DOI 10.1007/s00280-015-2938-x
ORIGINAL ARTICLE
A new animal model of intestinal mucositis induced
by the combination of irinotecan and 5-fluorouracil in mice
Venúcia B. M. Pereira1 · Anielle T. Melo1 · Eudmar M. Assis-Júnior1 ·
Deysi V. T. Wong1 · Gerly A. C. Brito2 · Paulo R. C. Almeida3 · Ronaldo A. Ribeiro1 · Roberto C. P. Lima-Júnior1
Received: 20 August 2015 / Accepted: 1 December 2015 / Published online: 14 December 2015 © Springer-Verlag Berlin Heidelberg 2015
Results The optimal dose combination that induced IM and presented no substantial mortality on the 7th day was IRI (45 mg/kg) + 5-FU (37.5 mg/kg), which was used for
subsequent studies. IRI and 5-FU in combination induced significant diarrhea, body weight loss, intestinal dam-age, inflammatory cell infiltration, and increased levels of cytokines when compared with other groups (P < 0.05). Neither IRI nor 5-FU alone induced IM.
Conclusions We developed a new experimental model of IM induced by combining irinotecan and 5-FU treatments, which will allow us to gain a better knowledge concerning the pathogenesis of this disease through the pharmacologi-cal modulation of key inflammatory mediators.
Keywords Irinotecan · 5-Fluorouracil · Mucositis · Intestine · Inflammation
Introduction
Colorectal cancer is the third most common cancer in men and the second most common cancer in women worldwide. In 2012, 1.4 million people were diagnosed with colorectal cancer, which is still a leading cause of death [1]. Accord-ing to the World Health Organization (WHO), 27 million new cancer cases are expected by 2030, with approximately 75–80 million survivors in total [2, 3], and many of these patients will suffer various side effects from the anticancer chemotherapy and radiotherapy.
Oral and intestinal mucositis is significant dose-lim-iting and costly side effects of anticancer therapy [4–7]. Mucositis is characterized by multiple signs and symptoms, including dysphagia, dyspepsia, diarrhea, nausea and vom-iting, abdominal pain, and oral ulcers, among others [8]. In the clinical setting, 80 % of patients subjected to standard Abstract
Purpose Intestinal mucositis (IM) is a common side effect of anticancer agents. Despite polychemotherapy use in clinical practice, the pathogenesis of IM has been investi-gated in single drug injection animal models. However, the progression of IM could vary according to drug regimens. Thus, we aimed to develop a new experimental mucositis model induced by combining irinotecan and 5-fluorouracil (5-FU) treatments.
Methods IM was induced in male C57BL/6 mice by the intraperitoneal administration of either 0.9 % saline (5 mL/ kg), irinotecan (IRI, 30 or 45 mg/kg), 5-FU (25, 37.5, or 50 mg/kg), or the combination of these doses (IRI + 5-FU)
for 4 days. Animal survival, body mass variation, and diar-rhea scores were evaluated daily. On the 7th day, the mice were euthanized, and intestinal samples were collected for histopathology and morphometric analysis, as well as for the determination of myeloperoxidase activity and cytokine dosage (TNF-α and IL-6).
Electronic supplementary material The online version of this article (doi:10.1007/s00280-015-2938-x) contains supplementary material, which is available to authorized users.
* Roberto C. P. Lima-Júnior robertocesarpljr@gmail.com
1
Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Rua Cel Nunes de Melo, 1315, Rodolfo Teófilo, Fortaleza, Ceará 60430-270, Brazil
2 Department of Morphology, Faculty of Medicine, Federal
University of Ceará, Fortaleza, Brazil
3
doses of chemotherapy have some degree of mucositis [5, 9, 10], which may reduce treatment efficacy mainly because of dose reductions and treatment interruption [11].
The chemotherapeutic agents irinotecan and 5-fluoro-uracil, which are employed as the first-line treatment of metastatic colorectal cancer in FOLFIRI and IFL proto-cols (irinotecan + 5-fluorouracil (5-FU) + leucovorin), are
commonly associated with the development of severe intes-tinal mucositis and diarrhea (grades 3/4), affecting up to 23 % of patients [7, 12]. However, severe diarrhea seems to affect different proportions of patients when 5-FU (6–13 % of patients) and irinotecan (31 % of patients) are used as single agents at varying dose schedules [12]. The difference in severity might suggest that the pathological mechanisms may vary according to the drug employed. Mucositis and its associated diarrhea management are still limited to anal-gesics, antibiotics, and antidiarrheal and mucosal protective agents. However, these are only palliative and frequently non-effective [7, 13, 14].
Knowledge concerning the underlying intestinal mucosi-tis and diarrhea pathogenesis is essential and might point to specific targets for modulation; therefore, it could effec-tively prevent the development of these side effects. In this context, several studies have shown the involvement of key cytokines, such as TNF-α, IL-1β, IL-4, IL-18, and IL-33, in the pathogenesis of intestinal mucositis [15–21]. In spite of recent important advances, the translation of these findings from the bench to the bedside has been somewhat limited. One possible explanation might involve the use of animal models that employ a single anticancer agent, unlike what is observed in the clinical setting. Therefore, we aimed to develop a new experimental model of mucositis induced by the combination of irinotecan and 5-FU treatments.
