RevistaBrasileiradeFarmacognosia26(2016)375–378
w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Solanum
paniculatum
root
extract
reduces
diarrhea
in
rats
Jonh
A.B.
Tenório
a,
Dulciana
S.
do
Monte
a,
Thelma
M.G.
da
Silva
a,
Teresinha
G.
da
Silva
b,
Clécio
S.
Ramos
a,∗aDepartmentofChemistry,UniversidadeFederalRuraldePernambuco,Recife,PE,Brazil bDepartmentofAntibiotics,UniversidadeFederaldePernambuco,Recife,PE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14September2015 Accepted7February2016 Availableonline23March2016
Keywords:
Antidiarrhealactivity Chronicdiarrhea Chlorogenicacid Gastrointestinalmobility Jurubeba
a
b
s
t
r
a
c
t
SolanumpaniculatumL.,Solanaceae,locallyknownas“jurubeba”,iswidelyusedinBrazilforculinary
purposes,andinfolkmedicinetotreatofdiversedisorderincludinggastricdysfunctions.Inthisstudy weinvestigatedtheantidiarrhealactivityofS.paniculatumrootsextractinratsatdifferent concen-trations(125,250and500mg/kg,p.o)usingdifferentexperimentalmodelssuchascastoroil-induced diarrhea,enteropoolingandgastrointestinalmotility,determinedbyinvivoexperimentalmodels.The majorcompoundofrootextractwascharacterizedaschlorogenicacidbasedintheIR,1Dand2DNMR analysis.Alltheextractdosesachievedantidiarrhealpotency,asindicatedbyreducedweightoffecesin castoroil-induceddiarrhea,decreasedintestinalmotilityandsignificantlyinhibitedcastoroil-induced enteropoolingcomparedtothevehiclegroup.Thehighestdose(500mg/kg)producedgreater anti-motilityeffectandbetterreductionofenteropooling,similartothereferencedrugLoperamide(5mg/kg). ExtractfromS.paniculatumL.rootshadantidiarrhealactivity,asshownbythelowerweightofthefeces aswellasdecreaseintheaccumulationofintestinalfluidandslowertransit,justifyingthetraditional useofplantfordiarrhea.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
ThefamilySolanaceaeA.L.Jussieucomprisesmorethan3000 speciesdistributed in106genera,and theirspecies havea cos-mopolitandistribution.Themosteconomicallyimportantgenus ofthe familyis Solanum,withapproximately1500 species dis-tributedallovertheworld(Nuritetal.,2007).Amongthespecies ofSolanumgenus,SolanumpaniculatumL.istobemorethat high-lightsforitsmanymedicinaluses,widedistributionandespecially forbeingrecognizedasherbalmedicinebytheBrazilian Pharma-copeia,whoserootsandstemsareindicatedinthetreatmentof anemiaandliveranddigestivedisorders.ThespeciesS. panicula-tumL.,knownpopularlyeitherasjurubebaorjurupeba,isused commonlyinBrazilianfolkmedicineforthetreatmentofdiverse disorders,suchasliverandgastricdysfunctionsandhangoveras wellasforculinarypurposes(Mesia-Velaetal.,2002;Botionetal., 2005;Agraetal.,2007;SabirandRocha,2008;Vieiraetal.,2013). Theplantisacomponentofavarietyofpharmaceutical formula-tionsincluding:syrups,infusionsanddecoctions,ethanolextracts,
∗ Correspondingauthor.
E-mail:clecio.ramos@ufrpe.br(C.S.Ramos).
andelixirs(Júnioretal.,2015).Ethnopharmacologicalstudieshave revealedthatleaves,roots,stemsandfruitsofS.paniculatumare usedtotreatdisordersoftheliverandkidney,anemia,tuberculosis, malariaand hypertension,andinanti-inflammatory,antipyretic and stimulant formulations (Joachimovits, 1954; Ribeiro et al., 1986; Agraet al.,2007).Chemicalstudies revealeda varietyof compoundgroupsinS.paniculatumtissues,suchassteroids, ter-penes, alkaloids (including jurubebina, jubebine and solanine), and saponins (including sojuripidine, isojurubidine, isopanicu-lidine and jurubidine) (Vieira et al., 2010; Ramos and Ramos, 2012).
