Clinical
Paper
Oral
Surgery
A
split-mouth,
randomized,
triple-blind,
placebo-controlled
study
to
analyze
the
pre-emptive
effect
of
etoricoxib
120
mg
on
inflammatory
events
following
removal
of
unerupted
mandibular
third
molars
F.W.G. Costa,E.C.S.Soares,D.F.S.Esses,P.G.deB.Silva,T.P.Bezerra,H.C. Scarparo, T.R.Ribeiro,C.S.R.Fonteles:Asplit-mouth,randomized,triple-blind, placebo-controlled studytoanalyzethepre-emptiveeffectofetoricoxib120mgon inflammatoryeventsfollowingremovalofuneruptedmandibularthirdmolars.Int.J. OralMaxillofac.Surg.2015;44:1166–1174.#2015InternationalAssociationof
OralandMaxillofacialSurgeons.PublishedbyElsevierLtd. Allrights reserved.
F.W.G.Costa1,E.C.S.Soares1, D.F.S.Esses2,P.G. deB.Silva2, T.P.Bezerra3,H.C.Scarparo1,4, T.R.Ribeiro5,C.S.R.Fonteles4,6 1
DivisionofOralSurgery,SchoolofDentistry, FederalUniversityofCeara´,Fortaleza,Ceara´, Brazil;2Post-graduatePrograminDentistry, SchoolofDentistry,FederalUniversityof Ceara´,Fortaleza,Ceara´,Brazil;3Divisionof OralSurgery,WalterCantidioUniversity Hospital,Fortaleza,Ceara´,Brazil;4Divisionof ClinicalPharmacology,SchoolofDentistry, FederalUniversityofCeara´,Fortaleza,Ceara´, Brazil;5DivisionofClinicalDentistry,Schoolof Dentistry,FederalUniversityofCeara´, Fortaleza,Ceara´,Brazil;6Divisionof Paediatrics,SchoolofDentistry,Federal UniversityofCeara´,Fortaleza,Ceara´,Brazil
Abstract. Painafterthirdmolarextractionhasbeenconsideredthemostsuitable
pharmaceuticalmodeltoevaluateacutepain.Thisstudyaimedtoevaluatethe
pre-emptiveanalgesic/anti-inflammatoryefficacyofetoricoxib120mgfollowing
mandibularthirdmolarsurgery.Asplit-mouth,randomized,triple-blind,
placebo-controlledstudywasconductedwithpatientsundergoingthe surgicalremovalof
mandibularthirdmolars.Allvolunteerswereallocatedrandomlytoreceiveeither
etoricoxib120mgorplacebo1hpreoperatively,andinflammatoryeventswere
evaluated.Anestimatedsampleof18surgicalunitspergroupwasrequiredbasedon
apilotstudy(95%confidenceleveland80%statisticalpower).Rescuemedication
wasanalyzedbyKaplan–Meiermethodthroughlog-rankMantel–Coxtestand
Pearsonlinearcorrelation(P<0.05).Pre-emptiveetoricoxibreduced
postoperativepainscoressignificantlyincomparisontoplacebo(P<0.001),witha
painscorepeakat6haftersurgery(P<0.001).Themeanrescuemedication
consumptionwaslowerintheetoricoxibgroupcomparedtotheplacebogroupover
the studyperiod(P<0.05).There wasnostatisticallysignificantdifference
betweengroupsrelatedtoswellingandtrismus.Thepre-emptiveadministrationof
etoricoxib120mgsignificantlyreducedthepostoperativepainintensityandthe
needforrescuemedication,butdidnotreduceswellingortrismus.
http://dx.doi.org/10.1016/j.ijom.2015.06.012,availableonlineathttp://www.sciencedirect.com
Keywords:pre-emptiveanalgesia;thirdmolar; non-steroidalanti-inflammatorydrugs; etoricox-ib.
Acceptedforpublication12June2015 Availableonline3July2015
Introduction
Third molar surgeriesare common pro-cedures that significantly affect patient quality oflife, especially in the 3 days followingsurgery,duetotheintensityof thepainexperienced1,2andthe inflamma-toryeventscausedbythistypeofsurgical intervention.3 Comparedto similar pro-cedures in the maxilla, the removal of mandibular third molars usually expresses a higher degree of surgical trauma and pain, requiring the removal ofgreateramountsofbonesecondaryto the presenceofmorecomplexlevels of dentalimpaction.Inadditiontopain,the most commonly observed postoperative complicationsassociatedwiththe remov-alofmandibularthirdmolarsaretrismus and swellingasa resultofthe local in-flammatoryprocess.4
In this context, pre-emptive analgesia represents an anti-nociceptive treatment that preventstheestablishment ofan al-tered afferent input process, something that would amplify postoperative pain.5 AccordingtoAl-Sukhunetal.,6this phar-macological strategy provides increased patientcomfortandreducestheingestion ofanalgesicmedicationsforpaincontrol inthe postoperativeperiod,reducingthe patient recovery time. Pre-emptive anal-gesiahasbecomeoneofthemost promis-ing strategies for pharmaceutical pain management.7,8
Several pharmacological methods to obtain pre-emptive analgesia have been described, such as regional blocks with local anaesthetics, the administration of intravenous opioids, and the use of N -methyl-D-aspartate receptor antagonists,
corticosteroids,andnon-steroidal anti-in-flammatory drugs (NSAIDs).5,9–13 NSAIDs inhibit prostaglandin synthesis and are commonly prescribed for pain reliefandthecontrolofswellingafteroral surgery.14Althoughsomeadverseeffects related to the use of NSAIDs such as gastrointestinal bleeding, renal function disturbances,areductioninplatelet func-tion, shortness of breath, and profound hypotension have been described in the literature,5,13 the oral intake ofNSAIDs has beenrecommendedbysome authors as an efficient pre-emptive therapeutic regimen.6,15,16Arecentstudyhas demon-strated etoricoxibtobe anefficient drug
forthemanagementofacutepain second-ary to primary dysmenorrhoea and oral and orthopaedic surgeries,17 and etori-coxib at 120mg has shown efficacy in several non-dental clinical trials as a pre-medicationtocontrolacute postoper-ative pain.18–22 Two recently published Cochranereviewsevaluatedtheanalgesic efficacyofasinglepostoperativedoseof etoricoxib in a dental pain model.23,24 However, evidence of the efficacy of pre-emptive analgesia after third molar surgeryremainsscarce,andtodate,there hasbeenonlyonenon-placebocontrolled study thathas evaluatedthepre-emptive analgesiceffectofthisdruginthirdmolar surgery.12 Etoricoxibis a potentand se-lectivecyclo-oxygenase2(COX-2) inhib-itorwithfewgastrointestinalsideeffects17 and with favourable pharmacological properties,andmaythusbeconsidereda promisingdrugforpre-emptiveanalgesia. Therefore,theaimofthepresent place-bo-controlledandtriple-blindstudywasto evaluate the pre-emptive analgesic and anti-inflammatory efficacy of etoricoxib 120mgfollowingmandibularthirdmolar surgery,usingasplit-mouthstudydesign.
