w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
article
In
vitro
photoprotective
effects
of
Marcetia
taxifolia
ethanolic
extract
and
its
potential
for
sunscreen
formulations
Sônia
C.C.
Costa,
Cassia
B.
Detoni,
Carla
R.C.
Branco,
Mariana
B.
Botura,
Alexsandro
Branco
∗DepartamentodeSaúde,UniversidadeEstadualdeFeiradeSantana,FeiradeSantana,BA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received31May2015
Accepted24July2015
Availableonline17August2015
Keywords:
Marcetiataxifolia
Flavonoid Photoprotective
Sunscreenformulation
a
b
s
t
r
a
c
t
ThespeciesMarcetiataxifolia(A.St.-Hil.)DC.,Melastomataceae,whichisendemicoftherupestrianfields ofnortheasternBrazil,containsasignificantamountofflavonoids.Inthiswork,thepotentialofthe ethanolicextractofM.taxifoliaastheactiveprincipleinasunscreenphotoprotection(UV-AandUV-B) formulationwasinvestigated.TheLiquidChromatographyHighPerformance-DiodeArrayDetector quan-tification(quercetin),totalflavonoidcontent,antioxidantactivitythrough2.2-diphenyl-1-picrylhydrazil method,photoprotectiveactivityagainstUV-BandUV-Aradiationinvitro(spectrophotometricmethod) andpotentialforeyeirritationusingthemethodologyoftheheneggtest-chorioallantoicmembranewere performedintheextract.Afterthat,theformulationswerepreparedusingdifferentconcentrationsof activeethanolicextract(5,10,20and30%)andtheevaluationofthesunprotectionfactorwascarriedout usingthesamemethodologyusedforthecrudeextract.ThecrudeextractshowedUV-Aphotoprotection andloweyeirritationintheheneggtest-chorioallantoicmembranetest.AllformulationscontainingM. taxifoliaextracthad≥6sunprotectionfactor.Itsshowsthepossibilitytousethisextractsasasunscreen inpharmaceuticalpreparations.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Theultraviolet radiation(UVR) isdivided intothree distinct
regions: ultraviolet A (UV-A, 315-400nm), ultraviolet B (UV-B,
280-315nm)andultravioletC(UV-C,100-280nm)(Poloninietal.,
2011).Reachingtheskin,withcumulativeaction,UVRcausesa
complexprocessassociatedwithmorphologicalandchemical
reac-tions.DNAisanimportantmacromoleculethatabsorbsUVRand
hencecanmutate,whatinthefuturecanresultinmalignant trans-formationofthecellskincancer(Baloghetal.,2011).Theeffective protectionagainstUVRisavailableaspreparationsfortopicaluse containingsolarfilters,knownassunscreens.Theefficacyofsuch productsisdependentontheircapacitytoabsorbradiantenergy. Theeffectivenessofasunscreenismeasuredasafunctionoftheir sunprotectionfactor(SPF).Thus,thenecessitytoprovidehighSPF andscreeningefficiencyagainstbothultravioletAandultraviolet Bwavelengthsisevident(Vilelaetal.,2011).
Flavonoids compounds are widely distributed in the plant
kingdom and possess a biological action, especially
antimicro-bial, antioxidant and photoprotective activities (Pietta, 2000;
∗ Correspondingauthor.
E-mail:branco@uefs.br(A.Branco).
Lacombeetal.,2010).Thedemandforactiveflavovoid-richextracts
hasbecomean important componentfor the discoveryof new
moleculesactivetohumanphotoprotection.Thatisduetoits struc-turalsimilaritytochemicalfilterswhichmakesitsusceptibleto radiationabsorptionintheultravioletregion(Agatietal.,2013). Plantextractsrichinflavonoidsarecapableofabsorbing ultravio-letlight,usuallytwomaximumpeaksofultravioletabsorptionin theUV-BandUV-Aregions,whatresultsinthepossibilityforthe useoftheseextractsinthedevelopmentofsunscreenformulations (Bobinetal.,1995).
TheMelastomataceaefamilycontainsaround170generaand
4,600speciesdistributedintropicalandsubtropicalareasofthe world(Reisetal.,2005).TheyareespeciallyabundantinBrazil, occurringinforests,savannahsandrupestrianfields.Several popu-lationsoftheMarcetiagenusarefoundinrockyfieldsintheMinas Gerais(SerradoEspinhac¸o)andBahia(ChapadaDiamantina)states. Thesefieldsareasetofelevationsaround1,200mwithhigh
irra-diation,highwinds,droughtandarockysoilquartznaturewith
small depthand low fertility (Giuliettiet al., 1987).The genus Marcetiacurrentlycomprehends29species,withapproximately 90%inhabitingBahia(Brazil).ThespeciesMarcetiataxifolia(A.St.
