ContentslistsavailableatScienceDirect
Journal
of
Ethnopharmacology
j o u r n al hom ep a g e :w w w . e l s e v i e r . c o m / l o c a t e / j e t h p h a r m
Antitumor
effect
of
laticifer
proteins
of
Himatanthus
drasticus
(Mart.)
Plumel
– Apocynaceae
Kristiana
C.
Mousinho
a,
Cecília
de
C.
Oliveira
a,
José
Roberto
de
O.
Ferreira
a,
Adriana
A.
Carvalho
a,
Hemerson
Iury
F.
Magalhães
a,
Daniel
P.
Bezerra
b,
Ana
Paula
N.N.
Alves
d,
Letícia
V.
Costa-Lotufo
a,
Claúdia
Pessoa
a,
Mayara
Patrícia
V.
de
Matos
c,
Márcio
V.
Ramos
c,
Manoel
O.
Moraes
a,∗ aDepartamentodeFisiologiaeFarmacologia,FaculdadedeMedicina,UniversidadeFederaldoCeará,Fortaleza,Ceará,BrazilbDepartamentodeFisiologia,UniversidadeFederaldeSergipe,SãoCristóvão,Sergipe,Brazil
cDepartamentodeBioquímicaeBiologiaMolecular,UniversidadeFederaldoCeará,Fortaleza,Ceará,Brazil dDepartamentodeClínicaOdontológica,UniversidadeFederaldoCeará,Fortaleza,Ceará,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received22December2010 Receivedinrevisedform27April2011 Accepted28April2011
Available online 6 June 2011
Keywords:
Himatanthusdrasticus Apocynaceae Janaguba Laticiferproteins Sarcoma180
Walker256carcinosarcoma
a
b
s
t
r
a
c
t
Ethnopharmacologicalrelevance:Himatanthusdrasticus(Mart.)Plumel–Apocynaceaeisamedicinalplant popularlyknownasJanaguba.Itsbarkandlatexhavebeenusedbythepublicforcancertreatment,among othermedicinaluses.However,thereisalmostnoscientificresearchreportonitsmedicinalproperties.
Aimofthestudy:TheaimofthisstudywastoinvestigatetheantitumoreffectsofHimatanthusdrasticus
latexproteins(HdLP)inexperimentalmodels.
Materialsandmethods:TheinvitrocytotoxicactivityoftheHdLPwasdeterminedonculturedtumorcells. HdLPwasalsotestedforitsabilitytoinducelysisofmouseerythrocytes.Invivoantitumoractivitywas assessedintwoexperimentalmodels,Sarcoma180andWalker256carcinosarcoma.Additionally,its effectsontheimmunologicalsystemwerealsoinvestigated.
Results:HdLPdidnotshowanysignificantinvitrocytotoxiceffectatexperimentalexposurelevels.When intraperitoneallyadministered,HdLPwasactiveagainstbothinvivoexperimentaltumors.However, itwasinactivebyoraladministration.Thehistopathologicalanalysisindicatesthattheliverandkidney wereonlyweaklyaffectedbyHdLPtreatment.ItwasalsodemonstratedthatHdLPactsasan immunomod-ulatoryagent,increasingtheproductionofOVA-specificantibodies.Additionally,itincreasedrelative spleenweightandtheincidenceofmegakaryocytecolonies.
Conclusion:Insummary,HdLPhassomeinterestinganticanceractivitythatcouldbeassociatedwithits immunostimulatingproperties.
© 2011 Published by Elsevier Ireland Ltd.
1. Introduction
Himatanthusis a small Apocynaceae genus composed of 14
species(Plumel,1991).TheimportanceofthegenusHimatanthus
intraditionalmedicineissupportedbyreportswherepreparations
wereevaluatedformainlyanti-tumor(Bolzanietal.,1999),
anti-inflammatory(Mirandaetal.,2000),anti-ulcerous(Baggioetal.,
Abbreviations: 5-FU, 5-fluorouracil;SBCAL,SociedadeBrasileiradeCiência em AnimaisdeLaboratório; HDLP, Himatanthusdrasticuslatexprotein;MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazoliumbromide;OVA, ovabul-min;PBMC, peripheralbloodmononuclearcells; TGF-, transforming growth factor-;TNF-␣,tumornuclearfactor-␣.
∗Correspondingauthorat:DepartamentodeFisiologiaeFarmacologia,UFC,Rua Cel.NunesdeMelo,1127,60430-270Fortaleza,Ceará,Brazil.Tel.:+558533668255; fax:+558533668333.
E-mailaddress:odorico@ufc.br(M.O.Moraes).
2005),anti-spasmodic(Rattmannetal.,2005),antimicrobial(Souza
etal.,2004),andantileishmanial(Castilloetal.,2007)activities.
Himatanthus drasticus (Mart.) Plumel – Apocynaceae is a
medium-sizedtreegrowingonfirmgroundinSouthAmerica.It
isamedicinalplantpopularlyknownasjanaguba,tiboma,
jasmim-manga, raivosa, pau-de-leite, joanaguba, and sucuúba (Plumel,
1991).Itsbarkandlatexhavebeenusedbythepublicmainlyfor
cancertreatment,asananti-inflammatorymedication,andto
stim-ulatetheimmunesystem(Amaroetal.,2006).Usually,latexplus
water(janagubamilk)ordecoctionsofthebarkorlatexaretakenat
adoseofonecupaboutthreetimesaday.Althoughseveral
medici-naluseshavebeendescribed,therearealmostnoscientificresearch
reportstothisregard.