Diarrheacontinuestobeamajorhealthproblemthroughout theworld,especiallycausingofmalnutritioninchildrenunderfive yearsold.Itisalsoamajorcauseofthehighmorbidityand mor-tality,particularlyamong children. In developing countries, the principalcausesof diarrheais associated withtheenterotoxins thatareproducedandsecretedfrombacterialorganismslike Vib-riocholerae,Salmonella,ShigellaandEscherichiacoli(Walkeretal., 2013).
ConsideringthepharmacologicalpotentialofS.paniculatumand theuseofplantsforthetreatmentofdiarrhea,thisstudy investi-gatedtheactivityofS.paniculatumextractagainstdiarrheainduced byvariousmethodsinrats.
http://dx.doi.org/10.1016/j.bjp.2016.02.003
376 J.A.B.Tenórioetal./RevistaBrasileiradeFarmacognosia26(2016)375–378
Materialsandmethods
Plantmaterials,extractionandisolation
SolanumpaniculatumL.,Solanaceae,specimenswerecollected inthecityofCamaragibe,Pernambucostate,northeasternBrazil, in January2012. Botanical identificationwas doneby Instituto AgronômicodePernambuco(IPA)and a voucherspecimenwas deposited at the Dárdano de Andrade Lima Herbarium of IPA (88503).Therootsweredriedat40◦Cby72handthedriedmaterial wasmilledtoafinepowderinaMACSALABmill.
Driedroots(46g)werefirstlysubmittedSoxhletextractionwith 200mlofdichloromethanefor2handfollowedbyextractionusing 300ml of ethanol for a period of 4h. The solvent wasfiltered andconcentratedinvacuotoyield4.4g(9.6%)offatty-freecrude extractswithpresenceofprecipitate.Partofextractwaswashed twicewithchloroform,filtratedanddriedtoyielda whitesolid identifiedaschlorogenicacid.
Instrumentationandchromatographymaterials
IRspectraweremeasuredinKBrpelletswithaVarianinfrared spectrometer.1Dand2DNMRspectrawererecordedusingBruker spectrometer NMR (300MHz and 500MHz). HPLC analyses of extractswereperformedinaShimadzuLC10instrumentusinga PhenomenexRP-18 column(250mm×4.7mm,5m),eluted in gradientmodestartingwith5%aqueousformicacid/methanol(8:2) for15min,raisingto30%methanolin20min,withdetectionat 325nmandflowrateof1ml/min.Fourmgofthecrudeextractwas suspendedin2mlofmethanolandappliedtoC18cartridges.The cartridgeswereelutedwith4mlofMeOHandtheeluentswere fil-tered(0.45mfilter)priortoanalysis.Thestandardofchlorogenic acidwasacquiredoftheSigma–Aldrich(Batch:SLBB6914V).
Experimentalanimals
Malealbinoratsweighingbetween200and250gwereusedfor theexperiments.Theanimalswereobtainedfromtheanimalhouse oftheAntibioticsDepartmentofFederalUniversityofPernambuco, whichisregisteredwiththeBrazilianCollegeofAnimal Experimen-tationunderno.18.AllexperimentswereauthorizedbytheEthical CommitteeforAnimalCare(protocolnumber: 23076.46150/2012-23CCB-UFPE).Theanimalswerekeptinpolypropyleneboxesata temperatureof22±3◦Cwithalight-darkcycleof12handreceived balancedfeedandwateradlibitum.Theanimalsweredeprivedof foodbeforeeachexperiment.