Hil.)DC.is representedbyshrubandsubshrubbetween15and
300cminheight,smallleaves,oppositeandsessileorshortly peti-ole(Martins,1989).
http://dx.doi.org/10.1016/j.bjp.2015.07.013
Thepreviousinvestigationmadeinourlaboratoryinthe Marce-tiataxifoliashowtheantimicrobialactivityandthepresence of flavonoidsusingHPLC-DADanalysis(Leiteetal.,2012).Inthiswork thepotentialofphotoprotectiveactivityoftheethanolicextract fromthesameplantinasunscreenformulationisinvestigated.
MaterialsAndMethods
Plantmaterial
Marcetiataxifolia (A.St.-Hil.) DC., Melastomataceae, was
col-lected in Rio-de-Contas (Chapada Diamantina, state of Bahia,
Brazil).VoucherspecimensweredepositedintheHerbariumofthe DepartmentofBiologyoftheStateUniversityofFeiradeSantana
(HUEFS)withthefollowingnumber:191053.
Preparationofplantextracts
Thedried powderedleavesofM.taxifolia(1500g)were
sub-jectedtothoroughmacerationin95%ethanol.Theextractionswere carriedoutatintervalsof72h.Theethanolicextractwasfiltered throughtavacuumfilterandthefiltratewasdefattedusinghexane. Theethanolicportionsweredriedandusedforthestudies.
HPLC-DADquantification
APurospher100RP-18(250mm×4.6mmi.d.,5m)column
(Merck)wasused.Themobilephasewascomposedbysolvent(A)
H2O/H3PO40.1%andsolvent(B)MeOH.Thesolventgradientwas
programmedasA(75-0%)andB(25-100%)for20min,then100%B
for4min,then75%Aand25%Bfor10min.Aflowrateof1.0l/min wasusedina30◦Coven,and20lofeachsamplewasinjected.
Theeluatewasmonitoredatadetectionwavelengthof360nm.
Precisely,weighedsampleweredissolvedinmethanol(10mg/l).
Themethodwasvalidatedbyanexternalcalibrationcurveusing
standardsolutionsofquercetin,preparedinmethanolinfive dif-ferentconcentrations,rangingfrom0.1to1.5mg/l.Thequercetin
standardsolutionswereinjectedthreetimes,andthecurvewas
constructedonMicrosoftOfficeExcel© usingtheaverageofthe
area.Theaccuracywasexpressedastheagreementbetweenthe
experimentallymeasuredvalueandthesetreferencevalue.The
precisionandaccuracywerecalculatedaccordingtotheformula,
respectively:RSD(%)=(SD×100)/C,whereRSD(%)isthe preci-sion,SDisthestandarddeviationandCisthemeanconcentrations calculated;Accuracy(%)=(Cexp×100)/TC,whereCexpisthetotal concentrationofquercetinfromtheextractandTCisthetheoretical concentrationofthestandardreference.Thedetectionlimit(LOD) andquantificationlimit(LOQ)wereestimatedbytheslopeandthe
meanstandarddeviationoftheconcentrationsusedtoconstruct
theanalyticalcurve.
Totalflavonoidcontent(TFC)
TheTFCwasdeterminedbyusingacolorimetricmethodas
pre-viouslydescribedwithafewmodifications(Gursoy,2009).Thedata wereexpressedasgofquercetinequivalents(Que)per10mgof ethanolicextractweight.
DPPHFreeradicalscavengingassay
Thefreeradicalscavengingactivitywasmeasuredusingthe
2.2-diphenyl-1-picrylhydrazil(DPPH)assay(Mensoretal.,2001).
Theabsorbancevaluesweremeasuredat518nmandconverted
into the percent antioxidant activity (AA) using the following
formula: AA%=[(absorbance of the control−absorbance of the
sample)/absorbanceof the control]×100. TheIC50 values were
calculatedfromalinearregressionofthedatausingtheGraphPad Prism®5.0program.
Sunprotectionfactor(SPF)ofthecrudeextract
Theethanolicextractwasdissolvedinethanoltoafinal concen-trationof12.5,25,50,125and250g/l.TheSPFmodelusedin
thisstudywasaccordingtothemethodologydescribedbyMansur
et al. (1986). The sample absorbanceswere measuredin UV-B
wavelengthrange(290-320nm),with5-nmincrementsandthree
determinationsweremadeateachpoint.TheSPFwascalculatedby applyingtheMansurequation:SPF=CFx290
320290EE()xI() xabs(),where:CF(correctionfactor)=10;EE()isthe erythe-malefficiencyspectrum;I()isthesolarintensityspectrum;abs ()istheabsorbanceofthesolution.ThevaluesofEE()xI()are constantaccordingSayreetal.(1979).UV-Ablockingactivityofthecrudeextract
A1mg/mlhydroalcoholicsolutionoftrans-resveratrolwas pre-pared.Petridishesof4.5cmdiameterwerefilledwith5lofthis solution.EachPetridishwascoveredwitha0.04-gevenlyspread layerofdryethanolicextractofM.taxifoliatobetestedfortheirUV blockingactivity.Petridisheswithcleanlidswereusedascontrol.
