w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Anti-caries
activity
of
selected
Sudanese
medicinal
plants
with
emphasis
on
Terminalia
laxiflora
Ebtihal
Abdalla
M.
Mohieldin
a,b,
Ali
M.
Muddathir
a,c,∗,
Kosei
Yamauchi
a,
Tohru
Mitsunaga
a aDepartmentofAppliedLifeScience,FacultyofAppliedBiologicalScience,GifuUniversity,Gifu,JapanbFacultyofPharmacy,UniversityofScienceandTechnology,Omdurman,Sudan
cDepartmentofHorticulture,FacultyofAgriculture,UniversityofKhartoum,KhartoumNorth-Shambat,Khartoum,Sudan
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received27November2016
Accepted19April2017
Availableonline11July2017
Keywords:
Glucosyltransferase
Dentalcavity
Antibacterial Terchebulin
a
b
s
t
r
a
c
t
InSudan,somemedicinalplants,suchasAcaciaseyal,CalotropisproceraandBalanitesaegyptiacahavebeen usedtopreventortreatoralhealthproblems.ThestemandstembarkofTerminalialaxifloraEngl., Combre-taceae,areusedasantisepticsformouthwashtopreventgingivitisandthrushinAfrica.Methanoland50% hydroethanolicextractsof25plantsthatareusedintraditionalSudanesemedicineforseveraldiseases andcavitydisorderswerescreenedforanti-cavityactivities.T.laxifloramethanolicwoodextracts,which exhibitedsuchactivity,wereinvestigated.Thecrudeextractswereassayedfortheirantimicrobial activi-tiesagainstStreptococcussobrinusintermsofminimuminhibitoryconcentrationandglucosyltransferase inhibition.TheactiveextractofT.laxiflorawoodwassubsequentlyfractionatedbydifferent chromato-graphictechniques.IsolatedcompoundswereidentifiedbyspectroscopicmethodsandassessedforS. sobrinusandglucosyltransferaseinhibitoryeffects.MethanolicextractsofTerminaliabrownii(bark),T. laxiflora(wood),A.seyal(bark),Persicariaglabra(leaves)andTamarixnilotica(stem)showedgood activi-tiesagainstbothS.sobrinusandglucosyltransferase(MIC≤1mg/ml,IC50values<50g/ml).Overallplant extracts,T.laxiflorademonstratedthegoodcombinedactivities(MIC0.5mg/ml,glucosyltransferase,IC50 10.3g/ml);therefore,itsmethanolicwoodextractswereselectedforfurtherphytochemicalstudies. Fourconstituentswereisolatedbychromatographictechniquesandidentifiedbyspectroscopic tech-niques.Pharmacologicalevaluationoftheobtainedcompoundsshowedthatflavogallonicaciddilactone hadcomparativelygoodantibacterialactivity.Intheglucosyltransferaseinhibitorytest,terchebulin dis-playedpotentactivitywithanIC50of7.5M.Thescreeningpresentedinthisstudyshowedthatmethanol extractsofT.laxiflorawoodpossessedpromisinganti-cavityeffects.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Dentalcariesisdefinedasanirreversiblemicrobialdiseaseof thecalcifiedtissuesoftheteeth,characterizedbydemineralization oftheinorganicportionanddestructionoftheorganicsubstance ofthetooth(Rajendranetal.,2009).
Bacterial plaque composed of native oral flora accumulated ondentalsurfacesembeddedinanextracellular polysaccharide (EPS)matrixand istheprimary etiologicagentofdental caries (Kolenbranderetal.,2006;Kooetal.,2009).Outofthesevenspecies ofmutansstreptococcigroup,Streptococcussobrinushavebeenone ofthemostcommonlyimplicatedinthepathogenesisofdental cavity;moreover,itproducesexoenzymesnamed glucosyltrans-ferases(GTF),whichplaycriticalrolesinthesynthesisofglucan
∗ Correspondingauthor.
E-mail:muddathir@uofk.edu(A.M.Muddathir).
andEPStoprovidingsitesondentalsurfacesformicrobial coloniza-tion,inadditiontoadherentglucanforbacterialcoherence(Paes Lemeetal.,2006;BowenandKoo,2011;Nishimuraetal.,2012; Hashizume-Takizawaetal.,2014).
Medicinalplantshavebeenagreatsourceofnoveldrug com-poundsfromlongtime.Plantderivedproductshavemadelarge contributionstothewellbeingofhumanhealth.Scientistsacross theglobehavereportedantimicrobialpropertiesofseveral medic-inalplantsbutstillaveryfewofthisenormouspotentialdrughas beenscientificallyscreened(SiqueiraandRocas,2005;Karuppiah andRajaram,2012;GauniyalandTeotia,2014).InSudan,various medicinalplantshavebeenusedtopreventor treatoralhealth problems.Thisstudyinvestigatedsomeplantsthatareusedin tra-ditionalSudanese medicineasmouthdetergentssuchasAcacia seyal,CalotropisproceraandBalanitesaegyptiaca(ElGhazalietal., 2003; Khalidetal., 2012).Different parts ofTerminalialaxiflora Engl.,Combretaceae,areusedtopreventgingivitisandthrushin Congo;thestemsareusedaschewingsticksandalsomacerated
http://dx.doi.org/10.1016/j.bjp.2017.04.002
0102-695X/©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
stem barkare usedas antiseptic towash mouth (Fasolaet al., 2013).
Inthepresentstudy,25plantspecieswereselectedand eval-uatedfortheiranti-cariogenicactivityintermsofinhibitionofS. sobrinusbacterialgrowthandGTFinhibitoryeffectsbyusingtheir methanolicand50%hydroethanolicextracts.Methanolicextracts ofT.laxiflorawooddemonstratedsignificantcombinedactivities; thus,itwasfurtherfractionatedinordertoidentifytheactive com-poundsresponsibleforthebiologicalactivities.