Castoroilinduceddiarrhea
The method described by Awouters et al. (1978) was fol-lowed.Healthyalbinoratsofeithersex(200–250g)wererandomly selectedand dividedintofivegroupsoffiveanimalseach.They weredeprivedoffoodfor12hpriortothetest,withfreeaccess towater.Group1receivedthevehiclealone(1%Tween80),while thoseingroup2receivedLoperamide(5mg/kg)aspositivecontrol. Animalsingroups3,4and5receivedS.paniculatumrootextracts (125,250,500mg/kg)respectively.Extractadministrationwasby theoralroute.Theanimalswerehousedsinglyincageslinedwith pre-weighedfilterpaper.Onehour afterpretreatmentwiththe extract,theanimalswerethengiven1mlofcastoroilorallyand thetimebetweenoiladministrationandappearanceoffirst diar-rhealdropwasnoted.Thereafter,theywereobservedfor4hfor thepresenceofdiarrheaandtotal weightoffecesexcretedwas obtained.
Gastrointestinalmotilitytest
Malealbinorats(200–250g)wererandomlydividedintofive groupsoffiveratseach.Theyweredeprivedoffoodfor12hbefore thetest,butwereallowedfreeaccesstowater.Group1rats (con-trol)weretreatedwiththevehicle(1%Tween80).Group2rats receivedLoperamide(5mg/kg),whilegroups3,4and5received differentdosesoftheS.paniculatumrootextracts(125,250and 500mg/kg),respectively.Thirtyminutesafterdrugadministration, 1mlofcharcoalmeal(5%)wasadministeredorallytoallanimals and30minlater,alltheratsweresacrificedandtheabdomenwas opened.Thesmallintestine wasdissectedout fromthepylorus tothececumandthetotaldistancetraveledbythecharcoalplug alongthesmallintestinewasestimatedforboththecontrolandthe treatedgroups.Thepercentagedistancetraveledbythecharcoal mealfromthepylorustothececumwasnoted.
Castoroil-inducedenteropooling
Inthismethod,asdescribedbyRobertetal.(1976),ratswere deprivedof foodfor12hbeforetheexperiment. Theratswere dividedintosixgroups offiveeach.Thevehicle(1%Tween80) wasgiventothefirstgroup.Thesecondgroupreceived Lopera-mide(5mg/kg),whilegroups3,4and5receivedgradeddosesof SPRE(125,250and500mg/kg).Thirtyminuteslater,alltherats weretreatedwith1mlofcastoroil.Ashamgroupdidnotreceive anytreatment.After30min,eachratwassacrificedandthewhole lengthoftheintestinefromthepylorustothececumwasdissected andthecontentsweighed.
Statisticalanalysis
Resultswereexpressedasmean±SEM.Thesignificanceof dif-ferencebetweenmeanswasdeterminedusingone-wayanalysis ofvarianceandthestatisticalsignificancewassetatp<0.05.All datawereanalyzedusingGraphPadPrismversion5.0(GraphPad SoftwareInc.,SanDiego,CA,USA).
Results
Chemicalprofile
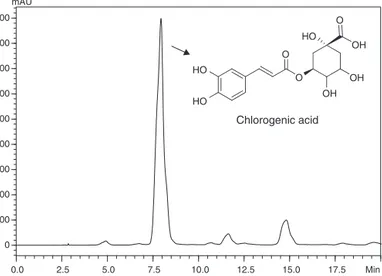
TheanalysisbyHPLCrootextractofS.paniculatumshoweda majorpeak withretentiontime at 8min(Fig.1).Theethanolic crudeextractoftheS.paniculatumrootswassubmittedto purifica-tionsteps,resultingintheisolationofmajorcompoundasayellow amorphouspowderdeterminedaschlorogenicacid.Structural elu-cidationofchlorogenicacidwasbasedoninterpretationofspectral data,mainlyIR,UV1Hand13CNMRand2D-NMR,including
com-parisonwithvaluesdescribedintheliterature(Agboetal.,2014).