In a preliminary study of the phytochemical and biological
effects of Himatanthus drasticus, the ethanolic extract of stem
bark was evaluated biologically. This extract was found to be
toxic against brine shrimp, but showedno antimicrobialeffect
422 137 (2011) 421–426
against the pathogens tested in vitro (Enterobacter,
Streptococ-cusandEscherichiacoli).Additionally, itshowedantinociceptive
(writhing test in rats) affect (Colares et al., 2008). Leite et al.
(2009)demonstratedthelatexfromHimatanthusdrasticustobe
acytoprotectiveagentagainstethanol-inducedulcerformationin
mice.
Theaimofthisstudywastoinvestigatetheantitumoreffectsof
Himatanthusdrasticuslatexproteins(HdLP)inexperimental
mod-els.InordertoevaluatethetoxicologicalaspectsrelatedtoHdLP
treatment,histopathologicalandmorphologicalanalysesoftreated
animalswerealsoperformed.
2. Materialandmethods
2.1. Reagents
5-Fluorouracil (5-FU), O-phenylenidiamine dihydrochloride,
Ficoll-Hypaque, phytohemagglutinin, resazurin, and ovalbumin
werepurchasedfromSigmaChemicalCo.(St.Louis,MO,USA);
rab-bitantimouse totalIg(IgG,A,M) wasfromSerotec(Kidlington,
Oxford,UK).Allotherreagentswereofanalyticalgrade.
2.2. Plantmaterial,latexcollectionandextractionof Himatanthusdrasticuslatexproteins(HdLP)
Latex was obtained in May 2010 from uncultivated plants
locatedinthevicinityofFortaleza,StateofCeará,Brazil.Botanical
materialwasidentifiedattheHerbariumPriscoBezerra
(Departa-mentodeBiologia,UniversidadeFederaldoCeará).Avoucherwas
registeredunderthecode40408.
Thelatexwasobtainedaftercuttingstemandallowingittoflow
intotapwaterinordertogiveanequalmixtureofvolumes.The
sampleswereinitiallycentrifuged(5000×g)at10◦Cfor25min.
Thepelletwasdiscardedandthesolublephasewasdialyzedagainst
distilledwaterfor60hat25◦Cwithwaterbeingrenewedthree
timesdaily.Finally,thedialyzedmaterialwascentrifugedas
previ-ouslydone,andcleansupernatantwasrecovered,freezedriedand
usedinallfurtherdeterminations.Thisfraction,comprisingalmost
allsolublelatexproteinwascalledHdLP.
2.3. Animals
Atotalof56Swissmice(female,25–30g)and40Wistarrats
(female,180–220g),obtainedfromthecentral animalhouse of
UniversidadeFederaldoCeará–Brazil,wereused.Animalswere
housedincageswithfree accesstofoodandwater. Allanimals
werekeptundera12:12hlight–darkcycle(lightsonat6:00a.m.).
Animalsweretreatedaccordingtotheethicalprinciplesfor
ani-malexperimentationofSBCAL(SociedadeBrasileiradeCiênciaem
AnimaisdeLaboratório),Brazil.TheAnimalStudiesCommitteeof
UniversidadeFederaldoCearáapprovedtheexperimentalprotocol
(number08/08).
2.4. Cells
ThecytotoxicityofHdLPwastestedagainstHL-60(leukemia),
MDA-MB-435 (melanoma), SF-295 (brain), and HCT-8 (colon)
humancancercelllines,allobtainedfromtheNationalCancer
Insti-tute,Bethesda,MD,USA.CellsweregrowninRPMI-1640medium
supplemented with 10% fetal bovine serum, 2mM glutamine,
100g/mlstreptomycinand100U/mlpenicillin,andincubatedat
37◦Cwitha5%CO2atmosphere.
Inordertogethealthyhumanperipheralbloodmononuclear
cells(PBMC),heparinizedblood(fromhealthy,non-smokerdonors
whohadnottakenanydrugatleast15dayspriortosampling)was
collected,andPBMCwereisolatedbyastandardmethodof
density-gradientcentrifugationoverFicoll-Hypaque.PBMCwerewashed
andresuspended.Cellsweregrownunderthesameconditionsas
aboveplustheadditionofphytohemagglutinin(4%).
Sarcoma180tumorcellshadbeenmaintainedintheperitoneal
cavityofSwissmiceandWalker256carcinosarcomatumorcells
hadbeenmaintainedbyintramuscularinoculationofthemedial
sideofthethighofWistarratsintheLaboratoryofExperimental
OncologyfromtheUniversidadeFederaldoCearásincethe
mid-1980s.
2.5. InvitrocytotoxicevaluationofHdLP
2.5.1. DeterminationoftheeffectofHdLPonculturedtumorcells
Tumorcellgrowthwasquantifiedbytheabilityoflivingcellsto
reducetheyellowdye
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a purple formazan product
(Mossman, 1983).For allexperiments,cellswereseededin
96-wellplates(105cells/wellforadherentcellsor0.5
×105cells/well
forsuspendedcellsin100lofmedium).After24h,thetest
sub-stance(0.39–50g/ml),dissolvedinsaline,wasaddedtoeachwell
(usingtheHTS—high-throughputscreening—Biomek3000;
Beck-manCoulterInc.,Fullerton,CA,USA)andincubatedfor72h.5-FU
wasusedasthepositivecontrol.Attheendofincubation,theplates
werecentrifugedandthemediumwasreplacedbyfreshmedium
(150l)containing0.5mg/mlMTT.Threehourslater,theformazan
productwasdissolvedin150lDMSOandtheabsorbancewas
measuredusingamultiplatereader(DTX880Multimode
Detec-tor,BeckmanCoulterInc.,Fullerton,CA,USA).Thedrugeffectwas
quantifiedasthepercentageofcontrolabsorbanceofreduceddye
at595nm.