Materialsandmethods
Reagents
All materials were purchased from Wako, Japan except p -iodonitrotetrazolium(INT)violet,whichwasfromSigma-Aldrich Co.Ltd,Japan.
Plantmaterialsandextraction
The plants were collectedfrom Khartoum state (Khartoum, Omdurman and Shambat cities) and Elgadarif state of Sudan. VoucherspecimensaredepositedintheHorticulturalLaboratory, DepartmentofHorticulture,FacultyofAgriculture,Universityof Khartoum(Table1).
Plantmaterialswereshadedriedandpowderedbefore extrac-tion;theywereeachextractedthreetimesfor12h,withmethanol and50%hydroethanol.Theextractswerefilteredandthesolvent wasremovedunderreducedpressureusingrotaryevaporator.The concentratedextractswerethendriedwithafreezedryer.
Fractionation,purificationandidentificationofpurecompounds fromTerminalialaxiflora
Fractionationandisolation ofTerminalialaxifloraEngl., Com-bretaceae, wood were performed by the method described by Muddathiretal.(2013).Methanoliccrudeextracts(5g)were chro-matographedonmediumpressureliquidchromatography(MPLC) usingoctadecyl-silica(ODS)column(YMC-DispoPackATODS-25, particlesize25m;columnsize120g,(40mm×188mm),Japan), chromatographypump(Co.No.540Yamazen,Osaka,Japan)with pressure of 1.2MPa, UV detector at 280 wavelength (UV-10V Yamazen,Osaka,Japan)andfractioncollector(SF-2120,Advantec TokyoLtd,Japan).
The column was conditioned with the first eluent used for separation for30minwithflow rateof 0.5ml/min. Thenwater containingincreasingproportionsofmethanolstepwiseelutionto obtaintwofractions(F1andF2).F1(0.89g)waspassedthrough columnchromatography(40mm×430mm)onaSephadexLH-20 (18–111m,GEHealthcareBio-SciencesCorp,Tokyo,Japan)that
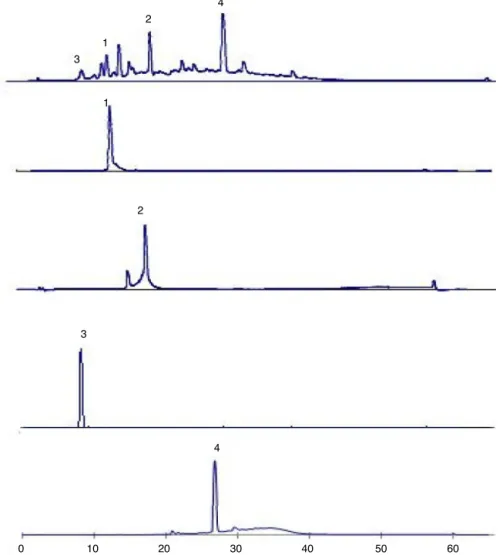
revealedfoursubfractions.Thesefoursubfractions,F(1a,1b,1c and1d)introducedthroughpreparativehighperformanceliquid chromatography(HPLC)withreversedphaseInertsilODS-3column (10mm×250mm,GLSciencesInc.,Tokyo,Japan)andmonitoredat 280nm.Solventsystemused10–100%gradientmethanolinwater with0.05%TFAprogrammedfor60minataflowrateof5ml/min togivecompound(1)(14mg),compound(2)(8.5mg)and com-pound(3)(5mg).F2(0.50g) was alsosubjected topreparative HPLCunderthesameconditiontoisolatecompound(4)(17.5mg). ThecompoundspuritywasconfirmedusinganalyticalHPLC (Shi-madzuSIL-20A)withreversedphaseInertsilODS-3Vcolumn(5m
(4.6mm×250mm), GL Sciences Inc., Tokyo, Japan), flow rate: 1ml/min,wavelength:280nm,gradientprogram:methanol:0.05% TFAaqueoussolutionfor60min(Fig.1).
The isolated compounds from T. laxiflora wood methanol extract,exactlycompounds(1)and(2)wereidentifiedusingthe
1H,13Cnuclearmagneticresonance(NMR).Spectrawererecorded
in methanol-d4 with a JEOL EC600MHz NMR (Tokyo, Japan).
Additionally, ultra-performanceliquid chromatography-time-of-flightmassspectrometry(UPLC-TOFMS,WatersXevoTMQTofMS,
Waters, Milford,MA, USA) was performed using a C18 column
(2.1mm×100mm,Waters)withMeOH/H2O=5/95(30min),100/0
(10min)withalineargradientaseluent.TheUPLC-TOFMSdata werecollectedinnegativeionizationmode(Fig.2).
Flavogallonicaciddilactone(1) Tanpowder.1HNMR(inCD
3OD):ı7.26(s),7.50(s).13CNMR(in
CD3OD):ı108.1(C-1,1′),110.1–114.4(C-6,6′),112.8(C-5),113.3
(C-6′′),117.5–120.2(C-5′,2′′),124.9(C-1′′),135.7(C-2),136.3 (C-3),136.5(C-4),137.8(C-2′),139.2(C-3′),143.2(C-4′),144.1(C-3′′), 145.9(C-4′′),147.8(C-5′′),158.9–160.4(C-7,7′),168.9(C-7′′); UPLC-TOFMSm/z469[M−H]−(calcd.forC
21H10O13470.2963).
