O
r
IG
IN
A
l
A
r
T
IC
lE
HBV, HCV and HIV seroprevalence among blood donors
in Istanbul, Turkey: how effective are the changes in the
national blood transfusion policies?
Authors AliAcar1 SabriKemahli2 HusnuAltunay3 ErdoganKosan3 OralOncul1 LeventGorenek1 SabanCavuslu1
1DepartmentofInfectious DiseaseandClinical Microbiology,Gulhane MilitaryMedicalAcademy HaydarpasaTraining Hospital,Uskudar-Istanbul. 2DepartmentofPediatrics (Ped.Hematology),Faculty ofMedicine,Ankara University,Ankara 3CapaRedCrescentCenter, Istanbul,Turkey.
Submittedon:08/14/2009 Approvedon:11/20/2009 Correspondence to: AliACAR,MD
GulhanemilitaryMedical Academy,Haydarpasa TrainingHospital DepartmentofInfectious DiseasesandClinical Microbiology 34668Uskudar-Istanbul Phone:+902165422020 Fax:+902163487880 E-mail:
draliacar@yahoo.com
Wedeclarenoconflict ofinterest.
ABSTRACT
ThenationalbloodtransfusionpolicieshavebeenchangedsignificantlyinrecentyearsinTurkey.The purposeofthisstudywastodeterminetheprevalenceofHBV,HCV,andHIVinblooddonorsatthe RedCrescentCenterinIstanbulandtoevaluatetheeffectofchangesinthenationalbloodtransfu-sionpoliciesontheprevalenceoftheseinfections.Thescreeningresultsof72695blooddonationsat theRedCrescentCenterinIstanbulbetweenJanuaryandDecember2007wereevaluatedretrospec-tively.HBsAg,anti-HCV,andanti-HIV-1/2werescreenedbymicroparticleenzymeimmunoassay (MEIA)method.Samplesfoundtobepositiveforanti-HIV1/2andanti-HCVwereconfirmedby Inno-LiaHCVAbIIIandInno-LiaHIVI/IIScore,respectively.TheseropositivityratesforHBsAg, anti-HCV,andanti-HIV-1/2weredeterminedas1.76%,0.07%,and0.008%,respectively.Compared tothepreviouslypublisheddatafromRedCrescentCentersinTurkey,itwasfoundthatHBVand HCVseroprevalancesdecreasedandHIVseroprevalanceincreasedinrecentyears.Inconclusion, webelievethatthedropinHBVandHCVprevalenceratesarelikelymultifactorialandmayhave resultedfrommorediligentdonorquestioninguponscreening,ahigherlevelofpublicawareness onviralhepatitisaswellastheexpansionofHBVvaccinationcoverageinTurkey.Anotherfactorto contributetothedecreasedprevalenceofHCVstemsfromtheuseofmoresensitiveconfirmation testingonallreactiveresults,therebyeliminatingafairamountoffalsepositivecases.Despitesimilar transmissionroutes,theincreaseinHIVprevalenceincontrasttoHBVandHCVmaybelinkedto theincreaseinAIDScasesinTurkeyinrecentyears.
Keywords:HBV,HCV,HIV,seroprevalence,blooddonors,Turkey.
[Braz J Infect Dis 2010;14(1):41-46]©ElsevierEditoraLtda.
INTRODUCTION
Anumberofviruses,bacteriaandparasitescan be transmitted through blood or blood prod-ucts.Amongstthese,thehepatitisBvirus(HBV), hepatitisCvirus(HCV),andthehumanimmu-nodeficiencyvirus(HIV)aremandatorilytested onblooddonorsworldwideduetothepotential seriouschronicclinicalsequelaeassociatedwith thesereadilytransmittedagents.1,2
Nevertheless,sincedonorhistoryquestion-ing is performed more diligently and due to theadvancesinscreeningtechniques,therisk of transmission has decreased considerably.3 However,smallrisksofinfectiontransmission persist due to several factors such as genetic variationsofinfectiousagents,presenceofan immunologicallysilentcarriage,laboratoryer-rors,andvariationsinthewindowperiodofan infectiousagentaswellaslimitationsinscreen-ingtestingmethodology.2,4
AsmandatedbytheTurkishlegalsystem, itisalawtoscreenallvoluntaryblooddo-norsforHBV,HCV,HIV-1/2,and
Trepone-ma palladium
MATERIAL AND METHODS
During the period of January to December 2007, the HBV, HCV,andHIV-1/2screeningtestresultsofblooddonorsand theconfirmatorytestresultsofHCVandHIV-1/2seropositive donorswereretrospectivelyobtainedfromlaboratoryrecords.
