w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Correlation
between
cellular
expression
of
complement
regulatory
proteins
with
depletion
and
repopulation
of
B-lymphocytes
in
peripheral
blood
of
patients
with
rheumatoid
arthritis
treated
with
rituximab
Daniela
Viecceli
a,∗,
Mariana
Pires
Garcia
b,
Laiana
Schneider
b,
Ana
Paula
Alegretti
b,
Cristiano
Kohler
Silva
a,
André
Lucas
Ribeiro
a,
Claiton
Viegas
Brenol
a,
Ricardo
Machado
Xavier
aaHospitaldeClínicasdePortoAlegre,Servic¸odeReumatologia,PortoAlegre,RS,Brazil bHospitaldeClínicasdePortoAlegre,Servic¸odePatologiaClínica,PortoAlegre,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received22December2015 Accepted31July2016
Availableonline18October2016
Keywords:
Rheumatoidarthritis
Complementregulatoryproteins Rituximab
Biomarkers
a
b
s
t
r
a
c
t
Objectives: Tocorrelatethebasalexpressionofcomplementregulatoryproteins(CRPs)CD55, CD59,CD35,andCD46inB-lymphocytesfromtheperipheralbloodofacohortof10patients withrheumatoidarthritis(RA)initiatingtreatmentwithrituximab(RTX)withdepletionand timerepopulationofsuchcells.
Methods:Ten patientswithRAreceivedtwoinfusionsof1g ofRTXwithanintervalof 14 days.ImmunophenotypicanalysisforthedetectionofCD55,CD59,CD35,andCD46 onB-lymphocyteswascarriedoutimmediatelybeforethefirstinfusion.Thepopulation ofB-lymphocyteswasanalyzedbymeansofbasalCD19expressionandafter1,2,and6 monthsaftertheinfusionofRTX,andthenquarterlyuntilclinicalrelapse.Depletionof B-lymphocytesinperipheralbloodwasdefinedasaCD19expression<0.005×109/L.
Results:Ten women witha median of49 years anda baselineDAS28=5.6were evalu-ated; 9wereseropositiveforrheumatoidfactor.Fivepatientsshowedarepopulationof B-lymphocytesafter2months,andtheotherfiveafter6months.Therewasacorrelation betweenthebasalexpressionofCD46andthe timeofrepopulation(correlation coeffi-cient=−0.733,p=0.0016).AsimilartrendwasobservedwithCD35,butwithoutstatistical
significance(correctioncoefficient=−0.522,p=0.12).
Conclusion: TheincreasedCD46expressionwaspredictiveofafasterrepopulationof B-lymphocytesinpatientstreatedwithRTX.Studiesinvolvingalargernumberofpatients willbeneededtoconfirmtheutilityofbasalexpressionofCRPsasapredictorofclinical response.
©2016ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:daniviecceli@gmail.com(D.Viecceli).
http://dx.doi.org/10.1016/j.rbre.2016.09.007
Correlac¸ão
entre
expressão
celular
de
proteínas
reguladoras
do
complemento
com
a
deplec¸ão
e
repopulac¸ão
de
linfócitos
B
no
sangue
periférico
de
pacientes
com
artrite
reumatoide
tratada
com
rituximabe
Palavras-chave:
Artritereumatoide Proteínasreguladorasdo complemento
Rituximabe Biomarcadores
r
e
s
u
m
o
Objetivos:Correlacionaraexpressãobasaldasproteínasreguladorasdocomplemento(PRC) CD55,CD59,CD35eCD46noslinfócitosBdosangueperiféricodeumacoortede10pacientes comartritereumatoide(AR)iniciandotratamentocomrituximabe(RTX)comadeplec¸ãoe tempoderepopulac¸ãodessascélulas.
Métodos: DezpacientescomARreceberamduasinfusõesde1gdeRTXcomintervalode14 dias.Análisesimunofenotípicasparadetecc¸ãodeCD55,CD59,CD35eCD46noslinfócitos Bforamfeitasimediatamenteantesdaprimeirainfusão.Apopulac¸ãodelinfócitosBfoi analisadapormeiodaexpressãodeCD19basaleapósum,doiseseismesesapósainfusão deRTXeentãotrimestralmenteatéarecaídaclínica.Deplec¸ãodelinfócitosBnosangue periféricofoidefinidacomoexpressãodeCD19<0,005×109/l.