Terchebulin(2)
Tanpowder.1HNMR(inCD
3OD):ı3.04(t,J=11.6Hz,oneof
theH-6′′),4.21(t,J=10.3Hz, H-5′′),4.48(t,J=8.9Hz,oneofthe H-6′′),4.78(t,J=11.0Hz,H-4′′),4.98(dd,J=3.5,9.7Hz,H-2′′),5.23 (d,J=2.8Hz,H-1′′),5.64(t,J=9.6Hz,H-3′′),6.37(s,H-B6),6.42(s, H-D6),6.56(s, H-A2),6.79(s,H-C2),7.48(s,H-5).13C NMR(in
CD3OD):ı63.4(C-6′′),68.5(C-4′′),69.0(C-5′′), 74.1(C-3′′), 74.2
(C-2′′),90.2(C-1′′),106.4(C-B6),106.5(C-D6),106.8(C-A2),108.5 (C-C2),112.0–114.0(C-A6,B2,5,5′,1,1′,2,2′,6,6′),116.0(C-C6), 122.2(C-D1),123.5(C-B1,C1),125.1(C-A1),135.9(C-B4),136.1 (C-A4),137.5(C-C4),137.6(C-D4),138.4(C-3),139.1(C-D3),140.7 (C-3′),141.7(C-D2),143.4–143.6(C-A5,B3,C5),144.5–144.6(C-A3, B5,C3,D5),147.4(C-4′),150.3(C-4),158.3(C-7′),159.5(C-7),166.9 (C-D7),167.0(C-C7),168.9(C-A7),169.5(C-B7);UPLC-TOFMSm/z 1083.07[M−H]−(calcd.forC
48H28O301084.7179).
Table1
Minimuminhibitoryconcentration(MIC)andglucosyltransferase(GTF)inhibitoryactivitiesofselectedSudanesemedicinalplantextracts.
No. Botanicalname Family Examinedpart Voucherspecimen Collectionplace Extracts MIC(mg/ml) GTF(%)
1 AristolochiabracteolataLam. Aristolochiaceae Wholeplant SD-SH-04 Shambat M –a –b
2 E 1.0 08.0±4.3h
3 Calotropisprocera(Aiton) Dryand.
Apocynaceae Leaves SD-SH-11 Shambat M 4.0 –
4 E – 06.7±8.9h
5 AmbrosiamaritimaL. Asteraceae Aerialpart SD-SH-03 Shambat M 4.0 –
6 E – 08.9±7.5h
7 XanthiumbrasilicumVell. Asteraceae Leaves SD-SH-12 Shambat M – –
8 E 4.0 –
9 Balanitesaegyptiaca(L.)Delile Zygophyllaceae Leaves SD-KH-15 Khartoum M 4.0 –
10 E 4.0 –
11 Bark M 4.0 –
12 E – –
13 AdansoniadigitataL. Malvaceae Fruitpulp SD-OD-27 Omdurman M – 57.0±1.0d
14 E – 13.7±4.8g,h
15 GuierasenegalensisJ.F.Gmel. Combretaceae Leaves SD-OD-40 Omdurman M 2.0 70.4±1.0c,d
16 E – 69.6±3.0c,d
17 TerminaliabrowniiFresen. Combretaceae Bark SD-GF-02 Elgadarif M 0.5 88.5±2.1a,b
18 E 4.0 90.1±2.1a,b
19 TerminalialaxifloraEngl. Combretaceae Wood SD-KH-03 Khartoum M 0.5 87.2±2.6a,b
20 E 4.0 80.5±1.7a,b,c
21 VernoniaamygdalinaDelile Asteraceae Leaves SD-KH-19 Khartoum M 4.0 –
22 E 4.0 –
23 EuphorbiahirtaL. Euphorbiaceae Aerialpart SD-SH-37 Shambat M – –
24 E – –
25 RicinuscommunisL. Euphorbiaceae Leaves SD-SH-36 Shambat M 4.0 –
26 E 4.0 –
27 Acaciatortilis(Forssk.)Hayne Fabaceae Bark SD-KH-07 Khartoum M 2.0 –
28 E 4.0 –
29 Wood M – –
30 E 4.0 11.2±9.0h
31 Acaciaseyalvar.fistula
(Schweinf.)Oliv.
Fabaceae Bark SD-KH-06 Khartoum M – 82.9±3.0a,b,c
32 E – 89.4±0.1a,b
33 Wood M – –
34 E – 50.3±1.0e
35 AcaciaseyalDelile Fabaceae Bark SD-GF-05 Elgadarif M 1.0 91.5±5.1a,b
36 E 1.0 85.2±3.1a,b,c
37 Wood M – –
38 E 4.0 34.8±4.3f
39 CassiaacutifoliaDelile Fabaceae Leaves SD-SH-24 Shambat M 2.0 –
40 E 2.0 –
41 ParkinsoniaaculeataL. Fabaceae Leaves SD-SH-02 Shambat M 4.0 –
42 E 4.0 07.1±5.0h
43 Khayasenegalensis(Desr.)A.Juss. Meliaceae Bark SD-SH-14 Shambat M 4.0 88.7±3.0a,b
44 E – 77.8±3.8b,c,d
45 PeganumharmalaL. Nitrariaceae Seed SD-OD-20 Omdurman M 2.0 –
46 E – –
47 ArgemonemexicanaL. Papaveraceae Leaves SD-KH-39 Khartoum M 4.0 –
48 E – –
49 Seed M 1.0 –
50 E – –
51 Persicariaglabra(Willd.)M. Gómez
Polygonaceae Leaves SD-SH-A-03 Shambat M 1.0 87.2±9.0a,b
52 E – 78.2±0.6b,c,d
53 Ziziphusspina–christi(L.)Desf. Rhamnaceae Bark SD-SH-06 Shambat M 4.0 94.5±2.3a
54 E 4.0 27.0±5.0f,g
55 Leaves M 2.0 –
56 E – –
57 SolanumdubiumDunal Solanaceae Fruits SD-SH-34 Shambat M – –
58 E – –
59 Tamarixnilotica(Ehrenb.)Bunge Tamaricaceae Stems SD-OD-10 Omdurman M 1.0 85.4±0.3a,b
60 E – 91.4±2.2a,b
61 TribulusterrestrisL. Zygophyllaceae Aerialpart SD-SH-33 Shambat M – –
62 E – –
M,methanol;E,50%hydroethanol.Valueswereexpressedasmean±SD;n=3.Meanswithdifferentlettersinthesamecolumnweresignificantlydifferentatthelevel
(p<0.05).
aNoinhibitoryactivityatconcentrationof4mg/ml.