HBsAg, anti-HCV, and anti-HIV-1/2 screening tests were performedontheautomaticEIAinstrument(Davinci,Biomer-ieux) with the microparticle enzyme immunoassay (MEIA) methodusingthemicro-Elisakit(Hepanostika,HBsAgUni-FormII,bioMerieux,NL)basedonthesinglestep“sandwich” principleforHBsAg,the4thgenerationEIAkit(Innotest,HCV Ab IV, Innogenetics N.V. Belgium) containing CORE, NS3, NS4,andNS5antigensofHCVforanti-HCV,andthemicro-Elisakit(Vironostika,HIVUni-FormIIAg/Ab,NL)basedon thesinglestep“sandwich”principleforanti-HIV-1/2.
Inthemethodsused,thesignal-to-cutoff(S/CO)ratio≥ 1
forHBsAg,anti-HCV,andanti-HIVwereconsideredasreac-tive.InitialS/COratiosintherangeof0.70-0.99forHCVand 0.75-0.99forHIVwereconsideredasbeinginthegreyzone. TherewasnogreyzoneestablishmentforHBV.Reactivesam-plesorsamplesingreyzoneinthescreeningtestswereretested using the same immunoassay. If one or both of the retested specimensreactedthen,itwasreferredasrepeatedlyreactive andwasconsideredscreeningtestspositive.
For samples that showed repeatedly reactive with HBsAg EIA,noconfirmatorytestswerecarriedoutandthoserepeatedly reactivesampleswereinterpretedaspositive.Repeatedlyreactive samplesintheanti-HCVEIAtestingwereconfirmedusingthe 3rdgenerationLineImmuneAssay(LIA)testmethod(INNO-LIA,HCVScore,Innogenetics,Belgium),whichincludedtheC1, C2,E2,NS3,NS4,NS5regionsoftheHCVgenome.Theanti-HIVEIArepeatedlyreactiveresultswereconfirmedbytheLIA kit(INNO-LIA,HIVI/IIScore,Innogenetics,Belgium)contain-ingHIV1.2andHIV1groupOantigens.LIAtestresultswere evaluatedaccordingtotherecommendationsoftheassaymanu-facturercompanyandresultsforbothanti-HCVandanti-HIV wereinterpretedaspositive,indeterminateornegative.
Statistical analysis
Allstatisticalanalyseswerecarriedoutwithcomputersoftware (SPSS;Version10.0;SPSS,Inc;Chicago,IL).Means,coefficients ofvariation,standarddeviationsandconfidenceintervalswere determined. Statistical studies were evaluated using Pearson chi-squareandFisher’sexacttest.Ap-value<0.05wasconsid-eredtobestatisticallysignificant.
RESULTS
Screening test results
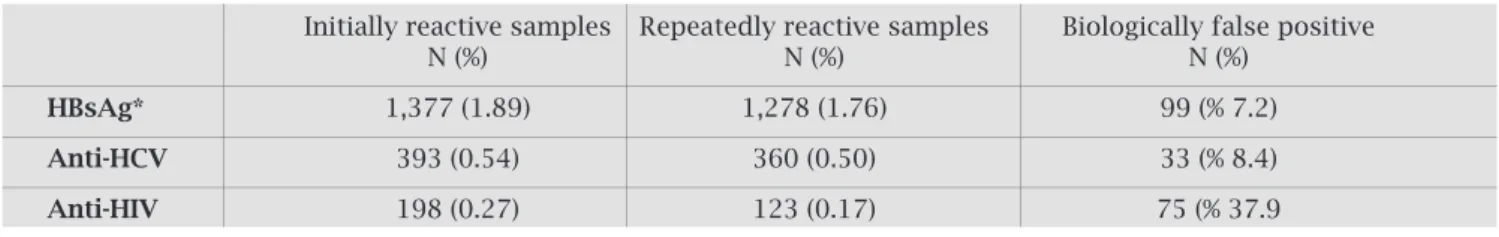
DuringtheperiodofJanuarytoDecember2007,EIAscreen-ingtestingwasperformedon72695blooddonorsattheRed Crescent Center in Istanbul, Turkey. At baseline screening testing,1377HBsAg,393anti-HCV,and198anti-HIVposi-tive results were detected (Table 1). Baseline tests revealed that130oftheanti-HCVpositivesamples(33.1%)and79 oftheanti-HIVsamples(39.9%)werewithinthegreyzone indexvalues(Table1).Forsamplesthatfellwithinthegrey zoneindexvaluefromtheinitialtesting,wedeterminedthe rateofrepeatedreactivityas80.8%(105/130)foranti-HCV and 65.8% (52/79) for anti-HIV, whereas those above the positivelimitindexvalueweredeterminedas97%(255/263) and59.7%(71/119),respectively.Whenthetotalsamplesize wasconsidered,therepeatedlyreactiverateforHBsAg,anti-HCV, and anti-HIV was found to be 92.8% (1278/1377), 