed in the acute and repeated-dose treatments when compared to the control group (acute treatment: NEOA F(7,77)=7.254, P\0.0001; TPOA F(7,76)=19.77,
P\0.0001; repeated administration: NEOA F(7,81)= 7.328, P\0.0001; TPOA F(7,78)=12.92, P\0.0001). Flumazenil (2.5 mg/kg) reversed the anxiolytic effects of HECN 100 mg/kg and DZP 1 mg/kg in all parameters analyzed (NEOA: acute treatment P\0.05, repeated administrationP\0.05; TPOA: acute treatmentP\0.05, repeated administrationP\0.05).
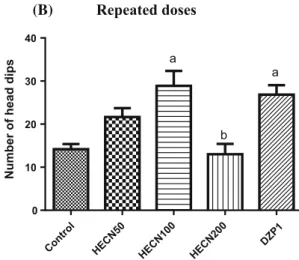
To better evaluate the anxiolytic activity of HECN, the hole-board test was performed. As shown in Fig.3a, b, HECN 100 mg/kg increased the number of dives (com-pared to the number made by the control animals) after acute and repeated doses [acute treatmentF(4,50)=8.371, P\0.0001; repeated administration: F(4,51)=11.00,
Acute treatment
Control HECN50
HECN100 HECN 200 DZP1
FLUM2.5
FLUM2.5+HECN100 FLUM2.5+DZP1
0 5 10 15
a
a
c
d
NEOA (5min)
Cont rol
HECN50
HECN100 HECN200 DZP1
FLUM2.5
FLUM2.5+H ECN100
FLUM2.5+DZP1 0
5 10 15
a
c a
c d
NEOA (5min)
Acute treatment Repeated administration
Control
HECN50 HECN100 HECN200 DZP1
FLUM 2.5
FLUM2.5+HECN100 FLUM2.5+DZP1 0
50 100 150 200
a
c c d
a,b
TPOA (5min)
Control HECN50 HECN
100 HECN200
DZP1 FLU
M2.5
FLUM2.5 +HE
CN 100
FLU M2.5+DZP1
0 50 100 150 200
a
c a
c d
TPOA (5min)
Repeated administration
(A) (B)
(C) (D)
Fig. 2 Plus maze test of groups of mice that received the vehicle only (control), HECN (50, 100, or 200 mg/kg, p.o.) in the acute and repeated treatments, diazepam (DZP 1 mg/kg), or flumazenil (FLUM 2.5 mg/kg). The parameters analyzed were NEOA (number of entries into open arms:a, b) and TPOA (time of permanence in the open
arms:c,d). The results are presented as the mean±SEM.aP\0.05
compared with control,bP\0.05 compared with HECN 50 mg/kg,
cP
\0.05 compared with HECN 100 mg/kg, dP\0.05 compared
P\0.0001]. These results were similar to those obtained with the positive control, DZP (1 mg/kg), a benzodiazepine already used clinically to treat anxiety, thereby confirming the anxiolytic activity of HECN.
Tail suspension and forced swimming tests
According to Fig.4a, b, only 50 mg/kg (acute:P\0.001; repeated administration:P\0.01) and 100 mg/kg (acute:
P\0.05; repeated administration: P\0.01) doses of HECN significantly decreased the time spent immobile in the tail suspension test when compared to the control group
in both acute [F(4,46) =8.905;P=0.0001] and repeated [F(4,50)=11.58;P\0.0001] treatments.