Materials and methods
Animals
Male C57BL/6 mice, weighing 20–25 g, from the Federal University of Ceará, were used in the present study. The animals had free access to drinking water and food. All ani-mal care and experimental procedures complied with the laboratory animal care and use principles outlined by the National Institutes of Health [22] and were approved by the local ethics committee for Animal Experiments (protocol number 76/2011).
Drugs
The following drugs were used: irinotecan hydrochloride (Evoterin®, 5 mL ampoules, 100 mg/mL, Evolabis, São
Paulo, Brazil) and 5-FU (5-fluoruracila®, 10 mL ampoules, 250 mg/mL, Eurofarma, São Paulo, Brazil).
Induction of experimental intestinal mucositis
This protocol was based on a model previously described by Lima-Júnior et al. [17] and modified for our experimen-tal conditions.
First, we performed a pilot study aiming to define the best dose combination to induce changes in clinical param-eters, such as diarrhea and body weight loss, without a significant mortality rate. The animals (n= 6/group) were administered either 0.9 % saline (5 mL/kg, i.p.), 5-FU (25, 37.5, or 50 mg/kg, i.p.), IRI (30 or 45 mg/kg, i.p.), or 5-FU (25, 37.5, or 50 mg/kg, i.p.) + IRI (30 or 45 mg/kg, i.p.) for
4 consecutive days.
After choosing the optimal combined doses of irinote-can and 5-FU in the pilot study, the mice were randomly assigned into the following groups (n = 6): 0.9 % saline
(0.5 mL/kg, i.p.), irinotecan (IRI 45 mg/kg for 4 days, i.p.), 5-FU (37.5 mg/kg for 4 days, i.p.), and the 5-FU + IRI
group (IRI 45 mg/kg + 5-FU 37,5 mg/kg for 4 days, i.p.)
with a 1-h interval between drug administration.
The first injection day was considered to be day one. On day seven, animals were anesthetized with a 2.5 % tri-bromoethanol solution (10 mL/kg, i.p.) for blood sample collection and killed by cervical dislocation. Duodenum, jejunum, ileum, and colon samples were collected for mor-phological and histopathological analyses, the determina-tion of myeloperoxidase activity (MPO), and the determi-nation of the levels of pro-inflammatory cytokines (TNF-α, IL-1, and IL-6)
Survival study
The survival rate (%) of the animals was noted daily up to day 9 after the administration of the first dose.
Weight loss evaluation
The animal body weight variation was measured in grams (g) and expressed as a percentage (%) in regard to day one.
Diarrhea assessment
Total leukocyte counts
Mice were lightly anesthetized with 2.5 % tribromoethanol (10 mL/kg, i.p), and blood samples (20 µL) were collected from the retro-orbital plexus of each animal and diluted (1:20) in Turk’s solution (380 µL). The blood leukocyte count/mm3 was then determined using a Neubauer cham-ber, as previously described [17].
Histopathological analysis and intestinal morphometry
On the 7th day, the animals were killed, and intestinal sam-ples (duodenum, jejunum, ileum, and colon) were dissected for the histopathological and morphometric analyses. The specimens were fixed in 10 % (v/v) neutral-buffered forma-lin, dehydrated, and embedded in paraffin. Sections (5 µm thick) were obtained for hematoxylin-eosin staining (H&E) and for a subsequent examination using light microscopy (100x). A blind method was used to avoid observer bias.
The severity of mucositis was assessed as previously described [24]: grade 0, no lesion; grade 1, <10 % of crypts contain individual necrotic cells, grade 2, >10 % of crypts contain necrotic cells, but the crypt architecture is intact; grade 3, >10 % crypts contain necrotic cells showing focal loss of crypt architecture (<20 %), villi are shortened, and variable hypertrophy/hyperbasophilia is apparent in the remaining crypt cells; and grade 4, the same as grade 3 except that the loss of crypt architecture and villous short-ening are more extensive.
For the morphometric analyses, the length of the intesti-nal villi (from villus tip to villus/crypt junction), the depth of the Lieberkuhn crypts (defined as invagination depth between adjacent villi), and the villus/crypt ratio were determined as previously described [17]. Between 5 and 10 villi and crypts were measured per slice (6 slices/group) using the software ImageJ 1.4 (NIH, Bethesda, MD, USA). Each slice was obtained from one intestinal sample of each mouse.