91.6%(360/393),and62.1%(123/198)respectively(Table 1).Basedontheseresults,theoverallseropositivityratefor theinitialtestresultswere1.89%forHBsAg,0.54%foranti-HCV, and 0.27% for anti-HIV. Based upon repeated reac-tive test results, the seropositivity rate dropped to 1.76% for HBsAg, 0.50% for anti-HCV, and 0.17% for anti-HIV. Whenrepeatreactivesamplesweresortedoutandtherate oftruepositivitywasdetermined,itwasfoundthat99ofthe 1377HBsAgresults(7.2%),33ofthe393anti-HCVresults (8.4%), and 75 of the 198 anti-HIV results (37.9%) were biologicallyfalsepositive(BFP)(Table2).
Table 1. HBs Ag, anti-HCV, anti-HIV EIA screening test results and repeating reactivity rate
Number of initially Number of repeatedly reactive samples
reactive samples
Grey zone Positive Total Grey zone Positive limit index Total
limit index
N N N N (%) N (%) N (%)
Number tested
HBsAg 72,695 - 1,377 1,377 - 1,278 (92.8) 1,278 (92.8)
Anti-HCV 72,695 130 263 393 105 (80.8) 255 (97) 360 (91.6)
Confirmatory test results HBsAg
FortheevaluationoftheHBsAgresults,samplesshowing repeatingreactivitywithEIAwereacceptedaspositivere-sultsandnofurtherconfirmatorytestswerecarriedout.As aresult,ourstudyrevealedtheHBsAgseroprevalencetobe 1.76%amongblooddonors.
HCV
In360serumsampleswithpositiveanti-HCVscreeningtest results(29.2%s/co=0.70-1;70.8%s/co≥ 1),54(15%)posi-tive,217(60.3%)negative,89(24.7%)indeterminateresults wereobtainedbyLIA.Whenindeterminateresultswereex-cluded,theanti-HCVseroprevalencewas0.07%(54/72695). Outofthe54LIApositivesamples,2werefromtheof (greyzone)0.70-0.99and52werefromsamplesthathada S/COvalueof≥ 1.Inotherwords,forinitialEIAtestsam-plesthatwereconsideredpositivebutwithinthegreyzone (S/COvaluebetween0.70and1.0),therateofconfirmation uponLIAtestingwasonly2%,whereassampleswithanini-tialS/COindexvalue>1%hadaconfirmationtestpositive rate of 20.4%. The difference in their rates of LIA
confir-mation positivity was statistically significant (p < 0.0001). Thus, samples within grey zone index values had signifi-cantlyhighernegativeoutcomeratesasdeterminedbyLIA confirmation, compared to samples with s/co rates above thepositivethresholdvalue(p<0.0001).However,among indeterminateresults,nodifferencewasfoundbetweenthe s/coratesinthegreyzoneandthosewithpositivethreshold values(p>0.05)uponLIAtesting(Table3).Whenindeter-minateresultswereexcluded,60.3%oftheanti-HCVEIA positiveresultswereconcludedtobeBFP.
HIV
In123serumsampleswithpositiveanti-HIVscreeningtest results(42.3%s/co=0.75-1;57.7%s/co≥ 1),8(6.5%)posi-tive and 115 (93.5%) nega1),8(6.5%)posi-tive results were obtained using LIA.AccordingtoresultsverifiedbyLIA,theHIVseropreva-lenceamongourblooddonorswas0.008%(6/72695).
Allsampleswithgreyzones/corateshadnegativeresults withLIA,comparedto8positiveand63negativeresultsin sampleswiths/co≥ 1(p=0.020),(Table4).Whenindeter-minate results were excluded, 95.1% of the anti-HIV EIA positiveresultswereconcludedtobeBFP.
