IgA Testing for Diagnosis of Retroviral
Infections in the Caribbean1
I? R. RIDEL,~ J. C. ARTus, & A. CAFRON~
In Guadeloupe, a Caribbean island with a high prevalence of HTLV-Z infected sub- jects, the percentage of false positive results for HIV IgG is high, requiring addi- tional time and expense for confirmatory tests. This article describes a simple way of overcoming this problem. First, all IgG and IgG immune complexes are removed by protein G treatment, and then IgA enzyme-linked immunosorbent assay (ELISA) and the Western blot test are performed with minor modification of commercially available kits.
0
ur experience in diagnosing human immunodeficiency virus (HIV) in- fection of man by means of the IgGenzyme-linked immunosorbent assay
(ELISA) indicates that about 5% of the sera tested yield false positive results. Moreover, IgG Western blot diagnosis
commonly yields one or more non-
specific bands, the classification of which is difficult-requiring further costly and time-consuming laboratory procedures.
One circumstance apparently aggra- vating this false positives problem on the Caribbean island of Guadeloupe is infec- tion of test subjects with another retro- virus, the human T-cell leukemia virus
*This article will also be published in Spanish in the
Bolefin de la Oficina Sanitaria Panamericana. vol. 112. 1992. This work was supported by funding fro6 the Institut Pasteur of Paris. Correspondence should be addressed to Dr. l? R. Ridel, Institut Pasteur de la Guadeloupe, PO. Box 484, Morne Joivere, P.O. Box 484, 97165, Pointe a Pitre Cedex, F.W.I.
ZInsiitut Pasteur de la Guadeloupe, Pointe-Hitre Cedex, F. W.I.
%stitut Pasteur de Lille, Centre de Biologie et d’humunologie Parasitaire, 8 rue Camille Guerin, 59019 Lille CEDEX, France.
(HTLV), which is highly prevalent on Guadeloupe and various other Caribbean islands. In this regard, we found that 35% to 45% of a group of subjects testing positively for HIV-l/2 by IgG ELISA and negatively for HIV-l/2 by IgG West- ern blot tested positively for HTLV-I in- fection by both IgG ELISA and IgG West- ern blot.
The study reported here was per- formed to determine whether we could easily perform a more specific ELISA HIV test directed at IgA rather than IgG anti- bodies, as described elsewhere (I), under the circumstances prevailing in Gua- deloupe. In contrast to IgG antibodies, neither IgA nor IgM antibodies cross the placenta, and so both are suitable for con- genital AIDS diagnosis (2). However, previous studies have found IgA HIV an- tibodies to persist longer than their IgM counterparts, and so we chose to seek a solution to the nonspecific results prob- lem by testing for IgA antibodies. By way of preparation, we used protein G to re- move IgG from the test sera (2, 3). Then, in order to save time and expense, we performed ELISA and Western blot pro- cedures with modified versions of com- mercially available kits.
MATERIALS AND METHODS
Patients
The serum samples tested were ob- tained from 10 Guadeloupe residents who were follow-up patients at the is- land’s Pasteur Institute. All 10 study sub- jects had yielded positive ELISA results for HIV-l/2 IgG (Rapid’ ELAVIA Mixt Di- agnostic Pasteur, Marnes la Coquette, France), and the sera of all but two had exhibited one or more nonspecific bands when tested for HIV-l/2 IgG by Western blot (New Lav-Blot I or II Diagnostic Pasteur). These two latter patients (whose cases are designated 9 and 10) were coinfected with HIV-l and HTLV-I.
None of the 10 study sera yielded posi- tive results when tested for HIV-2 (Pepti Lav or New Lav-Blot II, Diagnostic Pasteur). However, all of them yielded positive ELISA results for HTLV-I IgG (Abbott, Paris, France) and showed spe- cific HTLV-I IgG bands (P19, 21, and 24) when tested by Western blot (DuPont de Nemours, Paris, France). These positive ELISA and Western blot results (before removal of IgG) are indicated in Table 2.
Blood Samples
Blood for the investigation was ob- tained from the 10 study subjects by veni- puncture, and the resulting sera were stored at -20°C until used in the pro- cedures described below.