PBMCcellgrowthwasdeterminedbytheAlamar blueassay
(Ahmed et al.,1994).For all experiments,cells wereseeded in
96-wellplates(0.3×106cells/wellforsuspendedcellsin100lof
medium).After24h,thetestsubstance(0.39–50g/ml),dissolved
insaline,wasaddedtoeachwell(usingtheHTS–high-throughput
screening–Biomek3000–BeckmanCoulter,Inc.,Fullerton,CA,
USA) and incubated for 72h. 5-FU was used as the positive
control. Twenty-four hoursbefore theend of incubation, 10l
ofstock solution(0.312mg/ml) ofAlamar Blue (Resazurin) was
addedtoeachwell.Theabsorbancewasmeasuredusinga
mul-tiplatereader(DTX880MultimodeDetector,BeckmanCoulter®)
and the drug effect was quantified as the percentage of
con-trolabsorbanceat570nmand595nm.TheabsorbanceofAlamar
Blueinculturemediumismeasuredatahigherwavelengthand
lowerwavelength. Theabsorbance ofthemedium isalso
mea-sured atthe higher and lowerwavelengths. Theabsorbance of
themediumaloneissubtractedfromtheabsorbanceofmedium
plusAlamar Blueat thehigher wavelength.Thisvalueiscalled
AOHW.The absorbanceofthemediumalone issubtractedfrom
theabsorbanceofmediumplusAlamarBlueatthelower
wave-length.ThisvalueiscalledAOLW.Acorrectionfactor R0 canbe
calculated from AOHW and AOLW, where R0=AOLW/AOHW. The
percent Alamar Blue reduced is then expressed as follows: %
reduced=ALW−(AHW×R0)×100.
2.5.2. DeterminationoftheeffectofHdLPonmouseerythrocytes
Thetest wasperformedin 96-wellplates usinga 2%mouse
erythrocyte suspensionin 0.85% NaCl containing 10mm CaCl2,
following themethoddescribed byJimenez etal. (2003).HdLP
wastestedatconcentrationsrangingfrom8to200g/ml.After
incubation at room temperature for 30min and centrifugation,
the supernatant was removed and the hemoglobin released
was measured spectrophotometrically as the absorbance at
137 (2011) 421–426 423
2.6. InvivoantitumorevaluationofHdLP
2.6.1. DeterminationoftheeffectofHdLPontumorgrowthin miceandrats
2.6.1.1. Sarcoma 180 tumor protocol. Ten-day-old Sarcoma 180
ascitestumorcells(2×106cellsper500l)wereimplanted
subcu-taneouslyintothelefthindgroinofmice(asdescribedbyBezerra
etal.,2008).Onedayafterinoculation,HdLP(10or20mg/kg,by
intraperitonealadministration;50or100mg/kg,byoral
adminis-tration)wasdissolvedinsalineandadministeredfor7days.
2.6.1.2. Walker256carcinosarcomatumorprotocol.Eight-day-old
Walker256carcinosarcomatumorcells (2×106cellsper500l)
wereimplantedsubcutaneouslyintothelefthindgroinofrats(as
describedbyMoraesetal.,1997).Onedayafterinoculation,HdLP
(10or20mg/kg,byintraperitonealadministration)wasdissolved
insalineandadministereddailyfor7days.
Onday8,theanimalsweresacrificedbycervicaldislocation.The
tumors,livers,spleens,andkidneys wereexcised,weighed,and
fixedin10%formaldehyde.Percentinhibition(%)wascalculated
bythefollowingformula:percentinhibition(%)=[(A−B)/A]×100,
whereAisthetumorweightaverageofthenegativecontrol,and
Bisthatofthetreatedgroup.Bodyweightsweredeterminedat
thestartandonthelastdayoftreatment.5-FU(25mg/kg/day)was
usedasthepositivecontrol.Negativecontrolwastreatedwiththe
vehicleusedfordilutingthetestsubstance(saline).
2.6.2. Toxicologicalanalyses–histopathologyandmorphological analyses
Afterfixationwithformaldehyde,tumors,livers,spleens,and
kidneysweregrosslyexaminedforsizeorcolorchangesand
hem-orrhage. Next, portions of thetumor, liver, spleen, and kidney
werecutintosmallpieces,followedbystainingofthe
histologi-calsectionswithhematoxylinandeosin.Histologicalanalysiswas
performedbylightmicroscopy.Thepresenceandextentofliver,
kidneyorspleenlesionsattributedtodrugsweredetermined.
2.7. Immunomodulatoryanalyses
2.7.1. Subcutaneousimmunization
Two groups of eight Swiss mice were immunized
subcuta-neouslywithasingledoseofovabulmin(OVA)(2mg/kg)orOVA
(2mg/kg)plusHdLP(40mg/kg).Themicewerebledfromthe
retro-orbitalplexustoobtainserumsamplespriortoimmunizationand
at7,14and21daysafterimmunization.