Castoroil-induceddiarrhea
The highest total weight of feces was obtainedin the con-trolgroup (11.17±0.70g), whereas thelowest weight of feces wasrecordedforanimalsthatreceivedLoperamide(3.81±0.13g) (Table1).Alltheextractdosesachievedantidiarrhealpotencyby meansofreducedweightoffecescomparedtothecontrolgroup.
Gastrointestinalmotilitytest
J.A.B.Tenórioetal./RevistaBrasileiradeFarmacognosia26(2016)375–378 377
0.0 2.5 5.0 7.5 10.0 12.5 15.0 17.5 Min
0 100 200 300 400 500 600 700 800 900
mAU
O O HO
HO
HO
OH
OH OH O
Chlorogenic acid
Fig.1.ChemicalprofileobtainedbyHPLCofcrudeextractofSolanum panicula-tumroots.(C18column250mm×4.7mm,5m,elutedwith5%aqueousformic
acid/methanol8:2for15min,raisingto30%methanolin20min; =325nm;flow 1ml/min).
Table1
EffectoftheSolanumpaniculatumrootsextractoncastoroil-induceddiarrheain rats.
Group Dose(mg/kg) Totalweightof feces(g)
Inhibition(%)
Vehicle – 11.17±0.70 –
Loperamide 5 3.81±0.13a 65.9
125 6.54±0.16a 41.5
Extract 250 6.26±0.28a 44.0
500 5.59±0.10a 50.0
Valuesareexpressedasmean±SEM(n=5).
ap<0.01withrespecttothevehicle-treatedgroup,usingone-wayANOVA
fol-lowedTurkey’stest.
Table2
EffectoftheSolanumpaniculatumrootsextractongastrointestinalmotilitytest.
Group Dose(mg/kg) Distancetraveledby charcoal(%)
Inhibition(%)
Vehicle – 73.88±1.34 –
Loperamide 5 52.45±2.28b 47.5
125 64.62±1.87a 35.4
Extract 250 61.92±1.80a 38.0
500 55.86±3.40b 44.1
Valuesareexpressedasmean±SEM(n=5).
ap<0.05withrespecttothevehicle-treatedgroup,usingone-wayANOVA
fol-lowedTurkey’stest.
bp<0.01withrespecttothevehicle-treatedgroup,usingone-wayANOVA
fol-lowedTurkey’stest.
The highest dose of SPRE (500mg/kg) produced greater anti-motilityeffect,similartoLoperamide(5mg/kg).
Castoroil-inducedenteropooling
As shown in Table 3, the intestinal weight content was much higher in the control group (3.277±0.18g) compared withshamgroup(2.040±0.03g).Consistently,5mg/kgof Loper-amide reduced the intestinal content significantly compared with the control group (p<0.01). Treatments with SPRE (125, 250and500mg/kg)decreasedtheintestinalweightcontentsto 2.788±0.08g(p<0.05),2.657±0.08g(p<0.05)and2.402±0.14g (p<0.01),respectively.
Table3
EffectoftheSolanumpaniculatumrootsextractoncastoroil-inducedenteropooling.
Group Dose(mg/kg) Weightofintestinal content(g)
Inhibition(%)
Sham 2.040±0.03 –
Vehicle 3.277±0.18 –
Loperamide 5 2.392±0.05b 27.0
125 2.788±0.08a 14.9
Extract 250 2.657±0.08a 18.9
500 2.402±0.14b 26.7
Valuesareexpressedasmean±SEM(n=5).
ap<0.05withrespecttothevehicle-treatedgroup,usingone-wayANOVA
fol-lowedTurkey’stest.
bp<0.01withrespecttothevehicle-treatedgroup,usingone-wayANOVA
fol-lowedTurkey’stest.
