Evaluation of the efficacy and safety of endovascular
management for transplant renal artery stenosis
Leonardo G.M. Valle,I,* Rafael N. Cavalcante,IJoaquim M. Motta-Leal-Filho,IBreno B. Affonso,IFrancisco L. Galastri,I Marisa P. Doher,IINadia K. Guimara˜es-Souza,IIAna K.N. Cavalcanti,IIRodrigo G. Garcia,IA´ lvaro Pacheco-Silva,II Felipe NasserI
IDepartamento de Radiologia Intervencionista, Hospital Israelita Albert Einstein, Sao Paulo, SP, BR.IIDepartamento de Nefrologia, Hospital Israelita
Albert Einstein, Sao Paulo, SP, BR.
OBJECTIVES: To evaluate the safety and efficacy of endovascular intervention with angioplasty and stent placement in patients with transplant renal artery stenosis.
METHODS: All patients diagnosed with transplant renal artery stenosis and graft dysfunction or resistant systemic hypertension who underwent endovascular treatment with stenting from February 2011 to April 2016 were included in this study. The primary endpoint was clinical success, and the secondary endpoints were technical success, complication rate and stent patency.
RESULTS:Twenty-four patients with transplant renal artery stenosis underwent endovascular treatment, and three of them required reinterventions, resulting in a total of 27 procedures. The clinical success rate was 100%. All graft dysfunction patients showed decreased serum creatinine levels and improved estimated glomerular filtration rates and creatinine levels. Patients with high blood pressure also showed improved control of sys-temic blood pressure and decreased use of antihypertensive drugs. The technical success rate of the procedure was 97%. Primary patency and assisted primary patency rates at one year were 90.5% and 100%, respectively. The mean follow-up time of patients was 794.04 days after angioplasty.
CONCLUSION:Angioplasty with stent placement for the treatment of transplant renal artery stenosis is a safe and effective technique with good results in both the short and long term.
KEYWORDS: Transplant Renal Artery Stenosis; Endovascular Treatment; Stenting; Renal Transplantation.
Valle LG, Cavalcante RN, Motta-Leal-Filho JM, Affonso BB, Galastri FL, Doher MP, et al. Evaluation of the efficacy and safety of endovascular management for transplant renal artery stenosis. Clinics. 2017;72(12):773-779
Received for publication onJune 6, 2017;First review completed onSeptember 12, 2017;Accepted for publication onOctober 17, 2017 *Corresponding author. E-mail: dr.lgmvalle@gmail.com
’ INTRODUCTION
Renal transplantation is an important therapeutic option for patients with end-stage chronic kidney disease, and it is associated with increased rates of survival and a better quality of life in these patients (1).
However, certain complications following transplantation may affect the graft and patient survival. With advances in immunosuppressive drugs, graft loss due to rejection has decreased to rates of 20-30%. Additionally, other complica-tions have also gained major importance (2). Transplant renal artery stenosis (TRAS) is the most common vascular compli-cation that may occur after transplantation, affecting 1-23% of patients (3).
Vascular alterations in the renal artery of the transplanted kidney may be either asymptomatic and/or associated with cases of refractory hypertension during clinical treatment and/or graft dysfunction (6), and they are typically related to decreases in overall survival rates of patients who undergo renal transplantation (4-7).
The benefits of endovascular intervention for the correc-tion of TRAS remain under debate, however, and few results are currently available in the literature. It is believed that this technique may aid in the control of high blood pressure and may improve renal function, contributing substantially to better graft and patient survival (3,8-10).
This study aimed to evaluate the safety and efficacy of endovascular intervention with angioplasty and stent place-ment in patients with TRAS.
’ MATERIALS AND METHODS
Study design
This was a retrospective study carried out in the Depart-ment of Interventional Radiology and Nephrology, and it
DOI:10.6061/clinics/2017(12)09
Copyright&2017CLINICS–This is an Open Access article distributed under the terms of the Creative Commons License (http://creativecommons.org/licenses/by/ 4.0/) which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.
was approved by the Ethics and Research Committee of the institution.
Patients and eligibility
All patients diagnosed with TRAS who underwent endo-vascular treatment from February 2011 to April 2016 were included in this study.