Materialsandmethods
Studydesignandsample
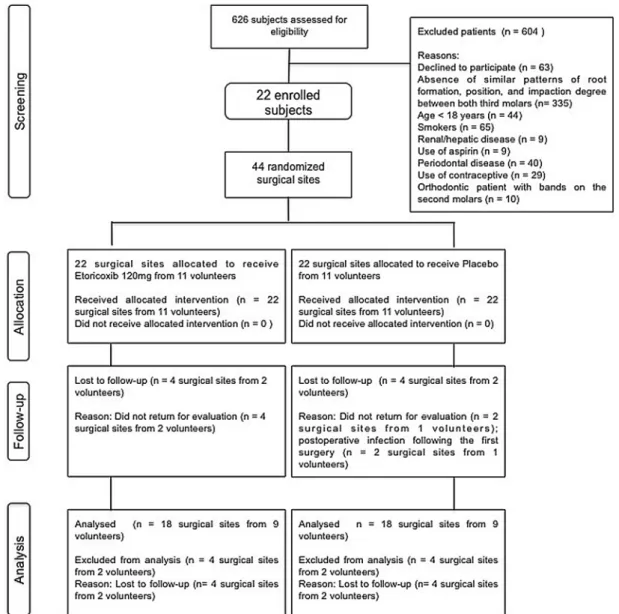
This study was approved by the Ethics CommitteeoftheAcademiaCearensede Odontologiaandwasperformedin accor-dance with the Helsinki statements. The researchprotocolfollowedaprospective, single-centre,split-mouth,randomized, tri-ple-blind,placebo-controlledstudydesign, anditwasconductedonpatientsrecruited fromtheDivisionofOralandMaxillofacial Surgery,WalterCantı´dioUniversity Hos-pital,FederalUniversityofCeara´(Brazil), whorequiredlowerthirdmolarextraction. Patientrecruitmentwasconductedbetween April2011andSeptember2012according totheCONSORTstatement.25Thesample unit used in the present study was the surgicalsite.
Healthysubjects(AmericanSocietyof Anesthesiologists (ASA)classification I) ofbothgenders,aged18–35years,witha clearindicationforremovaloftwolower thirdmolars,wereinvitedtoparticipatein thisstudy.Inordertocontrolforthelevel oftraumaticinjuryinflictedonthepatient,
thefollowinginclusioncriteriawerealso applied: (1)full coverage oflowerthird molarsbyosseoustissue, requiringbone removal and/ortoothsectioningfortheir extraction,and(2)similarpatternsofroot formation,position,anddegreeof impac-tionbetween theright and leftimpacted third molarsforallstudysubjects.Other inclusion criteria were the absence of periodontal disease, swelling, hyperther-mia, andtrismuspriortosurgery. Enrol-mentinthestudyrequiredtheabilityand willingnesstocooperatewiththeresearch protocolandtheprovisionofappropriate writteninformedconsent.
Patientswereexcludediftheyfulfilled anyofthefollowingcriteria:were smok-ers, pregnant or breast-feeding, using medicationsthatcouldpotentiallyinteract with the drugs used in the study, had orthodontic bandsonthe secondmolars, had a known allergyto NSAIDs, had a systemicchronicdisease,hadsignsofany pre-existingacuteinflammatoryor infec-tiouscondition,orhadusedNSAIDs with-inthe past21days.Individuals whodid notexpressaninterestinparticipatingin this clinical trial during subject recruit-ment were also excluded. Patients were removedfromthestudyiftherewas intol-erancetothepharmacologicalregimen,if theywereunabletofollowthestudy pro-tocol, if the surgical time exceeded a duration of 2h, or if they presented a postoperativeinfection.
Data were recorded preoperatively according to astandardized clinical ex-aminationandincludedgender,age, sys-temic conditions, periodontal status, haemogram parameters, platelet count, international normalized ratio (INR), andplasmaglucose.Apanoramic radio-graphwasrequiredtoevaluatevariables suchasthetoothpositionaccordingtothe PellandGregory26andWinter27 classifi-cations, degree of tooth/root develop-ment,andlevelofimpaction.
ibuprofen 300mg at 8-h intervals was allowedinthecasethatarescueanalgesic medicationwasneeded.
Samplesizecalculation
Initially, a placebo-controlledstudywith sixpatients(12mandibularthirdmolars) wasperformedtocalculatethesamplesize requiredtoconductthisclinicaltrial and statisticallyrejectthenullhypothesiswith 80%poweranda95%confidenceinterval. Basedonthemeanpainscoresofthepilot study (etoricoxib 0.30.8 and placebo 2.32.9), a minimum sample size of 18 surgicalsitesineachgroup was esti-mated.
Blinding
Information on the type of medication providedtoeachstudysubjectwas with-held from the patient, surgeon, clinical investigator (responsiblefor patient fol-low-upexaminationsand outcome mea-surements), and statistician. Prior to surgery, a list containing a randomized distributionofallsurgicalsitesandpain medicationstobeadministeredwasheld inasealedenvelopebyanexternalstudy collaborator, who was unaware of the studyprotocolandhadnofurther partic-ipation inthisclinicaltrialotherthanto guarantee a triple-blind study design. Thestatisticalanalysiswasinitially car-riedoutwithgroupscodedwiththeletter ‘A’ representing etoricoxib and ‘B’ representing placebo. The envelope decoding this information was only accessed once both the clinical trial andstatisticalanalysishadbeen conclud-ed.Atthistime,eachpatientandsurgical site assigned to receive etoricoxib or placebo wasidentified.
