RevistaBrasileiradeFarmacognosia25(2015)529–532
w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Short
communication
Microsomal
metabolism
of
erythraline:
an
anxiolitic
spiroalkaloid
Lucas
Maciel
Mauriz
Marques
a,b,
Fernando
Armani
Aguiar
a,
Denise
Brentan
da
Silva
a,b,
Daniel
Roberto
Callejon
b,
Anderson
Rodrigo
Moraes
de
Oliveira
c,
Norberto
Peporine
Lopes
a,
João
Luís
Callegari
Lopes
a,∗,
Thais
Guaratini
a,b,∗aNúcleodePesquisaemProdutosNaturaiseSintéticos,FaculdadedeCiênciasFarmacêuticasdeRibeirãoPreto,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil bLychnofloraPesquisaeDesenvolvimentoemProdutosNaturaisLtda,CampusUSP,RibeirãoPreto,SP,Brazil
cDepartamentodeQuímica,FaculdadedeFilosofia,CiênciaseLetrasdeRibeirãoPreto,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received25September2014 Accepted4May2015 Availableonline8July2015
Keywords:
Fabaceae Erythraline CYP450 Drugmetabolism Microsomalmodel
a
b
s
t
r
a
c
t
ThegenusErythrina,Fabaceae,iswidelydistributedintropicalandsubtropicalregions.Theirflowers, fruits,seedsandbarkarefrequentlyusedinfolkmedicineforitseffectsonthecentralnervoussystem suchasanticonvulsant,antidepressant,analgesic,sedative,andhypnoticeffects.Erythralinehasbeen reportedasoneoftheactivecompoundsfromErythrina,butuntilnowtherearenopharmacokinetics dataaboutthiscompoundandonlyfewresultsshowingaputativemetabolismwerereported.Toimprove theinformationabouterythralinemetabolism,thisarticlereportsanddiscusses,forthefirsttime,the
invitrometabolismbiotransformationoferythralinebycytochromeP450enzymes.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Naturalproductshavehistoricallyplayedamajorroleinthe dis-coveryanddevelopmentofadiversearrayoftherapeutics(Kurita and Linington,2015).Theyrepresent a rich sourceof chemical diversity,between25and50%ofapproveddrugsareoriginated fromnaturalproducts.However,discovery,isolation,and develop-mentofnaturalproductsaspharmaceuticaldrugsareexceptionally challenging,requiringamulti-disciplinaryapproachtounlocktheir potential(OgbourneandParsons,2014).
ThegenusErythrina,Fabaceae,iswidelydistributedintropical andsubtropicalregions(Aritaetal.,2014).ErythrinavernaVell.isa deciduoustreefoundmainlyinBraziliancerradosandother trop-icalregions.Itsflowers,fruits,seedsandbarkarefrequentlyused infolkmedicineduetotheireffectsonthecentralnervous sys-tem,suchas:anticonvulsant,antidepressant,analgesic,sedative andhypnoticeffects(Faggionetal.,2011;Flausinoetal.,2007a,b). Systematicphytochemicalinvestigationsreportedtheoccurrence ofoveronehundredstructuralErythrinaalkaloidsderivatives(Juma andMajinda,2004;Wanjalaetal.,2002).Biologicalstudies regard-ingitsanxiolyticpropertieshavesupportedthepopularuseand themechanismofactionwasdescribedas␣42nicotinic recep-torsantagonist.Inthelastdecade,initialpharmaceuticalproducts
∗ Correspondingauthors.
E-mails:joaoluis@usp.br(J.L.C.Lopes),thais@lychnoflora.com.br(T.Guaratini).
werecommerciallyavailable,butuntilnowthereareno pharma-cokineticsdataoftheactivecompoundsandonlyfewresultsabout putativemetabolismhavebeenreported(Guaratinietal.,2014; Perdigaoetal.,2013).
Metabolism of a drugcandidate often decides whetherit is safeand effective for clinicalapplication.The understandingof specificdrugmetabolitesisoftenakeyforthesuccessofdrug dis-covery(Cusacketal.,2013;Chenetal.,2014)anditiscrucialto informseveralimportantstructuralpropertiesasnew pharmaco-logicallyinactiveortoxicentities,poorbioavailability,efficacy,and safety-relatedevents.Therefore,suchanearlyscreeningindrug developmentisnecessary(Fragaetal.,2011).