Resultados: Dezmulherescommedianade 49anose DAS28basalde5,6foram avali-adas; nove eram soropositivas paraofator reumatoide.Cinco pacientesapresentaram repopulac¸ão delinfócitos B apósdois mesese as outrascinco aos seismeses. Houve correlac¸ão entrea expressãobasalde CD46 e otempode repopulac¸ão(coeficiente de correlac¸ão-0,733,p=0,0016).TendênciasemelhantefoiobservadacomCD35,porémsem significânciaestatística(coeficientedecorrec¸ão0,522,p=0,12).
Conclusão: ExpressãoaumentadadeCD46foipreditoraderepopulac¸ãomaisrápidade lin-fócitosBem pacientestratadoscomRTX.Estudoscomumnúmeromaiordepacientes serãonecessáriosparaconfirmarautilidadedaexpressãobasaldasPRCcomopreditorade respostaclínica.
©2016ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that affectsabout1%oftheadultpopulation, withaprevalence three times higher in women, that causes a chronic and persistent polyarthritis, mainly of peripheral joints.1 The inflammatoryinfiltratecomposedofmacrophages, CD4+ T-cells, B-cells, dendritic cells, granulocytes and mast cells leadstosynovialproliferation,resultinginswollenand ten-derjoints,withhighratesoffunctionallimitation.2Highlevels ofcomplementactivationproductssuchasmembraneattack complex(MAC),releaseofanaphylatoxinsC3aandC5a,and increasedC3andC4consumptioncanbedetectedinthe syno-vialfluidofpatientswithRA,suggestinganoveractivationof thecomplementsystem(CS)inthesepatients.3Normalcells areresistanttothecomplement-mediatedlysisbecausethey haveregulatorymechanismsconsistingofsolubleproteinsin biologicalfluids,suchasproperdinandfactorH,andanchored to the membrane, suchas CD55 (decay-accelerating factor [DAF]),CD59 (membrane inhibitor of reactive lysis [MIRL]), CD46(membranecofactorprotein[MCP])andCD35 (comple-mentreceptortype1[CR1]).4
Rituximab (RTX) is a chimeric monoclonal antibody directedtoCD20whichisexpressedonthesurfaceof pre-B-cellstomatureB-lymphocytes,leadingtoatransient,almost complete, depletion of B-cells in blood and to a partial depletioninbonemarrowandinthesynovialtissue.5–10 Its mechanismofactionisbasedonthesignalingandsubsequent
B-celldeathbyinductionofapoptosis,complement-mediated lysis,orcelldeathbymacrophages.11,12Theclinicalresponse seemstocorrelatetothelevelofB-celldepletionin periph-eral blood and synovium.6 Thetreatment of RAwith RTX showedsignificantefficacyincontrollingsignsandsymptoms, improvingphysicalfunction,inadditiontobenefitsinthe pre-ventionofradiologicaldamage.13–19
Althoughalargenumberofpatientspresentasatisfactory responsetotreatmentwithRTX,about40–50%arerefractory andthemechanismofthisfailureisnotfullyunderstood.5,18,19 In this context,thesearch forbiomarkers thatcan predict which subgroupsofpatientshave thegreatest potentialto bebenefitedfromthistherapyhasbeenoneofthegoalsof themostrecentstudiesrelatedtothedrug.Thisstrategy,in additiontoreducingcosts,couldminimizeperiodsofdisease activityandofexposuretopossiblesideeffectsofan ineffec-tivetreatment.20
lysistriggeredbyRTXinthefaceoftheincreasedexpression ofCD5523andCD59.24
Althoughtheliteratureincludesconsistentfindings sug-gestingthatCRPsexpression,particularlyCD55andCD59,can beusedasabiomarkerofresponsetotreatmentwithRTXon lymphoproliferativediseases,nostudy intheliteraturehas madethiscorrelationinpatientswithRA.
The aimof this study is to correlate the basal expres-sionofCRPsCD55,CD59,CD35,andCD46onBlymphocytes fromperipheralbloodfromacohortofRApatientsinitiating treatmentwithRTXwiththedepletionlevelofthesecellsin peripheralbloodandtheirtimeofrepopulation.Inthisstudy, thecorrelationbetweentheexpressionoftheseproteinswith aclinicalresponsewillbeevaluated,accordingtothe Ameri-canCollegeofRheumatology(ACR).