The diagnosis of TRAS was confirmed via Doppler ultra-sonography (peak systolic velocity4200-250 cm/s, resistance
index 40.8, pulsatility index 41.5, stenosis/prestenotic
velocity gradient42:1,tardus parvusin systolic acceleration 40.1 seconds, or acceleration in renal hilum4100 cm/s).
All patients included in the study had either graft dysfunc-tion or resistant systemic hypertension. Graft dysfuncdysfunc-tion was defined as delayed graft function in kidney transplants assessed by measuring the creatinine level and estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epi-demiology Collaboration (CKD-EPI) equation, while resistant systemic hypertension was defined as blood pressure4140/
90 mmHg, despite treatment with antihypertensive drugs.
Endovascular procedure
Patients with stenosis diagnosed using Doppler ultrasono-graphy underwent an angiographic study. The access route for the procedures was either the ipsilateral or contralateral common femoral artery. Pelvic and transplanted renal artery angiograms were performed for anatomical evaluation and lesion quantification.
Following angiographic confirmation of stenosis, systemic heparinization was performed with unfractionated heparin, followed by transposition of the lesion with a 0.014 guide-wire. All cases were treated with stent implantation: in one case, a self-expanding stent was used, and in the other cases, expandable balloon stents, such as Visions Cobalt Chro-mium (Abbott Laboratories, Chicago, IL, USA), TAXUSt
Libertét(Boston Scientific Corporation, Natick, MA, USA), Expresst Vascular Stent (Boston Scientific Corporation), Dynamic Stent (BIOTRONIK, Berlin, Germany), and/or Direct-Stents
(InSitu Technologies, St. Paul, MN, USA), were used. The materials were used without standardization at the discretion of the interventional physician. At the end of the procedure, hemostatic devices, such as the Perclose Proglide (Abbott) and/or the StarClose (Abbott) devices, were used at the puncture site.
Clinical variables and outcomes
Information such as sex, age, risk factors, technical data related to transplantation (i.e., from both the donor and recipient), laboratory tests, diagnostic methods, anatomical pattern of stenosis, and technical details related to treatment (e.g., contrast volume, tomographic imaging, material used, treatment methods, postoperative assessment with arterial patency, blood pressure control, renal function, and survival) was collected. Renal function was determined using serum creatinine levels, and the eGFR was obtained in the pre-operative and postpre-operative periods (i.e., 48 hours prior and 30, 60, and 90 days after) via the CKD-EPI equation.
Technical success was defined as revascularization with stent implantation without complications and/or with com-plete absence of oro30% residual stenosis. Clinical success
was defined as improved renal function (both creatinine levels and eGFR) in cases of renal dysfunction or improved blood pressure control, with a reduction in the use of
antihypertensive drugs, in patients with arterial hyperten-sion secondary to TRAS.
Statistical analysis
The quantitative characteristics evaluated in the patients are described using summary measures (i.e., mean, standard deviation, median and quartiles), and the qualitative vari-ables are described using absolute and relative frequencies.
The creatinine levels were compared preoperatively using the lowest value at follow-up via the paired Wilcoxon test. Blood pressure controls, measured mainly by the total number of medications used, were also compared between the pre-operative and follow-up periods using the paired Wilcoxon test. A Kaplan-Meier analysis was used to determine the graft survival curve and to evaluate patency rates and the probability of occurrence of events. A p-value p0.05 was
considered significant.
’ RESULTS
From February 2011 to April 2016, 29 patients who were diagnosed with TRAS using Doppler ultrasonography underwent angiography. Twenty-four of these cases under-went endovascular treatment, and three required reinterven-tions, resulting in a total of 27 procedures.
Five patients underwent only diagnostic angiography with-out intervention and, therefore, were excluded from the final analysis. In four of these cases, angiography showed only mild stenosis with no flow change, while the remaining patient demonstrated severe stenosis and thrombosis of segmental branches, with changes in the parenchymal phase of the angiographic study. No treatment approach was indicated for this patient.
Patient profile
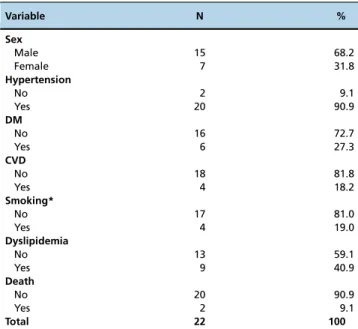
The profile of the study population and the data con-cerning the renal grafts, as well as the surgical procedures used, are described in Table 1 and Table 2.
