w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Interleukin-10
haplotypes
are
not
associated
with
acute
cerebral
ischemia
or
high-risk
transcranial
Doppler
in
a
newborn
cohort
of
395
children
with
sickle
cell
anemia
André
Rolim
Belisário
a,b,∗,
Rahyssa
Rodrigues
Sales
b,
Nayara
Evelin
Toledo
c,
Cibele
Velloso-Rodrigues
d,
Célia
Maria
Silva
c,
Marcos
Borato
Viana
baFundac¸ãoHemominas,CentrodeTecidosBiológicosdeMinasGerais,LagoaSanta,MG,Brazil
bUniversidadeFederaldeMinasGerais(UFMG),FaculdadedeMedicina,BeloHorizonte,MG,Brazil
cFundac¸ãoHemominas,Servic¸odePesquisa,BeloHorizonte,MG,Brazil
dUniversidadeFederaldeJuizdeFora(UFJF),GovernadorValadares,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received14July2016 Accepted22September2016 Availableonline21February2017
Keywords: Sicklecellanemia Stroke
Polymorphism Interleukin-10
TranscranialDopplerultrasound
a
b
s
t
r
a
c
t
Background:Theetiologyofstroke,a severecomplicationofsicklecellanemia, involves inflammatoryprocesses.However,thepathogeneticmechanismsareunknown.Theaimof thisstudywastoevaluatetheinfluenceofinterleukin-10polymorphismsandhaplotypes ontheriskofacutecerebralischemiaandhigh-risktranscranialDopplerin395children withsicklecellanemiafromthestateofMinasGerais,Brazil.
Methods:Interleukin-10 haplotypes were determined by polymerase chain reaction-restrictionfragmentlengthpolymorphismandsequencing.Theoutcomesstudiedwere acutecerebralischemiaandhigh-risktranscranialDoppler.Clinicaldatawereretrieved fromthechildren’srecords.
Results:Therewasno statistically significant differenceinthe frequencies of polymor-phismsand haplotypesbetweenchildrenwithandwithout acutecerebral ischemiaor childrenwithorwithouthigh-risktranscranialDoppler.Thesedataareconsistentwith apreviousreportthatshowedanabsenceofassociationbetweeninterleukin-10plasma levelsandhigh-risktranscranialDopplervelocityinchildrenwithsicklecellanemia. Conclusion:Interleukin-10haplotypeswerenotassociatedwiththeriskofacutecerebral ischemiaorhigh-risktranscranialDopplervelocityinchildrenwithsicklecellanemiafrom thestateofMinasGerais,Brazil.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:CentrodeTecidosBiológicosdeMinasGerais,Fundac¸ãoHemominas,RuadasGoiabeiras,779,33400-000Lagoa
Santa,MinasGerais,Brazil.
E-mailaddress:andrebelisario@yahoo.com.br(A.R.Belisário).
http://dx.doi.org/10.1016/j.bjhh.2016.09.017
Introduction
Cerebrovasculardiseaseisaseverecomplicationofsicklecell anemia(SCA),affectingalmostone-halfofchildrenwithSCA bythe ageof14.1 ThephenotypeofSCAisextremely
vari-able,andstrokeoccursin11%ofunder20-year-oldindividuals withoutpreemptivetreatment.2Despitethefactthat
transcra-nialDoppler(TCD)ultrasonography isvery useful,3 thereis
aneedtodevelopmoreaccurateprognostictoolstoidentify childrenwhoareatthehighestriskofdevelopingastroke.4
Inflammatoryprocessesplayanimportantroleinthe devel-opmentofocclusivedisease atthecircle ofWillis5 andare
clearlyinvolved inthe etiology ofstrokes inchildren with SCA.6Polymorphismsincytokineorcytokinereceptorgenes
havebeen shown to modulate the occurrenceofstroke in childrenwithSCA.7–9Interleukin-10(solubleinterleukin-10–
IL-10)isavitalimmunoregulatorycytokineinvolvedinboth immuneresponseandinflammation.TheeffectsofIL-10upon inflammationincludeinhibitionofproinflammatorycytokine production,includingIL-1,IL-6,IL-12,andtumornecrosis fac-tor(TNF),aswellasbothtypesofCCandCXCinflammatory chemokines.10Interleukin10gene(IL10)polymorphismsinthe
promoterregion(knownas−1082G/A,−819T/C,and−592
A/C)formhaplotypesthatarelinkedtodifferentexpression levelsofthiscytokine.11Inrecentyears,reportshave
demon-stratedthatIL10haplotypesareassociatedwithmanyaspects ofdifferentdiseasesand conditions,includingsurvivaland relapse in resected non-small cell lung cancer,12 systemic
lupuserythematosus,13asthma,14andinhibitordevelopment
inhemophiliaA.15Arecentreporthasshownanassociation
betweenreducedIL-10levelsandthefrequency,type,severity, anddurationofvaso-occlusivecrisesinchildrenwithSCA,16
suggestingapossibleinfluenceofIL-10onthe pathophysio-logyofstroke.StrokeinchildrenwithSCAhasbeenassociated withaproinflammatory statecharacterizedbyrecruitment ofwhite blood cells tothe endothelial lesion, microvascu-lar obstruction and ischemia, intimal hyperplasia, fibrosis, and finally, occlusion.17 Based on thesedata, we
hypothe-sizedthathighIL-10productionhaplotypescoulddecreasethe inflammatorystateand,consequently,reducetheoccurrence ofstrokeinchildrenwithSCA.