Surgicaloverview
Allpatientsunderwentastandardized sur-gicaltechniqueperformedinanoutpatient setting underlocalanaesthesia,followed by strict biosafety control. One surgeon with 10 years ofexperience in oral and maxillofacialsurgeryperformedallofthe surgical procedures. The same surgical procedure was adoptedfor bothsidesof themouth,aimingtoreducedifferencesin the level of intraoperative trauma. The extractionoflowerthirdmolarswas per-formed underlocalanaesthesiawith me-pivacaine 2%andepinephrine 1:200,000 (Mepivalem AD;Dentsply, USA), using two or three 1.8-ml cartridges. A full-thickness flap was raised, followed by bone removal using a drill cooled with
bi-distilledwater.Thesurgicalwoundwas closed usinga4–0silksuture.
Postoperative instructions were read and explained carefully to the patient, e.g. following a liquid andcold diet for 24h, performing rigorous oral hygiene, and avoiding mouthwashes to prevent the occurrenceofpost-surgicalbleeding. Patients were informed that they must contact the surgeon by telephone in the case ofpersistent bleeding or any other complications suchasfever.Inaddition, patients were also asked to report any physical symptoms experienced during the study period, e.g., nausea, vomiting, dizziness, headache,insomnia, andsigns ofinfection.
Outcomemeasures
Theprimaryoutcomeofthestudywasthe occurrence of postoperative pain. Mea-surementsofthisoutcomeconsideredpain intensityandtheneedforrescueanalgesia. Postoperativepainintensitywasmeasured using a 10-cm visual analogue scale (VAS), which consisted of an interval scalerangingfrom0(absenceofpainor discomfort)to10(maximumpainor dis-comfort).12,14,17,28,29Beforesurgery,each patientreceivedanexplanationonhowto measurepainintensityontheVAS.After surgery, patientsreceived a standardized VASformtorecordthevaluesof postop-erativepain,anditwasrequiredthatthis formbereturnedtotheresearcheronthe dayofsutureremoval.Studyparticipants were asked to record the pain intensity score at0, 2,4,6,8,10,12,24, 48,and 72handondays5and7following sur-gery. Additional analyses included the lengthoftimeelapseduntiltheintakeof a rescue analgesic bythe patient.30 The numberofpatientsrequiringarescue an-algesicwasalsorecorded.
Theoccurrenceofpostoperative inflam-matoryeventswasthesecondaryoutcome measure adopted in the present study. Measurements (Fig. 1) were performed to assess postoperative facial swelling onthesideofsurgery,includingthe dis-tancefromthemandibularangleto(1)the tragus(distanceMA–Tr),(2)theexternal corneroftheeye(distanceMA–ECE),(3) the nasal border (distanceMA–NB), (4) thelabialcommissure(distanceMA–LC), and(5)thesoftpogonion(distanceMA– SP). Differences between preoperative values (baseline) and those obtained at 24 and 72h and at 5 and 7 days after surgery werecompared. Inorder to esti-matetrismus,themaximummouth open-ing ability was measured pre- and postoperatively (after 24h, 72h, 5days,
and 7 days), by assessing the distance betweentheupperandlowercentral inci-sorsinmillimetresusingacalibratedruler.
Statisticalanalysis
Data were initially submitted to the Kolmogorov–Smirnov normality test. Parametric data were analyzed by one-way or two-way analysis of variance (ANOVA)/Bonferronitest. Non-paramet-ric data were analyzed with the Mann– WhitneyorWilcoxonandFriedman/Dunn posthoctests.Quantitativevariableswere expressedasthemeanstandard devia-tionofthemean(SD).TheKaplan–Meier methodwas used toevaluate the rescue medicationthroughthelog-rankMantel– Cox test.Pearson linear correlation was applied to correlatethe total number of rescuemedicationstakenandthesumof painscores.Allanalyseswereperformed with GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). The level of significance wassetasP<0.05forallofthe
evalua-tions.
Results
sampleconsistedof18volunteers(8males (44.4%)and10females(55.6%)), render-ing36surgicalsites.
Surgical and radiographic characteris-tics did not differ between the groups (Table1).Localanaesthetic(mepivacaine 2% and epinephrine 1:200,000) was injected at all appropriate sites before surgery, and the total amount of local anaesthetic injected ateach surgicalsite was measuredbasedonthetotalnumber of dental cartridges used per procedure. Themeannumberofdentalcartridgesdid not differ between groups (etoricoxib 2.20.4,placebo 2.10.2;P=0.265). Theaveragedurationofthesurgical pro-cedure was 16.29.4minfor the etori-coxib group and 16.79.5min for the placebogroup,andnostatistically signifi-cantdifferencewasdetectedbetweenthe groups(P=0.839).
Fig.2. Flowchartofpatientrecruitmentintothestudygroups,accordingtotheCONSORTstatement.
Table1. Comparisonofthesurgicalandradiographiccharacteristicsbetweentheplaceboand etoricoxibgroups.
Placebo Etoricoxib P-value Durationofsurgery,min 16.79.5 16.29.4 0.839*
Boneremoval,yes/no 18/0 18/0 1.000y
Toothsectioning,yes/no 9/9 12/6 0.310y
Numberofdentalcartridges 2.10.2 2.20.4 0.265* Postoperativebleeding,yes/noz 6/84 5/85 1.000y
Day0 0/18 0/18 1.000y
Day1 4/14 5/13 1.000y
Day3 1/17 0/18 1.000y
Day5 0/18 0/18 1.000y
Day7 1/17 0/18 1.000y
PellandGregoryposition,I/II/III 10/8/0 8/10/0 0.505y
PellandGregoryposition,A/B/C 0/15/3 0/15/3 1.000y
Winterposition,mesioangular/vertical 18/0 18/0 1.000y *
Wilcoxontest;dataexpressedasthemeanstandarddeviation.
y
x2testorFisher’sexacttest;dataexpressedastheabsolutefrequency.