Table 1-Demographic Profile of Patients.
Variable N %
Sex
Male 15 68.2
Female 7 31.8
Hypertension
No 2 9.1
Yes 20 90.9
DM
No 16 72.7
Yes 6 27.3
CVD
No 18 81.8
Yes 4 18.2
Smoking*
No 17 81.0
Yes 4 19.0
Dyslipidemia
No 13 59.1
Yes 9 40.9
Death
No 20 90.9
Yes 2 9.1
Total 22 100
* Information was not available for all patients. DM: Diabetes mellitus.
Of the 24 patients who were diagnosed with TRAS and underwent endovascular treatment, 21 had graft dysfunction and 3 had resistant systemic hypertension. Three of these patients underwent re-stenosis due to graft dysfunction.
All arterial anastomoses were performed on the iliac arteries. In one case, anastomosis was performed on the internal iliac artery (4.5% of cases), while anastomosis was performed on the external iliac artery in the remaining cases.
Of the 24 grafts used in the study, six (25%) grafts were from living donors. Furthermore, only one graft used during a reintervention was from a living donor (33% for reintervention).
Technical and clinical success
The technical success rate of the procedure was 97%. The mean pretreatment and post-treatment stenosis rates were 79.40% and 2.40%, respectively. No relationship between the postoperative period and the stenosis site was found (Table 3). The clinical success rate was 100%. All patients showed decreased serum creatinine levels (Figure 1) and improved eGFR and creatinine levels (Figure 2), with a statistically significant improvement noted following 30 days of treat-ment (po0.001), which was further maintained after 60
and 90 days (Table 4). Patients with high blood pressure also showed improved control of systemic blood pressure
and decreased use of antihypertensive drugs (po0.008)
(Table 5).
Procedures and patency
The median diameter and length of the stents used were 5 and 12 mm, respectively (Table 6). Intraoperative tomo-graphic imaging was performed in 37% of the cases, with no significant increase in contrast volume or radioscopy time noted (Table 7).
The primary patency and assisted primary patency rates in one year were 90.5% and 100%, respectively. Both rates were maintained until the end of the study, considering the mean follow-up time of patients of 794.04 days after angioplasty (Figure 3). During the evaluation period, two patients died due to non-renal causes; their grafts were patent and func-tioned properly for 587 days and 1,023 days, respectively.
Re-interventions
Of the three cases requiring re-intervention, one was due to in-stent re-stenosis after two months of treatment (Dynamic Stent 512, BIOTRONIK, Berlin, Germany).
In this case, the patient’s renal dysfunction remained after stent placement. A new Doppler ultrasonography exam showed increased resistance in the renal artery. Angioplasty
Table 3-Evaluation of the Stenosis Site.
Stenosis site Days until TRAS
Mean SD Median Minimum Maximum N p
Anastomosis 190.23 212.82 135 13 706 13 0.794
Distal 138.56 49.81 130 86 246 9
Total 169.09 165.84 132.5 13 706 22
Mann-WhitneyUtest.
Table 2-Data Analysis Associated with the Renal Graft and Surgical Procedure.
Variable Mean SD Median Minimum Maximum N
Age 49.9 14.3 51.5 20.0 75.0 22
Days until TRAS 169.1 165.8 132.5 13.0 706.0 22
Donor age 42.6 13.9 43.5 19.0 69.0 22
Graft survival rate 689.5 462.9 575.5 115 2069 22
Radioscopy time (min) 20.4 11.8 16.1 7.2 56 18
Contrast volume (ml) 185.1 52.0 180.0 100 300 17
Figure 1 -Preoperative and postoperative mean serum creatinine levels (in days).
using an IntraStents
balloon catheter (Covidien, Dublin, Ireland) was subsequently performed.
The second case of re-intervention was due to graft dysfunc-tion. Three renal arteries were transplanted, with one branch initially treated with a stent (Visions Cobalt Chromium 3.015, Abbott Laboratories) and two branches treated with
a pharmacological balloon catheter (FALCON 340).
Dur-ing the follow-up, five months after angioplasty, the patient’s serum creatinine levels were found to have increased, and a stent was placed in the two branches initially treated with a balloon (Visions
Cobalt Chromium 2.028, Abbott
Labora-tories), with progression occurring favorably.