Table 2. The biologically false positive rate in the first tests, after results with repeating reactivity in screening tests were accepted as true positive
Initially reactive samples Repeatedly reactive samples Biologically false positive
N (%) N (%) N (%)
HBsAg* 1,377 (1.89) 1,278 (1.76) 99 (% 7.2)
Anti-HCV 393 (0.54) 360 (0.50) 33 (% 8.4)
Anti-HIV 198 (0.27) 123 (0.17) 75 (% 37.9
Table 3. Confirmatory results of anti-HCV reactive results with LIA
Anti-HCV confirmatory test (lIA) results
Positive Negative Indeterminate
N % N % N %
Grey zone (105) 2 (1.9) 80 (76.2) 23 (21.9)
Positive index (255) 52 (20.4) 137 (53.7) 66 (25.9)
Total (360) 54 (15.0) 217 (60.3) 89 (24.7)
p < 0.0001 < 0.0001 > 0.05
Table 4. Confirmatory results of anti-HIV reactive results with LIA
Anti-HIV confirmatory test (lIA) results
Positive Negative Indeterminate
N % N % N %
Grey zone (105) 0 52 (100) 0
Positive index (255) 6 (8.5) 64 (91.5) 1
Total (360) 6 (4.9) 117 (95.1) 1
DISCUSSION
This study contains important results showing that, com-paredtopreviousyears,theinfectionrateshavedecreased among blood donors and that transfusions are being car-riedoutmoresafelyasaresultofvaccinationandtraining campaignslaunchedinTurkey’slargestmetropolitancityof Istanbul,allofwhichhavebeentheresultsoftheprivatesec- toreffortsaswellasgovernmentalagenciesunderthedirec-tiveoftheTurkishMinistryofHealth.
In screening tests performed on the general public in Turkey, the incidence of HBsAg positivity has ranged be-tween1.7-21%.5Accordingtostudiesconductedbyvarious bloodbankcenters,HBsAgpositivityamongstblooddonors was5.2%duringtheperiodsbetween1985and1999,which then decreased to 2.97% between 2000 and 2003.6 HBsAg positivityamongblooddonorsandthegeneralpopulation hasbeengraduallydecliningovertheyears.Reasonsforthis drophavebeenattributedtoheightenedpublicknowledge about HBV as well as the expansion and implementation of public vaccination policies.6-8 The most extensive data pertaining to HBV seroprevalence in blood donors is de-rived from the Red Crescent Centres. HBV seroprevalence
of 6,240,130 candidates giving blood at the Red Crescent Centres throughout Turkey in 1989-2004 was on average 4.9%.7Inthesamestudy,HBsAgpositivitywasfoundtobe 4.92%in1989,5.23%in1991,and2.10%in2004.Thede-crease in Hepatitis B seroprevalence was attributed to the decreaseinthenumberofmilitaryblooddonors(positive correlation)andtotheincreaseinvoluntarydonors(nega-tivecorrelation).7BasedontheresultsoftheRedCrescent Centers from Istanbul between 1987 and 2003, the mean HBsAgpositivitywas4%,withadecreaseinseroprevalence from 5% to 2.07%.8 This decrease in HBV seroprevalence wasemphasizedasbeingattributedtogreatercaretakenin donorquestioningandtotheuseofmoresensitivescreen-ingtestsfrom1997onwards.8OurstudydeterminedHBsAg positivity to be 1.7%, which is 2.6 times (p < 0.05) lower thantheHBVseroprevalencedetectedbytheRedCrescent Centre’sthroughoutTurkey7and2.3times(p<0.05)lower thantheresultsgivenforthecityofIstanbul.8Basedonour results,ageneraltrendtowardsadeclineinHBsAgpositiv-ityindonorpopulationwasobservedoverthepastseveral
years.Inadditiontoimprovedpublicawarenessandexpan-Table 5. Biologically false positive rate in anti-HCV and anti-HIV EIA screening results according to confirmatory test results
Prevalence in accordance Prevalence in accordancew Biologically false positive
with the screening test with the confirmatory test
N (%) N (%) N (%)
HCV 360 (0.50) 54 (0.07) 306 (% 85)
HIV1,2 123 (0.17) 6 (0.008) 117 (% 95)
Figure 1: Comparison of HBV, HCV and HIV seroprevalance among our blood donors with seroprevalence reported in the most extensively ranging studies conducted in the general Turkish population and the Red Crescent Centres. (*No HIV prevalence was reported at the Red Crescent Centre in Turkey results).