zDay0=dayofsurgery;day1=firstpostoperativeday;day3=thirdpostoperativeday;day
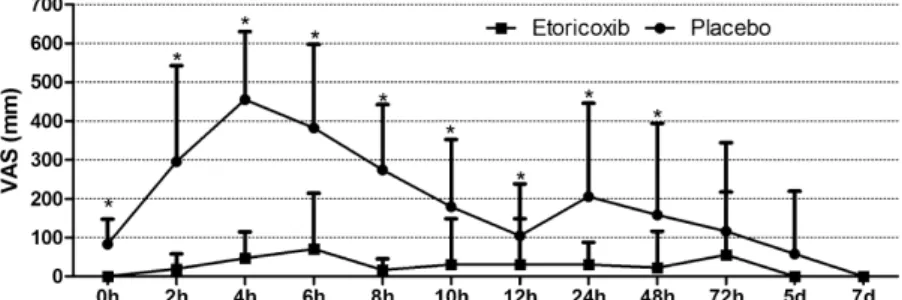
The postoperative peak pain score occurred at 4h in the placebo group and 6h in the etoricoxib group (Fig. 3), and a statistically significant difference was detected between the groups (P<0.001). The mean pain
score values immediately after (P=0.011) and at 2h (P=0.011), 4h (P<0.001), 6h (P<0.001), 8h
(P<0.001), 10h (P=0.013), 12h
(P=0.037), 24h (P=0.010), and 48h (P=0.048) after surgery were signifi-cantly lower in the etoricoxib group (Table 2). The placebo group showed a significant reduction in pain at 12h after surgery in comparison to the ob-servedmeanpeakpainscoreobservedat 4h (P=0.003), whereas the etoricoxib group demonstrated a significant reduc-tioninpain at8hpostoperativelywhen compared to the peakpain score at 6h postoperatively (P<0.001) (Table 2).
Intheplacebogroup,thetotalnumber ofingestedrescuecapsulesshoweda di-rectstatisticalcorrelationwiththesumof
painscores(P=0.007,r=0.377)(Fig.4). However, this correlation was not ob-served in the etoricoxib group (P=0.410, r= 0.109). In addition, the proportion of subjects requiring rescue analgesic medication was significantly higher in the placebo group at different timesofobservation(P=0.004)(Fig.5). At8haftersurgery,allpatientswhohad received placebo had consumed rescue analgesics, while 22.2%ofpatients who hadreceivedetoricoxibdidnotneed res-cue medication during the 7 days after surgery.
The time elapsed (meanSD) from theendofsurgerytotheintakeofthefirst rescue medication differed statistically between the etoricoxib group (27.6 48.7h) and the placebo group (4.01.9h)(P=0.033) (Table3). The mean number of rescue capsules con-sumed on day 0 (placebo 1.40.5 vs. etoricoxib 0.70.6;P=0.003) and on day 1 (placebo 1.30.9 vs. etoricoxib 0.30.4; P<0.001), and the overall
rescuemedicationconsumption(placebo 4.02.5 vs. etoricoxib 1.61.3;
P<0.001) were significantly higher in
theplacebogroup(Table3).
Postoperative facialmeasurements did notdifferbetweenthegroupsatanygiven time.However, differencesinindividual facial measurements and in the overall sumofthesemeasurementswereobserved withingroups(Table4).Inaddition, max-imum mouth openingdid not differ be-tweentheplaceboand etoricoxibgroups (P=0.662)(Table5).
Discussion
Thepresentstudyevaluatedtheefficacyof anoralNSAID,whichrepresentsaclassof drugs commonly prescribed following oral surgery procedures.31 For this pur-pose,mandibularthirdmolarsurgerywas chosenasaclinicalmodel.Thedifferent clinicalmodelsused toevaluate the effi-cacyoforal analgesicsarebasedon the relative frequency of the expression of chronic (i.e. cancer patients), postpar-tum/episiotomy,andpostsurgical(i.e. or-thopaedic and dental surgery) pain.32 According to Cooper,33 models to Fig.3. VAS painintensity(mm)intheplaceboand etoricoxibgroupsrecordedat specific
postoperativetimeintervals.Dataareexpressedasthemeanstandarddeviation.*P<0.05
comparedtotheplacebogroup(Mann–Whitneytest).
Table2. ComparisonoftheVASpostoperativepainintensitymeasurements(incentimetres) betweentheplaceboandetoricoxibgroups.
Period Druggroup P-value
Placebo Etoricoxib
0h 0.80.6* 0.00.0 0.011
2h 2.92.5* 0.20.4 0.011
4h 4.51.7*,y 0.50.7 <0.001
6h 3.82.1* 0.81.5y <0.001
8h 2.71.6* 0.2
0.1z <0.001
10h 1.81.7* 0.41.3 0.013
12h 1.01.3*,z 0.41.3 0.037
24h 2.02.4* 0.40.6 0.010
48h 1.62.4* 0.31.0 0.048
72h 1.22.3 0.71.8 0.285
5days 0.61.6 0.00.0 0.406
7days 0.00.0 0.00.0 1.000
Sum 17.112.8* 3.25.7 <0.001
P-value <0.001 0.003
VAS,visualanaloguescale.Dataareexpressedasthemeanstandarddeviation.
*
P<0.05comparedtoetoricoxib(Wilcoxontest).
yP<0.05inrelationtotheimmediatepostoperativeperiod(Friedman/Dunntest). z
P<0.05inrelationtothehourofpeakpain(placeboat4hpostoperative,etoricoxibat6h
postoperative)(Friedman/Dunntest).
Fig. 4. Pearson correlation analysis of the numberofingestedibuprofencapsules(rescue medication)andthesumofpainscoresduring the7-day evaluation period in the placebo group (P=0.007, r=0.377) and the etori-coxibgroup(P=0.410,r= 0.109).
evaluatedifferentformsofdentalpaincan bedividedintothreeoralsurgery catego-ries: complicated oral, periodontal, and impactedthirdmolarremoval.Sincethird molar surgery is associated with both moderate (40%)and severe (60%) post-operativepain,34–36thispharmacological model has become the most utilized in clinicaltrialsinvolvingacutepain, show-ing results comparable to non-dental postsurgicalmodels,regardlessofthe an-algesictested.37
Inthe present model of acute pain, a split-mouth,triple-blind,randomized, pla-cebo-controlled study was performed to controlforindividualbiologicalvariations andtoreducepossiblebiases.38The split-mouthstudydesignhastheadvantagesof allowing the surgical sides to be ‘mir-rored’,becauseofthesimilarityof radic-ular formation patterns and position/ degree of dental impaction in the same individual,andofhavingastudysubject Table3. Comparisonofrescueanalgesicconsumptionaftersurgicalproceduresintheplacebo
andetoricoxibgroups.