The third case underwent re-intervention initially due to graft dysfunction. The patient underwent endovascular treat-ment with stent placetreat-ment (Dynamic Renal Stent, BIOTRO-NIK, Berlin, Germany) and, after an initial improvement of symptoms, exhibited further worsening of renal function. A second intervention was performed in which renal stent patency was observed, although with dissection of the left
Table 4-Variation in Serum Creatinine Levels and Creatinine Clearance Rates (CKD-EPI) throughout the Study.
Variable Time Mean SD Median Minimum Maximum N p
Creatinine Preoperative period 3.05 1.61 2.76 1.34 6.88 22 o0.001
Immediate postoperative period 2.56 1.49 2.01 1.27 7.59 22
30 days 1.73 0.47 1.78 0.89 3.03 21
60 days 1.78 0.59 1.68 0.67 2.94 20
90 days 1.62 0.53 1.68 0.65 3.03 21
End of follow-up 1.67 0.68 1.68 0.80 3.49 22
CKD-EPI Preoperative period 28.39 17.18 23.26 7.48 68.15 22 o0.001
Immediate postoperative period 33.49 15.64 31.84 6.93 63.00 22
30 days 44.92 15.06 40.85 21.47 76.57 21
60 days 46.71 20.78 42.41 23.62 103.90 20
90 days 51.41 23.38 41.11 21.47 107.78 21
End of follow-up 51.91 21.71 45.50 18.00 100.00 22
Bonferroni correction.
Table 5-Preoperative and Postoperative Quantitative Analysis of Antihypertensive Drugs used by Patients.
Variable Mean SD Median Minimum Maximum N p
Preoperative antihypertensive drugs 2.09 1.15 2 0 4 22 0.008
Postoperative antihypertensive drugs 1.59 1.05 1.5 0 4 22
Wilcoxon signed-rank test.
Table 6-Evaluation of Patency Times and Stent Sizes.
Variable Mean SD Median Minimum Maximum N
Patency time (days) 488.91 450.09 345 21 1830 22
Mean diameter of stents 4.89 1.46 5 2 8 19
Mean length of stents 12.68 7.14 12 0 28 22
Table 7-Evaluation of Contrast Volume in Milliliters and Scoping Time Related to Cone-beam CT.
Variable CBCT Mean SD Median Minimum Maximum N p
Contrast volume (ml) No 195.11 58.01 200 130 300 9 0.481
Yes 173.75 45.336 175 100 250 8
Total 185.06 51.986 180 100 300 17
Radioscopy time (min) No 21.536 13.3556 17.765 10.5 56 10 0.762
Yes 19.08 10.2365 16.06 7.2 34.5 8
Total 20.444 11.7964 16.125 7.2 56 18
Mann-WhitneyUtest.
iliac artery, which was deemed to be a possible complication related to the first procedure. The patient underwent place-ment of a self-expanding stent (Everflext, Covidien, Dublin, Ireland) in the iliac artery, and renal function improved.
Complications
No immediate complications were observed after the pro-cedure. A case of dissection of the common iliac artery with extension to the external iliac artery was diagnosed 30 days after the initial procedure but was resolved with a self-expanding stent.
’ DISCUSSION
This retrospective study was carried out to evaluate the impact of endovascular treatment in patients diagnosed with TRAS, which was proven to be an effective and safe procedure.
The prevalence of TRAS observed in our study was 5.6%, similar to that found in other studies (3,5,6,11). Our analysis revealed renal dysfunction, associated with abnormalities in Doppler ultrasonography, to be a major clinical presentation and to be present in 90% of cases, a finding similar to that in other reviews (4,5).
A higher peak systolic velocity and velocity gradient between the transplanted renal artery and the iliac artery is a criterion of higher sensitivity in Doppler ultrasonography for TRAS (6). Although some authors cited the presence of a peak systolic pressure gradient410% or stenosis450% in
angiography as an indication for intervention, no consensus has been established in the literature regarding stenosis ranges and/or standards for intervention (12). In this study, Doppler ultrasonography was used as a screening method in patients with a clinical presentation of TRAS.