Myeloperoxidase assay
MPO is an enzyme that is found most abundantly in azuro-philic neutrophil granules. It was measured in this study as a neutrophil marker in inflamed tissue by following a previously described colorimetric method [25]. Briefly, an ileum sample (40–60 mg) was harvested and homog-enized in 0.02 M NaP04 buffer (pH 4,7) containing 0.1 M NaCl and 0.015 M Na2 EDTA and centrifuged at 800 g for 15 min at 4 °C. The pellet was then subjected to hypo-tonic lysis (0.2 % NaCl solution, followed 30 s later by the addition of an equal volume of a solution). After a further
centrifugation step, the pellet was resuspended in 300 µL of 0.05 M NaPO4 buffer, pH 5.4, containing 0.5 % hexa-decyltrimethylammonium bromide (HTAB, Sigma). MPO activity was developed with the color reagent tetrameth-ylbenzidine (1.6 mM) and H2O2 (0.5 mM). The reaction was stopped with a 2 M H2SO4 solution, and the absorb-ance at 450 nm was determined in a spectrophotometer. The readings were plotted on a standard curve of mouse peritoneal neutrophils processed in the same way, with the obtained data expressed as MPO activity (neutrophils/mg of tissue).
Detection of cytokines TNF-α and IL-6 in ileum tissue by ELISA
C57BL/6 mice had an intestinal sample removed on day 7 for the analysis of cytokines. The ileum was stored at
−70 °C until required for the assay. The collected tissue
was homogenized and processed [26]. The concentrations of TNF-α and IL-6 were determined using an enzyme-linked immunosorbent assay (ELISA) [27]. Briefly, micr-otiter plates were coated with an antibody against mouse
TNF-α and IL-6 (4 µg/mL, DuoSet ELISA Development kit R&D Systems) overnight at 4 °C. After blocking the plates, the sample and standard were added at various dilutions in duplicate and incubated at 4 °C for 2 h. The plates were washed three times with buffer. After wash-ing the plates, biotinylated goat antimouse (diluted 1:1000 with assay buffer 1 % BSA, R&D System, USA) was added to the wells. After a further incubation at room tem-perature for 2 h, the plates were washed, and 100 µl of streptavidin-HRP diluted 1:200 was added. To the plate, 100 µL of substrate solution (1:1 mixture of H2O2 and tetramethylbenzidine; R&D System, USA) was added, and the plate was incubated in the dark at room tempera-ture for 20 min. The enzyme reaction was stopped with H2SO42 N, and the absorbance was measured at 450 nm. The results are expressed as pg/g of tissue and reported as the mean ± SEM.
Statistical analyses
Data are expressed as the mean ± standard error of the
Results
Intestinal mucositis induction
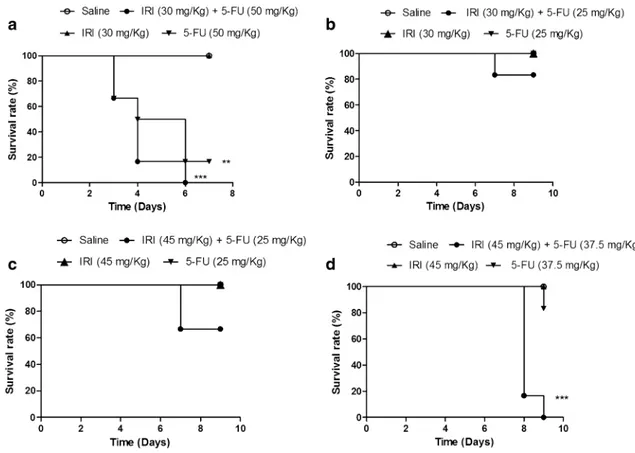
As shown in Fig. 1, the combination of irinotecan and 5-FU reduced the animal survival in all experimental pro-tocols (Fig. 1a–d), although a statistically reduced survival versus the drugs administered alone or the saline group (P < 0.05) was observed only when higher doses of irinote-can and 5-FU were used (Fig. 1a, d). In addition, as shown in Fig. 1d, no mortality was observed up to day seven of the experimental study.
The combination of irinotecan and 5-FU also caused body weight loss when compared to the drugs given alone or the saline group (Fig. 2a–d) with significant differences starting from day 5 (P < 0.05). Additionally, all groups that were injected with irinotecan or 5-FU alone did not pre-sent with a significant body weight loss compared to the saline group (P > 0.05, Fig. 2a–d). The exception was the group receiving 5-FU 50 mg/kg, which showed a severe body weight loss compared with the saline-treated mice (P < 0.05, Fig. 2a).