Discussion
Diarrheaisoneofthemaincausesofinfantmortalityin devel-opingcountries,causingabout5to8milliondeathsayear,mainly among children under five years of age. Medicinalplants have beenusedtraditionally,includingS.paniculatum,asantidiarrheal withoutanyscientificbase(Konatéetal.,2015).Diarrheainvolves increasedgastrointestinalmobility andsecretionand decreased absorptionofwaterandelectrolytes(Wangetal.,2015).
Thegastrointestinalmodelusingactivatedcharcoalasmarker hasbeenusedforover60yearsasasimpleandeffectivetoassess theeffectsoflaxatives.Thismethodindicatesthemaximum dis-tancetraveledbythemarkerinthesmallintestineinagiventime intervalafteritsadministration(Silvaetal.,2012).Accordingtothe model,theS.paniculatumextractsatthedifferentconcentrations evaluatedsignificantlyreducedtheintestinalmotility,witha dose-dependentpatternwhencomparedtothecontrolgroup(Table2). ThemotilityintheanimalsthatreceivedSPREattheconcentration of500mg/kgwasabout44%slower,demonstratingthattheextract hasapositiveeffectinreducingintestinaltransit.
Castoroilinducesdiarrheabycausinganincreaseinthe secre-tionoffluidsandelectrolytesintheintestinallumenthroughthe mucosa.Thisinturncausesa build-upof fluidandan aqueous lumencontentthatpassesquicklythroughthegut(Wangetal., 2015).Thereleaseofricinoleicacidproduceschangesinthe trans-portofwaterandelectrolytes,diminishingtheabsorptionofNa+
andK+,inturnreducingtheactivityofNa+,K+-ATPaseinthesmall
intestine and colon,resulting ina hypersecretoryresponse and fasterintestinaltransit(Dashetal.,2014).Castoroilincreasesthe productionorreleaseofprostaglandins(Saitoetal.,2002), chang-ingthepermeabilityandcausinginjuriestotheintestinalmucosa. Italsostimulatesbiosynthesisofplateletaggregationfactor(Izzo etal.,1998),whichcanresultininflammationofthemucosa.
TheextractfromS.paniculatumrootsinthecastoroil-induced diarrheamodelreducedthediarrheasignificantly,atallthetested doses,whencomparedtothecontrolgroupthatonlyreceivedthe vehicle(Table1).Theextractcausedadiarrheareductionofabout 50%,besidesmorefeceswithsolidconsistencyandreduced evac-uationfrequencyinrelationtothegroupcontrol.Anotherresult observedinthisworkwasthereductionoftheaccumulationof intestinalfluidinduced bycastoroil. TheS.paniculatumextract reducedtheaccumulationofintestinalfluidatalltheevaluated doses(125,250and500mg/kg),withinhibitionratesof14.9,18.9 and26.7%,respectively,showinga dose-dependentrelationship. Thedoseof500mg/kgproducedthebestresults,withsimilar inhi-bitionratetoLoperamide(Table2).
378 J.A.B.Tenórioetal./RevistaBrasileiradeFarmacognosia26(2016)375–378
inhibiteddiarrhealdroppingsby84.81%,resultsimilarto antidiar-rheal compounds Loperamide (72.65%) and diphenoxylate HCl (84.81%)(Tekeetal.,2010).Thediterpenoid19-deoxyicetexone iso-latedfromSalviaballotifloraatdosesof12.5and25mg/kgreduced diarrheafor61.8and89.6%,respectively (Pérez-Gutiérrezetal., 2013).A previousstudy,theethanolicextractfrom S. panicula-tumaerialpartsshowedantidiarrhealactivityatdoseof125,250, 500,and750mg/kg,andthechlorogenicacidalsowasidentified in theaerial parts of plant(Clementino-Netoet al., 2016).The reducedaccumulationofintestinalfluidpromotedbytheS. pan-iculatumextractfromrootsmaybeassociatedwiththepresence ofchlorogenicacid,aknownanti-inflammatorycompound,since theintestinal hypersecretion of fluid involves aninflammatory response(Izzoetal.,1998;Guoetal.,2015).