The anastomosis site and onset may be related to different risk factors. The mean onset of TRAS in our study was 169 days, and the most common site of stenosis was the anastomosis, which is similar to findings described in other studies, and was present in more than half of the cases in this study (4,6,13). This stenosis may be associated with mechan-ical lesions of the blood vessels during organ recruitment
or the surgical procedure and was found to present early after transplantation (14,15). The stenosis that occur later, sometimes several years after transplantation, usually reflect atherosclerotic disease and are also associated with rejection and/or cytomegalovirus infection (4,5,16,17). In our study, no relationship was found between the anastomosis site and time of onset. A review of the medical records showed that no vascular lesion was reported during organ recruitment or the surgical procedure, although other changes not described during the procedure, such as hyperflexion of blood vessels and endothelial injury during graft perfusion, are related to this type of stenosis.
The treatment of TRAS is crucial, as the presence of graft dysfunction and/or high blood pressure may alter the renal graft and compromise patient survival (10,18-20).
Concerning therapeutic options, clinical and medical care is limited to patients with TRAS, stable renal function, and non-significant lesions, while surgical intervention should be reserved as a salvage option in cases in which angioplasty is unsuccessful (9). Endovascular intervention, despite risks such as renal artery disease, re-stenosis, thromboembolism and complications related to the puncture site, may be considered a first-line therapy for TRAS (9,21,22).
Our results are similar to those in the literature, which describe a technical success rate ranging from 89 to 100% (4,16,21,23). In our study, the technical success rate was 97% and the clinical success rate was 100%.
Ngo et al. systematically reviewed 32 studies of TRAS and found a clinical success rate between 65.5 and 94% (24). Among the 28 studies that evaluated renal function, the mean creatinine level reduced 0.45 mg/dL after 30 days and 0.82 mg/dL after six months. Estimated GFR was evaluated in 11 studies, which showed an average gain of 8.6 mL/min/ 1.73 m2 three months after endovascular intervention. Our review showed that renal function significantly improved following intervention, with values higher than those found in these studies. The mean creatinine level decreased by 1.32 mg/dL and the eGFR increased by 16.53 mL/min/ 1.73 m2 after 30 days. These values were maintained after
90 days, and a short-term improvement in renal function was
predictive in the long run. The mean follow-up of patients was 794 days, and all of them maintained stable renal func-tion until the end of the follow-up period. The maintenance of satisfactory kidney function after transplantation is directly related to the graft and patient survival rates, and we expect that these factors may have an effect on our patients (18,19).
Among the patients undergoing treatment because of graft dysfunction, three underwent intervention due to delayed graft function (DGF), as they remained on dialysis even after renal transplantation. All of them left dialysis and main-tained adequate renal function after correction of TRAS, which had an important effect on graft and patient survival rate, as DGF is associated with poor outcomes (19,25).
Despite the heterogeneous results found following endo-vascular treatment of TRAS with balloon catheters, studies with stent placement showed that high blood pressure was improved following this treatment, with a reduction of anti-hypertensive drugs, which was also found in our study (24,26). Adequate blood pressure control is very important, as high blood pressure can result in a decreased graft survival rate and left ventricular hypertrophy, the latter of which is an independent risk factor for heart failure and death in both the general population and renal transplant recipients (20,27).
During follow-up, patency levels ranging from 63 to 82% were described one year after endovascular treatment, with a re-stenosis rate ranging from 10 to 36% (28). In our study, a primary patency of 90.5% was reached after three months, and assisted primary patency of 100% was achieved after two years following angioplasty with a balloon-expandable stent. In the literature, early complications are noted, including bleeding, pseudoaneurysm formation, thrombosis, arterial dissection, hematoma, and intimal flap, in approximately 10% of cases (6,29). In our experience, no cases of early complications were reported; however, it was observed that late complications occurred in 4% of patients, represented by one cases of iliac artery dissection.
No randomized trials comparing multiple treatment tech-niques for TRAS were found in the literature. This study is limited mainly because it is not a multicenter retrospective study, although our population was larger than that in many studies found in the literature.
Based on our experience, we conclude that angioplasty with stent placement for the treatment of TRAS is a safe and effective technique for improving eGFR, serum creatinine levels, high blood pressure control and graft survival rate, with excellent results in both the short and long term.