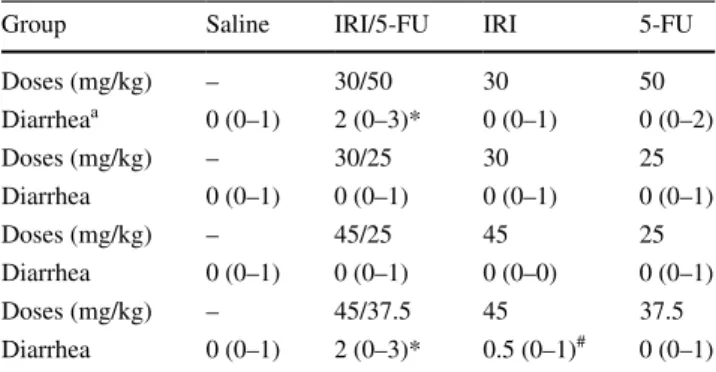
Table 1 shows that the diarrhea scores were increased when irinotecan and 5-FU treatments were combined, and the increase was more evident with the combination of higher doses of 5-FU and irinotecan. The groups that were injected with either irinotecan (30 mg/kg) + 5-FU (50 mg/
kg) or irinotecan (45 mg/kg) + 5-FU (37.5 mg/kg)
pre-sented with moderate-to-severe diarrhea [2(0-3)], which was significantly different (P < 0.05) than the saline-treated group [0(0–1)]. The groups injected with irinotecan or 5-FU alone showed no diarrhea (P > 0.05) versus the saline control group.
The combination of irinotecan and 5-FU induces intestinal damage
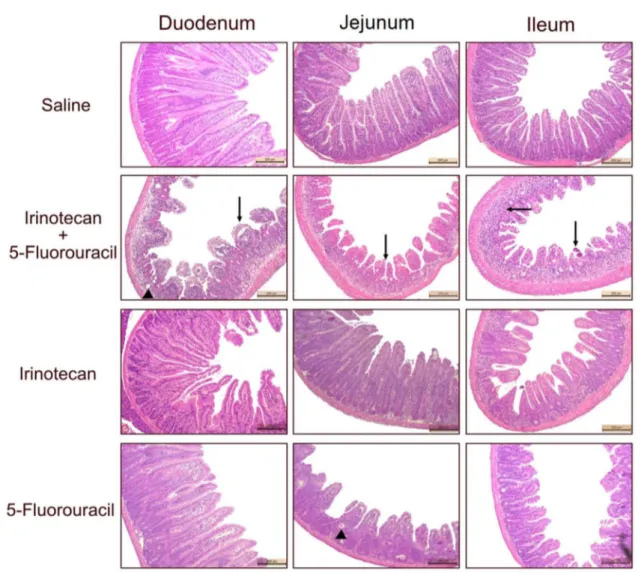
The combination of irinotecan and 5-FU induced pro-nounced histopathological changes in all segments of the smooth intestine [4(4–4)] when compared with the saline group [0(0–0), Fig. 3; supplemental Table 1, P < 0.05]. The altered intestinal architecture was characterized by short and flattened villi, partial crypt necrosis, entero-cyte vacuolization, and the presence of inflammatory
Fig. 1 The combination of irinotecan and 5-FU reduces animal sur-vival. The animals (n= 6 per group) were injected for four consecu-tive days with open circle saline, filled circle irinotecan + 5-fluoro-uracil, filled triangle irinotecan, or inverted filled triangle 5-FU
polymorphonuclear cell infiltrates in the mucosa. In addition, the groups injected with 5-FU or irinotecan alone showed mild-to-moderate intestinal injury (Fig. 3;
supplemental Table 1). On the other hand, the saline-injected mice showed a highly preserved intestinal struc-ture with intact villi and crypts, a cylindrical epithelial cell line covering the whole intestine, and intact Paneth cells (Fig. 3; supplemental Table 1). These results were con-firmed by the morphometric analysis of the duodenum, jejunum, and ileum samples, as observed in supplemental Fig. 1. The length of the villi in all intestinal portions was significantly reduced in the irinotecan + 5-FU group
ver-sus the saline-injected mice (P < 0.05). However, in the groups treated with only irinotecan or 5-fluorouracil, the villi height was partially reduced when compared with the saline group (P < 0.05) but was milder compared to the drug combination group (P < 0.05, supplemental Fig. 1). The crypt depth, which represents an attempt to recover the pronounced cell loss in the top of the villi, was unal-tered in the irinotecan + 5-FU group, but it was increased
in the single drug administration groups compared to the saline group (P < 0.05). The villus/crypt ratio normalized alterations independently described by the villus length and crypt depth in the whole smooth intestine, confirming the drug combination’s deleterious effect (supplemental Fig. 1).