Ratand humanlivermicrosomes(RLMandHLM)aremainly employedformetaboliteprofilingpurposes(Spaggiarietal.,2014; Yuanetal.,2014).Thesystemcontainsthemaindrug-metabolizing enzymes,suchasthecytochromeP450(CYP450)familyandflavin monooxygenase(Chenetal.,2014),PhaseIandPhaseIIenzymes, whichcangeneratevaluableinformationtopredictandavoid prob-lemsduringinvivostudies(Nowaketal.,2014).
Inthisway,ourgrouphasbeendedicatedtoinvestigatePhase I metabolites for a series of natural products (Marques et al., 2014; Messiano et al., 2013; Moreiraet al., 2013).Analysis of erythraline (1, ERT) metabolization by pig microbiota suggests a high stability in intestinal portion, but applying bioorganic catalysis,oneputativemetabolitewasformedand identifiedas 8-oxo-erythraline (2) (Guaratini et al., 2014). Considering ERT (1)asa promising drugcandidate,itsmetabolismstill requires
http://dx.doi.org/10.1016/j.bjp.2015.05.011
530 L.M.M.Marquesetal./RevistaBrasileiradeFarmacognosia25(2015)529–532
clarifications. Therefore, the aim of the present work was to investigateitsinvitrometabolismbylivermicrosomesobtained fromrats,aswellasfromhumanbeings,inordertopredictthe invivometabolism,comparingwithpreviousrelatedresultsand toimprove pre-clinical information of alkaloids from Erythrina genus.
Materialsandmethods
TheERT(1)wasextractedfromErythrinavernaVell.,Fabaceae, accordingtopublishedprocedures(Guaratinietal.,2014)andsome controlanalogswereobtainedaspreviouslydescribed(Callejon etal.,2014).Sodiumchlorideandsodiumdihydrogenphosphate wereobtainedfromMerck(Darmstadt,Germany).Sodium hydrox-ide and potassium chloride were obtained from Nuclear (São Paulo,Brazil).Glycerolandtris-(hydroxymethyl)-aminomethane wereobtainedfromJ.T.Baker (Phillipsburg,NJ, USA), ethylene-diaminetetraacetic acid (EDTA) from Carlo Erba (Milan, Italy). NADP+,glucose-6-phosphateandglucose-6-phosphate
dehydro-genasewereobtainedfromSigma–Aldrich(St.Louis,MO,USA). Male Wistar rats weighing 180–220g were obtained from the School of Pharmaceutical Sciences of Ribeirão Preto, Uni-versity of Sao Paulo. The Ethical Committee from University of São Paulo approved the studies. Microsomal preparation wasperformedaccordingpublishedprocedures(Marquesetal., 2014). Ratliver microsome incubations(shaking water bath at 37◦C) wereperformed in10mlamber tubes in atotal incuba-tion volume of 1000l. Incubations contained 250l cofactor solution,35lratlivermicrosomes(29.6mgml−1microsomal pro-tein), 715l phosphate buffer (pH 7.4; 0.25moll−1) and 25l ERT(1;500gml−1).The cofactor solutionconsistedof NADP+ (0.25mmoll−1),glucose-6-phosphate(5mmoll−1)and glucose-6-phosphatedehydrogenase(0.5units)inTris–HClbuffer(Tris–HCl 0.05moll−1–KCl0.15moll−1,pH7.4).
Humanlivermicrosomeincubations(BDGentestTM,Woburn,
MA,EUA)wereperformedaccordingClementsandLi(2011):to avoidmultiplefreeze–thawcyclesandmaintainenzymeactivity themicrosomesand NADPHregenerating system weredivided intosingleusefractionsandstoredat−94◦Cuntilneeded.Amber
tubesinatotalincubationvolumeof200lwereused.Incubations contained100lhumanlivermicrosome(4mgml−1microsomal protein),100lNADPHregenerating system(NRS)(40l0.5M phosphatebufferpH7.4,10lSolutionA,2lSolutionB, both fromBDGentestTM),5lERT(1;1.38mgml−1).