IgG Removal
IgG antibodies, including those pre- sent in autoantibodies or circulating im- mune complexes (CIC), tend to interfere with assays for IgA antibodies, reducing the latters’ sensitivity and specificity. Therefore, before testing the 10 study subjects’ sera for IgA, we treated those
sera with protein G (3) to remove all sub- classes of IgG.
The test sera were treated for 90 min- utes with protein G (Sepharose-Phar- macia, Uppsala, Sweden) at 37°C using a slightly modified version of the meth- odology described in Weiblen et al (1). Briefly, 25~1 of sera were incubated with 100,ul of a 50% suspension of protein G agarose in 0.02M phosphate-buffered sa- line at pH 7.6 and 37°C for 90 minutes under constant agitation. The sera were then diluted 125 (for Western blot test- ing) or 1:50 (for the ELISA) using the diluent reagent provided with each kit.4 The extent to which IgG had been re- moved was assessed in terms of the turbidity produced by an anti-human IgG serum (Behring Diagnostic, Rueil- Malmaison, France).
ELISA Procedures
The IgG ELISA testing for HIV-l/2 and HTLV-I was performed according to the
manufacturers’ recommendations (see
footnote 4).
In conducting the IgA ELISA testing for HIV-l/2 and HTLV-I, certain modifica- tions were made to the recommenda- tions. These were as follows: (1) The con- jugate was a 1:500 dilution of a F(ab’), phosphatase-conjugated anti-human IgA (Sigma, St. Louis, USA). (2) The sub- strate buffer was 1M diethanolamine (Sigma) containing 0.5M MgCl, (Sigma) and 0.02% NaN, (Sigma), pH 9.8 with HCl. (3) The chromogenic agent was P-nitrophenyl phosphate employed at lmg per ml of substrate buffer. (4) After 45 minutes of incubation at 37’C, absor- bance was determined using a 405nm fil- ter with a 62Onm reference filter.
4The makers of the kits used were as follows: ELISA HIV-l/2, Pasteur Diagnostic; ELISA HTLV-I, Ab- bott; Western blot Hw, Pasteur Diagnostic; West- ern blot HTLV-I, DuPont de Nemours.
Calculations and interpretation of the ELISA results were done using the pro- cedure described in the kit booklet. All IgG and IgA results exceeding the calcu- lated cutoff point by 10% or more were considered positive.
Western Blot Procedure
Western blot testing for HIV-l/2 IgG and HTLV-I IgG was done according to the manufacturers’ recommendations (see footnote 4).
IgA immunoblots were performed
with protein G depleted sera diluted 1:25 in the washing solution/diluent supplied with the kit. F(ab’), phosphatase-labeled anti-human IgA (Sigma) was substituted for that included in the kit. The substrate buffer was 1OOmM NaCI, 5mM MgCl,, 1OOrnM TRIS Cl, pH 9.5; and the chromo- genic agent was 66~1 of a 5% (W/V) solu- tion of nitro blue tetrazolium (Sigma) and 33~1 of a 5% solution of 5-bromo-4- chloro-3-indolyl phosphate (Sigma) in 10 ml of substrate buffer. The test sera were incubated for two hours at room tem- perature on a rocker platform, and the alkaline phosphatase-conjugated anti-
human IgA was incubated for one hour at room temperature.
RESULTS
Protein G Treatment
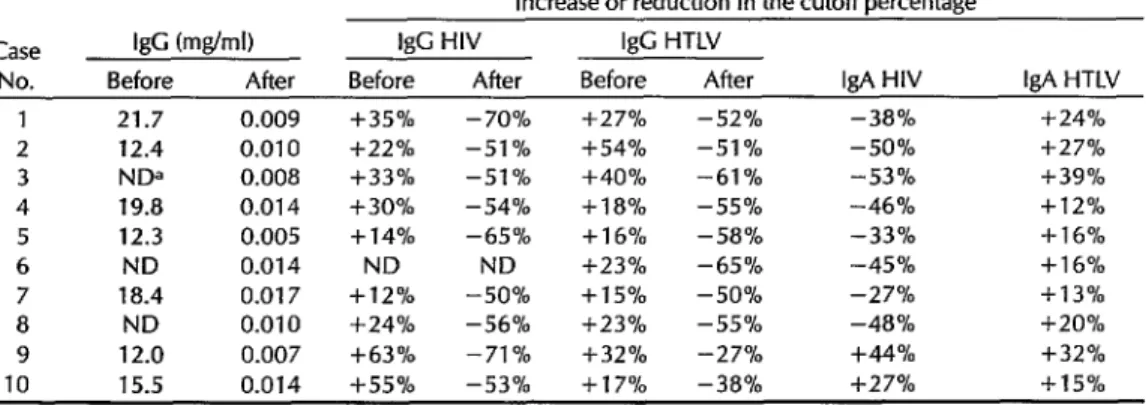
As indicated by the data in Table 1, the protein G treatment effectively reduced the IgG concentration in the test sera by 99.9%, from 12.0-21.7 mglml to 0.007-0.017 mglml.