Methods
Thiswasaretrospectivecohortstudythatincluded395 chil-dren with SCA from the state of Minas Gerais in Brazil. ChildrenwhowerebornbetweenJanuary1999andDecember 2008andfollowed-upatFundac¸ãoHemominasinBelo Hori-zonteuntilJune2015wererecruitedfromanewborncohortof 472subjectswithanHbFSelectrophoreticpattern.Ofthese 472children,77(16.3%)wereexcluded:32withtheHb S/beta-thalassemiagenotype,13withHbS/hereditarypersistenceof fetalhemoglobin,onechildwithahemorrhagicstroke,and 31 who could not bereached during the study or did not consenttoparticipate.Thestudyprotocolwasapprovedby theindividualinstitutionalreviewboards(Fundac¸ão Hemom-inas#295andUniversidadeFederaldeMinasGerais#154/11). Furthermore,written informed consentwas obtainedfrom parents or guardians, and children’s assent was obtained,
whenappropriate.Thestudy wasconductedinaccordance withtheguidelinesoftheDeclarationofHelsinki.
Genomic DNA extraction from blood samples was car-ried out using acommercial kit (QIAamp, DNA BloodMini Kit; Qiagen, Hilden, Germany). To confirm the presenceof SCA,theS allelegenotypeofallchildrenwasconfirmedby polymerasechainreaction-restrictionfragmentlength poly-morphism(PCR–RFLP)analysisaspreviouslydescribed.18IL10
promoterpolymorphisms,c.−627A>C(rs1800872;knownas −592A>C), c.−854 T>C (rs1800871; known as−819 T>C),
and c.−1117A>G(rs1800896; knownas −1082A>G),were
genotypedbyPCR–RFLPaspreviouslydescribed.19,20Atleast
5%ofthesampleswererandomlyselectedforDNA sequenc-ingtoconfirmPCR–RFLPresults.DNAsequencingwascarried outinanABIPrism3130Analyzer(AppliedBiosystems; Fos-terCity,CA,USA),usingstandardprotocols.Duetocomplete linkage disequilibrium(LD) between rs1800872(−592 A>C)
andrs1800871(−819T>C)polymorphisms,21only70children
weregenotypedforthers1800871polymorphismandinall, thelinkagebetweenthosetwopolymorphismswasdetected. Haplotypes of the single nucleotide polymorphisms (SNPs) werecodedinthefollowingorder:rs1800896,rs1800871,and rs1800872(forexample,GCC).