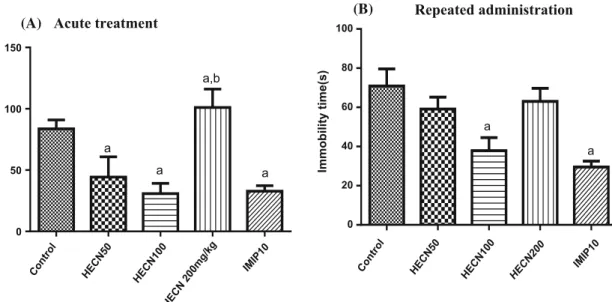
To better evaluate the antidepressant effect of HECN, the time spent immobile in the forced swimming test was also analyzed (Fig.5a, b). The results confirmed those obtained in the tail suspension test, with 50 (P\0.05) and 100 mg/kg (P\0.0001) doses of HECN in the acute treatment [F(4,43)=8.709; P\0.0001] reducing the time that the animals spent immobile. However, after repeated adminis-tration [F(4,49)=6.662; P=0.0003], only mice treated with 100 mg/kg HECN (P\0.01) showed a decrease in the time spent immobile in comparison to the control group. Acute doses
Control HECN50 HECN10
0
HECN2 00
DZP1 0
10 20 30 40
a
a
Number of head dips
Control HEC N50
HECN100 HECN200
DZP1 0
10 20 30 40
a
a
b
Number of head dips
Repeated doses
(A) (B)
Fig. 3 Hole-board test of groups of mice that received vehicle only (control), HECN (50, 100, or 200 mg/kg, p.o.) in the acute (a) and repeated (b) treatments, or diazepam (DZP 1 mg/kg). The parameter analyzed was the number of head dips. The results are presented as
the mean±SEM. aP\0.05 compared with control, bP\0.05
compared with HECN 100 mg/kg (ANOVA and Tukey’s post hoc multiple comparison test)
Acute treatment Repeated administration
Control HECN50
HECN100 HECN200
IMIP30 Control HECN50
HECN100 HECN200
IMIP30 0
50 100 150
a
a
a
time of immobility
0 50 100 150
a a
a
b,c
time of immobility
(A) (B)
Fig. 4 Tail suspension test of groups of mice that received vehicle only (control), HECN (50, 100, or 200 mg/kg, p.o.) in the acute (a) and repeated (b) treatments, or imipramine (Imip 30 mg/kg). The parameter analyzed was the time spent immobile. The results are
presented as the mean±SEM.aP\0.05 compared with the control,
bP
\0.05 compared with HECN 50 mg/kg,cP\0.05 compared with
Involvement of the monoaminergic system
Noradrenergic receptors. The involvement of noradrener-gic receptors in the effects of HECN was evaluated by submitting animals that had received pretreatment with prazosin (Fig.6a) or yohimbine (Fig.6b) to the forced swimming test. The results show that prazosin [F(5,63)=19.03; P\0.0001] significantly reversed the antidepressant effect of HECN (100 mg/kg). Yohimbine [F(5,66)=16.10; P\0.0001] alone decreased the time
spent immobile, and its antidepressant effect was main-tained when it was combined with HECN (100 mg/kg).
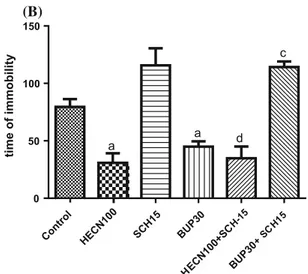
Dopaminergic receptors. The administration of SUL (Fig.7a) 30 min before the administration of HECN (100 mg/kg) completely reversed the effects of HECN (100 mg/kg) on the time spent immobile [F(5,57)=17.54; P\0.0001]. On the other hand, as presented in Fig. 7b, the anti-immobility effect of HECN (100 mg/kg) was not blocked by SCH23390 [F(5,62)=20.69; P\0.0001]. The reduction in the time spent immobile caused by the
Acute treatment
Control HECN50
HECN100
Contro l
HEC N50
HECN10 0
HECN 200mg/kg
IMIP10 IMIP10
0 50 100 150
a
a,b
a
Immobility time(s)
a
HECN200 0
20 40 60 80 100
a
a
Immobility time(s)
Repeated administration (A)
(B)
Fig. 5 Forced swimming test of groups of mice that received vehicle only (control), HECN (50, 100, or 200 mg/kg, p.o.) in the acute (a) and repeated (b) treatments, or imipramine (Imip 10 mg/kg). The parameter analyzed was the time spent immobile. The results are
presented as the mean±SEM. aP\0.05 compared with control,
bP
\0.05 compared with HECN 100 mg/kg (ANOVA and Tukey’s
post hoc multiple comparison test)
Control
HECN100 Control
HECN100 PRAZ1 IMIP10
IMIP10
PRAZ1+HECN100PRAZ1+IMIP10
0 50 100 150
a a
b c
time of immobility
YO H1
YO H1+HE
CN 100
YOH1+IMIP10
0 50 100 150
a a
a c
time of immobility
a
(A) (B)
Fig. 6 Effects of pretreatment of mice with prazosin (1 mg/kg, i.p.) (a) or YOH (1 mg/kg, i.p.) (b) on the HECN (100 mg/kg, p.o.)-induced reduction in time spent immobile in the forced swimming test. Each column represents the mean (s)±SEM. aP\0.05
compared with the control, bP\0.05 compared with HECN
100 mg/kg, cP\0.05 compared with IMIP 10 mg/kg (ANOVA
HECN treatment was reversed only by SUL, as shown in Fig.7.