Conclusion
OveralltheextractofS.paniculatumL.rootshadantidiarrheal activity,as shown bythe lower weightof thefeces as wellas decreaseintheaccumulationofintestinalfluidandslowertransit, justifyingthetraditionaluseofplantfordiarrhea.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contributions
JABT,DSMandTGSdevelopedtheanimalmodel.JABTandTMGS wereresponsibleforthecollectionofplantsampleandthe chem-icalstudies.CSRandJABTdevelopedtheanalyticalmethodology (HPLC).CSRandTGSdesignedthestudyandsupervisedthe labo-ratorywork.CSRandJABTwrotethemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
JABTthanksCAPESforprovidingascholarship.Theauthorsare indebtedtotheCentrodeApoioaPesquisa(CENAPESQ),UFRPE,for thelaboratoryfacilities.
References
Agbo,M.O.,Nnadi,C.O.,Ukwueze,N.N.,Okoye,F.B.C.,2014.Phenolicconstituents fromPlatyceriumbifurcatumandtheirantioxidatantproperties.J.Nat.Prod.7, 48–57.
Agra,M.F.,Baracho,G.S.,Nurit,K.,Basílio,I.J.L.D.,Coelho,V.P.M.,2007.Medicinal andpoisonousdiversityofthefloraofCaririParaibano.J.Ethnopharmacol.111, 383–395.
Awouters,F.,Niemegeers,C.J.E.,Lenaerts,F.N.,Janscen,P.A.,1978.Delayofcastoroil diarrhoeainrats:anewwaytoevaluateinhibitorsofprostaglandinbiosynthesis. J.Pharm.Pharmacol.30,41–45.
Botion,L.M.,Ferreira,A.V.M.,Côrtes,S.F.,Lemos,V.S.,Braga,F.C.,2005.Effectsof theBrazilianphytopharmaceuticalproductIerobina®onlipidmetabolismand intestinaltonus.J.Ethnopharmacol.102,137–142.
Clementino-Neto,J.,Pereira,J.C.,Vasconcelos,L.H.C.,deSouza,I.L.L.,Silva,A.D.S., Silva,T.M.G.,Ramos,N.S.M.,Pessôa,H.L.F.,Silva,T.M.S.,daSilva,B.A.,Cavalcante, F.A.,2016.Toxicological,antidiarrhealandspasmolyticactivitiesofSolanum paniculatum.PlantaMed.82,58–64.
Dash,P.R.,Nasrin,M.,Raihan,S.Z.,Ali,M.S.,2014.Studyofantidiarrhoealactivityof twomedicinalplantsofBangladeshincastor-oilinduceddiarrhea.Int.J.Pharm. Sci.Res.5,3864–3868.
Guo,Y.J.,Luo,T.,Wu,F.,Mei,Y.W.,Peng,J.,Liu,H.,Li,H.R.,Zhang,S.L.,Dong,J.H., Fang,Y.,Zhao,L.,2015.InvolvementofTLR2andTLR9intheanti-inflammatory effectsofchlorogenicacidinHSV-1-infectedmicroglia.LifeSci.127,12–18. Izzo,A.A.,Gaginella,T.S.,Mascolo,N.,Capasso,F.,1998.Recentfindingsonthemode
ofactionoflaxatives:theroleofplateletactivatingfactorandnitricoxide.Trends Pharmacol.Sci.19,403–405.
Joachimovits,R.,1954.TheuterotonicactivityofseveralBrazilianplantsincluding theBrazilianformofSpartiumjunceum.Sci.Pharm.22,7–18.
Júnior,G.M.V.,daRocha,C.Q.,deSouzaRodrigues,T.,Hiruma-Lima,C.A.,Vilegas,W., 2015.NewsteroidalsaponinsandantiulceractivityfromSolanumpaniculatum
L.FoodChem.186,160–167.