Materials
and
methods
Studypopulation
Weincludedinthis study 10consecutive RApatients with clinicalindicationforinitiationoftreatmentwithRTX, accord-ingtotheguidelinesoftheBrazilianSocietyofRheumatology (SBR):failureor intolerancetoatleasttwoschemesof tra-ditionaldisease-modifyingdrugs(DMARDs)andananti-TNF agent.25Theotherinclusioncriteriawere:ageunder18years; RAdiagnosisforatleast6monthsaccordingtoACRcriteria1; DAS28score(DiseaseActivityScore-28)greater ≥3.2;useof
an adequate contraception method to fertile patients; and desire to participate voluntarily and ability to understand theprotocol,documentedbythesigningtheConsentForm. Patients with overlapping of autoimmune rheumatic dis-ease,lymphoproliferativedisorders,andneoplasticdiseases; presenceofactiveinfection;useofcytotoxicdrugs; seropos-itivity for HIV, HBV, or HCV; active tuberculosis; allergy or hypersensitivity toRTX; pregnantor breastfeedingwomen; subjectsparticipatinginanotherinterventionalclinicalstudy; IVfunctionalclassdefinedbasedonSteinbrocker functional-itycriteriaforRA26andpriortherapywithRTX.Thepatients receivedtwo infusionsof RTX1gseparated byan interval of14 days. All patients were pretreated with methylpred-nisolone100mg,paracetamol1g,anddexchlorpheniramine 2mg. Patients were followed fora maximum periodof 24 months.
Clinicalassessment
All patientsunderwent abaselinevisit immediately before theinfusionofRTX,withamonthlyvisit6monthsafterthe infusions,andthenquarterly.Ateachvisit,routinelaboratory tests (blood count, transaminases, creatinine, routine uri-nalysis,erythrocytesedimentationrate(ESR),andC-reactive protein)werecarriedout.Onthefirstvisit,rheumatoidfactor (RF),antinuclear (ANA)factor, complement,anti-HCV, anti-HIV,anti-HBc,andHBsAgserology,chestX-raysofhandsand feet,and aMantoux testwerealsocarriedout.The follow-ingclinicalparameterswereevaluatedateachvisit:counting of28swollenand/orpainfuljoints(alwaysperformedbythe sameexaminer);overallscoreofdiseaseactivity(0–100mmon
avisualanalogscale[VAS]assignedbyboththeexaminerand thepatient);apainscore(0–100mm),theHealthAssessment Questionnaire(HAQ)andDAS28scoreusingESR.
Clinicalresponseassessment
Theclinical responsetotreatment wasassessed6months afterinfusionofRTX,beingconsideredaspositivewhenthe patient reached aresponse >20%,according to the criteria establishedbyACR.27Aresponsefailurewasconsideredwhen anonlyanimprovement<20% wasperceived,comparedto baseline parameters according to the same criteria. Clini-calrelapsewasconsideredwhentherewasalossofACR20 responseinpatientsconsideredresponders.
Immunophenotypicanalysis
Theanalyses ofperipheralblood flowcytometrywere per-formed immediately before the first infusion of RTX, and 1, 2and6monthsafterthe 2nd infusionofRTX andafter quarterlyintervals,accordingtoastandardizedtechniquein leukocytes,28 during a period of fewer than 24hours after thecollection.Briefly,100Lofwholebloodwereplacedinto polystyrenetubesandstainedwith8Lofeach fluorochrome-conjugated monoclonal antibody to CD19-PerCP, CD55PE, CD59FITC,CD35PE,andCD46FITC(BDBiosciences,SanDiego, CA,USA).Afterincubation,1.0mLofFACSlyse(BDBiosciences, SanDiego,CA,USA)wasadded,andlysiswasallowed dur-ing10minatroomtemperature.Sampleswerewashedand resuspended in 0.5mL of phosphate buffered saline (PBS) and analyzedinCellQuestTMsoftwareofFACSCaliburflow cytometer – BD (Becton Dickinson). The intensity of the membranefluorescencewascalculatedfromthemean fluo-rescenceintensity(MFI).Thedefinitionofpositiveornegative cells wasdefinedwhenthe stainingofcontrolisotypewas performedinordertodefine thegatesandtodistinguisha positive staining versus autofluorescence or of nonspecific antibodybinding.Ineachofthesecollections,CD19-marked cells werequantified.TheexpressionofCD55,CD59,CD35, andCD46wasinvestigatedbeforetheinfusionofRTX,onlyin CD19+subpopulations.