ThepetridisheswereplacedinsidetheUV-Achamberandwere
irradiatedwitharadiationintensityofabout830W/m2(between 320and400nm)foraperiodoftime(0-120min).Inpredetermined
intervals (20min) a sample was collected and diluted at 1:10.
Theabsorbancewasmeasuredspectrophotometrically(306nm)
usingaUV-VISVarian®
(Cary100BIO)(Kerrileeetal.,2009).The absorbanceofasampletotallyprotectedfromlightwasalso
mea-suredinpredeterminedintervalstoassurethatthedegradation
wasinducedbyUV-Alight;transisomer,whenexposedtolight,
becomesthecismakingconstantabsorbance(Kerrileeetal.,2009). Thisexperimentwasperformedintriplicate.
Invitroeyeirritationtests(HET-CAM)
Theirritatingpotentialofthecrudeextractwasperformedusing
HET-CAM(hen’seggtest-chorioallantoicmembrane)test
accord-ingtoICCVAM(ICCVAM,2010).The assayemploystheCAM of
a 10-day-oldfertilizedhen’s egg.TheCAM,a membrane which
surrounds the developing chick embryo, is highly vascularized
(Bagleyetal.,1991),andisregardedasbeinginsensitivetopain (Dannhardtetal.,1996).Forthistest,theethanolicextractofM taxifoliawasdissolvedindistilledwateratconcentrationsof250, 125,62.5g/l.Thefertilizedchicken eggswereobtainedfrom commercialsources.Onthe10thdayofincubation,theeggshell
wasremovedaroundthechamberair,showingtheshell
mem-brane.AfterCAMwasexposed,300loftheextractswasapplied.
Thepositivecontrols(NaOH 0.1M),andanegativecontrol
(dis-tilledwater)wereperformedtodemonstratethevalidityofthe
test.Foreachconcentrationandcontrols,threeeggswereused.
Aftertheapplicationoftheextracts,themembraneandblood ves-selswereexaminedfor5min.Thetimeofappearance,measuredin seconds,ofeachirritanteffect(haemorrhage,lysisandcoagulation)
wasrecorded.
Thefollowingformulaisusedtogenerateanirritationscore(IS):
IS=(301−H)×5
(300) +
(301−L)×7
300 +
(301−C)×9 300
WhereH=thetime takentostart thehemorrhagereactions;
Table1
PolowaxlotioncompositionfortheincorporationofMarcetiaextract.
Phase Rawmaterial Amount Properties
1 Methylparaben 0,15% Preservative
Glycerine 5% Moist
Deionizedwater q.s.p.100g Carrier
2 Propylparaben 0,05% Preservative
LiquidVaseline 3% Lubricant
Octylstearate 4% Emollient
Polowax 5% BasisandAuto-Emulsifier
3 Imidazolidinylurea 0,1% Preservative
Table2
Compositionofthesunscreenformulation.
Phase Rawmaterial Amount Properties
1 Methylparaben 0,15% Preservative
Glycerine 5% Moist
Deionizedwater q.s.p.100g Carrier
2 Propylparaben 0,05% Preservative
Vaselineliquid 3% Lubricant
Octylstearate 4% Emollient
Polowax 5% BasisAutoEmulsifier
Benzophenone-3 3% SunscreenUVA/UVB
3 Imidazolidinylurea 0,1% Preservative
The average score was calculated for each extract, and the extractswereclassifiedintofourcategories:non-irritant(IS>1), lowirritant(1≤IS<5),Moderateirritant(5≤IS<9)andirritant(IS
≥9)(Debbaschetal.,2005).
Preparationofsunscreenformulations
ThepharmaceuticalbaseforincorporatingMarcetiaextractand
sunscreenformulawereadaptedfromthePharmaceutical
Medi-calForm(Batistuzzoetal.,2006)asdescribedinTables1and2, respectively.
Thevehicle,polowaxlotion,usedfortheincorporationofherbal extracts,waspreparedbyheatingthephase1and2at75◦C, sep-arately(Proenc¸aetal.,2009).Afterthat,phase2waspouredinto phase1underconstantagitation,endingwiththeadditionofphase 3andthemixingtemperaturebelow40◦C.Theformulaconsistedof
astandardsunscreenemulsionofO/Wcontaining
benzophenone-3as chemical filter,inaccording toTable2. Theemulsionwas
preparedwiththesamemethoddescribedforpreparingtheO/W
emulsionwithoutbenzophenone-3.Theextractwasincorporated
tothelotionpolowaxvaryingintheirconcentrationsof5,10,20 and30%.Onceformulated,thepHwasmeasuredandtunedwith tri-ethanolaminetopH6.0to7.0,whichpHisdesirableforsunscreen accordingtoRibeiro(2010).