Serotonergic receptors. The antidepressant effect of HECN 100 mg/kg in mice was significantly blocked by the 5-HT synthesis inhibitor PCPA [F(5,61)=15.90; P\0.0001] (Fig.8). Similarly, pretreatment with PCPA reversed the antidepressant effect of FLX.
Discussion
In the present study, the behavioral effects of the hydroalcoholic extract of C. nucifera fiber were investi-gated using classical animal models and the open-field, plus maze, hole-board, forced swimming, and tail suspen-sion tests.
The spontaneous locomotor activity test (SLA) is used to investigate the central action of a drug. When the studied animal shows a decrease in locomotor activity upon being administered a drug, it means that the drug has a depressing effect on the CNS—a motor change that may interfere with other behavioral tests conducted in the same animal. Our results showed that HECN did not affect locomotor activity in the SLA test, suggesting that other tests of the animals administered HECN would not be compromised by the animal’s locomotor activity. However, there was a decrease in rearing and grooming activities upon the repeated administration of HECN at any of the dose levels considered here. These results suggest that the effect of HECN on rearing and grooming may be altered by the duration of treatment with the extract. According to Starr and Starr [27], these behavioral changes are related to the presence of different subtypes of dopaminergic receptors; the D2 receptors are involved with the locomotor and rearing mechanisms whereas the D1 receptors are related to the grooming behavior. Thus, HECN seems to have an action time that is dependent on the dopaminergic system, including both D1 and D2 receptor subtypes.
The anxiety tests involved exposing mice to a dilemma: whether to explore a new environment or to avoid it because of a fear of potential dangers in that new Control
HECN100
Con trol
HECN100 SULP50 BUP30
SULP50+HECN100SUL50 +BUP30
0 50 100 150
a a
time of immobility
b c
SCH 15
BUP30
HECN100+SC
H-15
BUP30
+SC H1
5 0
50 100 150
a
a
c
d
time of immobility
(B) (A)
Fig. 7 Effects of pretreatment of mice with sulpiride (50 mg/kg) (a) or SCH23390 (b) on the HECN (100 mg/kg, p.o.)-induced reduction in time spent immobile in the forced swimming test.Each columnrepresents the mean (s)±SEM.aP\0.05 compared with the
control, bP\0.05 compared with HECN 100 mg/kg, cP\0.05
compared with BUP 30 mg/kg,dP\0.05 compared with SCH 15lg/
kg (ANOVA and Tukey’s post hoc multiple comparison test)
Control
HECN100 PCPA1 00
FLX 35
PCPA100+HECN100 PCPA100+FLX35
0 50 100 150
a
a
time of immobility
b
c
Fig. 8 Effect of pretreatment of mice with p-chlorophenylalanine (PCPA) on the HECN (100 mg/kg, p.o.)-induced reduction in the time spent immobile in the forced swimming test. Each column
environment [28]. Mice exposed to the plus maze presented risk assessment behavior related to the state of hypervigi-lance demonstrated by anxious individuals [29]. Our results showed that HECN (100 mg/kg) in both the acute and repeated treatments was capable of significantly increasing all of the parameters of interest (NEOA and TPOA) in the plus maze test. This effect of HECN, and that of DZP (the positive control), was reversed by pretreatment with flumazenil, suggesting that this anxiolytic action of HECN occurs via the GABAergic system.