Twooutcomeswereanalyzed:acutecerebralischemiaand high-riskTCD.Acutecerebralischemiawasdefinedasa neu-rologicaldeficitlastingmorethan24h(stroke)orhistoryof transientischemicattack(TIA).2Thediagnosisofstrokewas
confirmedbyimagingassessmentsinallcases;magnetic reso-nanceangiographywasusedintwo(8.7%)children,computed tomographyin12(52.2%)children,andbothinnine(39.1%). Intracranialhemorrhagewasnotincludedinthecategoryof stroke.High-riskTCDwasdefinedasatime-averagedmean ofthemaximumvelocity(TAMMX)≥200cm/sintheinternal
carotidormiddlecerebralarteryasoriginallydefinedbythe STOPinvestigators.22Childrenwhohadsufferedacute
cere-bralischemiaaswellasthoseforwhomTCDscreeningwas inadequatewereexcludedfromthesecondanalysis.For indi-viduals who underwent multipleTCD tests, onlythe most recent wasincluded intheanalysis(High-risk TCDas out-come).Forchildrenundergoingchronictransfusiontherapy, treatmentwithhydroxyurea,orbonemarrowtransplantation, theresultofthelastTCDexampriortotheinitiationoftherapy wasincludedintheanalysis.
Statisticalanalysis
Nominalvariables areexpressedaspercentages.Univariate association between outcomes and single polymorphisms orhaplotypeswasevaluatedusingtwo-tailedchi-square or Fisher’s exact test. Initially, theinfluenceof each polymor-phismalonewasverified.Subsequently,onlychildrenwiththe homozygousformofeachhaplotype(GCC/GCC,ACC/ACC,or ATA/ATA)weretested.Finally,childrenweregroupedinto hap-lotypesdefined ashigh (GCC/GCC), intermediate (GCC/ACC andGCC/ATA)orlow(ACC/ACC,ATA/ATA,andATA/ACC)levels ofIL-10production.23Thecumulativeprobabilityofoutcomes
receivingchronicprophylacticbloodtransfusionsor hydrox-yurea, a patient undergoing bonemarrow transplantation, death from causes unrelated to outcomes,or no outcome eventuntilJune2015(endofstudy).
Statisticalsignificancewasdefinedasp-values≤0.05.
Sta-tisticalanalyseswereperformedusingtheStatisticalPackage for the Social Sciences (SPSS) v. 17.0 software (SPSS Inc., Chicago, IL, USA). Additionally, pairwise LD between SNPs was evaluated using the software HaploView (version 4.2;
http://www.broad.mit.edu/mpg/haploview/)andexpressedas D′ and R2. The haplotype blockstructures were calculated usingtheHaploView, accordingtothealgorithmpreviously described.24Ineachassociationtest,atotalof1000random
permutationswereperformedtointernallyvalidatethe asso-ciationbetweenhaplotypesandanalyzedoutcomes.
Results
Characteristics of children enrolled in this study were describedindetailelsewhere.25Briefly,meanfollow-upperiod
was 9.04 years (1.32–15.74), providing 3572 patient-years. Twenty-six(6.6%;95%CI:4.14–9.03)childrenhadacute cere-bralischemia;19 (4.8%)hada stroke,three(0.7%) hadTIA, and four(1%) hadboth strokeand TIA. Twenty-nine(8.6%; 95% CI:5.59–11.57) out of338 children had high-risk TCD; 57/395(14.4%)childrenhadacutecerebralischemiaor inade-quateTCDandwereexcludedfromtheanalysisforhigh-risk TCD.Thecumulativeprobability ofacute cerebralischemia bytheageof8.0yearswas7.4%(95%CI:4.66%–10.14%),and thecumulativeprobabilityofhigh-riskTCDbytheageof11.5 yearswas14.2%(95%CI:8.91%–19.49%).