2.7.2. Measurementofspecificantibody
Specificantibodiesinserumweredetectedbyenzyme-linked
immunosorbentassay(ELISA).Inordertoevaluatetheabilityof
HdLPtoincreasetheresponseelicitedbyOVA,total Ig
antibod-iesagainstOVAweredeterminedusingOVA(50g/well)-coated
plates.Theplateswereincubatedat37◦Cfor1handwashedthree
timeswithPBS-0.05%Tween.Theplateswereblockedwith5%
non-fatmilkin10mMpotassiumphosphatebuffer,pH7.2,with0.9%
NaCl(PBS)for2hat37◦Candwashedonce,afterwhich100lof
theappropriateserumdilutedinPBSwasaddedandtheplates
rein-cubatedfor2hat37◦C.Theplateswerewashedagainthreetimes
withPBS-0.05% Tween and treated withperoxidase-conjugated
rabbitantimousetotalimmunoglobulins(100l/well,1:1000final
dilution)for2hatroomtemperature.Theplatesweresubsequently
washedthreetimeswithPBS-Tween.Thereactionwasdeveloped
bytheadditionoforthophenylenediaminefollowedbyincubation
for10minat37◦C.Theintensityoftheresultingcolorwasreadat
450nmusingamultiplatereader(DTX880MultimodeDetector,
BeckmanCoulterInc.,Fullerton,CA,USA).
Table1
EffectofHimatanthusdrasticuslatexproteins(HdLP)onhumancelllines.
Cellline Histotype HdLPIC50(g/ml) 5-FUIC50(g/ml)
HL-60 Leukemia >50 12.599.72–16.29 HCT-8 Colon >50 0.36 0.18–0.71 SF295 Brain >50 0.38 0.23–0.63 MDA-MB-435 Melanoma >50 1.36 0.98–1.88 PBMC Lymphocytes >50 >5
DataarepresentedasIC50valuesand95%confidenceintervalfromthree indepen-dentexperiments,performedinduplicate.5-Fluorouracil(5-FU)wasusedasthe positivecontrol.
2.8. Statisticalanalysis
Dataarepresentedasmean±SEMorIC50valuesandtheir95% confidence intervals (CI 95%)obtainedby nonlinear regression.
Thedifferencesbetweenexperimentalgroupswerecomparedby
ANOVA(analysisofvariance)followedbyStudent–Newman–Keuls
(P<0.05).Allstatisticalanalyseswereperformedusingthe GRAPH-PADprogram(IntuitiveSoftwareforScience,SanDiego,CA,USA).
3. Results
3.1. InvitroevaluationofHdLPcytotoxicity
TheinvitroeffectsofHdLPagainsthumantumorcelllineswere determinedandshowednocytotoxicactivityatthetested con-centrations(Table1).5-FU,usedasthepositivecontrol,showed
IC50valuesrangingfrom0.36to12.59g/mlforHCT-8andHL-60,
respectively.
HdLPwasalsotestedfor itsability toinducelysis ofmouse
erythrocytes.HdLPwasnothemolyticevenatthehighest
concen-trationtested(200g/ml,datanotshown).
3.2. InvivoantitumorevaluationofHdLP
Theeffects ofHdLP onmicetransplanted withSarcoma180
tumororratstransplantedwithWalker256carcinosarcomaare
presentedinFig.1.HdLP,givenintraperitoneally,wasactiveagainst
bothexperimentaltumorsshowingaverysimilarprofile.However,
itwasinactivebyoraladministration.
InmiceinoculatedwithSarcoma180,theaveragetumorweight
of thecontrol was2.52±0.14gonday 8. Intraperitoneal HdLP
reduced tumor weightto 1.60±0.18 and 1.66±0.18g atdoses
of10and 20mg/kg/day,respectively(Fig.1A).Thesereductions
gaveinhibitionratesof36.46%and34.22%.At25mg/kg/day,5-FU
reducedtumorweightby48.02%withinthesameperiod.OralHdLP
hadnoeffectontumorweight(datanotshown).
InratsinoculatedwithWalker256carcinosarcoma,theaverage
tumorweightofthecontrolwas8.36±0.55gonday8.
Intraperi-tonealHdLPreducedtumorweightto3.52±0.94and3.74±0.95g
atdosesof10and20mg/kg/day,respectively(Fig.1B).These
reduc-tionsgaveinhibitionratesof57.91%and55.23%.At25mg/kg/day,
5-FUreducedtumorweightby56.34%withinthesameperiod.
Histopathologicalanalysisofthetumorsextirpatedfrom
Sar-coma180controlmiceorWalker256carcinosarcomacontrolrats
showedgroupsoflarge,roundandpolygonalcells,with
pleomor-phicshapes,hyperchromaticnucleiandbinucleation.Inthetumors
extirpated from treated animals,extensiveareas of coagulative
necrosiswereobserved(datanotshown).
3.3. Toxicologicalanalyses
AftertreatmentwithHdLP,nosignificantchangesintheweight
ofliversorkidneys wereseeninmiceinoculated withsarcoma
424 137 (2011) 421–426
0.0 0.5 1.0 1.5 2.0 2.5 3.0
0 10 20 30 40 50 60
a
a a
A
Saline 5-FU 10 20
HdLP
(mg/kg/day)
Tumor weight (g)
Inhibition (%)
0 2 4 6 8 10
0 20 40 60 80
Inhibition
a a a
Tumor weight
B
Saline 5-FU 10 20
HdLP
(mg/kg/day)
Tumor weight (g)
Inhibition (%)
Fig.1.EffectofHimatanthusdrasticuslatexproteins(HdLP)onmicetransplanted withsarcoma180tumor(A)andratstransplantedwithWalker256carcinosarcoma tumor(B).5-Fluorouracil(5-FU,25mg/kg/day)wasusedasthepositivecontrol. Negativecontrolwastreatedwiththevehicleusedfordilutingthetestedsubstance (saline).Theanimalsweretreatedbyintraperitonealadministration,startingone dayaftertumorimplantation,forsevenconsecutivedays.Dataarepresentedas mean±S.E.M.ofeightanimals.aP<0.05comparedwithsalinegroupbyANOVA followedbyStudent–Newman–Keuls.
