UTILIZATION OF BENTONITE AS AN
ADSPRPENT MATERIAL IN THE
REMOVAL OF IRON (III)
Ehssan M. N.
Petrochemical Departement, Pharos University, Alexandria, Egypt. ehssan.nassef@pua.edu.eg
Alexandria, Egypt.
Abstract:
In this study an Egyptian Bentonite clay has been used for the adsorption of iron salts from aqueous solutions over a concentration range of 50–100 mg/l, shaking time of 5–120 min, stirring rate from 50-200 r.p.m, adsorbent dosage from 0.1to 0.5 g, pH range(3-7), and temperature range from 25 C to 80 C. The experiments were carried out for the analysis of adsorption equilibrium capacities using a batch equilibrium technique. The process of uptake follows both the Langmuir and Freundlich isotherm models and also the second-order kinetics. The maximum removal of iron (100%) was observed with initial concentration of 40 mg/l and 0.5 g of bentonite, speed rate of 200 r.p.m. with 30 min time of contact and temperature of 25C. The paper also discusses the thermodynamic parameters of the adsorption (the Gibbs free energy, entropy, and enthalpy).
Key words: Adsorption; heavy metals; isotherms; kinetics; bentonite; thermodynamics;iron salts.
1. Introduction
Heavy metal ion pollution is currently of great concern due to the increased awareness of the potentially hazardous effects of elevated levels of these materials in the environment [Bhakta J.N et al. (2009) - Chantawong V. et al. (2003) - SenGupta S. and Bhattacharyya K.G. (2006)].Metal industries use substantial quantities of water in processes such as metal finishing and galvanized pipe manufacturing in order to produce corrosion-resistant products. Effluent wastewaters from such processes contain toxic substances, metal acids, alkalis, and other substances.
High concentrations of metals in the effluents may cause interference with biological treatment processes at sewage treatment plants [Lai C.H. et al.]. Iron ions cause serious problems in the aqueous streams especially at high levels concentration [Duffus J.H. (2002) - Weinberg E.D. (1999) - Das B. et al. (2007)]. In general, iron is regarded as one of the essential metal elements for humans.
Approximately3000–5000 mg of iron exists in the human body .Therefore; one can deduce thatas long as the quantity of iron in the environment is not too great, it may not be harmfulto the human body. However, iron can cause undesirable problems in industrial processesor ecosystems if its concentration in wastewater is not managed properly. For example, theprecipitates of iron hydroxide may block pipes and concentrated iron in the water can be accumulated biologically.
Also, iron can act as a source material for an unpleasant taste or odor in water. In addition, precipitates of iron form turbidity, which limits the usage of water for drinking or industrial processes. Since iron in the wastewater is able to induce several problems, it has been regarded as one of the major target materials to be removed [Bereket G. et al. (1997)]. In order to control iron content in water, many types of treatment technologies have been utilized, such as precipitation, electrochemical deposition, solvent extraction, ion exchange and adsorption, etc. [Uchida M. et al. (1999) - Pakula M. et al. (1998) - Huang C. and Cheng W.P. (1997) - Kato M. et al. (1998) - Belous A.G. et al. (2000) - Fahlman M. et al. (1998) - Voropanova L.A. and Velichko L.N. (1999) - Zarraa M.A. (1999)].
A review on the specific advantages and limitations of biological iron removal has been published recently [Sharma S.K. et al. (2005)]. Indeed, even in biological processes, physicochemical and biological mechanisms take place simultaneously [Dimitrakos Michalakos G. et al. (1997)]. These techniques were found not effective due to either being extremely expensive or too inefficient to reduce such high levels of ions from the large volumes of water [Ellis D. et al. (2000) - Hikmet K. et al. (2008)].
The effect of various parameters such as the effects of pH, bentonite doses, temperature, and contact time on the adsorption process has been investigated. This fundamental study will be helpful for further application in treatment of water containing heavy metal ions beyond their permissible limits.