5
4,5
4
3,5
3
2,5
2
1,5
1
0,5
0
1,76
0,07 0,08
5
1,5
4,9
0,38
4
0,5
0,001 0,046
Our results General
population in Turkey (%)
(5,10,12)
Blood donors in red Crescent Center/Turkey(7*)
Blood donors in red Crescent Center/Istanbul
(8)
HBV
HCV
sionofvaccinationpolicies,anotherreasonfortheobserved declineseeninourresultslikelystemsfromtheuseofhighly sensitive test kits by our group upon which samples were acceptedas“TruePositive’’onlyifclearcutrepeatreactiv-itywasdemonstrated,therebyexcludingfalsepositivesthat wouldhaveotherwisebeenacceptedaspositive.
In Turkey, HCV incidence in the general population rangesbetween1-2.4%.Incontrast,theratioamongblood donorsis0.3-1.8%.7,10UnlikeHBV,theincidenceofHCV infection does not show significant difference between regions.9,10 Anti-HCVpositivityinblooddonationsreport-edfromvariouscentreswas0.6%between1991and1999 and0.54%between2000and2006,showingnosignificant changeovertheyears.6BasedontheRedCrescentCenter’s resultsreflectingthegeneralpopulationacrossTurkey,an-ti-HCVseroprevalencebetween1996and2004was0.38%.7 Incomparison,anti-HCVpositivityfortheperiodof1996-2003was0.5%,basedontheIstanbul’sRedCrescentCenter results.8 Inourstudy,anti-HCVpositivityamongblooddo-norswasfoundtobe0.07%.Thisresultis7timeslowerthan theoverallRedCrescentCentre’sresultsthroughoutTur-key(p<0.05)and5timeslowerthantheresultsinIstanbul (p<0.05).Iftheincidenceintheoverallpopulationisac-ceptedas1%,itcanbesaidthat,comparedtothegeneral public,theHCVseroprevalenceofourblooddonorsisap-proximately14timeslower.Webelievethatthereasonfor theresultstovarytosuchadegreestemsfromthefactthat mostTurkishconductedstudiesreportreactivescreening resultsaspositivewithoutverifyinganti-HCVpositivityby confirmation testing. In comparison, in our study repeat reactivity was confirmed with the use of a sensitive con-firmatory test. Our view is further supported by the fact that85%ofthesamplesdeterminedaspositiveduringthe screeningtestwereactuallyBFPaccordingtotheconfirma-torytestresults.
Itisestimatedthat33.2millionpeoplethroughoutthe worldcarrytheHIVvirus.11Basedonthedataobtainedfrom
theMinistryofHealth,asof2006,2544casesofHIV/AIDS have been reported in Turkey.12 In 3% of these cases, the routeoftransmissionwasthroughbloodandbloodprod-ucttransfusions.12BasedupondataobtainedfromtheRed CrescentCenterbetweentheperiodsof1996and2003,An-ti-HIVpositivityon1,737,943screeneddonorswas0.001% andnosignificantchangeswereobservedinthisrateover theyears.8 BasedupondataobtainedfromtheTurkishMin-istryofHealth,HIV/AIDSincidenceratesbetween2000and 2005 were 0.023%, 0.027%, 0.027%, 0.028%, 0.030%, and 0.046%, respectively.12 Data from the Red Crescent Center revealedthatoverthepastsevenyears,theincidenceofHIV amongstdonorsremainedlowwithoutanincrease,whilethe incidenceinthegeneralpopulationgraduallyincreasedover the years. The rate of anti-HIV positivity amongst blood donors from our study was 0.008%, based upon our
con-firmatorytestresults.FromtheRedCrescentCenter’sdata, it was noted that the rate of anti-HIV positivity in Istan-bulhadincreasedeightfoldamongstscreenedblooddonors (p<0.05).Thismightbeattributabletothefactthatdonor candidates with risk factors for HIV tend to conceal their condition during donor questioning out of fear of associ-atedsociallabelling.