However,spleenweightsweresignificantlyincreasedwhen
com-paredtothecontrolgroup,inbothexperimentaltumors(P<0.05).
Bodyweightgainwassignificantlyreducedaftertreatmentwith
HdLP(20mg/kg/day)or5-FU(25mg/kg/day)inmiceinoculated
withsarcoma180.
Histopathologicalanalysesofkidneysremovedfrom
animals-treatedwithHdLPshowedintenseswellingoftubularepithelial
cells,presenceofhyalinecylinderandfocaltubularnecrosis,but
thestructureoftheglomeruliwasessentiallypreserved(datanot
shown).Intheliver,histopathologicalanalysisshowedthat
ani-malstreatedwithHdLPhadnumerousinflammatoryfoci,intense
ballooningdegenerationofhepatocytes,microvesicularsteatosis,
andnecrosis(datanotshown).Inthespleen,HdLP-treated
ani-malsshowedadiscreetincreaseinmegakaryocytecolonies,which
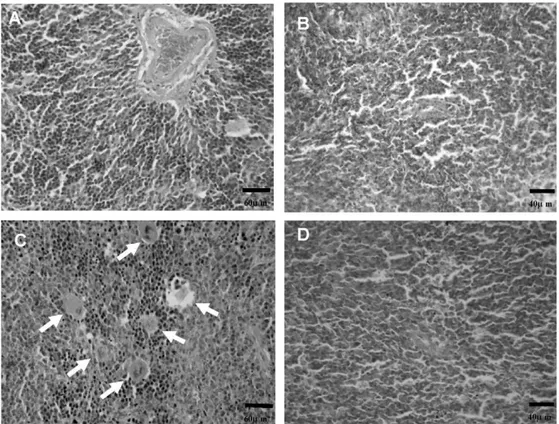
suggestsanimmunomodulatoryactivity(Fig.2).
3.4. Immunomodulatoryanalyses
ToinvestigatetheeffectofHdLPontheinductionofhumoral
immuneresponseinmiceimmunizedwithasingledoseofOVA
(2mg/kg)orOVA(2mg/kg)plusHdLP(40mg/kg),theOVA-specific
antibodylevelsin serumweremeasuredpriortoimmunization
andat7,14and21daysafter,atadilutionof1:3200byELISA.The
resultsareshowninFig.3.HdLPsignificantlyincreasedtheamount
ofOVA-specifictotalIgintheseraoftreatedanimalsatthedoseof
40mg/kgcomparedtotheOVAcontrol(P<0.05).
4. Discussion
The present work reports the antitumor effects of HdLP on
micetransplantedwithSarcoma180andratstransplantedwith
Walker256carcinosarcoma.Thesemodelsareanimal-originated
tumorsfrequentlyusedininvivocancertherapyresearch(Moraes
etal.,1997;Leeetal.,2003;Bezerraetal.,2008;Linsetal.,2009;
Chenetal.,2010).HdLPinhibitedthegrowthofbothexperimental
tumors,withnoeffectoncellproliferationinvitroatthe
concen-trationstested.Additionally,itsimmunoadjuvantactivitywasalso
demonstrated.Thisisthefirstreportdescribingtheantitumorand
immunomodulatorypropertiesofHdLP.
HdLPdidnotshowanysignificantinvitrocytotoxiceffectatthe
experimentalexposurelevels,butitdidshowaninvivoantitumor
effect.Inthepreclinicalanticancerdrug-screeningprogramusedin
thisstudy,anextractthatdisplayedIC50valuesbelow30g/mlwas
consideredpromising(SuffnessandPezzuto,1990).HdLPshowed
IC50values greaterthan50g/mlforalltumorcelllinestested,
suggestingthattheinvivoantitumoractivitywasnotrelatedto
directantiproliferativeeffects.
As previously cited, the ethanolic extract of stem bark of
Himatanthus drasticus was active in the brine shrimp lethality
test.Itsextractshowedahighlysignificantactivityinthisassay
(LC50=257ppm).Theauthorsattributedtheseeffectstothe
triter-penelupeolcinammate(Colaresetal.,2008).Ontheotherhand,the
effectofitslatexproteinshasneverbeenevaluatedagainsttumor
cellslines.
InthegenusHimatanthus,thelatexofHimatanthussucuubais
usedinitsrawformasanantitumoragent(VanderBerg,1984).
Chemicalstudieshave indicatedthepresence oflupeolacetate,
Table2
EffectofHimatanthusdrasticuslatexproteins(HdLP)onorganweights.