2. Materials and Methods
2.1.Chemicals
Ferric Chloride FeCl3.6H2O (Merck, USA) was used in the adsorption experiments. A stock solution of the heavy metal solution was prepared by using an analytical grade chemicals and dissolving them in distilled water. Experimental solutions of the desired concentrations were obtained by successive dilution with distilled water. pH adjustments were carried out using 0.1N hydrochloric acid (HCl) and 0.1N sodium hydroxide (NaOH). A digitally calibrated pH-meter (HaNNa, Model pH 211) was used to measure the pH of each solution.
2.2.Characterization of Adsorbent
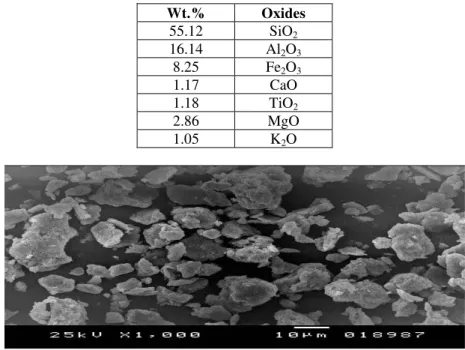
Bentonite clay was supplied from the Sphinx milling station Company (Alexandria free zone, Egypt). Bentonite is brown in color; it was characterized by x-ray florescence (XRF) using AXIOS PANalytical2005, The analysis was made in Central Metallurgical Research Institute in Alexandria. Table (1) shows the oxides constituents of the studied bentonite clay.
A scan electron microscope with different magnification was done on dry base for a sample of clay to specify the morphological features of bentonite. This was done in laboratory of faculty of science in Alexandria University as shown in Figure. (1). Natural bentonite has low alumina content (16.14%) and contains high silica contents (55.12%).Bentonites show higher distribution of alkali oxides. There is general agreement between the current obtained values for both major oxide contents and the high loss on ignition percentage (12-15%) with the calculated values and recorded results previously obtained for Egyptian kaolin and bentonite [Rashed M. and Soltan M. (2002) - Abollino O. et al. (2003) - Wahba M.M. and Zaghloul A.M. (2007)].
Table 1, Chemical characterization of bentonite
Wt.%
Oxides
55.12
SiO2 16.14
Al2O3 8.25
Fe2O3 1.17
CaO 1.18
TiO2 2.86
MgO
1.05 K2O
Fig. 1. Scanning electron microscope (SEM) of dry bentonite.
2.3. Adsorption Experiments
The adsorption experiments were conducted using Fe (III) salt solutions. The stock solution containing 1000 mg of Fe (III) per liter was prepared by dissolving 2.892 gm of FeCl3 in 1 L of distilled water. The sorption of Fe (III) on bentonite was studied by a batch technique. All the solutions were stored in glass bottles. The bottles were cleaned thoroughly with water, and then soaked in 0.01M nitric acid solution. Before use, the bottles were rinsed with distilled water.
with atomic adsorption flame photometer (Model ICE 3000, Thermo Fischer-USA). The percentage of removal of the heavy metal ions (%R) in solution was calculated using Equation (1)
*100 (1)
Where:
C0= Initial concentration of heavy metal (mg/L).
Ct= Heavy metals concentrations (mg/L) at a given time t. V= Volume of the heavy metals solutions (L).
Calculations were made by using these data and adsorption curves were obtained.
3. Results And Discussion
3.1.Effect of initial metal ion concentration
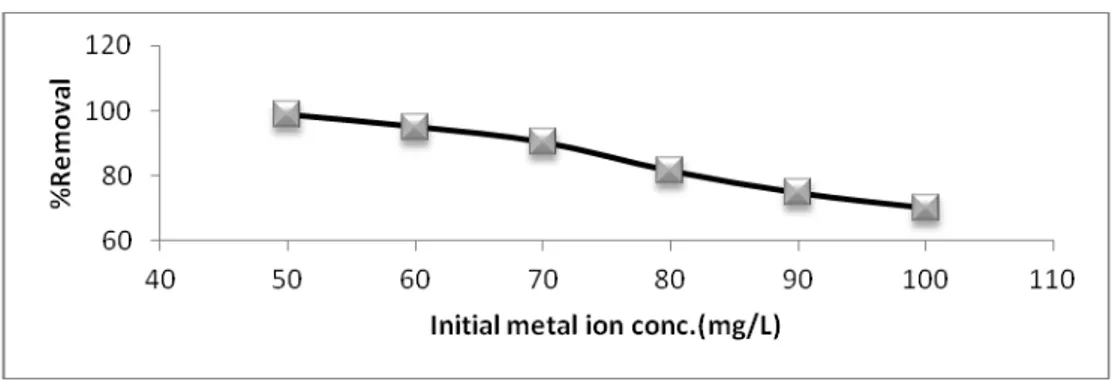
Figures (2 and 3) indicate the effect of initial concentration of Fe (III) ions on the residual concentration and percentage removal of iron respectively. It was found that as the initial concentration of Fe (III) increases from 50 mg/L to 100 mg /L, the percent removal of Fe (III) decreases from 98.6404 to 70.2028 with increase in residual concentration from 0.6798 mg/L to 29.7972 mg/L.