Placebo Etoricoxib P-value Sideeffects,yes/no 0/18 0/18 1.000* Rescuemedicationintake,yes/no 18/0 14/4 0.104* Timetofirstrescuemedication,h 4.01.9y 27.648.7 0.033 Numberofrescuemedicationsconsumed
Day0 1.40.5y 0.70.6 0.003
Day1 1.30.9y 0.3
0.4z <0.001
Day2 0.81.2 0.20.6 0.134
Day3 0.30.6z 0.10.4 0.389
Day4 0.10.2 0.10.2 1.000
Day5 0.10.2 0.10.2 1.000
Day6 0.00.0 0.10.3 0.584
Day7 0.10.2 0.00.0 0.791
Overallmedicationconsumption 4.02.5 1.61.3 <0.001
P-value <0.001 <0.001
Day0=dayofsurgery;day1=firstpostoperativeday;day2=secondpostoperativeday;day 3=thirdpostoperativeday;day4=fourthpostoperativeday;day5=fifthpostoperativeday; day6=sixthpostoperativeday;day7=seventhpostoperativeday.Dataareexpressedasthe absolutefrequency,orasthemeanstandarddeviation.
*Fisher’sexacttest. y
P<0.05comparedtoetoricoxib(Wilcoxontest). z
P<0.05relativetotheimmediatepostoperativeperiod(Friedman/Dunntest).
Table4. Comparisonofthefacialswellingvaluesmeasuredpre-andpostoperativelybetweentheplaceboandetoricoxibgroups.
Facialmeasurements Period* Druggroups P-value
Placebo Etoricoxib
MA–Tr Baseline 5.50.8 5.60.4 0.938y
24h 6.40.7z 6.30.6z
72h 6.20.7 6.10.6
5days 5.80.7§ 5.90.6§
7days 5.60.7 5.70.5
MA–ECE Baseline 9.80.8 10.00.6 0.807y
24h 10.81.0z 10.60.9z
72h 10.40.8 10.40.7
5days 10.10.8§ 10.00.7§
7days 9.90.8 10.00.6
MA–NB Baseline 10.30.8 10.50.6 0.878y
24h 11.40.9z 11.40.8z
72h 11.10.8§ 11.00.6§
5days 10.50.7 10.60.5 7days 10.30.6 10.50.5
MA–LC Baseline 8.30.5 8.60.7 0.731y
24h 9.20.6z 9.30.9z
72h 9.10.5 9.00.8§
5days 8.80.7§ 8.80.6
7days 8.40.2 8.70.8
MA–SP Baseline 9.90.6 10.10.8 0.722y
24h 11.11.0z 10.91.1z
72h 10.60.8§ 10.30.8§
5days 10.20.7 10.00.7
7days 9.90.6 10.00.7
Allmeasures Baseline 8.81.9 9.01.9 0.993y
24h 9.82.0z 9.72.0z
72h 9.51.9§ 9.31.9§
5days 9.11.9 9.11.8
7days 8.81.9 9.01.8
MA,mandibularangle;Tr,tragus;ECE,externalcorneroftheeye;NB,nasalborder;LC,labialcommissure;SP,softpogonion;ANOVA,analysis ofvariance.Dataareexpressedasthemeanstandarddeviation.
*
Baseline=preoperativevalue.
yP<0.05,repeated-measuresANOVA–two-way/Bonferroni. z
P<0.05relativetothebaselineperiod,repeated-measuresANOVA–one-way/Bonferroni. §
as his/her owncontrol for postoperative painperception.39Datahomogeneity be-tweenthetwogroupsstudiedinthe pres-entresearchwasfoundduetotheabsence of statistically significant differences in gender, age, number of surgical proce-dures, surgery duration, bone removal and/or tooth sectioning, postoperative bleeding,andtoothposition.
NSAIDs have beenconsidered effec-tive drugs in the management of pain after third molar removal,31 and have beenusedpreviouslytotesttheefficacy ofpre-emptiveanalgesiaasastrategyfor pain control.12,29,38–41 Pharmacological-ly, NSAIDs act by reducing peripheral and centralnociception, secondary toa reductionin thesensoryinflow of noci-ceptiveinputfromtheperipheralto cen-tral nervous system.10,13 Among NSAIDs, etoricoxibis considered a po-tent selectiveCOX-2inhibitor.42 Toivo-nen et al.22 and Boonriong et al.18 demonstrated that etoricoxib 120mg usedasapre-medicationinpatients un-dergoing arthroscopic shoulder surgery, significantlyreducedpostoperativepain. Puuraetal.20andSandhuetal.21reported the use of etoricoxib 120mg as a pre-emptive analgesic to decrease post-operative pain after laparoscopic chole-cystectomy. Liu et al.19 concluded that pre-medication with etoricoxib 120mg reduces the pain scores and need for postoperativefentanylafterminor gynae-cological surgery, without significant side effects. Inthe contextofdentistry, there have been no placebo-controlled studies to evaluate the efficacy of the pre-emptive analgesic effect of etori-coxib 120mgafter thirdmolarsurgery; thus, thepre-emptiveanalgesicvalueof etoricoxib 120mg remainsto be estab-lishedindentalstudies.Todate,onlyone study has used preoperative etoricoxib; however, the results were compared with a group in which a corticosteroid was used.12 In addition, no systematic
reviews concerningthe pre-emptiveuse of etoricoxib 120mg have been con-ducted.23,24
In the present study, the intensity of postoperativepainwasreduced consider-ablythroughthepreoperative administra-tionofetoricoxib.Morseetal.29compared thepre-emptiveanalgesicefficacyof ibu-profen,rofecoxib(NSAIDselective COX-2inhibitor),andplaceboandobservedthat inalloftheevaluationperiods,ibuprofen providedpainreliefsignificantlysuperior toplacebo.Rofecoxibalsoprovided simi-larresults,exceptforthepostoperative1, 3, and4-h periods,whenpainreliefwas not inferior to placebo. In the present study, etoricoxib showed a statistically significant difference overa48-h period when compared withplacebo. However, ChiuandCheung40performeda placebo-controlled study with pre-emptively ad-ministered ibuprofen and rofecoxib, and observed that rofecoxib showed signifi-cant pain reduction only in the first 6 postoperative hours in comparison with the preoperative use of placebo. In the presentclinicalresearch,thepre-emptive administration of etoricoxib showed a superior postoperativeanalgesiceffectin comparisontoplaceboat0,2,4,6,8,10, 12,24,and48hafterthirdmolarremoval. Incontrast, Sotto-Maioretal.12reported no significant difference in pain control when etoricoxib 120mg was used 1h before third molar surgery. Two recent systematicreviewsthatattemptedto mea-sure the postoperative efficacy of etori-coxib 120mg, showed that 72% of participantsinvolvedinfivedifferent stud-iesexperiencedsignificantpainreliefwith etoricoxib during the 4–6h after dental surgery.23,24
Therewasastatisticallysignificant dif-ference in peak pain between the two groups. The etoricoxib group displayed a pain peak at 6h after surgery, while the placebo group showed a pain peak at 4hpostoperatively. Thisdelay in the
onset of the pain peak reinforces the importanceofpre-emptiveanalgesiaand the efficacy of etoricoxib 120mg when compared with placebo. In two studies evaluating the pre-emptive analgesic effect of a selective COX-2 inhibitor (rofecoxib),29,40 the pain peak occurred 6h after the surgical procedure, which issimilartotheresultsofthepresentwork. Inthestudy bySotto-Maioret al.,12 the preoperativeadministration ofetoricoxib showed a total VAS score number of 6 (1.8);however,theauthorsdidnot men-tioninformationonthepainpeakreported bypatients.