ELISA
Before treatment with protein G, all the ELISA tests for HIV-l/2 IgG and HTLV-I IgG were positive, yielding values at least 10% above the calculated cutoff levels. As indicated in Table 1, these values ranged from 12% to 63% above the cutoff for HIV-l/2 IgG and from 15% to 54% above the cutoff for HTLV-I IgG .
After IgG depletion with protein G, none of the test sera were ELISA-positive for HIV-l/2 or HTLV-I IgG, all the values obtained being well below the cutoff levels. Specifically, the values obtained
Table 1. Detection by ELISA of IgG, HIV-l/2 IgC, HTLV-I IgG, HIV-l/2 IgA, and HTLV-I IgA anti- bodies in sera from the 10 study subjects before and after removal of IgG through protein G treat- ment. The detected levels of IgG are given in mg/ml, while those of the virus-specific antibodies are expressed in terms of a percentage above or below the cutoff value established for the test.
Increase or reduction in the cutoff percentage
Case IgG (mg/ml) I& HIV IgG HTLV
No. Before After Before After Before After IgA HIV IgA HTLV 1 21.7 0.009 l k35% - 70% f27% -52% -38% +24% 2 12.4 0.010 f22% -51% f54% -51 % -50% +27% 3 NDa 0.008 +33% -51 % +40% -61% -53% +39% 4 19.8 0.014 f30% -54% +las -55% -46% + 12 % 5 12.3 0.005 + 14% -65% +1 6% -58% -33% +16%
6 ND 0.014 ND ND +23% -65% -45% +l 6%
7 18.4 0.017 +12% -50% + 15 % -50% -27% + 1 3 % 8 ND 0.010 +24% -56% + 2 3 % -55% -48% +20% 9 12.0 0.007 +63% -71 % +32% -27% +44% +32% 10 15.5 0.014 i-55% -53% +17% -38% +27% + 15 % aND = Not determined.
for HIV-l/2 IgG were 50% to 71% below the cutoff level and those for HTLV-I IgG were 27% to 65% below the cutoff level- most of the values being close to the titers obtained with negative control sera (pro- vided with the kit or by uninfected subjects).
Regarding IgA, all the test sera except those of cases 9 and 10 yielded negative ELISA results for HIV-l/2 IgA, while all 10 sera tested positively for HTLV-I IgA (see Table 1). In most cases, the percent- age by which the HTLV-I IgA value ex- ceeded the cutoff was slightly below the percentage by which the HTLV-I IgG value exceeded the cutoff before protein G treatment (see columns 6 and 9 in Table l), but the differences observed in this small study sample were not statistically significant.
In contrast to the other eight sera, those from the two coinfected patients (cases 9 and 10) yielded ELISA results for HIV-l/2 IgA that were respectively 44% and 27% above the cutoff level-thereby indicating that specific anti-HIV-l/2 IgA was present in those patients.
Western Blot
We observed that treatment with pro-
tein G completely suppressed any
HIV-l/2 or HTLV-I IgG Western blot reac- tivity, thereby demonstrating the treat- ment’s effectiveness.