4 2
1 3
1
2
3
4
0 10 20 30 40 50 60
Fig.1.HPLCfingerprintofmethanolextractofTerminalialaxifloraandtheirisolatedcompounds.(1)Flavogallonicaciddilactone[retentiontime:13.6min],(2)terchebulin
[retentiontime:17.3min],(3)gallicacid[retentiontime:8.8min]and(4)ellagicacid[retentiontime:26.7min].
Biologicalactivitiesofthecrudeextractsandisolatedcompounds
Determinationoftheminimuminhibitoryconcentration(MIC) MICwasdeterminedbythebrothdilutionmethodaccordingto Iwakietal.(2006).S.sobrinus6715wasculturedinaBrain-Heart InfusionBroth.Thecrude extracts,fractionsorpurecompounds weretestedforantibacterialactivityinsterile96-wellplates.The inoculumswerepreparedbydilutingthebrothcultureto approx-imately 106cells/ml. To each wellcontaining sample,100
l of
microbialinoculumswereadded,followedbyadditionofmedium toachieveafinalvolumeof200l.Thetestedsamplewasprepared
inaconcentrationrangeof4000–31g/mlusingatwo-fold
dilu-tionmethod.Solventandmediumcontrolswereincludedineach testplate.Inordertodissolvethesampleextracts,20%dimethyl sulfoxide(DMSO)wasusedinthisstudy.Thefinalconcentrationof DMSOaloneinthewellshowednoinhibitoryeffectonS.sobrinus growth.Theexperimentswereperformedintriplicate. Chlorhex-idinewasincludedintheassaysaspositivecontrol.Thecultures wereincubatedfor24hat37◦Cunderanaerobicconditions. Micro-bialgrowthwasindicatedbytheadditionof50lof(0.2mg/ml)
INTtotheculture andincubated at37◦C for2h.The MICwas definedasthelowestconcentrationthatinhibitedthecolorchange ofINT(Eloff,2001).
AssayforGTFinhibitoryactivity
Streptococcussobrinus6715wasculturedfor20hat37◦Cin4l ofToddHewittbroth.Aftercentrifugationofthecultureat1300×g
for10minat4◦C,thecellswerecollectedandthenextractedwith 8Mureafor1hwhilestirring.Thecrudeenzymesolutionwas dia-lyzedagainst10mMsodiumphosphatebuffer(pH6.0).Thecrude enzymesolutionwasstoredinafreezerat−80◦C.
GTFwereincubatedin300lof0.1Mphosphatebuffer(pH
6.0)containing1%sucrose,0.5%dextranT-10,inthepresenceor absenceofasampleat37◦Cfor3h.ThevolumeofthecrudeGTF solutionusedintheassaywasdeterminedbymeasuredturbidity around1.0absorbanceat590nm(Mitsunagaetal.,1997).
Inhibition (%)
=Absorbanceofcontrol−Absorbanceofsample
Absorbanceofcontrol ×100
Statisticalanalysis
TheinhibitorypercentageandIC50valuesofGTFwereexpressed
asthemean(mean±standard deviation).Thesignificant differ-ences between samples were assessed by one-way analysis of variance(ANOVA)followedbypairwisecomparisonofthemean usingTukey’smultiplecomparisontest.Valuesweredetermined tobesignificantwhenpwaslessthan0.05(p<0.05).
Results
A
3W 13-320120901_1-4 110 (0.861)
3W 17
20120901_1-5 111 (0.868)
01-Sep-201220:21:10 1: TOF MS ES-3.33e4
01-Sep-201221:13:08 1: TOF MS ES-3.58e3 0
100
100
0
%
425.016
163.037
169.013
177.021298.985 301.000
423.018
392.996 425.018
450.997
495.023
539.011
595.093 626.112
627.129
729.207 781.069 861.258 993.304 1035.321 1081.055
1085.081 1084.073 1083.071
625.108
1086.073 1106.038
1167.355 245.155 249.041
335.024397.024 423.002
469.007
470.011 471.013
993.308
100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400
100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400
m/z
m/z
%
B
Table2
IC50values(g/ml)obtainedbythepotentmethanolicextractsagainst
glucosyl-transferase(GTF)enzyme.
Botanicalname Partused GTFIC50(g/ml)
A.seyal Bark 03.8±1.7a
T.brownii Bark 09.2±3.4a
T.laxiflora Wood 10.3±2.2a
K.senegalensis Bark 23.6±2.6b
P.glabra Leaves 27.1±4.1b,c,d
A.digitataL. Fruitpulp 35.9±3.2c,d,e
Z.spina–christi Bark 38.8±3.5d,e,f
A.seyalvar.fistula Bark 47.2±3.6e,f
T.nilotica Stems 49.8±4.9f
Meanswithdifferentlettersinthesamecolumnweresignificantlydifferentatthe level(p<0.05);n=3.
hydroethanolic extracts were prepared; and their antibacterial activitiesagainstS.sobrinusandGTFenzymeinhibitoryactivities wereinvestigated.