DeterminationoftheSPFofsunscreens
ForthedeterminationoftheSPFoftheformulationsextracts(5, 10,20and30%),theyweredissolvedinethanoltoafinal concen-trationof0.2,2.0,5.0,10,15,20,30and50mg/ltoevaluatethe
profileofthesunscreenformulationsprepared.Themethodology
usedforSPFevaluationoftheseformulationswasthesameused
forthecrudeextract.Theabsorbancereadingswereperformedin
triplicate.
Statisticalanalysis
The analyses were performed in triplicate, and the results
expressed as mean±standard deviation (SD).Differences were
considered significant when p<0.05. Multiple comparisons
betweenmore thantwo groups wereperformedwithone-way
5 10 15 20
Minutes
Quercetin standard
A
B
Que
25
Fig. 1.HPLCchromatogram of theethanolicextract ofMarcetia taxifolia (A):
flavonoidglycosylatedregion(Rt:10–15min),flavonoidnon-glycosilatedregion
(Rt:16-20min);HPLCchromatogram(B)ofthequercetinstandard(Rt:14.35min).
ANOVAsupplementedwithTukey’stest.Thedataobtainedwere
analyzedusingtheGraphPadPrism®version5.0.
ResultsandDiscussion
HPLCquantification
Thecrude extractof Marcetiataxifoliawasanalyzedthrough
HPLC-DAD (Fig. 1A), what shows the presence of the
non-glycosylated (peaks betweenRt 10 to15min) and glycosylated
(peaksbetweenRt16to20min)flavonoids.TheUV-spectraofthe peakatTr14.34minwassimilartotheflavonolquercetin.Thus,the
quercetinwasidentifiedfromtheretentiontime(Rt)comparison
usingstandard(Fig.1B).Duetothegoodphotoprotectiveactionof quercetin,thiscompoundwasHPLC-quantifiedintheM.taxifolia.
Thelinearitywasconfirmedbypreparing standardsolutions
of quercetin solutions in methanolat five concentrations.
Cali-brationcurve wasplotted and determinedusing thestandards
data:forquercetin,y=30000000x+14619andR2=0.9987,which
werelinearforspecifiedconcentrationranges.Thedetectionlimit was 0.0145mg/land quantification limit was 0.048mg/lfor
quercetin.The quantification resultsshow that theamounts of
quercetinpresentintheethanolicextractwashighabovethe detec-tionandquantificationlimits,furtheremphasizingthereliability
ofthemethod.Theprecisionandaccuracyofthemeasurements
werewithinallowablevalues;theaccuracydidnotallowvalues
that exceeded2.09%. Theconcentrationof quercetinpresent in
ethanolicextractfromM.taxifoliawas1.12mg/l.
Totalflavonoidcontentandantioxidantactivity
TheflavonoidcontentofM.taxifoliaextractcalculatedasthe quercetinequivalenceshows168±0.35g/lofquercetin,which isequivalentto10mgofeachethanolicextractweight.The antirad-icalactivityoftheantioxidantisshowintheTable3.Theseresults
showassociationbetweenantioxidativeactivitiesand flavonoid
Table3
AntioxidantactivityoftheethanolicextractobtainedfromMarcetiataxifolia.
250* 125 50 25 10 5
Extract 95.31 93.97 93.43 74.19 35.52 20.25
BHT* 94.64 94.07 86.58 57.16 30.20 18.38
AA** 97.03 96.62 96.98 96.57 74.32 24.60
* g/ml
** Butylatedhydroxytoluene
***Ascorbicacid
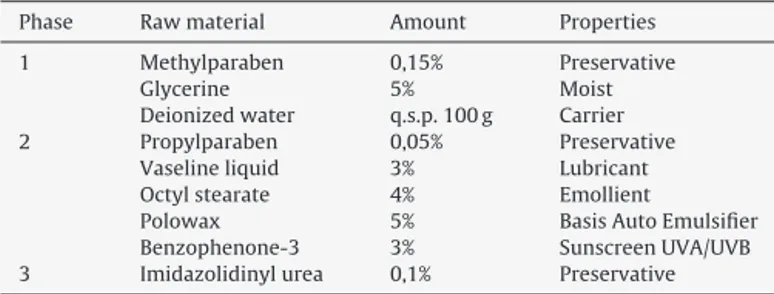
Sunprotectionfactor(SPF)ofthecrudeextract
TheethanolicextractofM.taxifoliahadthesunscreenactivity
evaluatedwiththemethoddevelopedbyMansuretal.,1986.The
resultsofthedeterminationsofinvitroSPFvaluesareshownin Fig.2.AccordingtotheBrazilianlaw,RDC30fromJune1,2012 (Anvisa,2012a),onlySPFgreaterthanorequalto6issuitablefor useincosmeticproductswithphotoprotectiveactivity.TheM. tax-ifoliaextractswithdifferentconcentrations(250and125g/l) hadsatisfactorysunscreenactivity(15.52and8.35,respectively), higherthantheminimumrequiredbyAnvisa.TheSPFvaluesofthe extractstestedwereconcentration-dependent,theincreasein con-centrationresultedinanincrementoftheSPF.Thisactivitycanbe attributedtotheflavonoidsfoundinspeciesoftheMarcetiafamily.