The results of the plus maze were confirmed in the hole-board test, another test that measures the anxiolytic action of a substance. Taken together, these results suggest that the anxiolytic effect of HECN is dose-dependent (100 mg/ kg) and does not vary with duration of treatment.
The observed anxiolytic action of HECN is probably due to the high levels of flavonoids in this extract. Flavo-noids are capable of influencing CNS activity through benzodiazepine GABA-A receptor modulation [30–32]. This probably occurred in our study, since the mechanism of action of HECN also involves the GABAergic system. Another study was performed with chrysin, a flavone extract fromPassiflora coeruleaL. (Passifloraceae), where the researchers noticed that there was an increase in both the number of entries and the time spent in the open arms in the plus maze test [33], thus confirming that the flavo-noids present in several species exert an anxiolytic action. Furthermore, it has been established that apigenin, hes-peridin, and (-)-epigallocatechin gallate (a catechin
tri-mer) increase the activity of diazepam by modulating the GABAergic system, although this process is not fully understood [34]. Given that HECN is rich in condensed tannins, we believe that it exerts a similar anxiolytic action through the GABAergic system.
The present study provides evidence that HECN pro-duces an antidepressant effect in the forced swimming and tail suspension tests, since the reduction in time spent immobile that occurs upon its administration does not seem to be related to any motor effects, given that the same doses of HECN (50, 100, and 200 mg/kg) did not alter locomotor activity in the open-field test. In the forced swimming and tail suspension tests, an antidepressant effect was observed only with the 50 and 100 mg/kg doses, and this effect was similar to that produced by imipramine (IMP), a clinically used tricyclic antidepressant, with an action mechanism based on the blockade of serotonin, norepinephrine, and dopamine reuptake [25,35,36].
Interestingly, this effect was not observed with the highest dose of HECN (200 mg/kg), suggesting that the antidepressant action of HECN is dose-dependent. This effect of HECN at a dose of 100 mg/kg has been confirmed by Lima et al. [18], who also observed an antidepressant-like effect of HECN that correlated with neurotrophic
pathways. Thus, the ineffectiveness of 200 mg/kg is probably associated with an inability to elevate the con-centrations of BDNF in the hippocampus, an important brain area related to depression.
The antidepressant action of HECN may be due to the presence of several chemical compounds in the extract. Studies of compounds rich in flavonoids have shown that they are potent inhibitors of monoamine oxidase (MAO) [37]. There are two isoforms of MAO: A and MAO-B. MAO-A preferably oxidizes serotonin (5-hydrox-ytryptamine) and noradrenaline, while MAO-B preferably oxidizes phenylethylamine [38]. Catechin, epicatechin, quercetin, and resveratrol are also polyphenolic inhibitors of MAO A and B; their inhibitory activities have been defined based on their ‘‘in vitro scores’’ [39–41]. It is believed that the pathology of depression involves impairment of 5-hydroxytryptamine and noradrenaline, so selective MAO-A inhibitors are used in the treatment of depression.
In order to identify the involvement of biogenic amines in the antidepressant effect of 100 mg/kg HECN, we applied the forced swimming test to mice adminis-tered with dopaminergic and noradrenergic system antagonists as well as a serotonin release inhibitor. The chosen dose (100 mg/kg HECN) yielded the strongest effects in both the forced swimming test and the tail suspension test.