Drug Dose(mg/kg/day) Liver(g/100gbodyweight) Kidney(g/100gbodyweight) Spleen(g/100gbodyweight)
Healthymice
Saline – 4.61±0.19 1.07±0.03 0.26±0.02
MicetransplantedwithS180
Saline – 4.66±0.06 1.08±0.02 0,73±0.02
5-FU 25 4.38±0.09 1.08±0.04 0.35±0.05a
HdLP 10 4.88±0.14 1.17±0.03 1.09±0.06a
HdLP 20 4.97±0.13 1.19±0.04 1.10±0.05a
Healthyrats
Saline – 4.54±0.11 1.01±0.06 0.50±0.01
RatstransplantedwithWalker256
Saline – 4.83±0.11 0.89±0.02 0.43±0.01
5-FU 25 4.95±0.06 1.04±0.04 0.34±0.03a
HdLP 10 4.84±0.30 0.88±0.06 0.50±0.06
HdLP 20 5.24±0.20 0.94±0.17 0.65±0.04a
Dataarepresentedasmean±SEMofeightanimals.5-Fluorouracil(5-FU)wasusedasthepositivecontrol.Negativecontrolwastreatedwiththevehicleusedfordiluting thetestedsubstance(saline).Theanimalsweretreatedbyintraperitonealadministration,startingonedayaftertumorimplantation,forsevenconsecutivedays.
137 (2011) 421–426 425
Fig.2. EffectofHimatanthusdrasticuslatexproteins(HdLP)onspleensofmicetransplantedwithsarcoma180tumor.Theanimalsweretreatedbyintraperitoneal admin-istration,startingonedayaftertumorimplantation,forsevenconsecutivedays.Photomicrographsshowthehistopathologyofthespleensfromsaline-treated(panelA), 5-FU-treated(25mg/kg/day,panelB),HdLP-treated(10mg/kg/day,panelC),andHdLP-treated(20mg/kg/day,panelD)animals,analyzedbylightmicroscopy(400×).White arrowshowsmegakaryocyte.
alpha-amyrin,andlupeolcinnamates(Mirandaetal.,2000).The
ethanolicextractofleavesofHimatanthusobovatusshowedstrong
cytotoxicactivityincancercelllines(Mesquitaetal.,2009).
Addi-tionally,theethanolicextractofleavesofHimatanthusattenuatus
showedsignificanttoxicityagainstArtemiasalina(Jiménezetal.,
2001).InthefamilyApocynaceae,thelaticiferproteinsofCalotropis
procera(Ait)wasshowntoexhibitselectivecytotoxiceffects on
humancancercelllines.Itwasshown toinhibitDNAsynthesis,
probablyaffectingtopoisomeraseIactivity,leadingtoapoptosisin
tumorcells(Oliveiraetal.,2007).Recently,itsinvivoanticancer
potentialwasalsoevaluated,reinforcingthepotentialoflaticifer
proteinsintreatingneoplasia(Oliveiraetal.,2010).
Furthermore,HdLPactsasanimmunomodulatoryagent,
rais-ingtheproductionofOVA-specificantibodies.Additionally,itwas
demonstrated that HdLP increased relative spleen weight, and
increasedmegakaryocyticnests,corroboratingthehypothesisthat
itactsthroughimmunestimulation.Thus,thesedatasuggestthat
themechanismofbeneficialtherapeuticeffectselicitedbyHdLP
seemedtobeattributedtothepotentiationofhost-defensethrough
theenhancementofimmunity,inagreementwithitstraditional
use.
Interestingly, the immunostimulant potential of the genus
Himatanthushasbeenevaluated.ThelatexofHimatanthus
sucu-ubaincreasednitricoxide(NO)andtumornuclearfactor-␣(TNF-␣)
anddecreasedtransforminggrowthfactor-(TGF-)productionin
macrophages,suggestinganimmunomodulatoryactivity(Soares
etal.,2010).InthefamilyApocynaceae,experimentalstudieswith
proteinsof thelatex of C. procerasuggested that theactive LP
fraction(PII)displaysinvivoinhibitionoftumorgrowth,
proba-blybymediatinganincreasedimmunopharmacologicalresponse
(Oliveiraetal.,2010).
Hepatotoxicityandnephrotoxicityareverycommonsideeffects
ofcancerchemotherapeuticdrugs.Hepaticdysfunctioninducedby
vincristineandetoposide(KingandPerry,2001)andrenal
toxic-ityinducedbycisplatin(Pinzanietal.,1994)aresuchexamples.
Inthepresentstudy,theintegrityofliverandkidneysinanimals
subjectedtoHdLPtreatmentwereevaluatedbyhistopathological
analyses.
TheliverofHdLP-treatedanimalsshowednumerous
inflam-matory foci, intense ballooning degeneration of hepatocytes,
microvesicularsteatosis,andnecrosis,suggestingintrinsic
hepato-toxicity.However,regenerationofhepatictissuesoccursinmany
diseases.Thisoccursevenwhenhepatocellularnecrosisispresent.
Preimmune 7 14 21
0.0 0.4 0.8 1.2 1.6
Days after immunization
*
*
*
Absorbance
426 137 (2011) 421–426
Inhepaticdegeneration,regenerationiscompletewhenthe
con-nectivetissueispreserved(ScheuerandLefkowitch,2000;Kummar
etal.,2004).ThehepaticalterationsobservedinHdLP-treated
ani-malscouldbeconsideredreversible(McGeeetal.,1992;Scheuer
andLefkowitch,2000;Kummaretal.,2004).
Thekidneysremovedfromanimals-treatedwithHdLPshowed
intense swelling of tubular epithelium cell, presence of
hya-linecylinderandfocaltubularnecrosis,butthestructureofthe
glomeruliwasessentiallypreserved.Necrosisoftherenaltubule
epitheliummayoftenoccurasaconsequenceofthe
administra-tionofvariouscancerchemotherapeuticdrugs(OlsenandSolez,
1994).Itisworthmentioningthatthehistopathologicalanalyses
ofHdLP-treatedanimalsdemonstratedthattheinterstitialtissues
werepreserved,suggestingthatregenerationispossible(Curran,
1990;OlsenandSolez,1994).