Afterprewarmingfor5minat37◦C, themetabolicreactions wereinitiatedbytheadditionoftheratandhumanliver micro-somes,separately.Tostopmetabolicreactionafter90min,itwas added4mland1mlofchloroform,respectively,toincubationswith ratandhumanlivermicrosomes.Then,thesamplepreparationwas performed.Controlincubationswereperformedintheabsenceof thecofactorsolutionandintheabsenceofthemicrosomal prepa-ration.Thedifferencebetween‘with’and ‘without’NADPHwas consideredCYP450-mediatedmetabolism.Toperformthe experi-ments,thesampleswerepooledfromtensingleincubations.
Aliquid–liquidextraction(LLE)procedurewasappliedtoextract theERT(1)fromtheratandhumanlivermicrosomes.Afterthe extractionprocedure,thesampleswereshakenfor15min(Vibrax
VXR agitator,IKA,Staufen, Germany)and centrifugedfor 5min at2860×g (HitachiCF16RXII,Himac,Tokyo,Japan). The
super-natantwascollected(3mland750l,respectively,fromratand humanmicrosomalpreparationsamples)andallowedto evapo-ratetodrynessunderagentlestreamofnitrogen.Then,theresidue wasreconstitutedin200lofmethanol,and1lwasinjectedinto thechromatographysystem.
In order to investigate the formation of metabolites, the samples were analyzed by a gas chromatograph (GC-MS-QP-2010, Shimadzu) coupled to a quadruple mass spectrometer with electron impact (EI) ionization at 70eV. The gas chro-matograph was equipped with an auto sampler AOC-20i, 1l splitinjections1/10wereperformedat250◦C.Separationswere carried out ona 5% phenyl methyl siloxane (DB-5ms) column (30m×0.25mm×0.25mfilmthickness).Theoventemperature
program for separation conditions were 100◦C for 0.8min fol-lowedbytemperatureincreasesto220◦Cat6◦Cmin−1,220◦Cfor 10min,temperatureincreasesto290◦Cat6◦Cmin−1,and290◦C for11min.Thecarriergaswasultrapureheliumhelium(grade5.5) atconstantflowratesof1.10mlmin−1.Amassrangeofm/z50–500 wasrecordedinthefull-scanmode.Finally,themetaboliteswere comparedwithauthenticallysamplespreviousisolated.
Toaccumulateenoughmetaboliteusedfor1HNMRanalysis,rat
livermicrosomeincubationwasperformedaspreviousdescribed to150individualtubes(ERT,1,2000gml−1).Then,thispoolwas submittedtosemipreparativeLCusingthefollowingconditions: C18 column (Shim-pack Prep-ODS, 5m, 20mm×25cm,
Shi-madzu),flowrate9mlmin−1andacetonitrile(B)andH2O(A)both withTFA0.02%(v/v)assolvents.Theelutionprofilewas0–25min (15–65%,B),25–30min(25–80%,B),30–32min(80–100%,B)and 32–35min(100%,B).The isolatedcompound wascharacterized by NMR (Bruker Avance® DRX-500) in a Shigemi symmetrical
susceptibility-matchedmicrotube.