Fig. 2 Mice injected with the combination of irinotecan and 5-FU show a pronounced loss of body weight. The animals (n = 6 per group) were injected for four consecutive days with open circle
saline, filled circle irinotecan + 5-fluorouracil, filled triangle irinote-can, or inverted filled triangle 5-FU at different doses, and the
vari-ations in body weight were recorded and expressed as a percentage in comparison with day 0. The points represent the mean ± standard error of the mean (SEM). Data were analyzed by two-way ANOVA/ Bonferroni’s test. *P < 0.05 and *** P < 0.001 versus the saline group
Table 1 Assessment of diarrhea scores
Data were analyzed using the Kruskal–Wallis test followed by Dunn’s test. The values are expressed as the median (mini-mum − maximum)
* P < 0.05 versus saline group. # P < 0.05 versus irinotecan (IRI) + 5-FU-treated group (n= 6 animals per group)
a Data shown correspond to the fourth day of analysis due to the high
mortality of the animals
Group Saline IRI/5-FU IRI 5-FU
Doses (mg/kg) – 30/50 30 50
Diarrheaa 0 (0–1) 2 (0–3)* 0 (0–1) 0 (0–2)
Doses (mg/kg) – 30/25 30 25
Diarrhea 0 (0–1) 0 (0–1) 0 (0–1) 0 (0–1)
Doses (mg/kg) – 45/25 45 25
Diarrhea 0 (0–1) 0 (0–1) 0 (0–0) 0 (0–1)
Doses (mg/kg) – 45/37.5 45 37.5
The injection of irinotecan and 5-FU causes an inflammatory intestinal reaction
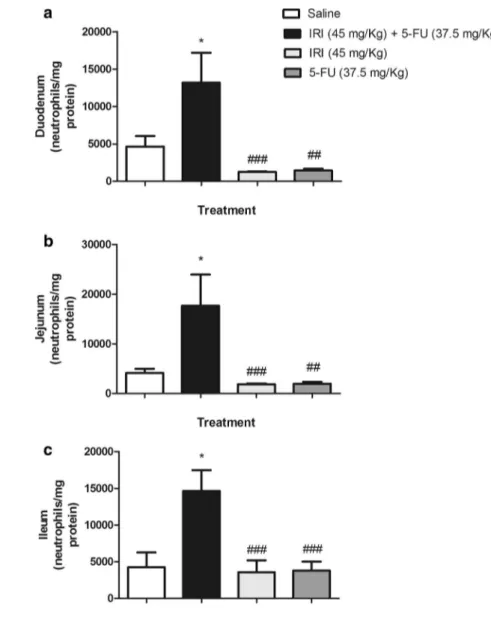
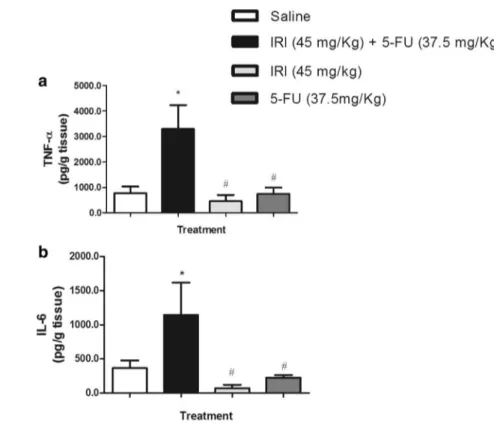
The irinotecan (45 mg/kg, i.p.) and 5-FU (37.5 mg/kg, i.p.) combination significantly (P < 0.05) increased the MPO activity in all of the intestinal segments (duodenum, jejunum, and ileum) compared to the saline-administered group (P < 0.001, Fig. 4a–c). However, the injection of irinotecan or 5-FU alone was ineffective in increasing the neutrophil infiltration compared to the saline control group (P > 0.05, Fig. 4a–c). These findings are in accordance with the augmented levels of pro-inflammatory cytokines TNF-α (3295 ± 941) and IL-6 (1146 ± 471) in the irinotecan
and 5-FU combined group versus the saline-treated mice
(TNF-α: 778 ± 254; IL-6: 337 ± 108, P < 0.05, Fig. 5). The injection of irinotecan or 5-FU alone showed basal lev-els of these cytokines (P > 0.05 vs. saline group, Fig. 5).
Discussion
In the present study, we elaborated on the experimental intestinal mucositis model by combining irinotecan and 5-fluorouracil, which was characterized by pronounced intestinal damage accompanied by an inflammatory reac-tion and diarrhea.
In the current research, we used much lower doses (sub-clinical doses) than those used in previous studies where
Fig. 3 The intestinal architecture is altered in mice treated with the combination of irinotecan and 5-fluorouracil. The animals (n= 6 per group) were injected for four consecutive days with saline, irinote-can + 5-fluorouracil, irinotecan, or 5-FU at different doses, and they were euthanized on the seventh experimental day. Samples from the duodenum, jejunum, and ileum were collected and processed for his-topathological analysis. H&E staining (× 100 magnification). The
the same chemotherapeutic agents were administered alone [15, 17, 18, 21, 28–30]. In those studies, irinotecan was commonly injected at doses ranging from 60 to 100 mg/ kg and 5-FU ranging from 150 to 450 mg/kg. The dose reductions were imperative due to the high mortality rate of the animals, which was probably associated with severe diarrhea. However, very low combined doses were inef-fective in establishing intestinal injury and diarrhea (data not shown). In addition, the subclinical doses of the drugs administered as single agents in our study did not induce appreciable toxicity. These low doses were needed in order to develop an optimal and well-tolerated dose combination. Each drug administration protocol was based on the fre-quency of irinotecan injections, as demonstrated by Lima-Júnior et al. [17]. Then, we adapted the 5-FU protocol to that experimental protocol. It is important to emphasize that most of the doses chosen did not lead to any considerable animal mortality when administered alone. Nevertheless,
5-FU seemed to be more deleterious because the dose of 37.5 mg/kg caused a residual animal mortality (20 % of animals) and even aggravated the diarrheic events when the dose was increased from 25 to 37.5 mg/kg and combined with irinotecan. For those reasons, we decided to use iri-notecan at 45 mg/kg and 5-FU at 37.5 mg/kg, because this combination did not cause a significant animal mortality on day seven but caused marked diarrhea and body weight loss.