From figures it is evident that at high concentration, the available sites of concentration adsorption become fewer. This behavior surface and prevent the metal ions from passing deeply inside the bentointe. The adsorption occurs on the surface only. Similar results were observed in recent studies using natural zeolite [Al-Anber M. and Al-[Al-Anber Z. (2008) - Al-[Al-Anber M. (2007)] and olive cake [Al-[Al-Anber Z. and Al-[Al-Anber M. (2008)], in addition to what have been found by Karthikeyan et al. [Karthikeyan G. et al. (2005)].
Fig. 2. Effect of initial metal ion concentration on Residual concentration of Fe (III).
(CO =50mg/L, pH = 4, time = 30 min, stirring rate = 300 r.p.m., bentonite dosage=0.5g, and temp. = 25 ˚C).
Fig. 3. Effect of initial metal ion concentration on percentage removal of Fe (III).
(CO =50mg/L, pH = 4, time = 30 min, stirring rate = 300 r.p.m., bentonite dosage=0.5g., and temp. = 25 ˚C).
3.2.Effect of adsorbent dosage
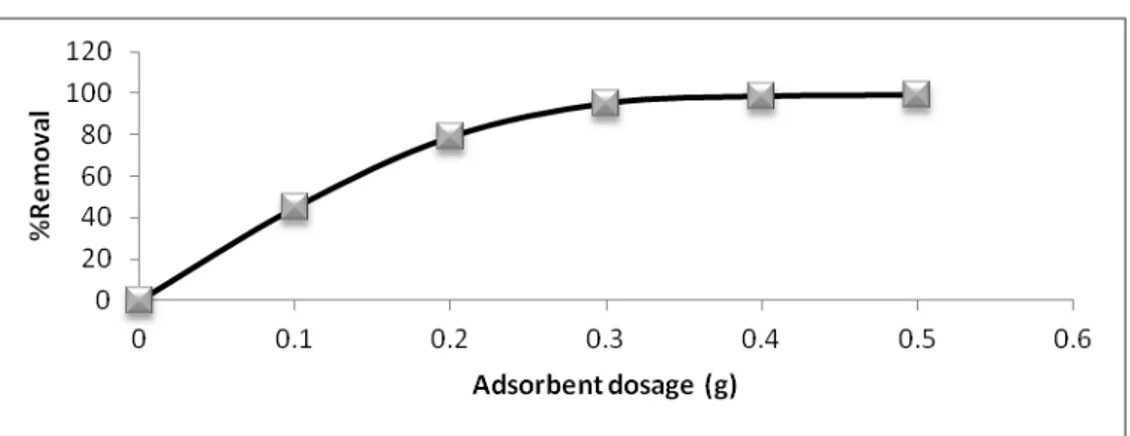
Other works have observed similar results for adsorption of metal ions on ion exchange resin [Rengaraj S. and Moon S.H. (2002)] and sawdust [Marin J. and Ayele J. (2002)] .The residual concentration of iron metal decreases as the dosage of bentonite increases. This is may be due to those higher amounts of bentonite means availability of a larger surface area or a larger number for adsorption sites [Awan M.A. et al. (2003)] and Therefore, higher capacity for adsorption.