Resultsanddiscussion
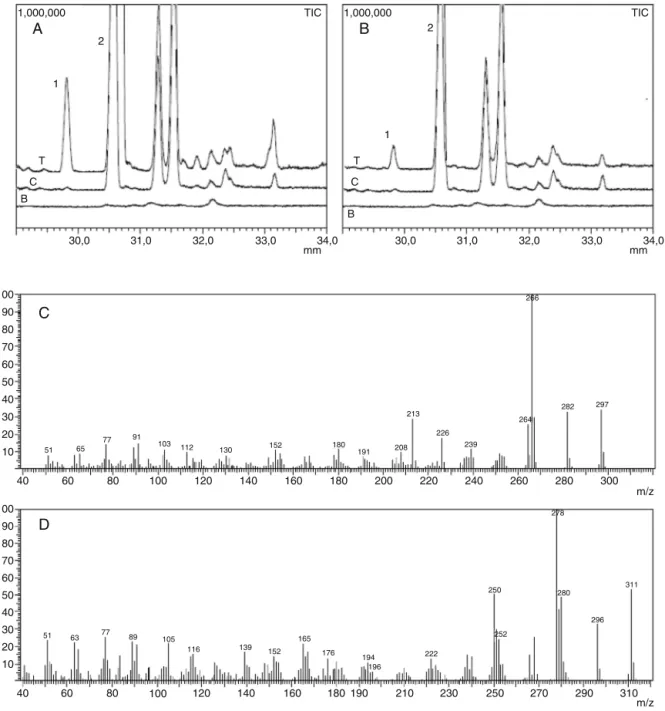
PureERT(1)elutes inGC–MSanalysisatRt 30.6min(Fig.1, charts A and B) and no other signal was observed. The chro-matogram of thetest reaction employing rat and humanliver microsomespresentedanewpeakatthesameRt29.81min show-ingthattherewasacorrelationbetweenbothmicrosomalmodels (Fig.1,chartsAandB).Theabsenceofsignalsintheblankand con-trolsamplesconfirmtheenzymaticconversionprobablythought anoxidativereaction.Theelectronionizationmassspectraofthe signal1atRt 29.81showanincrementof14massunits(Fig.1, chartD),relatedtoERT(Fig.1,chartC).Thisresultsuggestsan oxi-dationthroughacarbonylgroupformationaspreviousobserved forothernaturalproducts(Niehuesetal.,2012;Santosetal.,2005, 2008).Thefragmentationpatternofbothcompoundsshowed simi-larneutraleliminationsconfirmingthehomologybetweenthetwo structures.
Toconfirmthemetabolitestructurelarge-scaleratliver micro-someincubation wasperformedaspreviousdescribed (applied 150individualtubesofERTat2000gml−1).Then,thispoolwas submittedtosemipreparativeLCaffording900g.Afterthe sol-ventelimination,thesamplewascharacterizedbyNMR(Bruker Avance® DRX-500) in a Shigemi symmetrical
susceptibility-matchedmicrotube.The1HNMR(CDCl
3,500MHz)spectraofthe
isolatedmetaboliteexhibitedthesignals(seesupplementarydata associatedwiththisarticle):ıH6.33(m,H-1),6.72(s,H-14),6.72
(s,H-17),6.89(m,H-2),6.15(s,H-7),5.94(d,O-CH2-O),5.91(d,
O-CH2-O),3.90(m,H-3),3.78(m,H-10),3.68m(m,H-10),3.35(s,
OCH3),3.15(m,H-11),2.99(m,H-11),2.82(m,H-4),2.10(m,H-4).
L.M.M.Marquesetal./RevistaBrasileiradeFarmacognosia25(2015)529–532 531
30,0
100 90 80 70 60 50 40 30 20 10
40
51 65
77 91 103
112 130 152 180
191 208
213
226 239
264 266
282 297
60 80 100 120 140 160 180 200 220 240 260 280 300
100 90 80 70 60 50 40 30 20 10
51 63 77 89 105
116 139 152
165
176 194
196
222
250
252
280 278
296 311
40 60 80 100 120 140 160 180190 210 230 250 270 290 310 m/z m/z T
1 2
1,000,000 1,000,000
2
1
T
C
B
TIC TIC
A
B
C
D
C B
31,0 32,0 33,0 34,0
mm 30,0 31,0 32,0 33,0 mm34,0
Fig.1. RepresentativeGCchromatogramsofrat(chartA)andhumanmicrosomesextracts(chartB),signal1:8-oxo-erythraline(Rt=29.81min)andsignal2:ERT
(Rt=30.6min).LinesT(testsamples)ofERTmetabolismafterincubation;linesC(control)inbothmicrosomalpreparationwithoutNADPHcofactor;linesB(blank):
microsomalpreparationwithoutERT.Left:ratlivermicrosomes;right:humanlivermicrosomes.ChartCshowstheEI-MSdataofERT(Rt=30.6min)andchartDshowsthe
EI-MSdataof8-oxo-erythraline(Rt=29.81min).
ionmode)confirmedthemolecularstructure(observ.m/z312.1228 [M+H]+;calcd.forC
18H18NO4+312.1230,error=0.6ppm).