100,000eventsintheregionoflymphocytesbyflow cytom-etrywereacquired.DatawereanalyzedwithInfinicytsoftware intheregionoflymphocytes(intermsofrelativevaluesand offluorescenceintensity);todoso,weuseddatafromroutine bloodcountstocalculatetheabsolutevalues.
B-celldepletioninperipheralbloodwasdefinedbyavalue of CD19+ <0.005× 109/L of total leukocytes. B-cell
repopu-lation was defined when the concentration of B-cells was >0.005× 109/Loftotalleukocytes.
Statisticalanalysis
DatawereanalyzedusingtheSPSS16.0programfor Win-dows.Inordertocomparethemeansoftheparametricvalues, theStudent’st-testforpairedsamplestheMann–Whitneytest fornonparametricvalueswereperformed.Correlationswere analyzedusingthePearsontestforparametricdataandthe Spearmancorrelationfornonparametricdata.Thecorrelation wasconsideredasstatisticallysignificantwithap-value<0.05, andclinicallysignificant whenacorrelationcoefficient>0.4 wasobtained.
Ethicalaspects
ThisstudywasapprovedbytheHCPAResearchEthics Com-mittee under number 09–585 and was fundedthrough the FundodeApoioàPesquisaeEventos(FIPE)ofHCPA.All partici-patingpatientssignedanInformedConsentTerm.TheRoche LaboratorycontributedwithadonationofRTXforusein10 patientsthrougharesearchinitiativeprotocol,without inter-ferenceinthedesign,analysisandpreparationofthisstudy.
Results
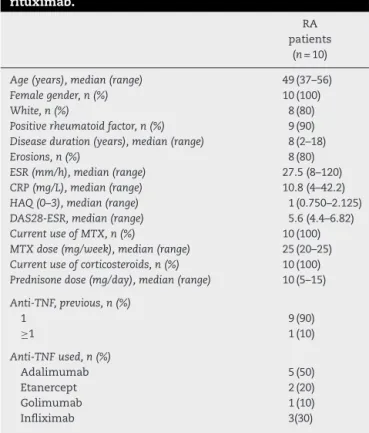
Intotal, 10femalepatientswithamedianof49yearswere included in this study. Their baseline characteristics are showninTable1.Themedianfordiseasedurationwas8years. Allpatientswerebeingmedicatedwithmethotrexate(median
Table1–Baselinecharacteristicsofpatientstaking rituximab.
RA patients
(n=10)
Age(years),median(range) 49(37–56)
Femalegender,n(%) 10(100)
White,n(%) 8(80)
Positiverheumatoidfactor,n(%) 9(90) Diseaseduration(years),median(range) 8(2–18)
Erosions,n(%) 8(80)
ESR(mm/h),median(range) 27.5(8–120)
CRP(mg/L),median(range) 10.8(4–42.2)
HAQ(0–3),median(range) 1(0.750–2.125)
DAS28-ESR,median(range) 5.6(4.4–6.82)
CurrentuseofMTX,n(%) 10(100)
MTXdose(mg/week),median(range) 25(20–25) Currentuseofcorticosteroids,n(%) 10(100) Prednisonedose(mg/day),median(range) 10(5–15)
Anti-TNF,previous,n(%)
1 9(90)
≥1 1(10)
Anti-TNFused,n(%)
Adalimumab 5(50)
Etanercept 2(20)
Golimumab 1(10)
Infliximab 3(30)
RA,rheumatoidarthritis;ESR,erythrocytesedimentationrate;CRP, C-reactiveprotein;HAQ,HealthAssesmentQuestionnaire; DAS28-ESR, Disease Activity Score in 28 joints using the erythrocyte sedimentationrate;MTX,methotrexate.
Range=minimumandmaximumvaluesfound.
25mg/week)andprednisone(median10mg/day);inaddition, thesepatientshadmadeuseofatleastoneanti-TNFagent. Ninepatients(90%)wereRF-positiveand8ofthem(80%)had jointerosions.