Fig. 4. Effect of adsorbent dosage on Residual concentration of Fe (III). (CO =50mg/L, pH = 4, time = 30 min, stirring rate = 300 r.p.m., and temp. = 25 ˚C).
Fig. 5. Effect of adsorbent dosage on percentage removal of Fe (III). (CO =50mg/L, pH = 4, time = 30 min, stirring rate = 300 r.p.m., and temp. = 25 ˚C).
3.3.Effect of contact time
Figures (6 &7) indicate the effect of contact time for Fe (III) on percentage removal and residual concentration for both respectively. It was found that as the contact time between adsorbent and adsorbate increases the percentage removal increases and the residual concentration of Fe (III) decreases. In the first 30 min, the rate of adsorption was apparently fast, which can be explained that the available adsorption sites available were sufficient in the beginning.
As the process goes on, the adsorption sites became saturated gradually. The uptake rate was controlled by the rate at which the adsorbate is transported from the exterior to the interior sites of the adsorbent particles, so the adsorption became much slower [AL-Anber A. (2010)].
Fig. 7. Effect of contact time on percentage removal of Fe (III). (CO =50mg/L, pH = 4, stirring rate = 300 r.p.m., and temp. = 25 ˚C).
3.4.Effect of pH
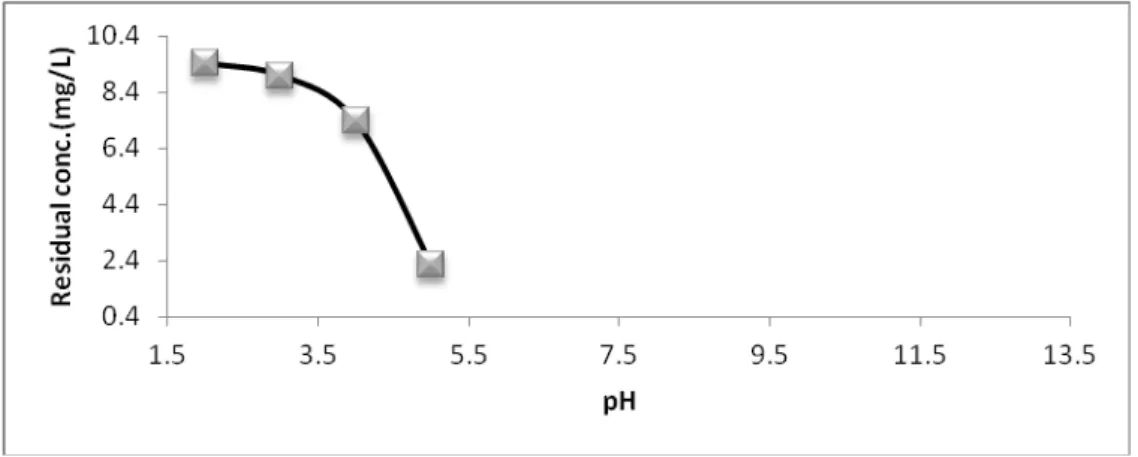
The pH of aqueous solution is an important parameter which controls the adsorption of metals. Therefore the effect of pH on the adsorption of Fe (III) was examined from pH (2 to 5) this is attributed to the fact that it was not possible to carry out adsorption experiment with Fe (III) at pH > 8 due to precipitation of metal hydroxide.
Figures (8&9) revealed that as pH increases from (2 to 5) the percentage removal of Fe (III) increases form 81.1492 % to 95.4934 % and the residual concentration decreases from 9.4254 mg/L to 2.2533 mg/L. From previous results it was found that the optimum pH for all experiment is 5. The decreased adsorption at low pH is obviously due to stiff competition faced by the metal ion from the large number of available hydrogen ions for adsorption sites on bentonite surface.
Fig. 8. Effect of pH on Residual concentration of Fe (III) .
(CO =50mg/L, pH = 4, time = 30 min, stirring rate = 300 r.p.m., and temp. = 25 ˚C).
Fig. 9. Effect of pH on percentage removal of Fe (III).
(CO =50mg/L, pH = 4, time = 30 min, stirring rate = 300 r.p.m., and temp. = 25 ˚C).