The plant extracts rich in flavonoids which are efficient in
absorbing ultraviolet light, usually show two maximum peaks
ofultravioletabsorption,one between240-280nmandanother
300-550nm(Bobinetal.,1995).Severalclassesofnatural
com-poundswereexaminedfortheirantioxidantandphotoprotective
activity.Recentstudies(Poloninietal.,2011)reviewedthe
impor-tanceof natural sunscreens in thecompositions ofcommercial
sunscreens and therefore analyzedtheir role in theprevention
of skincancer. Photoprotective propertieshave been evaluated
inextractsof agreat varietyof plants,extractsChrysanthemum
ramosumandCrocussativusL.provedtobegoodweather
reduc-ingagent-inducederythemaascomparedtobenzophenone4(Hu
andWang,1998).Violanteetal.(2009)havedevelopedstudieson thespeciesMacrosiphoniavelame,OxalishirsutissimaandLafoensia pacari,whichthesespeciesshowedabsorptionintheUVinmax.
318nm,324nmand356nm.Thephytochemicalstudiesdescribed
thepresenceofflavonoids,andotherconstituentsastanninsand
alkaloids,whicharesubstancesdescribedwithaphotoprotective
action.TheCalophylluminophyllumextractisrichinvariousnatural sunscreencompounds,suchasbiflavonoids,etocotrienols tocofer-ols,andbroadabsorbanceintheUV,aswellasantioxidantactivity (Saidetal.,2007).Studiesusingthedry extractfromtheleaves ofEncholiriumspectabile,Bromeliaceae,describedapotent antiox-idantactivityand significantphotoprotective activitycorrelated withflavonoidcompoundspresent(Oliveiraetal.,2013).
0 5 10 15 20
250 200
150 100
50 0
SPF
Concentration (μg/ml)
Fig.2.SunProtectionFactor(SPF)oftheethanolicextractofMarcetiataxifolia.
0 0 0.2 0.4 0.6 0.8 1.2 1.4 1.6
1
20 40 60
Time (min)
Absorbance (306 nm)
Control
M. taxifolia
80 100 120 140
Fig.3.EvaluationofthephotoprotectivepotentialagainstUVAradiationbythe
Marcetiaethanolicextract.
UV-Ablockingactivityofthecrudeextract
The plant extracts shown had the ability to protect a
pho-tolabile solution (trans-resveratrol) from UVA radiation. When
trans-resveratrolisexposedtoUVAradiation,itundergoes
degra-dationwhichcanbeseenbytheabsorbancedecreasesduringthe
timeintervals(Kerrilee,2009).Itwasfoundthatduringthecontrol
boardofexpositiontoUVA radiation,there wasphotobleaching
of50.73%trans-resveratrol.TheplatewascoatedwithM. taxifo-liaextractandphotobleachingwaslesspronounced;theendofthe 120minexposureconversionoftrans-resveratroltocis-resveratrol was9.48%.ThisstudyshowedthattheextractfromtheM. taxifo-liaiscapableofpreventingUV-Aradiationfrompermeatingacross aPetridishlid(Fig.3).ThisstudyofM.taxifoliashoweditsgood abilityofphotoprotectionagainstUV-A.
Invitroeyeirritationtests(HET-CAM)
TheHET-CAMtestshowedthatM.taxifolia(250and125g/l) contendlowirritationpotential:1.93.Theseresultsindicatedthat theethanolextractofM.taxifoliaissuitableforuseincosmetics (Table4).Reportsonthetoxicologicalevaluationsofspeciesofthe Marcetiagenerawerenotfoundintheliterature.
In addition to the protection against UV-B radiation,
prod-uctsbeingcurrentlyresearchedalsohavephotoprotectiveactivity againstUV-Arays.Initially,itwasbelievedthatUV-Aradiationdid
notcausedamagetotheskin.However,studieshaveshowedthat
thisprocesscontributestoradiationandpromotestheappearance
ofphoto-agingskintumors.TheUV-Aradiationpenetratesmore
deeplyintothedermallayersoftheskin,unlikeUV-Bwhich is
absorbedintotheskinepidermis.UV-Ageneratesmoreoxidative
stressthandoesUV-B,andatlevelsfoundinsunlight,itistentimes moreefficientthanisUV-Bincausinglipidperoxidationleading
toplasmamembranedamage(Damiani,2006).Somestudieshave
demonstratedthatsunscreenphoto-instabilityisprimarilya prob-lemconcerningtheUV-Aregion(Maieretal.,2001;Hojerovaetal., 2011).
Table4
Classificationofextractsaccordingtothescoreofthephenomena.
Concentration (g/ml)
IrritationScore (IS)
Classification Irritancy
M.taxifolia 250 1.93 lowirritant
125 1.08 lowirritant
62,5 0.14 non-irritant
NaOH0,1M* 0.1M 12.6 irritant
Distilledwater** 0.0 non-irritant
* Positivecontrol
Table5
Dilution(mg/l)andSPFfortheformulationswiththeMarcetiataxifoliaethanolic.