Konaté,K.,Yomalan,K.,Sytar,O.,Brestic,M.,2015.Antidiarrhealandantimicrobial profilesextractsoftheleavesfromTrichiliaemeticaVahl.(Meliaceae).AsianPac. J.Trop.Biomed.5,242–248.
Mesia-Vela,S.,Santos,M.T.,Souccar,C.,Lima-Landman,M.T.R.,Lapa,A.J.,2002.
SolanumpaniculatumL.(jurubeba):potentinhibitorofgastricacidsecretion inmice.Phytomedicine9,508–514.
Nurit,K.,Agra,M.F.,Basílio,I.J.L.D.,2007.Estudofarmacobotânicocomparativoentre
SolanumpaniculatumL.eSolanumrhytidoandrumSendtn.(Solanaceae).Rev. Bras.Bioci.5,243–245.
Pérez-Gutiérrez,S.,Zavala-Mendoza,D.,Hernández-Munive,A.,Mendoza-Martínez, A.,Pérez-González,C.,Sánchez-Mendoza,E.,2013. Antidiarrhealactivityof 19-deoxyicetexoneisolatedfrom SalviaballotifloraBenthinmiceandrats. Molecules18,8895–8905.
Ramos,N.S.M.,Ramos,C.S.,2012.VolatilesfromSolanumpaniculatumleavesin responsetomechanicaldamage.Chem.Nat.Compd.49,953–954.
Ribeiro,R.A.,Fiuza,DeMelo,M.M.R.,DeBarros,F.,Gomes,C.,Trolin,G.,1986.Acute antihypertensiveeffectinconsciousratsproducedbysomemedicinalplants usedinthestateofSaoPaulo.J.Ethnopharmacol.15,261–269.
Robert,A.,Nezamis,J.E.,Lancaster,C.,Hanchar,A.J.,Klepper,M.S.,1976. Enteropool-ingassay:atestfordiarrheaproducedbyprostaglandins.Prostaglandins5, 809–828.
Sabir,S.M.,Rocha,J.B.T.,2008.Antioxidantandhepatoprotectiveactivityof aque-ousextractofSolanumfastigiatum(falseJurubeba)againstparacetamol-induced liverdamageinmice.J.Ethnopharmacol.120,226–232.
Saito,T.,Mizutani,F.,Iwanaga,Y.,Morikawa,K.,Kato,H.,2002.Laxativeand anti-diarrhealactivity ofpolycarbophilinmiceand rats.Jpn. J.Pharmacol.89, 133–141.
Silva,P.C.B.,Neto,J.C.,Silva,A.D.S.,Silva,K.M.,Silva,T.M.S.,Agra,M.F.,Cavalcante, F.A.,2012.AntidiarrhealactivityofSolanumasterophoruminmice.Rev.Bras. Farmacogn.22,131–136.
Teke,G.N.,Kuiate,J-R.,Kueté,V.,Teponno,R.B.,Tapondjou,L.A.,Vilarem,G.,2010. AntidiarrhealactivityofextractsandcompoundfromTrilepisium madagas-cariensestembark.IndianJ.Pharmacol.42,157–163.
Vieira, P.M., Marinho, L.P.M., Ferri, S.C.S., Chen-Chen, L., 2013. Protective effectsofsteroidal alkaloidsisolatedfromSolanum paniculatumL.against mitomycin cytotoxic and genotoxic actions. An. Acad. Bras. Cienc. 85, 553–560.
Vieira,M.,Paula,J.R.,Chen-Chen,L.,2010.SolanumpaniculatumL.leafandfruit extracts:assessmentofmodulationofcytotoxicityandgenotoxicityby micro-nucleustestinmice.J.Med.Food13,1424–1430.
Walker,C.L.,Rudan,I.,Liu,L.,Nair,H.,Theodoratou,E.,Bhutta,Z.A.,O’Brien,K.L., Campbell,H.,Black,R.E.,2013.Globalburdenofchildhoodpneumoniaand diar-rhoea.Lancet381,1405–1416.