3.5.Effect of temperature
Fig. 10. Effect of temperature on Residual concentration of Fe (III). (CO =50mg/L, pH = 4, time = 30 min, bentonite dosage=0.5g, stirring rate = 300 r.p.m).
Fig. 11. Effect of temperature on Percentage removal of Fe (III).
(CO =50mg/L, pH = 4, time = 30 min, bentonite dosage=0.5g, stirring rate = 300 r.p.m., and temp. = 25 ˚C).
3.6.Adsorption isotherm
Adsorption isotherms of bentonite for Fe (III) ion were expressed mathematically in terms of the Langmuir and Freundlich models. The obtained experimental data are commonly well fitted with the Langmuir (Eq. (2)) and Freundlich (Eq. (3)) models. The linearization of these two equations gives equation (4 and 5), [Lu C.Y. et al. (2009)]
(2)
(3) If these equations are rearranged to the linear form: then,
(4)
(5)
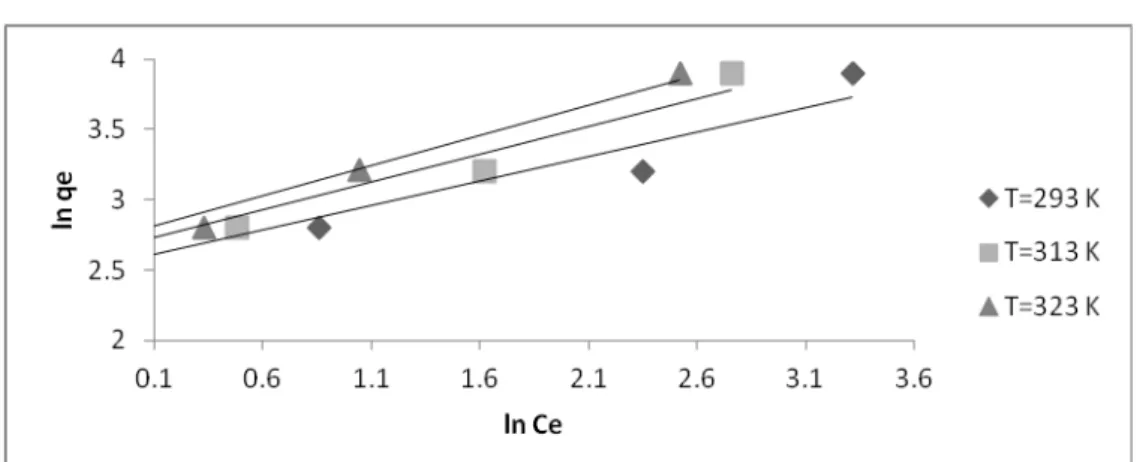
Where KL, a and KF, n are the constants for Langmuir and Freundlich models, respectively. In addition to the experimental data, the linear zed forms of Langmuir and Freundlich isotherms using the above equation for Fe (III) ion removal by bentonite are given in table (2).and figs.( 12&13).
Table 2. Parameters of Langmuir and freundlich adsorption isotherm models for Fe (III) on bentonite at different temperature.
Langmuir isotherm Freundlich
isotherm Heavy
metals T (K)
qm
(mg/g)
KL (L/g) R2 Kf mg(1-1/n) n R2
Fe(III)
Fig. 12. Linearized Langmuir isotherm of Fe(III) at different temperatures.
Fig. 13. Linerized freundlich isotherm of Fe(III) at different temperature.
3.7.Adsorption Thermodynamics
The thermodynamic parameters of the adsorption process that is, the standard enthalpy H⁰, Gibbs free energy G⁰ and entropy S⁰ were calculated using the equations (6 and 7),[ Cheung W.H. et al. (2003)]
(6)
(7)
Where R is the ideal gas constant (kJ mol-1K-1), KL=kads / kd is the Langmuir constant and T is the temperature (K). H and S values can be obtained from the slope and intercept of Van’t Hoff plots of LnKL (from the Langmuir isotherm) versus 1/T. The results of these thermodynamic calculations for iron ions are shown in Figure14and table (3).