Insummary,thehistopathologicalanalysisindicatesthatthe
liverandkidneywereonlyweaklyaffectedbyHdLPtreatment.
Anyway,the HdLP-inducedsystemic toxicity effectneedstobe
moreextensivelyevaluatedtoassessthesafety.Then,further
stud-iesmustbedonetowell-definedandunderstandtheunderline
mechanisminvolvedinHdLPtoxiceffect.
5. Conclusion
Briefly,thisworkshowedthatHdLPexhibitedantitumoreffects
againstexperimentaltumorswithoutsubstantialtoxicity.In
addi-tion, this activity seems to be related to its immunostimulant
properties.
Acknowledgments
TheauthorsaregratefultotheBrazilianagenciesFINEP,CNPq,
BNB/FUNDECI,PRONEX,andCAPESforfellowshipsandfinancial
support.SilvanaFranc¸a dos Santosprovidedexcellent technical
assistanceand Dr. A. Leyvahelped withEnglish editing of the
manuscript.
References
Ahmed,S.A.,Gogal,R.M.,Walsh,J.E.,1994.Anewrapidandsimplenon-radioactive assaytomonitoranddeterminetheproliferationoflymphocytes:analternative to[3H]thymidineincorporationassay.JournalofImmunologicalMethods170, 211–224.
Amaro,M.S.,Filho,S.M.,Guimarães,R.M.,Teófilo,E.M.,2006.Morfologiadefrutos, sementesedeplântulasdejanaguba(Himatanthusdrasticus(Mart.)Plumel– Apocynaceae).RevistaBrasileiradeSementes28,63–71.
Baggio,C.H.,Otofuji, G.M.,Souza, W.M.,Santos,C.A.M.,Torres, L.M.,Rieck,L., Marques,M.C.A.,Mesia-Vela,S.,2005.Gastroprotectivemechanismsofindole alkaloidsfromHimatanthuslancifolius.PlantaMedica71,733–738.
Bezerra,D.P.,Castro,F.O.,Alves,A.P.,Pessoa,C.,Moraes,M.O.,Silveira,E.R.,Lima, M.A.,Elmiro,F.J.,Alencar,N.M.,Mesquita,R.O.,Lima,M.W.,Costa-Lotufo,L.V., 2008.Invitroandinvivoantitumoreffectof5-FUcombinedwithpiplartineand piperine.JournalofAppliedToxicology28,156–163.
Bolzani,V.S.,Young,M.C.,Furlan,M.,Cavalheiro,A.J.,Araújo,A.R.,Silva,D.H.,Lopes, M.N.,1999.Searchforantifungalandanticancercompoundsfromnativeplant speciesofCerradoandAtlanticforest.AnaisdaAcademiaBrasileiradeCiências 71,181–187.
Castillo,D.,Arevalo,J.,Herrera,F.,Ruiz,C.,Rojas,R.,Rengifo,E.,Vaisberg,A.,Lock, O.,Lemesre,J.L.,Gornitzka,H.,Sauvain,M.,2007.Spirolactoneiridoidsmight beresponsiblefortheantileishmanialactivityofaPeruviantraditionalremedy madewithHimatanthussucuuba(Apocynaceae).JournalofEthnopharmacology 112,410–414.
Chen,X.,Zhang,L.,Cheung,P.C.,2010.Immunopotentiationandanti-tumoractivity ofcarboxymethylated-sulfatedbeta-(1→3)-d-glucanfromPoriacocos. Interna-tionalImmunopharmacology10,398–405.
Colares,A.V.,Cordeiro,L.N.,Costa,J.G.M.,Silveira,E.R.,Campos,A.R.,Cardoso,A.H., 2008.PhytochemicalandbiologicalpreliminarystudyofHimatanthusdrasticus (Mart.)Plumel(Janaguba).PhcognosyMagazine4,73–77.
Curran, R.C., 1990. Color Atlas of Histopathology. Oxford University Press, NewYork.
Jiménez,G.,Hasegawa,M., Rodríguez,M.,Estrada, O.,Méndez,J.,Castillo, A., Gonzalez-Mujica, F., Motta, N., Vásquez, J., Romero-Vecchione, E., 2001. BiologicalscreeningofplantsoftheVenezuelanAmazons.Journalof Ethnophar-macology77,77–83.
Jimenez,P.C.,Fortier,S.C.,Lotufo,T.M.C.,Pessoa,C.,Moraes,M.E.A.,Moraes,M.O., Costa-Lotufo,L.V.,2003.Biologicalactivityinextractsofascidians(Tunicata, Ascidiacea)fromthenortheastern Braziliancoast.JournalofExperimental MarineBiologyandEcology287,93–101.
King, P.D., Perry, M.C., 2001. Hepatotoxicity of chemotherapy. Oncologist 6, 162–176.
Kummar,V.,Abbas,A.,Fausto,N.,2004.RobbinsandCotranPathologicBasisof Disease.W.B.Saunders,China.
Lee,Y.L.,Kim,H.J.,Lee,M.S.,Kim,J.M.,Han,J.S.,Hong,E.K.,Kwon,M.S.,Lee,M.J.,2003. OraladministrationofAgaricusblazei(H1strain)inhibitedtumorgrowthina Sarcoma180inoculationmodel.ExperimentalAnimal52,371–375.