Saltz and colleagues [31] have indicated that the com-bination of irinotecan, leucovorin, and 5-FU induces significant diarrhea. In a review by Keefe et al. [32], the severity of diarrhea is reported to be markedly increased to grades 3 or 4 (severe diarrhea) when drugs commonly used for the treatment of colorectal cancer are combined, including irinotecan, 5-fluorouracil, and oxaliplatin [12,
32]. Despite the high incidence, the mechanisms of diar-rhea development seem to vary according to the drug
Fig. 4 The combination of irinotecan and 5-FU induces a pronounced neutrophil accu-mulation in the intestine. The animals (n= 6 per group) were injected for four consecutive days with 0.9 % saline (0.5 mL/ kg, i.p) (white bar), irinote-can (45 mg/kg, i.p) + 5-FU (37.5 mg/kg i.p) (black bar), irinotecan (45 mg/kg, i.p) (light gray bar), or 5-FU (37.5 mg/ kg, i.p) (dark gray bar) and were euthanized on the seventh experimental day. Samples from the duodenum (a), jejunum (b), and ileum (c) were removed and frozen for a later determination of myeloperoxidase activity (MPO). The values represent the mean ± SEM of neutrophils/ mg protein and were analyzed by one-way ANOVA/Bonfer-roni’s test. *P < 0.05 versus the saline group, ##P < 0.01 and
###P
and are partially unclear. In the case of irinotecan, early- and late-onset diarrheas are reported to occur [33]. The early form exhibits a cholinergic-dependent mechanism because the use of atropine prevents its establishment [33]. On the other hand, late diarrhea apparently depends on the underlying inflammatory reaction with the partici-pation of TNF-α, IL-1β, KC, IL-18, and nitric oxide [15,
17, 18, 34], which may contribute to the reduced expres-sion of claudin-1 and occludin [35]. In regard to 5-fluoro-uracil, Sakai et al. [36] reported that neutrophil elastase alters the levels of aquaporin 4 and 8 in the colon of mice treated with 5-FU. In addition, similar to irinotecan, 5-FU activates pro-inflammatory cytokines, causing a reduction in the expression of tight junction proteins, such as occlu-din and clauocclu-din-1, and leaocclu-ding to diarrhea [37]. Further-more, the modulation of key pro-inflammatory cytokines, such as IL-1, prevents the development of diarrhea induced by either 5-FU [38] or irinotecan [34]. However, the inflammatory players that cause the intestinal dam-age in each case may be different. For instance, a genetic deletion of Toll-like receptor 2 prevents the irinotecan-related intestinal mucositis [39], but aggravates the del-eterious methotrexate effect on the gut [40]. Accordingly, Logan et al. [41] reported that the production of several cytokines during mucositis followed a different time course depending on the chemotherapeutic agent used. Such findings reinforce the need for new animal models that mimic the polychemotherapy regimens employed in the clinical setting.
One important mechanism that accounts for diarrheic events is the intestinal injury that is locally caused by the anticancer agents’ antiproliferative and apoptotic effects [42]. In our experimental model, we also verified a marked intestinal injury, as detected by the loss of epithelial cells and changes in the intestinal architecture due to villi short-ening, cell vacuolization, and intestinal crypt necrosis. Our findings are in line with those published by our group and others [15, 17, 18, 30, 34, 38]. In addition to the direct toxic effect on the intestinal architecture associated with antican-cer agents, the inflammatory reaction is clearly recognized as a pivotal component of gut injury. Here, we showed a pronounced accumulation of neutrophils in all smooth intestinal segments and increased levels of cytokines, such as TNF-α and IL-6, which suggests that polymor-phonuclear inflammatory cells recruited by inflammatory mediators might be a relevant component of chemother-apy-related intestinal injury. Accordingly, Sakai et al. [36] reported that the modulation of neutrophil recruitment with a CXCR2 antagonist prevented the development of 5-fluo-rouracil-induced mucositis. Moreover, the data obtained by some studies clearly suggest that myeloperoxidase activity, cytokines production, and morphometric analysis can be used as an indication of the level of intestinal damage [30,
43, 44]. Currently, the role of each inflammatory compo-nent in our animal model is not clear, which merits further investigation.