Experimental and clinical studies indicate that a decrease in the activity of the noradrenergic system is associated with the pathophysiology of depression. Some antidepressants increase synaptic levels of norepinephrine (NE) or act directly on the noradrenergic receptors [42]. To elucidate the possible mechanism for the action of HECN on noradrenergic activity, mice were pretreated with pra-zosin (PRA) (an a1-adrenergic antagonist) or yohimbine
(an a2-adrenergic antagonist). We found that PRA was
able to reverse the antidepressant effect of the extract, but the same effect was not observed with yohimbine. This result suggests that HECN may exert its effect in the forced swimming test by interacting with thea1 adrenoceptor but
not thea2 adrenoceptor. However, it is possible that HECN
did not have an effect when the mice were pretreated with yohimbine, as treatment with YOH alone yielded an antidepressant effect.
It should be noted that our results did not point to an antidepressant effect when both YOH and IMIP were used at the same time. We believe that there is a modulatory process in this case which interferes with any antidepres-sant effects, but further studies are needed to investigate this phenomenon.
system may underlie symptoms associated with depression, such as anhedonia. Neuroimaging studies in depressed patients without medication have shown that extracellular dopamine is impaired in individuals who present major depressive episodes, which is consistent with the interpre-tation that depressive patients have a functional deficiency of dopamine [44,45].
In this study, the antidepressant effects of HECN (100 mg/kg) were blocked by SUL (D2 antagonist) but not by SCH23390 (D1 antagonist), indicating that the antide-pressant effect of HECN (100 mg/kg) is probably mediated by the action of the dopamine D2 receptors. It is worth emphasizing that, in other studies, antidepressant action observed in the forced swimming test has been attributed to D2 activation, not to D1 [46].
The involvement of the 5-HT system was investigated by inhibiting 5-HT synthesis with p-chlorophenylalanine (PCPA), an inhibitor of the enzyme tryptophan hydroxy-lase. In our study, pretreating the mice with PCPA before applying the acute HECN (100 mg/kg) treatment blocked the antidepressant effect of HECN in the forced swimming test. A similar effect was observed when fluoxetine (35 mg/kg)—a serotonin reuptake inhibitor that is used clinically to treat depression—was administered. These results suggest that the serotonergic system is involved in the effect induced by HECN in the FST. Furthermore, evidence suggests that PCPA depletes stores of endogenous 5HT in mice by 60 %, and prevents the antidepressant effect of serotonin reuptake inhibitors [47–49].
The present study showed that acute or repeated-dose HECN treatment has anxiolytic and antidepressant effects. The anxiolytic effect seems to be related to the GABAergic system. On the other hand, the antidepressant effect of HECN is related to its action on noradrenergic, dopamin-ergic, and serotonergic receptors, suggesting that HECN acts similarly to tricyclic antidepressants, with low action selectivity.
Acknowledgments The authors would like to thank the National Council for Technological and Scientific Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the Ceara´ Foundation for the Support of Scientific and Technological Development (FUNCAP).
References
1. DebMandal M, Mandal S (2011) Coconut (Cocos nuciferaL.: Arecaceae): in health promotion and disease prevention. Asian Pac J Trop Med 4(3):241–247
2. Araga˜o W (2002) Coco: po´s-colheita. Se´rie frutas do Brasil. Embrapa Informac¸a˜o tecnolo´gica, Brası´lia
3. Senhoras E (2004) Agroindustrial green coconut chain opportu-nities: from green coconut nothing is lost, everything is used. Uruta´gua 5:1–10(in Portuguese)