Inevaluatingtheclinicalresponse,8patients(80%)reached ACR20four monthsafterinfusionofRTX;but only3(30%) maintainedthisresponseafter6months.Whenconsidering theDAS28activityindex,fourmonthsafterinfusionofRTX3 patients(30%)hadclinicalremission,2patients(20%)showed lowclinical activity,and 5patients(50%)showedmoderate diseaseactivity.Intheevaluationperformed6monthsafter infusionofRTX, only1patient(10%)exhibitedlow clinical activity.
TherepopulationofB-cellsinperipheralbloodwas identi-fiedintheanalysiscarriedout2monthsafterinfusionofRTX in5patients(50%),andintheotherpatientsatthetimeofthe 6-monthanalysis.
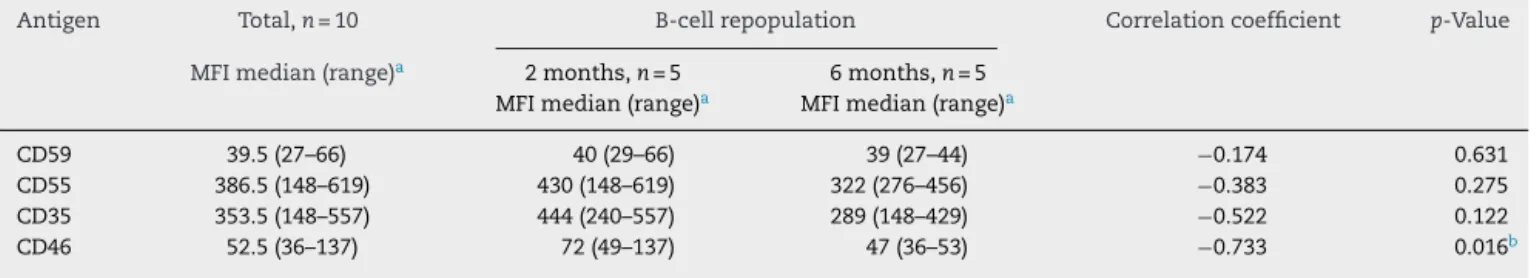
Table2showsthemedianofCD55,CD59,CD35,andCD46 expressions,asmeasuredbyMFIinB-cellsofthewholegroup ofpatientsandofsubsetswithrepopulation identifiedin2 and6months.Patientswithrepopulation ofBlymphocytes in the peripheral blood at2 monthsshowed anincreased expressionofCD46(median ofMFI=72)comparedtothose with repopulation at 6 months (median of MFI=47), con-firmingacorrelationbetweenanincreasedexpressionofCD46 withanearlierrepopulationofBlymphocytesinthe periph-eralblood(correlationcoefficient=−0733,p=0.016).Asimilar
trendwasfoundwithCD35,butwithnostatisticalsignificance (median ofMFI=444 forpatients with earlier repopulation versus MFI=289forthose withalaterrepopulation,witha correlationcoefficient=−0522,p=0:12).Nocorrelationswere
foundbetweentheexpressionsofCD55andCD59onB lym-phocytesandthetimeofrepopulationofBlymphocytesin this sampleofpatients(correlationcoefficients=−0383and −0174,andp=0.275and0.631,respectively).
AlthoughavariationintheexpressionofCD55,CD59,CD35, andCD46onBlymphocytesofthepatientsstudiedhasbeen observed, wefoundnocorrelationbetweenthisexpression andtheclinicalresponsemeasuredbyACR20at6months.The expressionsofCRPsmeasuredbythemedianofMFIamong responders(CD59=39;CD55=456;CD35=444;andCD46=79) werenotstatisticallydifferentcomparedtonon-responders (CD59=40;CD55=322;CD35=346;andCD46=49).Thesedata arepresentedinTable3.
Discussion
Table2–CorrelationbetweenbaselineexpressionofCD55,CD59,CD35,andCD46inB-lymphocytesbeforetreatment withrituximabandrepopulationtimeofthesecells.
Antigen Total,n=10 B-cellrepopulation Correlationcoefficient p-Value
MFImedian(range)a 2months,n=5 6months,n=5
MFImedian(range)a MFImedian(range)a
CD59 39.5(27–66) 40(29–66) 39(27–44) −0.174 0.631
CD55 386.5(148–619) 430(148–619) 322(276–456) −0.383 0.275
CD35 353.5(148–557) 444(240–557) 289(148–429) −0.522 0.122
CD46 52.5(36–137) 72(49–137) 47(36–53) −0.733 0.016b
MFI,meanoffluorescenceintensity.
a Rangebetweenminimumandmaximumvaluesfound. b p<0.05=statisticallysignificant.