Antibacterialassay
To evaluate the antibacterial activity of selected Sudanese medicinalplantextractsagainstS.sobrinususingdilution meth-ods.TheMICweredeterminedinTable1.Among62plantextracts 35 extracts showed antibacterial activity. Alsonoteworthy the methanolicextractsofCombretaceae family;Terminaliabrownii (bark)andT.laxiflora(wood)demonstratedhighestantibacterial activity(MICof0.5mg/ml)amongthem.
GTFenzymeinhibitoryactivity
Inhibitoryeffectsover55%onGTFenzymeactivitywere demon-stratedby18ofthe62plantextractsat100g/ml(Table1);of
thesethirteenextractsexhibitedinhibitoryactivitymorethan80%. Table2showstheIC50 valuesof GTFinhibitoryactivityof nine
methanolicextractsrangedbetween3.8and49.8g/ml.A.seyal
(bark),T.brownii(bark)andT.laxiflora(wood)displayasignificant inhibitoryactivity.
Combinedactivitiesofthecrudeextractsandisolatedcompounds
Methanolicextracts of T. brownii (bark),T. laxiflora, A. seyal (bark), Persicaria glabra (leaves) and Tamarix nilotica (stems) demonstratedMIC≤1mg/mlagainstS.sobrinusandIC50lessthan
50g/mlagainstGTFenzyme.
Methanolicwoodextractsof T. laxiflorawhich showedgood combinedactivities(MIC0.5mg/ml,GTF,IC5010.3g/ml),were
selectedfor furtherpurification,crudemethanolicextractsafter subjectedtoMPLCresultedin twofractions F1(MIC0.5mg/ml, GTF96.4%) and F2(MIC1mg/ml, GTF93.1%); moreover purifi-cationrevealingthepresence offlavogallonicaciddilactone(1), terchebulin(2), gallicacid(3),and ellagicacid(4).Antibacterial andGTFinhibitoryactivitiesforisolatedcompoundsareshownin Table3.Flavogallonicaciddilactone(1)demonstratedrelatively goodantibacterialactivity,withMICof0.5mg/ml.Terchebulin(2) andellagicacid(4)showedmoderateantibacterialactivity, how-ever,terchebulin(2)displayedpotentactivityagainstGTFenzyme.
Discussion
AntibacterialandGTFenzymeinhibitoryactivitiesofextracts
Inthisstudysolventselectionreliedonpreviousstudies men-tioningthatmethanolisclassifiedasa polarsolventdue tothe presence of hydroxyl group.Nevertheless, there is alsomethyl
Table3
Anti-S.sobrinusandglucosyltransferase(GTF)inhibitoryactivityofcompounds
iso-latedfromT.laxiflorawood.
Compound MIC(mg/ml) GTFIC50(M)
Terchebulin 1 7.5±2.7a
Gallicacid –a NC
Ellagicacid 1 1017.5±4.1c
Flavogallonicaciddilactone 0.5 149±1.6b
Chlorhexidineb 0.0004 5.8±3.3a
NC,theIC50couldnotbecalculatedbecausetheactivitywaslessthan50%athighest
concentration.Meanswithdifferentlettersinthesamecolumnweresignificantly
differentatthelevel(p<0.05);n=3.
aNoinhibitoryactivityatconcentrationof4mg/ml.
bPositivecontrol.
group presence in methanol, which is sort of non-polar, so it hasability toextract varioustypes ofcompounds and increase extract’syield.Furthermore,itcangivehigherconcentrationsof bioactivemoleculesfromplantssuchasdifferentclassesof phe-noliccompounds.50%hydroethanolyieldhighcontentofphenolic andflavonoidcompounds(Ahmadetal.,2009;Cauniietal.,2012; Thanhetal.,2016).Somestudiesmentionedthatpolyphenols,such as flavonoids,phenolic acidsand tanninsshowed anti-enzyme, antibacterialand/oranti-biofilmactivities(Gulatietal.,2012;Livia etal.,2016).
TheMICvaluesofthemethanolextractswererelativelylower thanthoseofthe50%hydroethanolicextracts,implyingmoreactive antibacterialcompositioninmethanolthanin50% hydroethano-lic extracts. These findings were similar to those reported by Samuelsen (2000).Ncube et al. (2012) believed that the crude extractwillbeactivewhenhavingMICvalueslessthan8mg/ml, whilst Gibbons (2005) suggested that isolated phytochemicals shoulddemonstrateatleastMIC<1mg/ml.Inthisstudy,MICvalues of0.5mg/mlwereconsideredanindicationofgoodantibacterial activity.
Zhietal.(2016)statedthatinhibitionofGTFactivityandthe con-sequential polysaccharidesynthesis maydiminishthevirulence ofcariogenicbiofilms, whichcouldbeanalternativestrategyto eradicatedentalcaries.MethanolicextractsofA.seyal(bark),T. brownii(bark)andT.laxiflora(wood)showedsignificantinhibitory activitieson GTF.A previous studymentioned that methanolic extractsoftheseplantscontaincondensedandhydrolysable tan-nins (Muddathir and Mitsunaga, 2013).Yamauchi et al. (2016) reportedthatT.brownii(bark)containedgallicacid,punicalagin, terchebulin,ellagicacid4-O-␣-l-rhamnopyranoside,ellagicacid and3,4,3′-tri-O-methylellagicacid.Plantpolyphenolssharedwith catechin-basedoligomericforms(condensedtannins)and/or gal-lateesterformcompounds(hydrolysabletannins)displaystrong anti-GTFactivities(Yanagidaetal.,2000).