Leite,G.D.,Penha,A.S.,daSilva,G.Q.,Colares,A.V.,Rodrigues,F.G.,Costa,J.G., Car-doso,A.L.,Campos,A.R.,2009.Gastroprotectiveeffectofmedicinalplantsfrom ChapadadoAraripe,Brazil.JournalofYoungPharmacists1,54–56.
Lins,K.O.,Bezerra,D.P.,Alves,A.P.,Alencar,N.M.,Lima,M.W.,Torres,V.M.,Farias, W.R.,Pessoa,C.,deMoraes,M.O.,Costa-Lotufo,L.V.,2009.Antitumorproperties ofasulfatedpolysaccharidefromtheredseaweedChampiafeldmannii (Diaz-Pifferer).JournalofAppliedToxicology29,20–26.
McGee,J.O.D.,Isaacson,P.A.,Wright,N.A.,1992.OxfordTextbookofPathology: PathologyofSystems.OxfordUniversityPress,NewYork.
Mesquita,M.L.,dePaula,J.E.,Pessoa,C.,deMoraes,M.O.,Costa-Lotufo,L.V.,Grougnet, R.,Michel,S.,Tillequin,F.,Espindola,L.S.,2009.CytotoxicactivityofBrazilian Cerradoplantsusedintraditionalmedicineagainstcancercelllines.Journalof Ethnopharmacology123,439–445.
Miranda,A.L.,Silva,J.R., Rezende,C.M.,Neves,J.S.,Parrini, S.C.,Pinheiro,M.L., Cordeiro,M.C.,Tamborini,E.,Pinto,A.C.,2000.Anti-inflammatoryandanalgesic activitiesofthelatexcontainingtriterpenesfromHimatanthussucuuba.Planta Medica66,284–286.
Moraes,M.O.,Fonteles,M.C.,Moraes,M.E.A.,Machado,I.I.,Matos,F.J.A.,1997. Screen-ingforanticanceractivityofplantsfromtheNortheastofBrazil.Fitoterapia68, 235–239.
Mossman,T., 1983.Rapidcolorimetric assayforcellular growthandsurvival: applicationtoproliferationandcytotoxicityassays.JournalofImmunological Methods65,55–63.
Oliveira, J.S., Costa-Lotufo, L.V., Bezerra, D.P., Alencar, N.M., Marinho-Filho, J.D., Figueiredo, I.S., Moraes, M.O., Pessoa, C., Alves, A.P., Ramos, M.V., 2010. In vivo growth inhibition of sarcoma 180 by latex proteins from Calotropis procera. Naunyn-Schmiedeberg’s Archives of Pharmacology 382, 139–149.
Oliveira,J.S.,Pereira,D.B.,Freitas,C.D.T.,Marinho-Filho,J.D.B.,Moraes,M.O.,Pessoa, C.,Costa-Lotufo,L.V.,Ramos,M.V.,2007.Invitrocytotoxicityagainstdifferent humancancercelllinesoflaticiferproteinsofCalotropisprocera(Ait.)R.Br. ToxicologyInVitro21,1563–1573.
Olsen,S.,Solez,K.,1994.Acutetubularnecrosisandtoxicrenalinjury.In:Tisher,C.C., Brenner,B.M.(Eds.),RenalPathology:WithClinicalandFunctionalCorrelations. JBLippincottCompany,Philadelphia,pp.769–809.
Pinzani,V.,Bressolle,F.,Haug,I.J.,Galtier,M.,Blayac,J.P.,Balmès,P.,1994. Cisplatin-inducedrenaltoxicityandtoxicity-modulatingstrategies:areview.Cancer ChemotherapyandPharmacology35,1–9.
Plumel,M.M.,1991.LegenreHimatanthus(Apocinaceae).Revisióntaxonomique: bradea.BoletimdoHerbariumBradeanum5,1–20.
Rattmann,Y.D.,Terluk,M.R.,Souza,W.M.,Santos,C.A.,Biavatti,M.W.,Torres,L.B., Mesia-Vela,S.,Rieck,L.,daSilva-Santos,J.E.,Marques,M.C.,2005.Effectsof alka-loidsofHimatanthuslancifolius(Muell.Arg.)Woodson,Apocynaceae,onsmooth muscleresponsiveness.JournalofEthnopharmacology100,268–275. Scheuer,P.J.,Lefkowitch,J.H.,2000.Drugsandtoxins.In:Scheuer,PJ,Lefkowitch,JH
(Eds.),LiverBiopsyInterpretation.W.B.Saunders,London,pp.134–150. Soares,D.C.,Andrade,A.L.,Delorenzi,J.C.,Silva,J.R.,Freire-de-Lima,L.,Falcão,C.A.,
Pinto,A.C.,Rossi-Bergmann,B.,Saraiva,E.M.,2010.Leishmanicidalactivityof HimatanthussucuubalatexagainstLeishmaniaamazonensis.Parasitology Inter-national59,173–177.
Souza,W.M.,Stinghen,A.E.,Santos,C.A.,2004.Antimicrobialactivityofalkaloidal fractionfrombarksofHimatanthuslancifolius.Fitoterapia75,750–753. Suffness,M., Pezzuto,J.M., 1990.Assays related tocancer drugdiscovery. In:
Hostettmann,K.(Ed.),MethodsinPlantBiochemistry:AssaysforBioactivity. AcademicPress,London,pp.71–133.