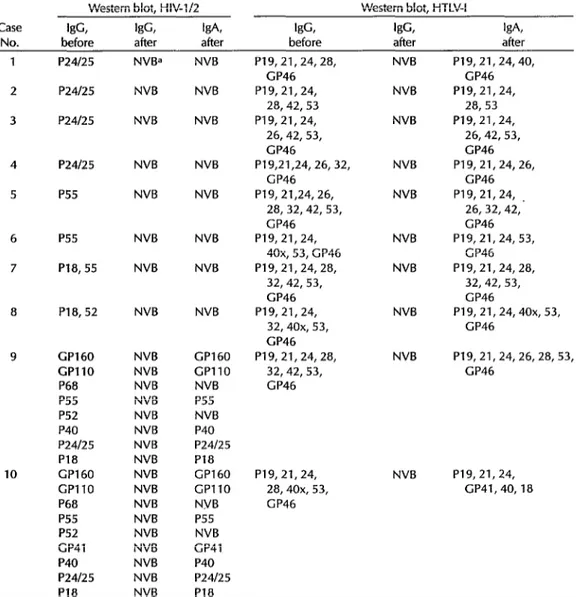
Also, as indicated in Table 2, the HIV IgG bands observed in the eight false positive sera (cases 1 to 8) completely dis- appeared, indicating that the IgG reac- tivity observed was probably nonspecific.
As in the case of IgA testing for HIV-l/2 by ELISA, sera from the two coinfected patients tested positively for HIV-l/2 IgA by Western blot. Some of the bands ini- tially observed were not detected by IgA testing, but the principal bands (GPllO, GP160, and P24/25) remained. In contrast to the HIV IgA results, all 10 sera tested
positively for HTLV-I, showing most or all of the specific bands previously ob- served.
DISCUSSION
AND CONCLUSIONS
Our findings clearly indicate that the presence of nonspecific HIV-l/2 IgG pro- duced the observed false positive HIV-l/2 IgG ELISA results.
Since our test subjects were all infected with HTLV-I, and since HIV and HTLV are known to exhibit cross-reactivity with one another, it seems likely that some of the false HIV-l/2 IgG ELISA results ob- served were due to antibodies directed against HTLV-I antigen. This is hard to prove, because depletion of specific HTLV-I antibodies would require a large supply of HTIV-I antigens and because, as reported elsewhere, circulating im- mune complexes as well as many types of autoantibodies can yield nonspecific re- sults (4, 5).
However, when all the IgG was re- moved with the streptococcal protein G that binds all classes of IgG (3) (including circulating immune complexes), this en- abled us to test the sera for IgA. And when the sera were tested by ELISA for IgA, all the false positive HIV-l/2 results disappeared-indicating that this pro- cedure is more specific than testing for IgG. It should be noted, however, that this IgA methodology is useful only after removal of IgG. (When we first tested the study sera for IgA before protein G treat-
ment, we found that 45% yielded
positive ELISA results for HIV-l/2 or HTLV-I IgA .)
Regarding the Western blot test re- sults, we never observed HIV-specific bands in eight of the 10 study sera. How- ever, our results clearly showed that use of protein G treatment and IgA ELISA testing permitted quick and cost-efficient screening of patients while avoiding any need for additional testing. Moreover,
Table 2. Data from Western blot testing of the 10 study sera, showing the HIV-l/2 and HTLV-I IgG antibody bands seen before protein G treatment and IgG and IgA bands seen after treatment.