Table (3): Thermodynamic constants for the adsorption of Fe (III) at various temperatures
Fe(III) T(K) 1/T
(K-1)
Ln(KL)
(L/g)
G⁰ (KJ.mol-1)
H⁰ (KJ.mol-1)
S⁰ (KJ.K-1mol-1)
Ea (KJ.mol-1)
293 0.003413 2.506184 -6.12526 13.5543 0.067133 13.5543 313 0.003195 2.85625 -7.45735
323 0.003096 3.064457 -8.22986 333 0.003003 3.154671 -8.73443
Fe (III) adsorption followed an endothermic path as adsorption capacity increase with increasing temperature range from 293 to333 K. Thus, adsorption of Fe (III) on bentonite has to overcome a small activation barrier and increasing energy supply makes it easier for Fe (III) to adsorb to the bentonite surface. The endothermic enthalpy change (H⁰) for Fe (III) adsorption varied significantly: 13.5 and 26.05 kJ mol-1.
The endothermic interactions between bentonite and metals ions had an average entropy increase of 0.115 and 0.06 kJ.K-1mol-1, which is therefore responsible for driving the interactions towards equilibrium despite being an endothermic process. The entropy increase is due to increased randomness at the solid-solution interface.All the interactions between the bentonite and Fe (III) decreased the Gibbs energy, with G⁰ varying from (-6.12526 kJ mol-1 to -8.73443) for bentonite –Fe interactions, respectively.
3.8.Adsorption kinetics
The kinetic study is an important issue in order to examine the order of the reaction. In the previous section emphasis was placed on the percentage removal of iron ions .The present section is concerned with the process kinetics.
In an attempt to throw some light on the kinetics of the present process which is difficult to quantify owing to its complexity and it’s being controlled by many interacting variables. In order to clarify the adsorption kinetics of Fe (III) and ions onto bentonite, Lagergren’s pseudo-first-order and pseudo-second-order kinetic models were applied to the experimental data. The linearized form of the pseudo-first order rate equation by Lagergren is given as [Cheung W.H. et al. (2003)]:
(8)
Where qt and qe (mg/g) are the amounts of the metal ions adsorbed at equilibrium (mg/g) and t (min), respectively and k1 is the rate constant of the equation (min-1). The adsorption rate constants (k1) can be determined experimentally by plotting of ln (qe - qt) versus t. The plots of ln (qe - qt) versus t for the Lagergren-first-order model (was not shown here) does not fit a pseudo-Lagergren-first-order kinetic model and the R2 value it shown in Table (4) and (5) for this model is very low (R2 equal 0.11 for Fe (III)) for the adsorption by bentonite. The similar results were found for the adsorption of same metal ions on various adsorbents by several authors. [Gu¨ndogan R. et al. (2004) - King P. et al. (2006)]Experimental data were also applied to the pseudo-second- order kinetic model, which is given in the following form [Wen D. et al. (2006) - Ho Y.S. and McKay G. (1999)].
(9)
Fig. 15. The pseudo- second-order adsorption kinetics of Fe (III).
Table 4.Parameters for adsorption of Fe (III) onto bentonite derived from the first- and second-order kinetic models:
First-order
Heavy metals Time (min) qe(mg/g) K1 (min-1) R2
Fe (III) 30 27.01791 0.0006 0.433
60 5.696774 -0.0008 0.0704 90 0.636863 -0.0029 0.1891
Se Second-order -order
Heavy metals
Time (min) qe (mg/g) K2 (min-1) h (mg/g*min) R2
Fe (III) 30 25.06266 0.010663 6.697924 0.9967
60 17.92115 0.015064 4.837929 0.991
90 15.4321 0.011138 2.65252 0.9989
4. Conclusions
Local Egyptian bentonite can be successfully used as adsorbent for removal of iron(III) ions from waste waterThe maximum adsorption percentages of iron(III) ions using bentonite was achieved at contact time of 30 min,pH=5,intial ion concentration of 50mg/L ,adsorbent dosage of 0.3 mg/L and temperature of 25 C.