Currently described mucositis animal models have a par-tial limitation, which is the use of a single agent to simulate
clinical mucositis [15, 17, 18, 21, 28–30]. In addition, the translation of experimental knowledge to the clinical set-ting might be restricted and influenced by a variety of fac-tors, including the experimental conditions in which those are performed, invariably higher drug doses to reproduce the pathology in all the animals under investigation, and interspecies differences [13]. However, those models pro-vide important information on how each drug contributes to the development of intestinal mucositis because they reveal specific molecular targets to be pharmacologically modulated.
Therefore, in this context, we contributed with the elab-oration of an animal model combining two drugs that are commonly used in the same anticancer regimens in clinical practice.
Acknowledgments We are grateful to Maria Silvandira Freire for her technical assistance. This study was supported by CNPq (Con-selho Nacional de Desenvolvimento Científico e Tecnológico, Grant Nos 308879/2009-0, 307143/2014-7) and FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico, Grant No. 11.01.00/08). G.A.C.B., R.A.R., and R.C.P.L.J. are CNPq fellowship holders.
Compliance with ethical standards
Conflict of interest None.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
2. World Health Organization. Projections of mortality and causes of death, 2015 and 2030. http://www.who.int/healthinfo/global_ burden_disease/projections/en/, accessed on June 01st, 2015 3. Sutcliffe SB (2012) Cancer control: life and death in an unequal
world. Curr Oncol 19(1):12–15
4. Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284
5. Rubenstein EB, Peterson DE, Schubert M, Keefe D, Mcguire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST (2004) Mucositis study section of the multinational association for sup-portive care in cancer; international society for oral oncology. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Can-cer 100:2026–2046
6. Gibson RJ, Keefe DM, Lalla RV, Bateman E, Blijlevens N, Fijl-stra M, King EE, Stringer AM, Van Der Velden WJ, Yazbeck R, Elad S, Bowen JM (2013) Mucositis study group of the multi-national association of supportive care in cancer/intermulti-national society of oral oncology (MASCC/ISOO). Systematic review of agents for the management of gastrointestinal mucositis in can-cer patients. Support Care Cancan-cer 21:313–326
7. Lee SH, Son MH, Sung KW, Choi YB, Lee NH, Yoo KH, Koo HH, do Lim H, Shin HJ (2014) Toxicity of tandem high-dose chemotherapy and autologous stem cell transplantation using carboplatin–thiotepa–etoposide and cyclophosphamide–melpha-lan regimens for malignant brain tumors in children and young adults. J Neurooncol 120(3):507–513
8. Gibson RJ, Keefe DM (2006) Cancer chemotherapy-induced diarrhea and constipation: mechanisms of damage and preven-tion strategies. Support Care Cancer 14:890–900
9. Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB (2003) The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98:1531–1539
10. Elting LS, Cooksley CD, Chambers MS, Garden AS (2007) Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 68:1110–1120
11. Scully C, Sonis S, Diz PD (2006) Oral mucositis. Oral Dis 12:229–241
12. Saltz LB, Douillard JY, Pirotta N, Alakl M, Gruia G, Awad L, Elfring GL, Locker PK, Miller LL (2001) Irinotecan plus fluoro-uracil/leucovorin for metastatic colorectal cancer: a new survival standard. Oncologist 6:81–91
13. Bowen JM, Gibson RJ, Keefe DMK (2011) Animal mod-els of mucositis: implications for therapy. J Support Oncol 9:161–168
14. Lalla RV (2014) Alleviating mucositis: are we on track for a novel therapeutic? Expert Rev Gastroenterol Hepatol 9(2):127–128
15. Melo ML, Brito GA, Soares RC, Carvalho SB, Silva JV, Soares PM, Vale ML, Souza MH, Cunha FQ, Ribeiro RA (2008) Role of cytokines (TNF-alpha, IL-1beta and KC) in the pathogen-esis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide. Cancer Chemother Pharmacol 61:775–784
16. Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM (2008) Serum levels of NF KappaB and drug pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer BiolTher 7:1139–1145
17. Lima-Junior RC, Figueiredo AA, Freitas HC, Melo ML, Wong DV, Leite CA, Medeiros RP, Marques-Neto RD, Vale ML, Brito GA, Oriá RB, Souza MH, Cunha FQ, Ribeiro RA (2012) Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother Pharmacol 69:931–942
18. Lima-Júnior RC, Freitas HC, Wong DV, Wanderley CW, Nunes LG, Leite LL, Miranda SP, Souza MH, Brito GA, Magalhães PJ, Teixeira MM, Cunha FQ, Ribeiro RA (2014) Targeted inhibition of IL-18 attenuates irinotecan-induced intestinal mucositis in mice. Br J Pharmacol 171:2335–2350
19. Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS, Braun H, Lima-Junior RC, Ribeiro RA, Cunha FQ, Teixeira MM, Beyaert R, Graham GJ, Liew FY (2014) IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol 7:1079–1093. doi:10.1038/mi.2013.124
20. Soares PM, Mota JM, Gomes AS, Oliveira RB, Assreuy AM, Brito GA, Santos AA, Ribeiro RA, Souza MH (2008) Gastroin-testinal dysmotility in 5-fluorouracil-induced inGastroin-testinal mucosi-tis outlasts inflammatory process resolution. Cancer Chemother Pharmacol 63:91–98
21. Soares PM, Lima-Junior RC, Mota JM, Justino PF, Brito GA, Ribeiro RA, Cunha FQ, Souza MH (2011) Role of platelet-activating factor in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Cancer Chemother Pharmacol 68:713–720
22. National Institutes of Health (2011) Guide for the Care and Use of Laboratory Animals, National Academies Press (US), Wash-ington (DC)
administration schedule improves induction of delayed-onset diarrhea in rats. Cancer Chemother Pharmacol 46:211–220 24. Woo PCY, Ng WF, Leung HCH, Tsoi HW, Yuen KY (2000)
Clarithomycin attenuates cyclophosphamide-induced mucositis in mice. Pharmacol Res 41:526–532
25. Alves-Filho JC, de Freitas A, Russo M, Cunha FQ (2006) Toll-like receptor 4 signaling leads to neutrophil migration impair-ment in polymicrobial sepsis. Crit Care Med 34:461–470 26. Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ
(1995) Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol 115:1265–1275
27. Cunha FQ, Boukili MA, Motta JIB, Vargaftig BB, Ferreira SH (1993) Blockade by fenspiride of endotoxin-induced neutrophil migration in the rat. Eur J Pharmacol 238:47–52
28. Ballerini G, Bosi L, Castoldi GL, Ricci N (1961) Experimental enteropathy induced by 5-fluorouracil in the rat. Boll Soc Ital Biol Sper 30(37):578–580
29. Ikuno N, Soda H, Watanabe M, Oka M (1995) Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and caecum. J Natl Cancer Inst 87:1876–1888
30. Mashtoub S, Tran CD, Howarth GS (2013) Emu oil expedites small intestinal repair following 5-fluorouracil-induced mucosi-tis in rats. Exp Biol Med (Maywood) 238:1305–1317
31. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
32. Keefe DM et al (2007) Mucositis study section of the multina-tional association of supportive care in cancer and the interna-tional society for oral oncology. Cancer 109:820–831
33. Hyatt JL, Tsurkan L, Morton CL, Yoon KJ, Harel M, Brumshtein B, Silman I, Sussman JL, Wadkins RM, Potter PM (2005) Inhibi-tion of acetylcholinesterase by the anticancer prodrug CPT-11. Chem Biol Interact 157–158:247–252
34. Arifa RD, Madeira MF, de Paula TP, Lima RL, Tavares LD, Menezes-Garcia Z, Fagundes CT, Rachid MA, Ryffel B, Zam-boni DS, Teixeira MM, Souza DG (2014) Inflammasome activa-tion is reactive oxygen species dependent and mediates irinote-can-induced mucositis through IL-1β and IL-18 in mice. Am J Pathol 184:2023–2034
35. Wardill HR, Bowen JM, Al-Dasooqi N, Sultani M, Bateman E, Stansborough R, Shirren J, Gibson RJ (2014) Irinotecan disrupts tight junction proteins within the gut: implications for chemo-therapy-induced gut toxicity. Cancer Biol Ther 15:236–244 36. Sakai H, Sagara A, Matsumoto K, Jo A, Hirosaki A, Takase K,
Sugiyama R, Sato K, Ikegami D, Horie S, Matoba M, Narita M (2014) Neutrophil recruitment is critical for 5-fluorouracil-induced diarrhea and the decrease in aquaporins in the colon. Pharmacol Res 87:71–79
37. Song MK, Park MY, Sung MK (2013) 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J Cancer Prev 18:322–329
38. Wang X, Gao J, Qian L, Gao J, Zhu S, Wu M, Zhang Y, Guan W, Ye H, Yu Y, Han W (2015) Exogenous IL-1Ra attenuates intesti-nal mucositis induced by oxaliplatin and 5-fluorouracil through suppression of p53-dependent apoptosis. Anticancer Drugs 26:35–45
39. Wong DV, Lima-Júnior RC, Carvalho CB, Borges VF, Wander-ley CW, Bem AX, Leite CA, Teixeira MA, Batista GL, Silva RL, Cunha TM, Brito GA, Almeida PR, Cunha FQ, Ribeiro RA (2015) The adaptor protein Myd88 is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS ONE 10:e0139985
40. Frank M, Hennenberg EM, Eyking A, Rünzi M, Gerken G, Scott P, Parkhill J, Walker AW, Cario E (2015) TLR signaling modu-lates side effects of anticancer therapy in the small intestine. J Immunol 194(4):1983–1995
41. Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM (2009) Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother Pharmacol 63:239–251 42. Keefe DM, Brealey J, Goland GJ, Cummins AG (2000)
Chemo-therapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47:632–637 43. Cheah KY, Howarth GS, Bastian SE (2014) Grape seed extract
dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effective-ness in colon cancer cells. PLoS ONE 9:e85184