Inthe present study, the timeelapsed fromtheendofthesurgicalprocedureto thefirstuseofrescuemedicationdiffered statisticallybetweenthegroupsanalyzed. Theaveragetimetothestartof postoper-ativerescuemedicationintheetoricoxib groupwasstatisticallylongerthanforthe placebo group; thus, those patients who used etoricoxib took longer to require postoperativeanalgesicmedication. This factmayreflectthedrugpharmacokinetics ofthepre-emptiveanalgesicaction. Etor-icoxibis a drug thathas a fastonset of actionand acts for a long period in the organism.43 The maximum plasma con-centrationis1.36mg/ml.43Thetimetaken foretoricoxibtoreachthemaximum plas-maconcentrationis1handitselimination half-lifeis 24.9h43;thesearefavourable pharmacokinetic properties for a drug usedtoproducelong-lastingandeffective pre-emptiveanalgesiaintheoralsurgery setting.Similarly,thepostoperative etor-icoxib-relateddentalstudiesevaluatedby Clarkeetal.23,24showedthattheweighted meanofthemediantimerequiredforthe useofrescuemedicationexceeded24h.
Theapparentanalgesicefficacyof etor-icoxib is also reflected in the reduced numberofrescuemedications consumed duringtheevaluationperiod.Asexpected, day 0 was the period with the highest rescue medication consumptionfor both groups,andduringtheoverall postopera-tiveperiod,theetoricoxibgroupingested thelowestnumberofrescue medications when compared to the placebo group. Morse et al.29 also noted that the pre-emptiveuse ofNSAIDssignificantly re-duced the need for rescue medication. Chiu and Cheung40 observed a smaller quantity of medication required by the patients whoutilized a selectiveCOX-2 inhibitor when compared with patients who utilized ibuprofen. In the present research,thepre-emptiveanalgesic effica-cy of etoricoxib was reinforced by the observance of a positive correlation between average pain intensity and the Table5. Comparisonofmaximummouthopeningvaluesintheplaceboandetoricoxibgroups.
Period Druggroups P-value
Placebo Etoricoxib
0h 44.05.0 47.280 0.662*
24h 34.96.6y 38.48.3y
72h 37.06.0 42.08.6y
5days 41.58.2z 45.18.2 7days 43.75.0 47.28.3
ANOVA,analysisofvariance.Dataareexpressedasthemeanstandarddeviation.
*
P<0.05,repeated-measuresANOVA–two-way/Bonferroni. y
P<0.05relativetotheimmediatepostoperativeperiod,repeated-measures
ANOVA–one-way/Bonferroni.
z
P<0.05 relative to thefirst 24h after surgery, repeated-measures ANOVA–one-way/
number of rescue medication capsules thatwereingestedintheplacebogroup. Thisfindingisconsistentwiththeresults of the dental postoperative studies de-scribed by Clark et al.23,24 The authors reported that 17%ofparticipants inthe etoricoxib120mggroupand68%of in-dividuals in the placebo grouprequired rescue medication within the initial 6h after the respective ingestion of either etoricoxiborplacebo.Inaddition,during a24-hevaluationperiod,50%of partici-pantsintheetoricoxib120mggroup re-quiredrescuemedication,comparedwith 89%ofindividualsintheplacebogroup, demonstratingasatisfactoryanalgesic ef-ficacywiththeuseofetoricoxib120mg overplacebo.
It is recognizedthat the inflammatory response is mediated by prostaglandins, andtheir synthesisisinitiatedbythe re-leaseofarachidonicacidfromthecellular membrane phospholipids through the action of cyclo-oxygenase. The conse-quences of this physiological process includeinterlinked eventsthatare repre-sentedbypain,swelling,andtrismus.44It isimportanttoobservethatinthepresent research,althoughthegrouptreatedwith etoricoxibshowedasignificantreduction in pain scores,these individualsdid not demonstrateareductioninswellingoran improvement in maximum mouth open-ing.Hence,nodirectcorrelationbetween painandswelling/trismuswasnoted.