The experimental data are well fitted to both the linear zed Langmuir isotherm and the linear zed Freundlich isotherm. Finally thermodynamic parameters are determined at three different temperatures and it has been found that the adsorption process is endothermic because of positive ΔH0 accompanied by a decrease inm entropy change and Gibbs free energy change (ΔG0) respectively.
5. References
[1] Abollino O. et al. (2003),"Adsorption of Heavy Metals on Na-Montmorillonite Effect of pH and Organic Substances", Water Research, Vol. 37, pp. 1619–1627.
[2] AL-Anber A. (2010), “Removal of high level Fe (III) from aqueous solution using natural inorganic materials”, Desalination , Vol. 250, pp. 885-891.
[3] Al-Anber M. (2007), “Removal of Iron(III) from Model Solution Using Jordanian Natural Zeolite: Magnetic Study, Asian J. Chem., Vol. 19, Iss. 5, pp.3493–3501.
[4] Al-Anber M. and Al-Anber Z. (2008), “Utilization of natural zeolite as ion-exchange and sorbent material in the removal of iron”, Desalination, Vol. 255, pp. 70–81.
[5] Al-Anber Z. and Al-Anber M. (2008), “Thermodynamics and Kinetic Studies of Iron(III)Adsorption by Olive Cake in a Batch System”, J. Mex. Chem. Soc., Vol. 52, Iss 2, pp. 108–115.
[6] Awan M.A. et al. (2003), “Removal of heavy metals through adsorption using sand.”, Journal of Environmental Sciences-China, Vol.15 , Iss.3, pp. 413-416.
[7] Belous A.G. et al. (2000),” Effect of precipitation conditions on the phase composition, particle morphology, and properties of iron(III, II) hydroxide precipitates”, Inorg. Mater. , Vol. 36, pp. 343–351.
[8] Berbenni P. et al. (2000), “Removal of iron and manganese from hydrocarbon-contaminated groundwaters”, Bioresour. Technol. , Vol. 74 , pp. 109–114.
[9] Bereket G. et al. (1997), “Removal of Pb(II), Cd(II), Cu(II), and Zn(II) from aqueous solutions by adsorption on bentonite”, J. Colloid Interf. Sci., Vol.187, pp. 338.
[10] Bhakta J.N. et al. (2009), “Mercury(II) Adsorption onto the Magnesium Oxide Impregnated Volcanic Ash Soil Derived Ceramic from Aqueous Phase”, ARPN J. Eng. Appl. Sci., Vol. 4, Iss 6, pp. 52–59.
[11] Chantawong V. et al. (2003), “Removal of Mercury from aqueous solution by waste brick”, Water Air Soil Pollut., Vol., 148, p.p.111– 125.
[13] Das B. et al. (2007),”Removal of iron from groundwater by ash: A systematic study of a traditional method”, J. Hazard. Mater, Vol. 141, pp. 834–841.
[14] Dimitrakos Michalakos G. et al. (1997), “Removal of iron from potable water using a trickling filter”, Water Res., Vol. 31, pp.991– 996.
[15] Duffus J.H. (2002), “Heavy metals a Meaningless Term IUPAC Technical Report”, Vol. 74, Iss 5, pp. 793–807.
[16] Ellis D. et al. (2000), “Removal of iron and manganese from groundwater by oxidation and microfiltration”, Desalination, Vol. 130, pp. 255–264.
[17] Fahlman M. et al. (1998), “The iron/polyaniline interface and its effect on corrosion protection of iron and cold rolled steel in aqueous and salt environments”, in: Proceedings of the US Society of Plastics Engineers, ANTEC 1998, Atlanta, GA, May, pp. 1238–1241. [18] Gu¨ndogan, R. et al. (2004), “Determination of Heavy Metals in Domestic, Commercial and Industrial Soot Samples”, J. Colloid
Interface Sci., pp. 269- 303.
[19] Hikmet K. et al. (2008), “Removal of cadmium(II) ion from aqueous system by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from freshwater (Mogan Lake)”, Bioresour. Technol. , Vol. 99, pp. 4185–4191.