Conclusion
Thispaperdescribes,forthefirsttime,theCYP450-mediated metabolismofapromisingnaturalproduct,ERT(1),usingratand humanlivermicrosomes.Itsmetabolismshowedtheformationof thepreviouslyformed8-oxo-erythraline(2)bybioinorganic cataly-sis(Guaratinietal.,2014).Thiscompoundwaspreviouslyisolated asaminorcompoundinsomecultivarofE.vernaandbiological evaluationapplyingmacrophageandLeishmaniacellsrevealedlow cytotoxicactivityfortheERTanalogalkaloidsinvestigated.Inthis manner,thiscompoundshouldbeconsideredaspossible metabo-liteintheinvivometabolismanditstoxicologicaleffectmustbe furtherinvestigated.
Authors’contributions
LMMM,FAA,TG,DBS,DRCconductedextractionandisolation oftheeritralinandmetabolitesfromplantandinmicrosomal bio-transformation, respectively and the interpretation of all these data. LMMM, ARMO, NPL, JLCL and TG wrote and revised the manuscript.Allauthorsreadandapprovedthefinalmanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
532 L.M.M.Marquesetal./RevistaBrasileiradeFarmacognosia25(2015)529–532
CNPq(168114/2014-3),INCTifandRHAEforfellowshipsand finan-cialsupport.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2015.05.011.
References
Arita,T.,Miyazaki,S.,Teramoto,S.,Yoshitama,K.,2014.Majoranthocyanin biosyn-thesisinthebrilliantcrimsonpetalsfromErythrinacrista-galliL.Sci.Hortic.168, 272–280.
Callejon,D.R.,Riul,T.B.,Feitosa,L.G.P.,Guaratini,T.,Silva,D.B.,Adhikari,A.,Shrestha, R.L.S.,Marques,L.M.M.,Baruffi,M.D.,Lopes,J.L.C.,Lopes,N.P.,2014. Leishmanici-dalevaluationoftetrahydroprotoberberineandspirocyclicerythrina-alkaloids. Molecules19,5692–5703.
Chen,T.L.,Zhang,Y.B.,Xu,W.,Kang,T.G.,Yang,X.W.,2014.Biotransformationof isoimperatorinbyratlivermicrosomesanditsquantificationbyLC–MS/MS method.Fitoterapia93,88–97.
Clements,M.,Li,L.,2011.Strategyofusingmicrosome-basedmetaboliteproduction tofacilitatetheidentificationofendogenousmetabolitesbyliquid chromatog-raphymassspectrometry.Anal.Chim.Acta685,36–44.
Cusack,K.P.,Koolman,H.F.,Lange,U.E.W.,Peltier,H.M.,Piel,I.,Vasudevan,A.,2013.
Emergingtechnologiesformetabolitegenerationandstructuraldiversification. Bioorg.Med.Chem.Lett.23,5471–5483.
Faggion,S.A.,Cunha,A.O.S.,Fachim,H.A.,Gavin,A.S.,Santos,W.F.,Pereira,A.M.S., Beleboni,R.O.,2011.Anticonvulsantprofileofthealkaloids(+)-erythravineand (+)-11-␣-hydroxy-erythravineisolatedfromtheflowersofErythrinamulungu
MartexBenth(Leguminosae–Papilionaceae).EpilepsyBehav.20,441–446.
FlausinoJr.,O.,Pereira,A.M.,Bolzani,V.S.,deSouza,R.L.N.,2007a.Effectsof ery-thrinianalkaloidsisolatedfromErythrina mulungu(Papilionaceae) inmice submittedtoanimalmodelsofanxiety.Biol.Pharm.Bull.30,375–378.
FlausinoJr.,O.,Santos,L.A.,Verli,H.,Pereira,A.M.,Bolzani,V.S.,deSouza,R.L.N., 2007b.AnxiolyticeffectsoferythrinianalkaloidsfromErythrinamulungu.J.Nat. Prod.70,48–53.
Fraga, A.G.M., Silva, L.L., Fraga, C.A.M., Barreiro, E.J., 2011.
CYP1A2-mediated biotransformation of cardioactive 2-thienylidene-3,4-methylenedioxybenzoylhydrazine(LASSBio-294)byratlivermicrosomesand humanrecombinantCYPenzymes.Eur.J.Med.Chem.46,349–355.
Guaratini,T.,Silva,D.B.,Bizaro,A.C.,Sartori,L.R.,Humpf,H.U.,Lopes,N.P.,Lotufo, L.V.C.,Lopes,J.L.C.,2014.Invitrometabolismstudiesoferythraline,themajor spiroalkaloidfromErythrinaverna.BMCComplement.Altern.Med.14(61),2–5.