4. Carrijo O (2002) Fiber from the bark of green coconut as an agricultural substrate. Hortic Bras 20(4):533–535 (in Portuguese)
5. Esquenazi D, Wigg M, Miranda M, Rodrigues H, Tostes J, Rozental S, da Silva A, Alviano C (2002) Antimicrobial and antiviral activities of polyphenolics from Cocos nuciferaLinn. (Palmae) husk fiber extract. Res Microbiol 153(10):647–652 6. Calzada F, Ye´pez-Mulia L, Tapia-Contreras A (2007) Effect of
Mexican medicinal plant used to treat trichomoniasis on Tri-chomonas vaginalis trophozoites. J Ethnopharmacol 113(2):248–251
7. Costa C, Bevilaqua C, Morais S, Camurc¸a-Vasconcelos A, Maciel M, Braga R, Oliveira L (2010) Anthelmintic activity of
Cocos nuciferaL. on intestinal nematodes of mice. Res Vet Sci 88(1):101–103
8. Huang Y, Nassar B, Horrobin D (1989) The prostaglandin out-flow from perfused mesenteric vasculature of rats fed different fats. Prostaglandins Leukot Essent Fatty Acids 35(2):73–79 9. Koschek P, Alviano D, Alviano C, Gattass C (2007) The husk
fiber ofCocos nuciferaL. (Palmae) is a source of anti-neoplastic activity. Braz J Med Biol Res 40(10):1339–1343
10. Rinaldi S, Silva D, Bello F, Alviano C, Alviano D, Matheus M, Fernandes P (2009) Characterization of the antinociceptive and anti-inflammatory activities from Cocos nucifera L. (Palmae). J Ethnopharmacol 122(3):541–546
11. Lima E, Sousa C, Meneses L, Ximenes N, Santos Ju´nior M, Vasconcelos G, Lima N, Patrocı´nio M, Macedo D, Vasconcelos S (2015) Cocos nucifera (L.) (Arecaceae): a phytochemical and pharmacological review. Braz J Med Biol Res 48(11):953–964 12. Mendonc¸a-Filho Rodrigues I, Alviano D, Santos A, Soares R,
Alviano C, Lopes A, Rosa Mdo S (2004) Leishmanicidal activity of polyphenolic-rich extract from husk fiber ofCocos nucifera.
Linn (Palmae). Res Microbiol 155(3):136–143
13. Silva R, Oliveira e Silva D, Fontes H, Alviano C, Fernandes P, Alviano D (2013) Anti-inflammatory, antioxidant, and antimi-crobial activities ofCocos nuciferavar typica. BMC Complement Altern Med 13:107
14. Assini F (2013) Pharmacological effects of aqueous extract of
Solidago chilensis Meyen in mice. Rev Bras Plant Med 15(1):130–134(in Portuguese)
15. Brochet D, Chermat R, DeFeudis F, Drieu K (1999) Effects of single intraperitoneal injections of an extract ofGinkgo biloba
(EGb 761) and its terpene trilactone constituents on barbital-in-duced narcosis in the mouse. Gen Pharmacol 33(3):249–256 16. Cito´ M, Silva M, Santos L, Fernandes M, Melo F, Aguiar J,
Lopes I, Sousa P, Vasconcelos S, Maceˆdo D, Sousa F (2015) Antidepressant-like effect ofHoodia gordoniiin a forced swim-ming test in mice: evidence for involvement of the monoamin-ergic system. Braz J Med Biol Res 48(1):57–64
17. Shah P, Trivedi N, Bhatt J, Hemavathi K (2006) Effect of
Withania somniferaon forced swimming test induced immobility in mice and its interaction with various drugs. Indian J Physiol Pharmacol 50(4):409–415
18. Lima E, de Sousa C, Vasconcelos G, Meneses L, E Silva Pereira Y, Ximenes N, Santos Ju´nior M, Matos N, Brito R, Miron D, Leal L, Maceˆdo D, Vasconcelos S (2016) Antidepressant, antioxidant and neurotrophic properties of the standardized extract ofCocos nuciferahusk fiber in mice. J Nat Med 70(3):510–521
19. Kaster M, Santos A, Rodrigues A (2005) Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull 67(1–2):53–61 20. Machado D, Kaster M, Binfare´ R, Dias M, Santos A, Pizzolatti
21. Archer J (1973) Tests for emotionality in rats and mice: a review. Anim Behav 21(2):205–235
22. Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacol 92(2):180–185
23. Clark G, Koster A, Person D (1971) Exploratory behavior in chronic disulfoton poisoning in mice. Psychopharmacology 20:169–171