Similarly, the etoricoxib and placebo groupsshowedanapparentlypositive an-ti-inflammatoryeffectinsome postopera-tiveperiods.Thisresultmaybeexplained as follows: (1) preoperative etoricoxib doesnotexhibitanimportant anti-inflam-matoryeffect,and(2)sinceareductionin inflammation was observed intra-group, without any significant differences be-tween groups, this effect was probably due to ibuprofen alone. Since the pre-scribed rescue medication regimen con-sisted of ibuprofen 300mg every 8h (whenrequired forpain control), the in-take of ibuprofen was higher within the first72h(speciallyonday0),andwithin these initial postoperative days patients would frequently consume two to three capsules(600–900mg)atatimeinsteadof followingtheregular prescriptionofone single capsule (300mg), an anti-inflam-matoryeffectgeneratedbyibuprofen can-notatpresentbediscarded.Inbothgroups, allmeasurementsshowedapeakswelling at24handthereductioninswellingwas onlysignificantfromthefifthtothe sev-enth postoperative day. Sotto-Maior et al.12 didnotobserveasignificant dif-ferenceinswellingwiththepre-emptive
use of etoricoxib. In addition, these authors observed that during the first 48hofthepostoperativeevaluation,there wasanincreaseinfacialswellingdespitea reductionintrismus,whichwasnotfound inthepresentstudy.
In the present study there were no reportedsideeffectswiththeuseof etor-icoxib. According to the published Cochrane reviews,23,24 the use of etori-coxib120mghasbeendemonstratedtobe relativelysafe.Informationrelatedto ad-verse events collected 6h to 14 days postoperativelyshowednosignificant dif-ference in the number of participants reporting at least one adverse effect in theetoricoxibandplacebogroups.In ad-dition, no serious adverse events were reported followingthe use ofetoricoxib 120mg.However,theCommitteefor Me-dicinal Products for Human Use of the EuropeanMedicinesAgencyhasupdated the previously existing contraindications totheuse ofetoricoxibandhas issueda warningfornon-adequatelycontrolled hy-pertensivepatients:(1)etoricoxib should notbeusedinpatientswithhypertension whose bloodpressureispersistently ele-vated above 140/90mmHg and has not beenadequatelycontrolled,and(2)blood pressureshouldbemonitoredfor2weeks after the startoftreatmentandregularly thereafter.45Inspiteoftheevidence sug-gestingthatthebenefitsofthisdrug out-weigh the risks, the US Food and Drug Administration has notyetapprovedthe useofetoricoxibintheUSA.24
In conclusion, etoricoxib significantly reducedtheintensityofpostoperativepain andtheneedforrescue medication com-paredtotheplacebo.However,etoricoxib didnotshowanysignificant anti-inflam-matory effect on swelling or trismus in comparisontotheplacebogroup.
Ethicalapproval
This study was approved by the Ethics Committee of the Academia Cearense deOdontologia(Fortaleza,Brazil; proto-colNo.132),andwasinagreementwith theHelsinkistatements.
Funding
None.
Competinginterests
None.
Patientconsent
Writtenpatientconsentwasobtained.
References
1. BenediktsdottirIS,WenzelA,PetersenJK, HintzeH.Mandibularthirdmolarremoval: riskindicatorsforextendedoperationtime, postoperativepain,andcomplications.Oral Surg Oral Med Oral Pathol Oral Radiol Endod2004;97:438–46.
2. MollerPL,Sindet-PedersenS,PettersenCT, JuhlGI,DillenschneiderA,SkoglundLA. Onsetofacetaminophenanalgesia: compar-ison of oralintravenousroutes after third molarsurgery.BrJAnaesth2005;94:642–8. 3. Colorado-Boninn M, Valmaseda-Castellon E, Birini-AytesL, Gay-Escoda C. Quality oflifefollowinglowerthirdmolarremoval. IntJOralMaxillofacSurg2006;35:343–7. 4. Van Gool AV, Ten Bosch JJ, Boering G. Clinicalconsequencesandcomplaintsafter removalofthemandibularthirdmolar.IntJ OralSurg1977;6:29–37.
5. KissinI.Preemptiveanalgesia. Anesthesiol-ogy2000;93:1138–43.
6. Al-Sukhun J, Al-Sukhun S, Penttila¨ H, AshammakhiN,Al-Sukhun R.Preemptive analgesiceffectoflowdosesofcelecoxibis superiortolowdosesoftraditional nonste-roidalanti-inflammatorydrugs.JCraniofac Surg2010;23:526–9.
7. LiporaciJuniorJL.Assessmentof preemp-tiveanalgesiaefficacyinsurgicalextraction or third molars. Rev Bras Anestesiol 2012;62:502–10.
8. CampigliaL,ConsalesG,DeGaudioAR. Pre-emptiveanalgesiaforpostoperativepain control: a review. Clin Drug Investig 2010;30:15–26.
9. DahlJB,MøinicheS.Pre-emptiveanalgesia. BrMedBull2004;71:13–27.
10. KellyDJ,AhmadM,BrullSJ.Preemptive analgesia I: physiological pathways and pharmacologicalmodalities.CanJAnaesth 2010;48:1000–10.
11. Pozos-GuillenA,Martinez-RiderR, Aguirre-BanuelosP,Perez-UrizarJ.Pre-emptive anal-gesiceffectoftramadolaftermandibularthird molarextraction:apilotstudy.JOral Max-illofacSurg2007;65:1315–20.
12. Sotto-MaiorBS,SennaPM,deSouza Picor-elliAssisNM.Corticosteroidsor cyclooxy-genase2-selectiveinhibitormedication for themanagementofpainandswellingafter third-molarsurgery.JCraniofacSurg2011;
22:758–62.
13. WoolfCJ,ChongMS.Preemptiveanalgesia –treatingpostoperativepainbypreventing the establishment of central sensitization. AnesthAnalg1993;77:362–79.
14. DeMenezesSA,CuryPR.Efficacyof nime-sulid versus meloxicam in the control of pain,swellingandtrismusfollowing extrac-tionofimpactedlowerthirdmolar.IntJOral MaxillofacSurg2010;39:580–4.
16. SiskAL,MosleyRO,MartinRP. Compari-sonofpreoperativeandpostoperative diflu-nisalforsuppressionofpostoperativepain.J OralMaxillofacSurg1989;47:464–8. 17. Daniels SE, Bandy DP, Christensen SE,
BoiceJ,LosadaMC,LiuH,etal.Evaluation ofthedoserangeofetoricoxibinanacute painsettingusingthepostoperativedental painmodel.ClinJPain2011;27:1–8. 18. BoonriongT,TangtrakulwanichB,Glabglay
P,NimmaanratS.Comparingetoricoxiband celecoxibforpreemptiveanalgesiaforacute postoperative pain in patients undergoing arthroscopic anterior cruciate ligament re-construction:arandomizedcontrolledtrial. BMCMusculoskeletDisord2010;11:246. 19. LiuW,LooCC,ChiuJW,TanHM,RenHZ,
LimY.Analgesicefficacyofpre-operative etoricoxibforterminationofpregnancyinan ambulatorycentre.SingapMedJ2005;46:
397–400.