Juma,B.F.,Majinda,R.R.T.,2004.Erythrinalinealkaloidsfromtheflowersandpodsof
ErythrinalysistemonandtheirDPPHradicalscavengingproperties.
Phytochem-istry65,1397–1404.
Kurita,K.L.,Linington,R.G.,2015.Connectingphenotypeandchemotype: high-contentdiscoverystrategiesfornaturalproductsresearch.J.Nat.Prod.78, 587–596.
Mantle,P.G.,Laws, I.,Widdowson, D.A.,1984. 8-Oxo-erythraline, a naturally-occurringprincipal alkaloid fromErythrina crista-galli. Phytochemistry 23, 1336–1338.
Marques,L.M.M.,SilvaJr,E.A.,Gouvea,D.R.,Vessecchi,R.,Pupo,M.T.,Lopes,N.P.,Kato, M.J.,DeOliveira,A.R.M.,2014.Invitrometabolismofthealkaloidpiplartineby ratlivermicrosomes.J.Pharm.Biomed.95,113–120.
Messiano,G.B.,Santos,R.A.S.,Ferreira,L.S.,Simoes,R.A.,Jabor,V.A.P.,Kato,M.J., Lopes,N.P.,Pupo,M.T.,deOliveira,A.R.M.,2013.Invitrometabolismstudyof thepromisinganticanceragentthelignan(-)-grandisin.J.Pharm.Biomed.72, 240–244.
Moreira,F.L.,Souza,G.H.B.,Rodrigues,I.V.,Lopes,N.P.,DeOliveira,A.R.M.,2013.A non-michaelianbehavioroftheinvitrometabolismofthepentacyclictriterpene alfaandbetaamyrinsbyemployingratlivermicrosomes.J.Pharm.Biomed.84, 14–19.
Niehues,M.,Barros,V.P.M.,Emery,F.S.,Dias-Baruffi,M.,Assis,M.D.,Lopes,N.P., 2012.Biomimeticinvitrooxidationoflapachol:amodeltopredictandanalyse theinvivophaseImetabolismofbioactivecompounds.Eur.J.Med.Chem.54, 804–812.
Nowak,P.,Wozniakiewicz,M.,Koscielniak,P.,2014.Simulationofdrugmetabolism. TrendsAnal.Chem.59,42–49.
Ogbourne,S.M.,Parsons,P.G.,2014.Thevalueofnature’snaturalproductlibrary forthediscoveryofnewchemicalentities:thediscoveryofingenolmebutate. Fitoterapia98,36–44.
Perdigao,P.S.,Serrano,M.A.R.,FlausinoJr.,O.,Bolzani,V.S.,Guimaraes,M.Z.P., Castro, N.G., 2013. Erythrina mulungu alkaloids are potent inhibitors of neuronal nicotinic receptor currents in mammalian. PLoS ONE 8, e82726.
Santos,M.D.,Martins,P.R.,Santos,P.A.,Bortocan,R.,Iamamoto,Y.,Lopes,N.P.,2005.
Oxidativemetabolismof5-O-caffeoylquinicacid(chlorogenicacid),abioactive naturalproduct,bymetalloporphyrinandratlivermitochondria.Eur.J.Pharm. Sci.26,62–70.
Santos,M.D.,Iamamoto,Y.,Lopes,N.P.,2008.HPLC–ESI-MS/MSanalysisofoxidizes di-caffeoylquinic acids generated by metalloporphyrin-catalydes reactions. Quim.Nova31,767–770.
Spaggiari,D.,Geiser,L.,Serge,R.,2014.Couplingultra-high-pressureliquid chro-matographywithmass spectrometryfor in-vitrodrug-metabolismstudies. TrendsAnal.Chem.63,129–139.
Wanjala,C.C.W.,Juma,B.F.,Bojase,G.,Gache,B.A.,Majinda,R.R.T.,2002. Erythri-nalinealkaloidsandantimicrobialflavonoidsfromErythrinalatissima.Planta Med.68,640–642.
Yuan,L.,Jia,P.,Sun,Y.,Zhao,C.,Zhi,X.,Sheng,N.,Zhang,L.,2014.Studyofinvitro