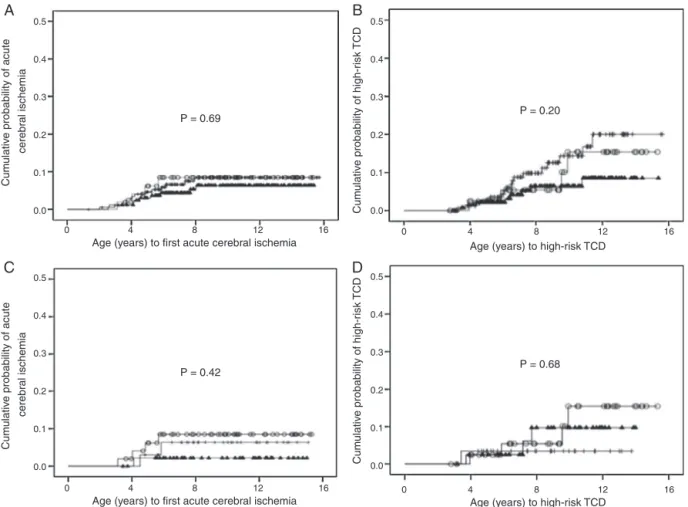
AnanalysisofIL10haplotypesinthiscohortshowedthat99 (25.1%)childrenwereGCC/ATA,85(21.5%)ACC/ATA,79(20%) GCC/ACC,50 (12.7%)GCC/GCC, 47(11.9%) ATA/ATA,and 34 (8.6%) were ACC/ACC. One (0.3%) child had anuncommon heterozygoushaplotype(GCC/GTA).TheSNPs were inhigh LD(Figure1).Therewasnosignificantdifferenceinthe fre-quenciesofpolymorphismsandhaplotypesbetweenchildren withandwithoutacute cerebralischemia(Table1).Similar results were obtained between children with and without high-riskTCD(Table2).Thecumulativeprobabilitiesofacute cerebralischemiaorhigh-riskTCDforIL-10expression hap-lotypegroupswerenotsignificantlydifferent(Figure2Aand B,respectively).Similarresultswereobtainedwhenonly chil-drenthatwerehomozygousforeachhaplotypeweretested (Figure2Cand D). Likewise,HaploViewanalysis (haplotype associationsand permutationtests)showed noassociation betweenhaplotypesandoutcomes(datanotshown).
Discussion
ThisisthefirstreporttoevaluatetheinfluenceofIL10 hap-lotypesupontheriskofcerebrovascular diseaseinchildren withSCA.Contrarytoourinitialhypothesis,thisstudyshowed noassociation betweenIL10 haplotypesand acutecerebral ischemiaorhigh-riskTCDinchildrenwithSCA.
Substantial advancesinthepast years haveestablished the naturalhistory of stroke inindividuals with SCA2 and
haveenabledearlyidentification andtreatmentofchildren
rs1800872
rs1800871
rs1800896
Block1 (0 kb)
1
2
3
98
98
Figure1–Linkagedisequilibrium(LD)plotofthethree singlenucleotidepolymorphisms(SNPs)intheIL10
promoterregiongene.LDplot,eachsquare(withD′values writtenwithinthebox)representsapairwiseLD
relationshipbetweenthetwoSNPs.White,D′<1and logarithmoftheoddsratio(LOD)<2;gray,D′<1and LOD≥2;black,D′=1andLOD≥2.
whoareatthehighestrisk.22However,thepathophysiology
ofischemicstrokeisnotcompletelyunderstood.Many can-didategenesandpolymorphismshavebeenassociatedwith stroke inchildrenwithSCA.8,9,26–28 Thecombinationofthe
TNFA −308 GG genotype and IL4R 503Pvariant was
signif-icantly associatedwith apredisposition for stroke inlarge vessels,determinedbytheanalysesofchildreninanewborn cohortaspartoftheCooperativeStudyofSickleCellDisease (CSSCD).9Thers284875polymorphismlocatedintheTGFBR3
genewasassociated27andvalidated8asariskfactorforstroke
developmentinindividualswithSCA.Recently,anassociation betweentheTNFA−308Aalleleandstrokeinchildrenwith
SCAwasproposed.7
The overall frequency of IL10 haplotypes found in this studywassimilartothatreportedinarecentstudyconducted inadultindividuals withSCAfromthenortheasternregion ofBrazil.29Thepresentstudyfoundslightlyhigher
frequen-ciesofhaplotypesassociatedwithhighandintermediateIL10 expressions.
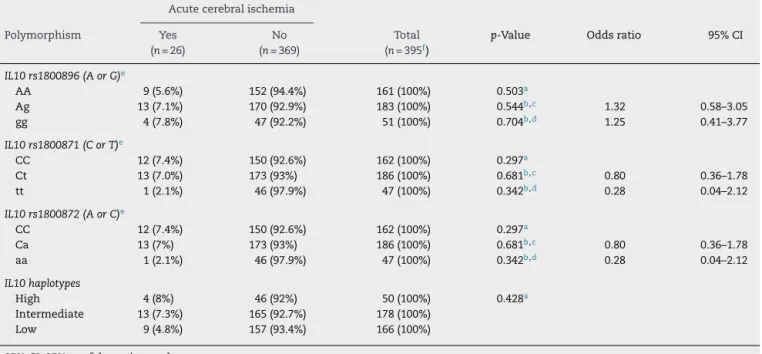
Table1–IL10polymorphismsandhaplotypesofsicklecellanemiachildrenwithandwithoutacutecerebralischemia.