MT5% MT10% MT20% MT30% Benzophenone5%
0.2mg/ml
1.241±0.119d 1.796±0.0800c 2.231±0.0494c 2.860±0.0655b 4.823±0.0907a
2.0mg/ml
6.397±0.0626e 7.336±0.2748d 13.360±0.0625c 14.530±0.0598b 38.530±0.3256a
5.0mg/ml
10.200 ± 0.3160e 15.810± 0.2379d 17.170± 0.0511c 27.460 ± 01150b 38.800 ± 0.01464a
10.0mg/ml
25.430±0.1806e 28.480±0.0265d 30.54±0.0505c 33.780±0754b 38.730±0.01201a
15.0mg/ml
32.620±0.1931e 33.150±0.0775d 34.590±0.3471c 35.340±0987b 39.100±0.0808a
20.0mg/ml
32.730± 0.1561d 33.30± 0.1103c 33.730± 0.0657b 33.890 ± 0887b 39.20 ± 0.0624a
30.0mg/ml
33.250±0.1402e 34.140±0.0340d 24.610±0.0209c 36.550±0417b 42.370±0.2960a
50.0mg/ml
37.310±0.1708c 38.430±0.0377b 42.550±0.2215a 43.110±0.0800a 39.840±0.2343a
Thelevelofsignificativewasp<0.05.Theequallettersrepresentstatisticallyequalvaluesforthesamedilution.
0 5 10 15 20 25 30 35 40 45 50
50 45 40 35 30 25 20 15 10 5 0
SPF
Diluition (mg/ml)
5% 10% 20% 30%
Benzophenone 5%
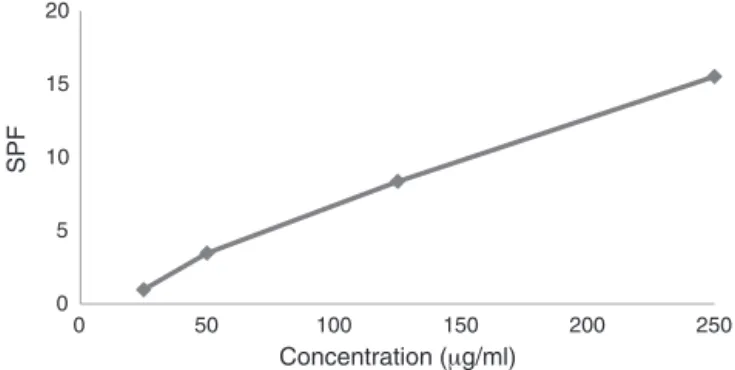
Fig.4. ProfileofsunscreenformulationswiththeMarcetiataxifoliaethanolicextract.
Currently,thepharmaceuticalandcosmeticindustries, along withgovernmentregulatoryandqualitycontrolofproducts,have usedalternativemethodologiesforassessingthepotentialforeye irritation. In this perspective, the National Health Surveillance Agency(Anvisa)hasadoptedtheGuidelinefortheSafetyEvaluation ofCosmeticProducts(Anvisa,2012b).TheinvitroHET-CAM,which providesinformationontheeye-irritatingproperties,isan essen-tialpartofthehazardidentificationofchemicalsandproducts.The HET-CAMbasicallyassessesvascularchangesandgeneratesresults which,inturn,comparedtotheanimalmodel,haveagreat relation-shipwithphenomenaobservedinconjunctiva,eveniftheyare,as arule,lighterweightthanchangesdetectedinothersystemssuch as,forexample,thoseinducedinisolatedorgans(Nóbregaetal., 2008)
DeterminationoftheSPFofsunscreens
Tukey’smultiple-comparisontestwasusedtoevaluatewhether
theSFPinthedilutionswasthesame,thereby formingaUV-B
sunscreenprofileforformulationsofM.taxifoliaextract(Fig.4).
TheSFPevaluationshowgoodresultsforformulationscontent
M.taxifoliaextracts(5,10,20and30%)whichwereobservedSFP≥6 dilutionfrom2mg/l(Table5).Forthespeciesinvestigated,itwas observedthatthedilutionof50mg/lwasclosetotheSFPstandard ofbenzophenone5%,inthefixedprotectivefactorof30-50,which
indicatesthat,accordingtotheRDC30/12,theformulationsmay
besuitableforverysensitiveskinsunburn,becauseoftheirhigh sunprotection,asdemonstratedintheinvitromethodology.
AnalyzingstatisticallytheSPFvaluesispossiblenotedthatthe formulationswithM.taxifoliaextract10%and20%(Table5)showed thesameSPFvaluesforthedilutionof0.2mg/l.Theformulations at20%and30%ataconcentrationof20mg/lwerealsostatistically equal.Thesameistruefortheconcentrationof50mg/landthe
standardformulationof5%ofbenzophenone.