Therearehoweversomeshortcomingsinourstudy.One of these is the lack of confirmation testing of our HBsAg results.AlthoughthespecificityoftheEIAtestsusedduring HBsAgscreeningisnearly100%,therewasaneedtoconfirm thepositiveresultswithaneutralizationtest,particularlyin communitieswithalowprevalenceofHepatitisB,suchas voluntaryblooddonors.13,14Inastudyrecentlyconductedin Turkey,nearly70%oftheweakpositiveHBsAgresults(with aS/COratiobetween1and2.5)weredeterminedtobenega-tive(falsepositive)intheconfirmatoryneutralizationtests.15 Anotherstudyfoundthefalsepositivityrateinweakpositive samplestobesignificantlyhigherthanthoseofthestrong positivesamplesandrecommendedthatconfirmatorytests should only be performed on weakly positive samples.16 Basedontheseresults,ifconfirmatorytestingwerecarried outinourHBsAgpositiveresults,apartoftheweakpositive samplesinparticularwouldhavehadanegativeresultand the subsequent HBV prevalence among our blood donors wouldhavebeenevenlower.Afurthershortcomingwasthat indeterminate results for anti-HCV and anti-HIV results werenotfinalizedwithfurthertesting.Whenanti-HCVEIA positiveresultsareshowntobeindeterminateuponimmu-noblot confirmatory testing, it is recommended to either repeattheanti-HCVEIAtestorperformaHCVRNAquan-tificationforthesamedonor.17Amongstblooddonors,most indeterminate anti-HCV immunoblot results test negative uponrepeattesting,17however,occasionallyserumsamples thathadbeeninthepre-seroconversionwindowperiodcan laterseroconverttopositive.18Similarly,duringthewindow periodforindividualsrecentlyinfected,HIVconfirmatory testingmayhaveanindeterminateoutcome.Forthesecases, repeat HIV testing (screen with confirmation) are recom-mendedafter1-2months.19 InlowriskHIV/HCVpopula-tions, it has been hypothesized that repeat indeterminate resultsarelargelyduetothedevelopmentofautoantibod- iesorfromthecross-reactionofantibodieswithcertainir-relevant antigens.20 Donors with indeterminate or positive anti-HIV/anti-HCV confirmatory results are directed to a healthcarefacilityforfurtherevaluationandmanagement, butunfortunatelynofeedbackhasbeenreceivedaboutthe definiteclinicalstatusofthesecases.
sensitivityofanti-HCVandanti-HIVscreeningtests.Posi-tiveresultswereobtainedinonly2outofthe105(1.9%) anti-HCV reactive samples that had fallen within the grey zoneindex(s/covalue0.70-0.99).Forbothsamples,HCV RNA testing was performed simultaneously, however in bothsamplestheresultswerefoundtobenegative.Incom-parison,allconfirmatorytestresultsoftheanti-HIVpositive samples that were within the grey zone index value range weredeterminedtobenegative.Inanti-HIVandanti-HCV EIApositivesampleswhosevalueswerewithinthegreyzone index (s/co 0.70-0.99), significantly more samples tested negative upon confirmation than those samples above the positive threshold value (s/co > 1.0). This suggests a con-tradictionwithregardsto2issues,thefirstisthenegative zoneapplicationrequirement,theotherishowtodealwith donors whose test result is positive, but HCV RNA tests negative.Inthiscase,itcanbeconsideredthatthegreyzone application is at least not required in anti-HIV screening tests.Nevertheless,furtherresearchisrequiredtoestablish whetherthegreyzoneapplicationleadstounnecessarydo-norlossandincreaseincosts.
Inconclusion,webelievethatthedecreaseinHBVand HCVprevalenceovertimehasbeenmultifactorialandhas been attributed to a higher public awareness compared to previous years as a result of intensive training and cam-paigns,anincreaseinthevolunteerdonornumbers,more diligentdonorquestioningandexamination,expansionof HBV vaccination coverage as well as confirmation of our screeningresultswithsensitiveconfirmatorytesting.While adecreaseinHBVandHCVprevalencehasbeenobserved, the increase in HIV prevalence rate, despite similar trans-missionroutes,maybelinkedtoarecentincreasedtrendof HIV/AIDScasesobservedinTurkeyoverthepastfewyears. AnotherfactortoconsiderwithregardstoHIVdonorques-tioningisthefactthatmanydonorsstillhavereservations in answering questionnaires to the full extent out of fear thatriskbehaviourquestionsmaysomehowbeusedagainst them.Webelievethatthehealthcaresystemwouldbebetter servedbydevelopingstrategieswithimproveddonorques- tioningthataddressthepsychosocialaspectsofHIVscreen- ing.Wehopethatthecreationofthesechangescanmini-mizethefearofsocialstigmataassociatedwithHIVdonor questioningwhilepreservingdonorconfidentiality.