Acutecerebralischemia
Polymorphism Yes (n=26)
No (n=369)
Total (n=395f)
p-Value Oddsratio 95%CI
IL10rs1800896(AorG)e
AA 9(5.6%) 152(94.4%) 161(100%) 0.503a
Ag 13(7.1%) 170(92.9%) 183(100%) 0.544b,c 1.32 0.58–3.05
gg 4(7.8%) 47(92.2%) 51(100%) 0.704b,d 1.25 0.41–3.77
IL10rs1800871(CorT)e
CC 12(7.4%) 150(92.6%) 162(100%) 0.297a
Ct 13(7.0%) 173(93%) 186(100%) 0.681b,c 0.80 0.36–1.78
tt 1(2.1%) 46(97.9%) 47(100%) 0.342b,d 0.28 0.04–2.12
IL10rs1800872(AorC)e
CC 12(7.4%) 150(92.6%) 162(100%) 0.297a
Ca 13(7%) 173(93%) 186(100%) 0.681b,c 0.80 0.36–1.78
aa 1(2.1%) 46(97.9%) 47(100%) 0.342b,d 0.28 0.04–2.12
IL10haplotypes
High 4(8%) 46(92%) 50(100%) 0.428a
Intermediate 13(7.3%) 165(92.7%) 178(100%) Low 9(4.8%) 157(93.4%) 166(100%) 95%CI:95%confidenceinterval.
a X2linear-by-linearassociation. b Fisher’sexacttest.
c Dominantassociation(XXversusXxorxx). d Recessiveassociation(XXorXxversusxx).
e Minoralleles,asrecordedbydbSNP,arerepresentedbylowercaseletters.
f n=394forIL10haplotypesbecauseonechildhadanunclassifiablehaplotype(GCC/GTA).
Table2–IL10polymorphismsandhaplotypesamongsicklecellanemiachildrenwithandwithouthigh-risktranscranial Doppler(TCD).
High-riskTCD
Polymorphism Yes (n=29)
No (n=309)
Total (n=338f)
p-Value Oddsratio 95%CI
IL10rs1800896(AorG)e
AA 7(5.1%) 130(94.9%) 137(100%) 0.151a
Ag 18(11.5%) 139(88.5%) 157(100%) 0.075b,c 2.28 0.95–5.50
gg 4(9.1%) 40(9.1%) 44(100%) 0.78b,d 1.08 0.36–3.25
IL10rs1800871(CorT)e
CC 15(10.7%) 125(89.3%) 140(100%) 0.311a
Ct 11(7.0%) 146(93%) 157(100%) 0.244b,c 0.63 0.30–1.36
tt 3(7.3%) 38(92.7%) 41(100%) 1.00b,d 0.82 0.24–2.85
IL10rs1800872(AorC)e
CC 15(10.7%) 125(89.3%) 140(100%) 0.311a
Ca 11(7.0%) 146(93%) 157(100%) 0.244b,c 0.63 0.30–1.36
aa 3(7.3%) 38(92.7%) 41(100%) 1.00b,d 0.82 0.24–2.85
IL10haplotypes
High 4(9.3%) 39(90.7%) 43(100%) 0.198a
Intermediate 17(11.2%) 135(88.8%) 152(100%) Low 8(5.6%) 134(94.4%) 142(100%) 95%CI:95%confidenceinterval.
a X2Linear-by-linearassociation. b Fisher’sexacttest.
c Dominantassociation(XXversusXxorxx). d Recessiveassociation(XXorXxversusxx).
e Minoralleles,asrecordedbydbSNP,arerepresentedbylowercaseletters.