BiologicalactivitiesofcompoundsisolatedfromTerminalia laxiflorawood
OurchemicalprofilingofthemethanolextractsoftheT.laxiflora wooddisclosedthepresencesoffourcompoundsaswedescribed inexperimentalpart.Flavogallonicaciddilactone(1)showed1H
NMRspectral patternwithtwo1Hsinglets(ıH7.26and7.50). 13CNMRspectrumdisplayedsignalsofninenon-oxygenated
excellentlyreportedbyseveralauthors(GrimshawandHaworth, 1956;Tanakaetal.,1986,1996;Kinjoetal.,2001;Hiranoetal., 2003;Shuaibuetal.,2008;Ibrahimetal.,2014;Orabietal.,2015). Flavogallonicaciddilactone(1)wasisolatedearlierfrom Termina-liacatappainfreeformandasasingle-bondedacylunitontheir tanninpyranosecores,suchasterflavinsA–D(Tanakaetal.,1986). The1Hand13CNMRdataofterchebulin(2)weresimilartothose
reportedbyLinetal.(1990).TheUPLC-TOFMS(m/z1083[M−H]−) datawasinagreementwithSilvaetal.(2000).PreviouslySilvaetal. (2000)reportedthatterchebulinwasthemaincompoundpresent inTerminaliamacropteraroot.Gallicacid(3)andellagicacid(4) werealsodetectedin T.laxiflorawoodextract;thesetwo com-poundswereisolatedfromdifferentplantsofthegenusTerminalia (Silvaetal.,2000;Shuaibuetal.,2008).
Data in Table3 showedthat flavogallonicaciddilactone (1) exhibitedgood antibacterialactivity(0.5mg/ml).From previous study,theactivityofPropionibacteriumacneswasinhibitedat con-centration0.25mg/ml(MuddathirandMitsunaga,2013).TheMIC forgallicacid(3)hasnoactivitytowardS.sobrinusupto4mg/ml. Kangetal.(2008)statedthattheMICofgallicacidagainstS. sobri-nuswas8mg/ml.TheMICofellagicacid(4)was1mg/ml.Sarabhai etal.(2013)mentionedthatpureellagicaciddidnotexertmuch biofilminhibitingeffect.
Accordingtoourfindings, ellagicacid(4)showedweakGTF inhibitoryactivity(IC501017.5M).EventhoughSawamuraetal.
(1992)suggestedthat theuseofellagicacidwillnotaffectthe ecologicalbalanceoforalbacterialflora,butitcouldbeapossible anti-carriesagentthroughitsGTFinhibitoryaction.
Terchebulin(2)exhibitedsignificanceactivityagainstGTF(IC50
7.5M),whencomparedtothepositivecontrolofchlorhexidine
(IC505.8M).Oolongteafractionrichinhighmolecularweight
polyphenolsinhibitedthesynthesisofglucannon-competitively (Matsumotoetal.,2003).
Chlorhexidinedemonstrated thestrongest activity againstS. sobrinus and in addition, it showed good activity against GTF (Table 3).Nonetheless, it hasbeenreported that chlorhexidine wasfoundtobeacytotoxicagenttomurinefibroblastcelllines, humanalveolarbonecellsandhumanosteoblasticcellline.These resultsapprovethatchlorhexidineisnotcelltypespecific(Cabral andFernandes,2007;Giannellietal.,2008;Fariaetal.,2009;Li etal.,2014).Toxicityassaywithmousefibroblastsshowedthat ellagicacid,terchebulinandflavogallonicacidisolatedfrom Termi-naliaavicennoidesstembark,hadIC50≥1500g/mlaswellasthey
didnotaffecttheintegrityofhumanerythrocytemembraneofthe human(Shuaibuetal.,2008).
Conclusion
Inthepresentstudy,T.laxiflorawooddemonstratedsignificant anti-cavityactivity.Theseresultsjustifytheuseofthisplantfor oral carein traditional African medicine.The promising results ofantimicrobialandGTFinhibitoryactivityshownhere,suggests terchebulin(2)andflavogallonicaciddilactone(1)couldbe con-sideredforfurtherpharmacologicalstudies,evaluatingthetoxicity anddevelopmentofanaturalanticariogenicagentfordentalcaries.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethattheyhave fol-lowed theprotocolsof theirworkcenter onthe publicationof patientdata.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contributions
EAMM,AMM,KYandTMallcontributedtothewritingofthis article.EAMMandAMMobtainedsamples.TMdesignedthestudy andsupervisedthelaboratorywork.EAMMandAMMperformed thedifferentassaysandstatisticalanalysis.KMcontributedto com-poundidentification.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
The authorsthank Dr.Ashraf Mohamedfrom theFaculty of Forestry,Mrs.HamzaTagEL-Sir,Botanist,FacultyofAgriculture, DepartmentofBotany(Herbarium),UniversityofKhartoum,Sudan fortheirassistanceintheplantsidentificationandauthentications. EAMMacknowledgeforUniversityofGifu,Japan.
References
Ahmad,A.,Alkarkhi,A.A.,Hena,S.,Lim,H.K.,2009.Extraction,separationand
identi-ficationofchemicalingredientsofElephantopusscaber(L)usingfactorialdesign ofexperiment.Int.J.Chem.1,36–49.
Bowen,W.H.,Koo,H.,2011.BiologyofStreptococcusmutans-derived
glucosyltrans-ferases:roleinextracellularmatrixformationofcariogenicbiofilms.CariesRes. 45,69–86.
Cabral, C.T.,Fernandes, M.H., 2007. Invitrocomparison ofchlorhexidine and
povidone-iodineonthelong-termproliferationandfunctionalactivityofhuman alveolarbonecells.Clin.OralInvest.11,155–164.
Caunii,A.,Pribac,G.,Grozea,I.,Gaitin,D.,Samfira,I.,2012.Designofoptimal
sol-ventforextractionofbioactiveingredientsfromsixvarietiesofMedicagosativa. Chem.Cent.J.6,123.