20. PuuraA,PuolakkaP,RorariusM,Salmelin R, Lindgren L. Etoricoxib pre-medication for post-operative pain after laparoscopic cholecystectomy. ActaAnaesthesiol Scand 2006;50:688–93.
21. SandhuT, PaiboonworachatS,Ko-iamW. Effectsof preemptiveanalgesiain laparo-scopiccholecystectomy:adouble-blind ran-domized controlled trial. Surg Endosc 2011;25:23–7.
22. ToivonenJ,PitkoVM,RosenbergPH. Etor-icoxibpre-medicationcombinedwith intra-operative subacromialblockfor painafter arthroscopicacromioplasty.Acta Anaesthe-siolScand2007;51:316–21.
23. ClarkeR,DerryS,MooreRA.Singledose oraletoricoxibforacutepostoperativepain in adults. Cochrane Database Syst Rev 2012;4.CD004309.
24. ClarkeR,DerryS,MooreRA.Singledose oraletoricoxibforacutepostoperativepain in adults. Cochrane Database Syst Rev 2014;5.CD004309.
25. MoherD,HopewellS,SchulzKF,Montori V,GøtzschePC,DevereauxPJ,etal. CON-SORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomisedtrials. IntJ Surg2012;
10:28–55.
26. PellGJ,GregoryBT.Impactedmandibular third molars; classification and modified
techniquesforremoval.DentDigest1933;
39:330–8.
27. WinterGB.Principlesofexodontiaapplied to the impacted mandibular third molar. SaintLouis:AmericanBooks;1926. 28. MerryAF,GibbsRD,EdwardsJ,TingGS,
Frampton C, Davies E, et al. Combined acetaminophenandibuprofenforpainrelief after oralsurgeryin adults:a randomized controlled trial. Br J Anaesth 2010;104:
80–8.
29. MorseZ,TumpA,KevelhamE.Ibuprofenas a pre-emptiveanalgesic is aseffective as rofecoxib for mandibular thirdmolar sur-gery.Odontology2006;94:59–63. 30. OngKS,SeymourRA,ChenFG,HoVC.
Preoperativeketorolachasapreemptive ef-fect forpostoperative thirdmolar surgical pain. Int J Oral Maxillofac Surg 2004;
33:771–6.
31. SavageMG,HenryMA.Preoperative non-steroidal anti-inflammatory agents: review oftheliterature.OralSurgOralMedOral PatholOralRadiolEndod2004;98:146–52. 32. CooperSA.Modelsforclinicalassessment oforalanalgesics.AmJMed1983;75:24–9. 33. Cooper SA. Fivestudies onibuprofen for postsurgicaldentalpain.AmJMed1984;77:
70–7.
34. AverbuchM,KatzperM.Severityof base-linepainanddegreeofanalgesiainthethird molar post-extraction dental pain model. AnesthAnalg2003;97:163–7.
35. Forbes JA. Oralsurgery,advancesin pain researchandtherapy:thedesignofanalgesic clinical trials. In: Max MB, Portenoy R, Laska EM, editors.Advances in pain re-searchandtherapy:thedesignofanalgesic clinicaltrials.NewYork:RavenPress;1991. p.347–74.
36. Saito K, Kaneko A, Machii K, Ohta H, OhkuraM,SuzukiM.Efficacyandsafety ofadditional200-mgdoseofcelecoxibin adult patients with postoperativepain fol-lowingextractionofimpactedthird mandib-ular molar: a multicenter, randomized, double-blind, placebo-controlled, phase II studyinJapan.ClinTher2012;34:314–28. 37. BardenJ,EdwardsJE,McQuayHJ,Andrew MooreR.Painandanalgesicresponseafter thirdmolarextractionandotherpostsurgical pain.Pain2004;107:86–90.
38.LauSL,ChowRL,YeungRW,SammanN. Pre-emptiveibuprofenarginateinthird mo-larsurgery:adouble-blindrandomized con-trolledcrossover clinicaltrial.AustDentJ 2009;54:355–60.
39.Lo¨kkenP,OlsenI,BruasetI, Norman-Ped-ersen K.Bilateralsurgicalremovalof im-pactedlowerthirdmolarteethasamodelfor drugevaluation:atestwithibuprofen.EurJ ClinPharmacol1975;8:209–16.
40.ChiuWK,CheungLK.Efficacyof preoper-ativeoralrofecoxibinpaincontrolforthird molar surgery. OralSurg OralMed Oral PatholOralRadiolEndod2005;99:e47–53. 41.LustenbergerFD,Gra¨tzKW,MutzbauerTS. Efficacyofibuprofenversuslornoxicam af-terthirdmolarsurgery:arandomized, dou-ble-blind, crossover pilot study. Oral MaxillofacSurg2011;15:57–62.
42.ShiS,KlotzU.Clinicaluseand pharma-cological properties of selective COX-2 inhibitors. Eur J Clin Pharmacol 2008;
64:233–52.
43.RodriguesAD,HalpinRA,GeerLA,CuiD, WoolfEJ,MatthewsCZ,etal.Absorption, metabolism,and excretionofetoricoxib,a potent and selectivecyclooxygenase-2 in-hibitor, in healthy male volunteers. Drug MetabDispos2003;31:224–32.
44.LipskyPE.Roleofcyclooxygenase-1and-2 inhealthanddisease.AmJOrthop1999;28:
8–12.
45. Liote´ F, Terkeltaub R. Overviewof gout therapystrategyandtargets,andthe man-agementofrefractorydisease.In: Terkel-taub R, editor. Gout and other crystal arthropathies. ElsevierInc;2012.p.198.
Address:
Fa´bioWildsonGurgelCosta
Post-GraduationPrograminDentistry FederalUniversityofCeara´
St.MonsenhorFurtado RodolfoTeo´filo Fortaleza Ceara´ 60.430-350 Brazil
Tel:+558599981426