0.5
0.4
0.3
0.2
0.1
0.0
0.5
0.4
0.3
0.2
0.1
0.0
0.5
0.4
0.3
0.2
0.1
0.0 0.5
0.4
0.3
0.2
0.1
0.0
0 4 8 12 16
P = 0.69
P = 0.42
P = 0.20
P = 0.68 Age (years) to first acute cerebral ischemia
0 4 8 12 16
Age (years) to high-risk TCD
0 4 8 12 16
Age (years) to first acute cerebral ischemia
0 4 8 12 16
Age (years) to high-risk TCD
Cum
ulativ
e probability of acute
cerebr
al ischemia
Cum
ulativ
e probability of acute
cerebr
al ischemia
Cum
ulativ
e probability of high-r
isk
TCD
Cum
ulativ
e probability of high-r
isk
TCD
A
B
C
D
Figure2–CumulativeprobabilityofcerebrovascularcomplicationsaccordingtoIL10expressionhaplotypes.(A)Cumulative probabilityofacutecerebralischemiainchildrenwithHbSSaccordingtoIL10haplotypeslinkedwithIL10expression.() high(n=50);(+)intermediate(n=178);and()lowIL10expression(n=166;p-value=0.69).(B)Cumulativeprobabilityof high-risktranscranialDopplerinchildrenwithHbSSaccordingtoIL10haplotypeslinkedwithIL10expression.()high (n=43);(+)intermediate(n=152);and()lowIL10expression(n=142;p-value=0.20).(C)Cumulativeprobabilityofacute cerebralischemiainchildrenwithsicklecellanemiaaccordingtohomozygousIL10haplotypes.()GCC/GCC(n=50);(+) ACC/ACC(n=34);and()ATA/ATA(n=47)genotypes(p-value=0.42).(D)Cumulativeprobabilityofhigh-risktranscranial DopplerinchildrenwithHbSSaccordingtohomozygousIL10haplotypes.()GCC/GCC(n=43);(+)ACC/ACC(n=29);and() ATA/ATA(n=41)genotypes(p-value=0.68).
signalingpathwaymightnotbeaviabletargetfor pharmaco-logicalinterventionstopreventortreatstrokeinSCA.
Although not statistically significant, survival analysis (Figure2)suggestedatrendtowardacutecerebralischemia protectionin children carrying the haplotypes linked with lowIL10expression.However,thisfindingdiffersfrom pre-vious published studies, associating low IL-10 levels with vaso-occlusive crises,16 osteomyelitis,31 and acute chest
syndrome29inpatientswithSCA.Furthermore,this
contra-dictsapreviousstudyshowingthatalowerserumIL-10level isassociatedwithischemicstrokeinthegeneralpopulation.32
Due to the relatively small number of acute cerebral ischemiaandhigh-riskTCDevents,the statisticalpowerin thepresentstudymayhavebeeninsufficienttorobustly ana-lyzethe associationofIL10 polymorphismsand haplotypes withtheriskoftheseevents.Additionally,theuseof medi-calrecordsasthesolesourceofretrospectivedatamayhave placedfurtherlimitationsonthepossibleconclusionsofthis study.Furthermore,confoundingfactorsmaynothavebeen
recognized,suchassilentinfarcts,whichisoneimportantrisk factorforstrokeinchildrenwithSCA.33,34
Conclusion
TheseresultsdonotsupportthehypothesisthatIL10 haplo-typesareassociatedwiththeriskofacutecerebralischemiaor high-riskTCDinchildrenwithSCA.Furtherlargerscale stud-iesarewarrantedtoconfirmtheabsenceofanyassociation betweenIL10haplotypesandstrokesusceptibilityinchildren withSCA.
Funding
grant # PPM-00266-13), “Conselho Nacional de Desenvolvi-mentoCientíficoeTecnológico”(CNPq;grant#304530/2011-5), and“Coordenac¸ãode Aperfeic¸oamento dePessoalde Nível Superior”(CAPES;absenceofgrantnumber).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
Theauthors acknowledgeall subjectsandparentsfortheir cooperationinthestudy.TheauthorsalsothanktheFundac¸ão Hemominas,NúcleodeAc¸õesePesquisaemApoio Diagnós-tico(Nupad-UFMG),Fundac¸ãodeAmparoàPesquisadeMinas Gerais(FAPEMIG;grant#PPM-00266-13), ConselhoNacional deDesenvolvimentoCientíficoeTecnológico(CNPq;grant# 304530/2011-5),andCoordenac¸ãodeAperfeic¸oamentode Pes-soaldeNívelSuperior(CAPES)fortheirfinancialsupport.
r
e
f
e
r
e
n
c
e
s
1. BernaudinF,VerlhacS,ArnaudC,KamdemA,ChevretS,Hau I,etal.ImpactofearlytranscranialDopplerscreeningand intensivetherapyoncerebralvasculopathyoutcomeina newbornsicklecellanemiacohort.Blood.2011;117(4):1130–40 [quiz1436].