Western blot, HIV-l/2 Western blot, HTLV-I
Case &s, w, kA kfG kG kA,
No. before after after before after after
1 P24l25
2 P24l2.s
3 P24f2.5
NVB= NVB
NVB NVB
NVB NV&
4 P24125
5 P.55
NVB NVB
NV& NVB
6 P55 NVB NVB
7 P18,55 NVB NVB
8 P18,52 NVB NVB
9
10
GP160 NVB CP160 GPllO NVB CPllO P68 NVB NV&
P55 NVB P55
P52 NVB NVB
P40 NVB P40
P24125 NVB P24/25
P18 NVB PI8
CP160 NVB GP160 GPllO NVB GPllO
P68 NVB NVB
P55 NVB P55
P52 NVB NVB
CP41 NVB GP41
P40 NVB P40
P24/25 NVB P24125
P19,21,24,28, GP46 P19,21,24,
28,42, 53 P19,21,24,
26,42, 53, GP46
P19,21,24, 26, 32, GP46
P19, 21,24,26, 28, 32,42, 53, GP46 P19,21,24,
40x, 53, G P46 P19,21,24,28,
32,42, 53, GP46 P19,21,24,
32,40x, 53, GP46 P19,21,24, 28,
32,42, 53, GP46
P19, 21,24, 28,40x, 53, GP46
NVB
NVB
NVB
NVB
NVB
NVB
NVB
NV&
NVB
NVB
P19,21,24,40, GP46 P19,21,24,
28,53 P19,21,24,
26,42,53, GP46 P19,21,24, 26,
GP46 P19, 21,24,
26, 32,42, GP46 P19, 21, 24, 53,
GP46 P19, 21,24,28,
32, 42, 53, GP46
P19,21,24,4Ox, 53, GP46
P19,21,24, 26,28, 53, GP46
P19,21,24, GP41,40,18
P18 NVB P18
aNVB = No visible band(s).
the IgA Western blot showed clear bands specific for HTLV-I in all 10 sera and also showed clear bands specific for HIV-l in the two coinfected sera-suggesting that IgA is at least as useful if not more useful than IgG for confirming retroviral infections.
In general, the presence of IgM and IgA antibodies indicates a recently ac-
quired infection, though IgA HIV anti- bodies appear to persist longer than their IgM counterparts. The IgA class of im- munoglobulins, which like the IgM class of immunoglobulins does not cross the placenta, is more useful than IgM for di- agnosing intrauterine infection-includ- ing acquired intrauterine toxoplasmosis, cytomegalovirus infections, and AIDS (2,
6, 7). Our experience to date suggests that HIV-l IgA may appear somewhat be- fore HIV-l IgG. Specifically, we found that a patient yielding positive Poly- merase Chain Reaction test results for the presence of HIV-l Gag and Pol antigens had a negative HIV-l/2 IgG ELISA but a positive HIV-l/2 IgA ELISA (l? R. Ridel, R. Goursaud, M. Chalcou, and J. C. Artus, personal communication).
Modification of commercially available kits appears to provide a simple cost-effi- cient means of testing for HIV IgA. In the Caribbean, where a high prevalence of HTLV-I infection is reported (up to 13% of the population in some countries), the percentage of false positive HIV IgG ELISA results is relatively high. Our re- sults clearly indicate that switching from the HIV IgG ELISA to the more specific HIV IgA ELISA would save time and money, and would also permit more gen- eralized testing for HTLV antibodies. Nevertheless, it also appears advisable that the recommended methodology be further tested elsewhere in the Caribbean in order to confirm the initial findings re- ported here.
Acknowledgments.
The authors would like to thank Mirette Chalcou and Marie- France Gamblin for technical and secre- tarial assistance, and R@is Goursaud forproviding serum samples used in the
study.
REFERENCES
1.
2.
3.
4.
5.
6.
7.
Weiblen BJ, Schumacher RT, Hoff R. Detec- tion of IgM and IgA HIV antibodies after removal of IgG with recombinant protein G. 1 Immunol Methods. 1990;126:199-204. Jendis JB, Tomasik Z, Hunziker U, et al. Evaluation of diagnostic tests for HIV in- fection in infants born to HIV-infected mothers in Switzerland. AIDS. 1988; 2:273-79.
Bjijrck L, Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984;133:969-74.
Euler HH, Kern P, Ltiffler H, Dietrich M. Precipitable immune complexes in healthy homosexual men with acquired immu- nodeficiency syndrome. Clin Exp Immunol. 1985;59:267-75.
Lane HC, Fauci AS. Immunological anom- alies in the acquired immunodeficiency
syndrome. Ann Rev lmmunol. 1985;
3:477500.
Decoster A, Caron A, Darcy F, Capron A. IgA antibodies against P30 as markers of congenital and acute toxoplasmosis. Lan- cer. 1988;2:1104-07.
Stagno S, Pass RF, Reynolds DW, Moore MA, Nahmias AJ, Alford CA. Comparative study of the diagnostic procedures for con- genital cytomegalovirus infection. Pedi- atrics. 1980;62:251-60.