Conclusion
TheethanolicextractofM.taxifoliashowgoodantioxidantand
photoprotectiveactivityagainstUV-BandUV-Aradiation.These
effects canbeattributedtoflavonoidderivatives presentin the
plant including quercetin.Sunscreens were developed and SPF
wasevaluated.TheformulationscontainingM.taxifoliaethanolic extractshowedSPFvaluesnearachemicalfilterfrequentlyusedin thepharmaceuticalindustry,benzophenone-3.TheM.taxifoliahas greatapotentialforuseasanactiveingredientforsunscreens.
Authors’Contributions
SCCChelpedwiththemeasurementoftheinvitroantioxidant
activity,totalflavonoidandHPLCanalysis.Shealsoparticipatedof thepreparationoftheformulations,interpretationofdataand sta-tisticalanalysis,evaluationoftheinvitrophotoprotectiveactivityof sunscreensandtheevaluationstabilitytests.CBDwasinchargeof theevaluationoftheinvitrophotoprotectiveactivityofsunscreens.
MBBhelpedwiththemeasurement oftheinvitroEye Irritation
Tests(HET-CAM)and carriedouttheinterpretationofdataand
statisticalanalysis.CRCBconductedstudiesoftheHPLCandthe
developmentofthesunscreenformulation.ABparticipatedinthe
coordinationoftheproject.Allauthorshavereadandapprovedthe finalmanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Agati,G.,Brunetti,C.,DiFerdinando,M.,Ferrini,F.,Pollastri,S.,Tattini,M.,2013.
Functionalrolesofflavonoidsinphotoprotection:newevidence,lessonsfrom thepast.PlantPhysiol.Biochem.72,35–45.
Anvisa,2012a.Resoluc¸ãoRDCn.30de1◦
.dejunhode2012.AgênciaNacionalde
Anvisa, 2012b. Guia para avaliac¸ão de seguranc¸a de produtos
cosméti-cos. Agência Nacional de Vigilância Sanitária,Brasília, DF. Disponível em
http://www.anvisa.gov.br
Bagley,D.M.,Rizvi,P.Y.,Kong,B.M.,1991.Factorsaffectinguseofthehen’segg
chorioallantoicmembraneasamodelforpredictingeyeirritationpotential.J. Toxicol-Cutan.Ocul.10,95–104.
Balogh,T.S.,Velasco,M.V.R.,Pedrial,C.A.,Kaneko,T.M.,2011.Protec¸ãoàradiac¸ão
ultravioleta:recursosdisponíveisnaatualidadeemfotoprotec¸ão.An.Bras. Der-matol.86,732–742.
Batistuzzo,J.A.O.,Eto,Y.,Itaya,M.,2006.Formuláriomédico-farmacêutico,3thed.
Pharmabooks,SãoPaulo.
Bobin,M.F.,Raymond,M.,Martini,M.C.,1995.Propriedadesdeabsorc¸ãoUVA/UVB
deprodutosnaturais.CosmetToiletries7,44–50.
Damiani,E.,Rosati,L.,Castagna,R.,Carloni,P.,Greci,L.,2006.Changesinultraviolet
absorbanceandhenceinprotectiveefficacyagainstlipidperoxidationoforganic sunscreensafterUVAirradiation.J.Photochem.Photobiol.B:Biol.82,204–213.
Dannhardt,G., Kreher, M., Nowe,U., 1996. Method for testing non-steroidal
anti-inflammatories:themodifedhen’seggchorioallantoicmembranetest (HET-CAMtest)comparedtootherprocedures.Arch.Pharm.(Weinheim)329, 301–310.
Debbasch,C.,Ebenhahn,C.,Dami,N.,Pericoi,M.,VanDenBerghe,C.,2005.Eye
irritationoflow-irritantcosmeticformulations:correlationofinvitroresults withclinicaldataandproductcomposition.FoodChem.Toxicol.43,155–165.
Giulietti,A.M.,Menezes,N.L.,Pirani,J.R.,Meguro,M.,Wanderley,M.G.L.,1987.Flora
daSerradoCipó,MinasGerais:caracterizac¸ãoelistadeespécies.Bol.Bot. Uni-versidadedeSãoPaulo96,1–151.
Gursoy,N.,Sarikurkcu,C.,Cengiz,M.,Solak,M.H.,2009.Antioxidantactivities,metal
contents,totalphenolicsandflavonoidsofsevenMorchellaspecies.FoodChem. Toxicol.47,2381–2388.
Hojerova,J.,Movcikova,A.,Mikul,M.,2011.Photoprotectiveefficacyand
photo-stabilityoffifteensunscreenproductshavingthesamelabelSPFsubjectedto naturalsunlight.Int.J.Pharm.408,27–38.
Hu,G.,Wang,X.,1998.ResearchonanaturalsunscreenfromChineseherbs.Int.J.
Cosmet.Sci.20,175–181.
ICCVAM,2010.RecommendedTestMethodProtocol.“Hen’seggtest-
chorioallan-toicmembrane(HET-CAM)testmethod”.