2. Ohene-FrempongK,WeinerSJ,SleeperLA,MillerST,Embury S,MoohrJW,etal.Cerebrovascularaccidentsinsicklecell disease:ratesandriskfactors.Blood.1998;91(1):288–94.
3. AdamsR,McKieV,NicholsF,CarlE,ZhangDL,McKieK,etal. Theuseoftranscranialultrasonographytopredictstrokein sicklecelldisease.NEnglJMed.1992;326(9):605–10.
4. JordanLC,CasellaJF,DebaunMR.Prospectsforprimary strokepreventioninchildrenwithsicklecellanaemia.BrJ Haematol.2012;157(1):14–25.
5. ChangMilbauerL,WeiP,EnensteinJ,JiangA,HilleryCA,Scott JP,etal.Geneticendothelialsystemsbiologyofsicklestroke risk.Blood.2008;111(7):3872–9.
6. AdamsRJ,McKieVC,CarlEM,NicholsFT,PerryR,BrockK, etal.Long-termstrokeriskinchildrenwithsicklecelldisease screenedwithtranscranialDoppler.AnnNeurol.
1997;42(5):699–704.
7. BelisarioAR,NogueiraFL,RodriguesRS,ToledoNE,Cattabriga AL,Velloso-RodriguesC,etal.Associationof
alpha-thalassemia,TNF-alpha(-308G>A)andVCAM-1 (c.1238G>C)genepolymorphismswithcerebrovascular diseaseinanewborncohortof411childrenwithsicklecell anemia.BloodCellsMolDis.2015;54(1):44–50.
8. FlanaganJM,FrohlichDM,HowardTA,SchultzWH,DriscollC, NagasubramanianR,etal.Geneticpredictorsforstrokein childrenwithsicklecellanemia.Blood.2011;117(24):6681–4.
9. HoppeC,KlitzW,ChengS,AppleR,SteinerL,RoblesL,etal. Geneinteractionsandstrokeriskinchildrenwithsicklecell anemia.Blood.2004;103(6):2391–6.
10.MosserDM,ZhangX.Interleukin-10:newperspectivesonan oldcytokine.ImmunolRev.2008;226:205–18.
11.Edwards-SmithCJ,JonssonJR,PurdieDM,BansalA, ShorthouseC,PowellEE.Interleukin-10promoter
polymorphismpredictsinitialresponseofchronichepatitisC tointerferonalfa.Hepatology.1999;30(2):526–30.
12.WangYC,SungWW,WuTC,WangL,ChienWP,ChengYW, etal.Interleukin-10haplotypemaypredictsurvivaland
relapseinresectednon-smallcelllungcancer.PLoSONE. 2012;7(7):e39525.
13.Palafox-SanchezCA,Oregon-RomeroE,Salazar-Camarena DC,ValleYM,Machado-ContrerasJR,CruzA,etal. Associationofinterleukin-10promoterhaplotypeswith diseasesusceptibilityandIL-10levelsinMexicanpatients withsystemiclupuserythematosus.ClinExpMed. 2015;15(4):439–46.
14.ZhengXY,GuanWJ,MaoC,ChenHF,DingH,ZhengJP,etal. Interleukin-10promoter1082/-819/-592polymorphismsare associatedwithasthmasusceptibilityinAsiansandatopic asthma:ameta-analysis.Lung.2014;192(1):65–73.
15.ChavesD,BelisarioA,CastroG,SantoroM,RodriguesC. Analysisofcytokinegenespolymorphismasmarkersfor inhibitordevelopmentinhaemophiliaA.IntJImmunogenet. 2010;37(2):79–82.
16.SarrayS,SalehLR,LisaSaldanhaF,Al-HabboubiHH,MahdiN, AlmawiWY.SerumIL-6,IL-10,andTNFalphalevelsin pediatricsicklecelldiseasepatientsduringvasoocclusive crisisandsteadystatecondition.Cytokine.2015;72(1): 43–7.
17.PlattOS.Preventingstrokeinsicklecellanemia.NEnglJMed. 2005;353(26):2743–5.