REFERENCES
1. Kaur P, Basu S. Transfusion-transmitted infections: existing andemergingpathogens.JPostgradMed2005;51(2):146-51. 2.
BihlF,CastelliD,MarincolaF,DoddRY,BranderC.(2007).Trans-fusion-transmittedinfections.JTranslMed2007;6;5:25. 3.
AlterHJ,HoughtonM.ClinicalMedicalResearchAward.Hep-atitisCvirusandeliminatingpost-transfusionhepatitis.Nat Med2000;6(10):1082-6.
4. HeperY.TransfüzyondaMikrobiyolojikTaramaTestleri.Anti-biyotikveKemoterapiDergisi2007;21(Ek2):146-52. 5.
ÖzdemirD,KurtH.HepatitBVirüsüEnfeksiyonlarınınEpi-demiyolojisi. In: Tabak F, Balıkİ, Tekeli E. ed.Viral Hepatit 2007,1.Baskı.İstanbul:ViralHepatitleSavaşımDerneği,2007; s.:108-17.
6. Mıstık R. Türkiye’de viral hepatit epidemiyolojisi yayınlarının
İrdelenmesi.In:TabakF,Balıkİ,TekeliE.ed.ViralHepatit2007,1. Baskı.İstanbul:ViralHepatitleSavaşımDerneği,2007;s.:10-50. 7. GurolE,SabanC,OralO,CigdemA,ArmaganA.Trendsin
hepatitisBandhepatitisCvirusamongblooddonorsover16 yearsinTurkey.EurJEpidemiol2006;21(4):299-305
8. KocakN,HepgulS,OzbayburtluSet al .Trendsinmajortrans-fusion-transmissibleinfectionsamongblooddonorsover17 yearsinIstanbul,Turkey.JIntMedRes2004;32(6):671-5. 9. Sakarya S, Tuncer G, Yaşar H, Cicek C, Kadıkoylu G,
Yukse-len V. Aydın Bölgesi Kan Dönerlerinde HBsAg ve Anti-HCV Seroprevalansı ve Yaş ve Cinsiyetleİlişkisi. Klimik Dergisi 2001; 14:22-4
10. SünbülM.HCVEnfeksiyonunepidemiyolojisi.ViralHepatit 2007, 1. Baskı. Ed., Tabak F, Balıkİ, Tekeli E.İstanbul:Viral HepatitleSavaşımDerneği,2007;s.:208-19.
11. AccessedJan2,2009.
12. http://www.saglik.gov.tr/istatistikler/istatistik yıllıkları/Temel SağlıkHizmetleriGenelMüdürlüğüÇalışmaYıllığ ı2006.Ac-cessedJan2,2009.
13. Public Health Service inter-agency guidelines for screening donorsofblood,plasma,organs,tissues,andsemenforevi-dence of hepatitis B and hepatitis C. MMWR Recomm Rep 1991;19;40(RR-4):1-17.
14. Dufour DR. Hepatitis B surface antigen (HBsAg) assays: are they good enough for their current uses? Clin Chem 2006; 52(8):1457-9.
15. Chen D, Kaplan L. The performance of a new generation chemiluminescent assay for hepatitis B surface antigen. Clin Chem2006;52:1592-8.
16. O’BrienJ.HepatitisBsurfaceantigen:decreasedneedforcon-firmationofreactiveresults.ClinChem2000;46:582. 17.
KielyP,KayD,ParkerS,PiscitelliL.Thesignificanceofthird-generationHCVRIBA-indeterminate,RNA-negativeresultsin voluntaryblooddonorsscreenedwithsequentialthird-gener-ationimmunoassays.Transfusion2004;44(3):349-58. 18.
CavazzaS,LaggingM.Indeterminatethird-generationhepati-tisCrecombinantimmunoblotassayandHCVRNAanalysis: isolatedreactivityagainstNS5associatedwithHCVviraemia inclinicalpatientsbutnotblooddonors.ScandJInfectDis 2005;37(6-7):488-92.
19. Rothman RE, Merchant RC. Update on emerging infections fromtheCentersforDiseaseControlandPrevention.Revised recommendationsforHIVtestingofadults,adolescents,and pregnant women in health-care settings. Ann Emerg Med 2007;49(5):575-9.