Kerrilee,E.,Allan,A.,Claire,E.,Lenehan,A.,Amanda,V.,2009.UVlightstabilityof␣
-cyclodextrin/resveratrolhost–guestcomplexesandisomerstabilityatvarying pH.Aust.J.Chem.62,921–926.
Lacombe,A.,Wu,V.C.H.,Tyler,S.,Edwards,K.,2010.Antimicrobialactionofthe
Americancranberryconstituents;phenolic,anthocyanins,andorganicacids, againstEscherichiacoliO157:H7.Int.J.FoodMicrobiol.139,102–107.
Leite,T.C.C.,Sena,A.R.,Silva,T.R.S.,Santos,A.K.A.,Uetanabaro,A.P.T.,Branco,A.,
2012.AntimicrobialactivityofMarcetiaDCspecies(Melastomataceae)and
analysisofitsflavonoidsbyreversephase-highperformanceliquid chromatog-raphycoupled-diodearraydetector.Pharmacogn.Mag.8,209–214.
Maier,H.,Schauberger,G.,Brunnhofer,K.,Honigsmann,H.,2001.Changeof
ultravio-letabsorbanceofsunscreensbyexposuretosolar-simulatedradiation.J.Invest. Dermatol.117,256–262.
Mansur,J.D.S.,Breder,M.N.R.,Mansur,M.C.D.A.,Azulay,R.D.,1986.Determinac¸ão
dofatordeprotec¸ãosolarporespectrofotometria.An.Bras.Dermatol.61,121– 124.
Martins,A.B.1989.RevisãotaxonômicadogêneroMarcetiaDC.(Melastomataceae).
Tesededoutorado.InstitutodeBiologiaVegetaldaUniversidadedeCampinas,
Campinas.
Mensor,L.L.,Menezes,F.S.,Leitão,G.G.,Reis,A.S.,Santos,T.C.D.,Coube,C.,Leitão,
S.G.,2001.ScrenningofBrazilianplantextractsforantioxidantactivitybythe
useofDPPHfreeradicalmethod.Phytother.Res.15,127–130.
Nóbrega,A.M.,Alves,E.N.,Presgrave,R.F.,Delgado,I.F.,2008.Avaliac¸ãoda
irri-tabilidadeocularinduzidaporingredientesdecosméticosatravésdotestede DraizeedosMétodosHET-CAMeRBC.Universitas:CiênciasdaSaúde.6,103– 120.
Oliveira,R.G.,Souza,G.R.,Guimarães,A.L.,Oliveira,A.P.,Morais,A.C.S.,CruzAraújo,
E.C.,Almeida,J.R.G.S.,2013.DriedextractsofEncholiriumspectabile
(Bromeli-aceae)presentantioxidantandphotoprotectiveactivitiesinvitro. J.Young Pharmacis.5,102–105.
Pietta,P.G.,2000.Flavonoidsasantioxidants.J.Nat.Prod.63,1035–1042.
Polonini,H.C.,Raposo,N.R.B.,Brandão,M.A.,2011.Fotoprotetoresnaturaisno
con-textodasaúdepúblicabrasileira.RevistadeAPS14,216–233.
Proenc¸a,K.D.S.,Oliveira,R.,Gonc¸alves,M.,Vila,M.,Chaud,M.,2009.Avaliac¸ãoda
estabilidadedeemulsõesO/Acomfotoprotetores.Rev.Bras.Ciênc.Farm.90, 132–136.
Reis,C.,Bieras,A.C.,Sajo,M.G.,2005.AnatomiafoliardeMelastomataceaedoCerrado
doestadodeSãoPaulo.Rev.Bras.Bot.28,451–466.
Ribeiro,C.,2010.CosmetologiaaplicadaàDermoestética,2thed.Pharmabooks,São
Paulo.
Said,T.,Dutot,M.,Martin,C.,Beaudeux,J.L.,Boucher,C.,Enee,E.,Rat,P.,2007.
Cyto-protectiveeffectagainstUV-inducedDNAdamageandoxidativestress:roleof newbiologicalUVfilter.Eur.J.Pharm.Sci.30,203–210.
Sayre,R.M.,Agin,P.P.,Levee,G.J.,Marlowe,E.,1979.Comparisonofinvivoandinvitro
testingofsunscreeningformulas.Photochem.Photobiol.29,559–565.
Vilela,F.M.P.,Fonseca,Y.M.,Vicentini,F.T.,Fonseca,M.J.V.,Amaral,M.D.P.H.D.,2011.
Determinationofthreeultravioletfiltersinsunscreenformulationsandfrom skinpenetrationstudiesbyhigh-performanceliquidchromatography.Quim. Nova34,879–883.
Violante,I.M.,Souza,I.M.,Venturini,C.L.,Ramalho,A.F.,Santos,R.A.,Ferrari,M.,
2009.InvitrosunscreenactivityevaluationofplantsextractsfromMatoGrosso