’ AUTHOR CONTRIBUTIONS
Valle LG conceived and designed the study and was responsible for the analysis and interpretation, data collection, manuscript writing, critical revision of the manuscript, statistical analysis, final approval of the manuscript and overall responsibility. Cavalcante RN and Motta-Leal-Filho JM were responsible for the analysis and interpretation, manuscript writing, critical revision of the manuscript, statistical analysis,final approval of the manuscript and overall responsibility. Affonso BB conceived and designed the study and was responsible for the critical revision of the manuscript, final approval of the manuscript and overall responsibility. Galastri FL conceived and designed the study and was responsible for the analysis and interpretation, manuscript writing,final approval of the manuscript and overall responsibility. Doher MP was responsible for the analysis and interpretation, data collection, manuscript writing, critical revision of the manuscript, statistical analysis,final approval of the manu-script and overall responsibility. Guimarães-Souza NK was responsible for
the analysis and interpretation, critical revision of the manuscript, final approval of the manuscript and overall responsibility. Cavalcanti AK was responsible for the data collection, manuscript writing,final approval of the manuscript and overall responsibility. Garcia RG conceived and designed the study and was responsible for the critical revision of the manuscript, final approval of the manuscript and overall responsibility. Pacheco-Silva A was responsible for the critical revision of the manuscript,final approval of the manuscript and overall responsibility. Nasser F Conceived and designed the study and was responsible for the critical revision of the manuscript, final approval of the manuscript and overall responsibility.
’ REFERENCES
1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725-30, http://dx.doi.org/10.1056/NEJM 199912023412303.
2. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605-12, http://dx. doi.org/10.1056/NEJM200003023420901.
3. Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15(1):134-41, http://dx.doi.org/10.1097/01.ASN.0000 099379.61001.F8.
4. Audard V, Matignon M, Hemery F, Snanoudj R, Desgranges P, Anglade MC, et al. Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant. 2006;6(1):95-9, http://dx.doi.org/10.1111/ j.1600-6143.2005.01136.x.
5. Ghirardo G, De Franceschi M, Vidal E, Vidoni A, Ramondo G, Benetti E, et al. Transplant renal artery stenosis in children: risk factors and outcome after endovascular treatment. Pediatr Nephrol. 2014;29(3):461-7. 6. Braga AF, Catto RC, Dalio MB, Tenório EJ, Ribeiro MS, Piccinato CE, et al.
Endovascular approach to transplant renal artery stenosis. Ann Trans-plant. 2015;20:698-706.
7. Glebova NO, Brooke BS, Desai NM, Lum YW. Endovascular interventions for managing vascular complication of renal transplantation. Semin Vasc Surg. 2013;26(4):205-12, http://dx.doi.org/10.1053/j.semvascsurg.2014. 06.013.
8. Biederman DM, Fischman AM, Titano JJ, Kim E, Patel RS, Nowakowski FS, et al. Tailoring the endovascular management of transplant renal artery stenosis. Am J Transplant. 2015;15(4):1039-49, http://dx.doi.org/ 10.1111/ajt.13105.
9. Chen W, Kayler LK, Zand MS, Muttana R, Chernyak V, DeBoccardo GO. Transplant renal artery stenosis: clinical manifestations. diagnosis and therapy. Clin Kidney J. 2015;8(1):71-8.
10. Feldman HI, Gayner R, Berlin JA, Roth DA, Silibovsky R, Kushner S, et al. Delayed function reduces renal allograft survival independent of acute rejection. Nephrol Dial Transplant. 1996;11(7):1306-13, http://dx.doi.org/ 10.1093/ndt/11.7.1306.
11. Nasserala JC, Oliveira CM, Cerqueira JB, Souza S, Silva SL, Santos LC, et al. Artery Stenosis of the Renal Graft: Experience of a Center of Northeastern Brazil. Transplant Proc. 2016;48(1):74-80, http://dx.doi.org/ 10.1016/j.transproceed.2015.11.004.
12. Beecroft JR, Rajan DK, Clark TW, Robinette M, Stavropoulos SW. Trans-plant renal artery stenosis: outcome after percutaneous intervention. J Vasc Interv Radiol. 2004;15(12):1407-13, http://dx.doi.org/10.1097/01. RVI.0000141338.62574.F4.
13. Taylan Ozgur Sezer and Cuneyt Hoscoskun. Vascular Complications After Renal Transplantation, Vascular Surgery, Dr. Dai Yamanouchi [Internet], InTech 2012 [cited 09 June 2017]. Available from: https://www. intechopen.com/books/vascular-surgery/vascular-complications-after-renal-transplantation.