24. Steru L, Chermat R, Thierry B, Simon P (1985) The tail sus-pension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370
25. Porsolt R, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229(2):327–336
26. Russell J, Douglas A, Brunton P (2008) Reduced hypothalamo-pituitary-adrenal axis stress responses in late pregnancy: central opioid inhibition and noradrenergic mechanisms. Ann N Y Acad Sci 1148:428–438
27. Starr B, Starr M (1986) Differential effects of dopamine D1 and D2 agonists and antagonists on velocity of movement, rearing and grooming in the mouse. Implications for the roles of D1 and D2 receptors. Neuropharmacology 25(5):455–463
28. Takeda H, Tsuji M, Matsumiya T (1998) Changes in head-dip-ping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350(1):21–29 29. Blanchard D, Griebel G, Blanchard R (2001) Mouse defensive
behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev 25(3):205–218
30. Hanrahan J, Chebib M, Johnston G (2011) Flavonoid modulation of GABA(A) receptors. Br J Pharmacol 163(2):234–245 31. Ja¨ger A, Saaby L (2011) Flavonoids and the CNS. Molecules
16(2):1471–1485
32. Nilsson J, Sterner O (2011) Modulation of GABA(A) receptors by natural products and the development of novel synthetic ligands for the benzodiazepine binding site. Curr Drug Targets 12(11):1674–1688
33. Wolfman C, Viola H, Paladini A, Dajas F, Medina J (1994) Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol Biochem Behav 47(1):1–4
34. Campbell E, Chebib M, Johnston G (2004) The dietary flavonoids apigenin and (-)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recom-binant GABA(A) receptors. Biochem Pharmacol 68(8):1631–1638
35. Willner P (1990) Animal models of depression: an overview. Pharmacol Ther 45(3):425–455
36. Ramireza K, Sheridana JF (2016) Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav Immun 57:293–303
37. Ren L, Wang F, Xu Z, Chan W, Zhao C, Xue H (2010) GABA(A) receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavone. Biochem Pharmacol 79(9):1337–1344
38. Waldmeier P (1987) Amine oxidases and their endogenous sub-strates (with special reference to monoamine oxidase and the brain). J Neural Transm Suppl 23:55–72
39. De Boer A, Gaillard P (2007) Drug targeting to the brain. Annu Rev Pharmacol Toxicol 47:323–355
40. Ne´meth K, Plumb G, Berrin J, Juge N, Jacob R, Naim H, Wil-liamson G, Swallow D, Kroon P (2003) Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr 42(1):29–42
41. Youdim K, Dobbie M, Kuhnle G, Proteggente A, Abbott N, Rice-Evans C (2003) Interaction between flavonoids and the blood– brain barrier: in vitro studies. J Neurochem 85(1):180–192 42. Maletic V, Robinson M, Oakes T, Iyengar S, Ball S, Russell J
(2007) Neurobiology of depression: an integrated view of key findings. Int J Clin Pract 61(12):2030–2040
43. Dailly E, Chenu F, Renard C, Bourin M (2004) Dopamine, depression and antidepressants. Fundam Clin Pharmacol 18(6):601–607
44. Martin-Soelch C (2009) Is depression associated with dysfunc-tion of the central reward system? Biochem Soc Trans 37(1):313–317
45. Meyer J, McNeely H, Sagrati S, Boovariwala A, Martin K, Verhoeff N, Wilson A, Houle S (2006) Elevated putamen D(2) receptor binding potential in major depression with motor retar-dation: an [11C]raclopride positron emission tomography study. Am J Psychiatry 163(9):1594–1602
46. Vaugeois J, Pouhe´ D, Zuccaro F, Costentin J (1996) Indirect dopamine agonists effects on despair test: dissociation from hyperactivity. Pharmacol Biochem Behav 54(1):235–239 47. O’Leary O, Bechtholt A, Crowley J, Hill T, Page M, Lucki I
(2007) Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology 192(3):357–371
48. Redrobe J, Bourin M, Colombel M, Baker G (1998) Dose-de-pendent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psy-chopharmacology 138(1):1–8