[20] Ho, Y.S. and McKay, G. (1999), “Biosorption of lead(II) and chromium(VI) on groundnut hull: Equilibrium, kinetics and thermodynamics study”, Process Biochem., Vol. 34, p. 451.
[21] Huang C. and Cheng W.P. (1997), “Thermodynamic parameters of iron-cyanide adsorption onto Al2O3”, J. Colloid Interf. Sci., Vol. 188, pp. 270–274.
[22] Kalin M. et al. (2005), “The removal of uranium from mining waste water using algal/microbial biomass”, J. Environ. Radioact., Vol. 78 , pp. 151–177.
[23] Karthikeyan G. et al. (2005), “Adsorption studies of iron(III) on chitin”, J. Chem. Sci. , Vol. 117, Iss 6, pp. 663–672.
[24] Kato M. et al. (1998), “Adsorption equilibria of 1,10-phenanthroline and its Fe(II) complex on octadecyl-bonded silica gel”, Bull. Chem. Soc. Jpn., Vol. 49, p. 267.
[25] King P. et al (2006), “Sorption of copper(II) ion from aqueous solution by Tectona grandis l.f. (teak leaves powder)” J. Hazard. Mater., Vol. 136, p. 560.
[26] Lai C.H. et al. “Evaluating an iron coated sand for removing copper from water”, Wat. Sci. Technol., Vol. 30, p.175.
[27] Lu, C.Y. et al. (2009), “Sorption kinetics, thermodynamics and competition of Ni2+ from aqueous solutions onto surface oxidized carbon nanotubes”, Desalination, Vol.249, Iss.1, PP.18-23.
[28] Marin, J. and Ayele, J. (2002), “Removal of some heavy metal cations from aqueous solutions by spruce sawdust. I. Study of the binding mechanism through batch experiments”, Environmental Technology, Vol. 23, Iss 10, pp. 1157-1171.
[29] Pakula M. et al. (1998), “Chemical and electrochemical studies of interactions between iron(III) ions and activated carbon surface”, Langmuir, Vol. 14, pp.3082–3089.
[30] Pakula M. et al. (1998), “Chemical and electrochemical studies of interactions between iron (III) ions and activated carbon surface”, Langmuir, Vol. 14, pp. 3082–3089.
[31] Rashed M. and Soltan M. (2002), "Removal of Nutrients and Heavy Metals from Urban Wastewater Using Aeration", Alum and Kaolin Ore., Proceeding EPCOWM, pp. 621-627.
[32] Rengaraj, S. and Moon, S.H. (2002),”Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins”, Water Research, Vol. 36, Iss. 7, pp. 1783-1793.
[33] SenGupta S. and Bhattacharyya K.G. (2006),” Adsorption of Ni(II) on clays”, J. Colloid Interface Sci., Vol. 295, pp. 21–32.
[34] Sharma S.K. et al. (2005), “Biological iron removal from groundwater: a review”, J. Water Supply: Res. Technol. Aqua, Vol. 54, pp. 239–247.
[35] Uchida M. et al. (1999), “Competitive adsorption of chloroform and iron ion onto activated carbon fiber”, J. Colloid Interface Sci., Vol. 220, pp.406–409.
[36] Uchida M. et al. (1999), “Competitive adsorption of chloroform and iron ion onto activated carbon fiber”, J. Colloid Interf. Sci., Vol. 220, pp. 406–409.
[37] Voropanova L.A. and Velichko L.N. (1999), “Extraction of copper(II), nickel(II), cobalt(II), chromium(II), and iron(II, III) ions from aqueous solutions with a technical lubricant”, Russ. J. Appl. Chem., Vol. 72, pp.1970–1975.
[38] Wahba M.M. and Zaghloul A.M. (2007),"Adsorption Characteristics of Some Heavy Metals by Some Soil Minerals", Journal of Applied Sciences Research, Vol. 3, No. 6, pp. 421-426.
[39] Weinberg E.D. (1999), “Iron Loading and Disease Surveillance emerg”, Infect. Dis., Vol. 5, Iss 3.
[40] Wen D. et al. (2006), “Adsorption of ammonium ions onto natural zeolite”, J. Hazard. Mater., Vol. 133, pp. 252.