14. Fervenza FC, Lafayette RA, Alfrey EJ, Petersen J. Renal artery stenosis in kidney transplants. Am J Kidney Dis. 1998;31(1):142-8, http://dx.doi. org/10.1053/ajkd.1998.v31.pm9428466.
15. Roberts JP, Ascher NL, Fryd DS, Hunter DW, Dunn DL, Payne WD, et al. Transplant renal artery stenosis. Transplantation. 1989;48(4):580-3. 16. Becker BN, Odorico JS, Becker YT, Leveson G, McDermott JC, Grist T,
et al. Peripheral vascular disease and renal transplant artery stenosis: a reappraisal of transplant renovascular disease. Clin Transplant. 1999; 13(4):349-55, http://dx.doi.org/10.1034/j.1399-0012.1999.130412.x. 17. Rengel M, Gomes-Da-Silva G, Incháustegui L, Lampreave JL, Robledo R,
Echenagusia A, et al. Renal artery stenosis after kidney transplantation: diagnostic and therapeutic approach. Kidney Int Suppl. 1998;68:S99-106, http://dx.doi.org/10.1038/sj.ki.4490573.
long-term kidney transplant survival. Kidney Int. 2002;62(1):311-8, http://dx.doi.org/10.1046/j.1523-1755.2002.00424.x.
19. Harada KM, Sampaio EL, Freitas TV, Felipe CR, Park SI, Machado PG. Fatores de risco associados à perda do enxerto e óbito após o transplante renal. J Bras Nefrol. 2008;30(3):213-20.
20. Mange KC, Feldman HI, Joffe MM, Fa K, Bloom RD. Blood pressure and the survival of renal allografts from living donors. J Am Soc Nephrol. 2004;15(1):187-93, http://dx.doi.org/10.1097/01.ASN.000010 4574.04006.08.
21. Salsamendi J, Pereira K, Baker R, Bhatia SS, Narayana G. Successful technical and clinical outcome using a second generation balloon expandable coronary stent for transplant renal artery stenosis: our experience. J Radiol Case Rep. 2015;9(10):9-17, http://dx.doi.org/ 10.3941/jrcr.v9i10.2535.
22. Seratnahaei A, Shah A, Bodiwala K, Mukherjee D. Management of transplant renal artery stenosis. Angiology. 2011;62(3):219-24, http://dx. doi.org/10.1177/0003319710377076.
23. Sankari BR, Geisinger M, Zelch M, Brouhard B, Cunningham R, Novick AC. Post-transplant renal artery stenosis: impact of therapy on long-term kidney function and blood pressure control. J Urol. 1996;155(6):1860-4, http://dx.doi.org/10.1016/S0022-5347(01)66030-0.
24. Ngo AT, Markar SR, Lijster MS, Duncan N, Taube D, Hamady MS. A systematic review of outcomes following percutaneous transluminal
angioplasty and stenting in the treatment of transplant renal artery ste-nosis. Cardiovasc Intervent Radiol. 2015;38(6):1573-88, http://dx.doi. org/10.1007/s00270-015-1134-z.
25. Sandes-Freitas TV, Felipe CR, Aguiar WF, Cristelli MP, Tedesco-Silva H, Medina-Pestana JO. Prolonged delayed graft function is associated with inferior patient and kidney allograft survivals. PLoS One. 2015;10(12): e0144188, http://dx.doi.org/10.1371/journal.pone.0144188.
26. Abate MT, Kaur J, Suh H, Darras F, Mani A, Nord EP. The use of drug-eluting stents in management of transplant renal artery stenosis. Am J Transplant. 2011;11(10):2235-41, http://dx.doi.org/10.1111/j.1600-6143. 2011.03652.x.
27. Fernández-Fresnedo G, Escallada R, Martin de Francisco AL, Ruiz JC, Rodrigo E, Castro SS, et al. Association between pulse pressure and car-diovascular disease in renal transplant patients. Am J Transplant. 2005; 5(2):394-8, http://dx.doi.org/10.1111/j.1600-6143.2004.00694.x. 28. Bruno S, Ferrari S, Remuzzi G, Ruggenenti P. Doppler ultrasonography
in post-transplant renal artery stenosis: a reliable tool for assessing effectiveness of revascularization? Transplantation. 2003;76(1):147-53, http://dx.doi.org/10.1097/01.TP.0000071849.78031.13.