ElGhazali,G.B.,Abdalla,W.E.,Khalid,H.E.,Khalafalla,M.M.,Hamad,A.A.,2003.
MedicinalPlantsofSudan.PartV.MedicinalPlantsofIngassanaArea.National CouncilforResearch,KhartoumPress,Khartoum,Sudan.
Eloff,J.N.,2001.AntibacterialactivityofMarula(Sclerocaryabirrea(A.rich)Hochst.
subsp.caffra(Sond.)Kokwaro)(Anacardiaceae)barkandleaves.J. Ethnophar-macol.76,305–308.
Faria,G.,Cardoso,C.R.B.,Larson,R.E.,Silva,J.S.,Rossi,M.A.,2009.
Chlorhexidine-inducedapoptosis ornecrosisinL929 fibroblasts:arole forendoplasmic reticulumstress.Toxicol.Appl.Pharmacol.234,256–265.
Fasola,T.R.,Oluwole,M.E.,Olaniyi,I.F.,Adeboye,I.E.,2013.Thephytochemicaland
antimicrobialactivitiesofTerminalialaxifloraEngl.&Dielsrootbarkextract.Nat. Sci.11,122–127.
Gauniyal,P.,Teotia,U.V.S.,2014.Phytochemicalscreeningandantimicrobialactivity
ofsomemedicinalplantsagainstoralflora.AsianPac.J.HealthSci.1,236–255.
Giannelli,M.,Chellini,F.,Margheri,M.,Tonelli,P.,Tani,A.,2008.Effectof
chlorhex-idine digluconateon differentcell types: a molecular and ultrastructural investigation.Toxicol.InVitro22,308–317.
Gibbons,S.,2005.Plantsasasourceofbacterialresistancemodulatorsand
anti-infectiveagents.Phytochem.Rev.4,63–78.
Grimshaw,J.,Haworth,R.D.,1956.Flavogallol.J.Chem.Soc.,4225–4232.
Gulati, V., Harding, I.H., Palombo, E.A., 2012. Enzyme inhibitoryand
antiox-idant activities of traditional medicinal plants: potential application
in the management of hyperglycemia. BMC Complement. Altern. Med.,
http://dx.doi.org/10.1186/1472-6882-12-77.
Hashizume-Takizawa,T.,Shinozaki-Kuwahara, N.,Tomita,N.,Kurita-Ochiai,T.,
2014.Establishmentofaconvenientsandwich-ELISAfordirectquantificationof
glucosyltransferase-I:applicationfordualdiagnosisofdentalcaries.Monoclon. Antib.Immunodiagn.Immunother.33,89–93.
Hirano,Y.,Kondo,R.,Sakai,K.,2003.5␣-Reductaseinhibitorytannin-related
com-poundisolatedfromShorealaevifolia.J.WoodSci.49,339–342.
Ibrahim,M.A.,Mohammed,A.,Isah,M.B.,Aliyu,A.B.,2014.Anti-trypanosomal
activ-ityofAfricanmedicinalplants:areviewupdate.J.Ethnopharmacol.154,26–54.
Iwaki,K.,Koya-Miyata,S.,Kohno,K.,Ushio,S.,Fukuda,S.,2006.Antimicrobialactivity
ofPolygonumtinctoriumLour:extractagainstoralpathogenicbacteria.J.Nat. Med.60,121–125.
Kang,M.S.,Oh,J.S.,Kang,I.C.,Hong,S.J.,Choi,C.H.,2008.Inhibitoryeffectofmethyl
gallateandgallicacidonoralbacteria.J.Microbiol.(Seoul,Korea)46,744–750.
Karuppiah,P.,Rajaram,S.,2012.AntibacterialeffectofAlliumsativumclovesand
Khalid,H.E.,Abdalla,W.E.,Haider,A.,Till,O.,Thomas,E.,2012.Gemsfromtraditional north-Africanmedicine:medicinalandaromaticplantsfromSudan.Nat.Prod. Bioprospect.2,92–103.
Kinjo,J.,Nagao,T.,Tanaka,T.,Nonaka,G.I.,Okabe,H.,2001.Antiproliferative
con-stituentsintheplant8.SeedsofRhynchosiavolubilis.Biol.Pharm.Bull.24, 1443–1445.
Kolenbrander,P.E.,Palmer,R.J.,Rickard,A.H.,Jakubovics,N.S.,Chalmers,N.I.,Diaz,
P.I.,2006.Bacterialinteractionsandsuccessionsduringplaquedevelopment.
Periodontology42,47–79.
Koo, H., Xiao,J., Klein, M.I., 2009. Extracellular polysaccharidesmatrix – an
oftenforgottenvirulencefactorinoralbiofilmresearch.Int.J.Oral Sci.1, 229–234.
Li,Y.C.,Kuan,Y.H.,Lee,T.H.,Huang,F.M.,Chang,Y.C.,2014.Assessmentofthe
cyto-toxicityofchlorhexidinebyemployinganinvitromammaliantestsystem.J. Dent.Sci.9,130–135.
Lin,T.C.,Nonaka,G.I.,Nishioka,I.,Ho,F.C.,1990.Tanninsandrelatedcompounds,
CII.Structuresofterchebulin,anellagitanninhavinganovel tetraphenylcar-boxylicacid(terchebulicacid)moiety,andbiogeneticallyrelatedtanninsfrom TerminaliachebulaRetz.L.Chem.Pharm.Bull.38,3004–3008.
Livia,S.,Silvia,F.,Katarina,R.,Jan,K.,Pavel,M.,2016.Antibiofilmactivityofplant
polyphenols.Molecules21,1717.
Matsumoto,M.,Hamada,S.,Ooshima,T.,2003.Molecularanalysisoftheinhibitory
effectsofoolongteapolyphenolsonglucan-bindingdomainofrecombinant glucosyltransferasesfromStreptococcusmutansMT8148.FEMSMicrobiol.Lett. 228,73–80.