18.BelisarioAR,MartinsML,BritoAM,RodriguesCV,SilvaCM, VianaMB.beta-Globingeneclusterhaplotypesinacohortof 221childrenwithsicklecellanemiaorSbeta-thalassemiaand theirassociationwithclinicalandhematologicalfeatures. ActaHaematol.2010;124(3):162–70.
19.JohnsonVJ,YucesoyB,LusterMI.Genotypingofsingle nucleotidepolymorphismsincytokinegenesusingreal-time PCRallelicdiscriminationtechnology.Cytokine.
2004;27(6):135–41.
20.HoebeeB,BontL,RietveldE,vanOostenM,Hodemaekers HM,NagelkerkeNJ,etal.Influenceofpromotervariantsof interleukin-10,interleukin-9,andtumornecrosisfactor-alpha genesonrespiratorysyncytialvirusbronchiolitis.JInfectDis. 2004;189(2):239–47.
21.ShinHD,ParkBL,KimLH,JungJH,KimJY,YoonJH,etal. Interleukin10haplotypeassociatedwithincreasedriskof hepatocellularcarcinoma.HumMolGenet.2003;12(8):901–6.
22.AdamsRJ,McKieVC,HsuL,FilesB,VichinskyE,PegelowC, etal.Preventionofafirststrokebytransfusionsinchildren withsicklecellanemiaandabnormalresultsontranscranial Dopplerultrasonography.NEnglJMed.1998;339(1):5–11.
23.EskdaleJ,GallagherG,VerweijCL,KeijsersV,WestendorpRG, HuizingaTW.Interleukin10secretioninrelationtohuman IL-10locushaplotypes.ProcNatlAcadSciUSA.
1998;95(16):9465–70.
24.GabrielSB,SchaffnerSF,NguyenH,MooreJM,RoyJ, BlumenstielB,etal.Thestructureofhaplotypeblocksinthe humangenome.Science.2002;296(5576):2225–9.
25.BelisarioAR,SalesRR,ToledoNE,MunizMB,
Velloso-RodriguesC,SilvaCM,etal.Reticulocytecountisthe mostimportantpredictorofacutecerebralischemiaand high-risktranscranialDopplerinanewborncohortof395 childrenwithsicklecellanemia.AnnHematol.
2016;95(11):1869–80.
26.HoppeC,ChengS,GrowM,SilbergleitA,KlitzW,
TrachtenbergE,etal.Anovelmultilocusgenotypingassayto identifygeneticpredictorsofstrokeinsicklecellanaemia.Br JHaematol.2001;114(3):718–20.
27.SebastianiP,RamoniMF,NolanV,BaldwinCT,SteinbergMH. Geneticdissectionandprognosticmodelingofovertstrokein sicklecellanemia.NatGenet.2005;37(4):435–40.
29.SommetJ,AlbertiC,CouqueN,VerlhacS,HaouariZ,
MohamedD,etal.Clinicalandhaematologicalriskfactorsfor cerebralmacrovasculopathyinasicklecelldiseasenewborn cohort:aprospectivestudy.BrJHaematol.2016;172(6):966–77.
30.HyacinthHI,GeeBE,AdamkiewiczTV,AdamsRJ,KutlarA, StilesJK,etal.PlasmaBDNFandPDGF-AAlevelsare
associatedwithhighTCDvelocityandstrokeinchildrenwith sicklecellanemia.Cytokine.2012;60(1):302–8.
31.SarrayS,AlmawiWY.ContributionofreducedInterleukin-10 levelstothepathogenesisofosteomyelitisinchildrenwith SickleCellDisease.ClinVaccineImmunol.2015;22(9):1020–4.
32.XieG,MyintPK,ZamanMJ,LiY,ZhaoL,ShiP,etal. Relationshipofseruminterleukin-10anditsgenetic
variationswithischemicstrokeinaChinesegeneral population.PLOSONE.2013;8(9):e74126.
33.ThangarajhM,YangG,FuchsD,PonisioMR,McKinstryRC, JajuA,etal.Magneticresonanceangiography-defined intracranialvasculopathyisassociatedwithsilentcerebral infarctsandglucose-6-phosphatedehydrogenasemutationin childrenwithsicklecellanaemia.BrJHaematol.
2012;159(3):352–9.