Mitsunaga,T.,Abe,I.,Kontani,M.,Ono,H.,Tanaka,T.,1997.Inhibitoryeffectsof
barkproanthocyanidinsontheactivitiesofglucosyltransferasesofStreptococcus sobrinus.J.WoodChem.Technol.17,327–340.
Muddathir,A.M.,Mitsunaga,T.,2013.Evaluationofanti-acneactivityofselected
Sudanesemedicinalplants.J.WoodSci.59,73–79.
Muddathir,A.M.,Mitsunaga,T.,Yamauchi,K.,2013.Anti-acneactivityof
tannin-relatedcompoundsisolatedfromTerminalialaxiflora.J.WoodSci.59,426–431.
Ncube, B., Finnie, J.F., Van Staden,J., 2012. Invitro antimicrobial synergism
withinplantextractcombinationsfromthreeSouthAfricanmedicinalbulbs. J.Ethnopharmacol.139,81–89.
Nishimura,J.,Saito,T.,Yoneyama,H.,Bai,L.L.,Okumura,K.,Isogai,E.,2012.Biofilm
formationby Streptococcusmutansandrelated bacteria.Adv.Microbiol. 2, 208–215.
Orabi,M.A.A.,Yoshimura,M.,Amakura,Y.,Hatano,T.,2015.Ellagitannins,
gallotan-nins,andgallo-ellagitanninsfromthegallsofTamarixaphylla.Fitoterapia104, 55–63.
PaesLeme,A.F.,Koo,H.,Bellato,C.M.,Bedi,G.,Cury,J.A.,2006.Theroleofsucrosein
cariogenicdentalbiofilmformation–newinsight.J.Dent.Res.85,878–887.
Rajendran,R.,Sivapathasundharam,B.,Shafer,Hine,Levy,2009.Shafer’sTextbook
ofOralPathology,6thed.Elsevier,India,pp.409.
Samuelsen,A.B.,2000.Thetraditionaluses,chemicalconstituentsandbiological
activitiesofPlantagomajorL.Areview.J.Ethnopharmacol.71,1–21.
Sarabhai,S.,Sharma,P.,Capalash,N.,2013.EllagicacidderivativesfromTerminalia
chebulaRetz.downregulatetheexpressionofquorumsensinggenestoattenuate PseudomonasaeruginosaPAO1virulence.PLoSOne8,1–12.
Sawamura,S.,Tonosaki,Y.,Hamada,S.,1992.Inhibitoryeffectofellagicacidon
glucosyltransferasefrommutansstreptococci.Biosci.Biotechnol.Biochem.56, 766–768.
Shuaibu,M.N.,Wuyep,P.A.,Yanagi,T.,Hirayama,K.,Tanaka,T.,Kouno,I.,2008.The
useofmicrofluorometricmethodforactivity-guidedisolationofantiplasmodial compoundfromplantextracts.J.Parasitol.Res.102,1119–1127.
Silva,O.,Gomes,E.T.,Wolfender,J.L.,Marston,A.,Hostettmann,K.,2000.
Appli-cationofhighperformanceliquidchromatographycoupledwithultraviolet spectroscopyandelectrospraymassspectrometrytothecharacterisationof ellagitanninsfromTerminaliamacropteraroots.Pharm.Res.17,1396–1401.
Siqueira,J.F.,Rocas,I.N.,2005.Exploitingmolecularmethodstoexploreendodontic
infection:Part2–redefiningtheendodonticmicrobiota.J.Endod.3,488–498.
Tanaka,T.,Nonaka,G.,Nishioka,I.,1986.Tanninsandrelatedcompounds.XLII.
Iso-lationandcharacterizationoffournewhydrolysabletannins,terflavinsAandB, tegallaginandtercatainfromtheleavesofTerminaliacatappaL.Chem.Pharm. Bull.34,1039–1049.
Tanaka,T.,Ueda,N.,Shinohara,H.,Nonaka,G.,Fujioka,T.,Mihashi,K.,Kouno,I.,1996.
C-glycosidicellagitanninmetabolitesintheheartwoodofJapanesechestnuttree (CastaneacrenataSieb.etZucc.).Chem.Pharm.Bull.44,2236–2242.
Thanh,V.N.,Christopher,J.S.,Michael,C.B.,Phuong,D.N.,Quan,V.,2016.Impactof
differentextractionsolventsonbioactivecompoundsandantioxidantcapacity fromtherootofSalaciachinensisL.J.FoodQual.2017,8.
Yamauchi,K.,Mitsunaga,T.,Muddathir,A.M.,2016.Screeningfor
melanogenesis-controlledagentsusingSudanesemedicinalplantsandidentificationofactive compoundsinthemethanolextractofTerminaliabrowniibark.J.WoodSci.62, 285–293.
Yanagida,A.,Kanda,T.,Tanabe,M.,Matsudaira,F.,OliveiraCordeiro,J.G.,2000.
Inhibitoryeffectsofapplepolyphenolsandrelatedcompoundsoncariogenic factorsofmutansstreptococci.J.Agric.FoodChem.48,5666–5671.
Zhi,R.,Lulu,C.,Jiyao,L.,Yuqing,L.,2016.InhibitionofStreptococcusmutans
![Fig. 2. Mass spectra of flavogallonic acid dilactone (A) [m/z 469] and terchebulin (B) [m/z 1083] by UPLC-TOFMS in negative ionization mode.](https://thumb-eu.123doks.com/thumbv2/123dok_br/14931903.501511/5.918.104.832.77.1029/mass-spectra-flavogallonic-dilactone-terchebulin-tofms-negative-ionization.webp)