Table3–CorrelationbetweenbaselineexpressionofCD55,CD59,CD35,andCD46inB-cellsbeforetreatmentwith rituximabandclinicalresponseat6months.
Antigen Clinicalresponseat6months(ACR20) p-Value
Responders,n=3 Nonresponders,n=7
MFImedian(range)a MFImedian(range)a
CD59 39(29–66) 40(27–60) 1.0
CD55 456(430–619) 322(148–551) 0.067
CD35 444(289–456) 346(148–557) 0.383
CD46 79(53–137) 49(36–72) 0.067
MFI,meanoffluorescenceintensity.
a Rangebetweenminimumandmaximumvaluesfound.
earlierlymphoidrepopulationandalowerclinicalresponseto therapy,inourstudy,wefoundatendencyforhigher expres-sionofCD46andCD55onperipherallymphocytesBofpatients thatreachedACR20at6months,comparedtononresponders, butwithoutstatisticalsignificance(p=0.067).Webelievethat thisseeminglycontradictoryfindingisduetothesmall sam-plesizeleadingtoalackofpowerofthisstudy,inorderto properlyevaluatethis outcome.Thislimitationalso proba-blyaffectedtheresultsthatshowednocorrelationbetween CD55andCD59expressiononperipheralBlymphocyteswith theearlierrepopulationofthesecellsinperipheralbloodafter treatmentwithRTX.
Anotherimportantissue inthis studywas thelow sus-tainedresponserateat6months(30%). Thisresponsewas lower than previous studies evaluating patients with RA whose prior anti-TNF treatment failed,18,19 where ACR20 responseswereobtainedatameanofabout50%at6months. Oursample,whencompared tothepopulationsstudied in thesestudies,had lowerratesofHAQ(1.0×1.8–1.9,
respec-tively),DAS28(5.6×6.8),anddiseaseduration(8years×10–12
years),butusedhigherdosesofmethotrexate(25mg× 15mg)
andglucocorticoids.Allourpatientsweretaking glucocorti-coids,whileonly65%usedthisdrugclassinthestudyofCohen etal.19Wedonotknow,however,whetherthesedifferences haveinfluencedsomehowourresults.
Despitethelimitationsrelatedtosamplesizeandthelow rateoftherapeuticresponseat6months,thisisthefirststudy tocorrelatetheexpressionofCRPsofCD55,CD59,CD35,and CD46withtherepopulationtimeofBlymphocytesin periph-eralblood and clinicalresponse inacohortofRApatients treated with RTX. Previous studies of lymphoproliferative
diseaseshadalreadyhypothesizedthatanincreased
expres-sion of CRPs could reduce the complement-mediated
cytotoxicityandinfluenceintheresponsetotreatmentwith RTX.21,23,24,29Thesestudieshaveinvolvedinvitromodels, ani-mals, andhumans.Dalle et al.investigatedtheexpression ofCD55,CD59,and CD46inratsthatreceivedcellsderived fromhumanfollicularlymphomaanddetectedanincreased expression of CD59 in those animals with RTX-resistant lymphoma.24 Terui et al. studied the expression of CD55 innon-Hodgkin’sLymphoma (NHLs)cellsfrom 30 patients, showing that this proteincontributed to the resistance to complement-mediatedcytotoxicityandtotheresistanceto RTX.23 Although there is evidence that CRPs may have a response-biomarker role toRTXinlymphoproliferative dis-eases,thisassociationhasneverbeenstudiedinpatientswith RA.
recombinantproteinwhichinducesCD46internalizationand degradation)inmonkeystreatedwithsubclinicalRTX, observ-ingacompletecelldepletionofperipheralBlymphocytes.33
WecanconcludethattheincreaseofCD46expressioninB lymphocytesfromRApatientscanpredictanearlier repopu-lationofsuchcellsintheperipheralbloodofpatientstreated withRTX.Furtherstudiesareneededtoconfirmthisresult andtoevaluatethecorrelationofotherCRPswith repopula-tionandclinicalresponsetotreatment,thusenablingtheuse oftheseproteinsasbiomarkersofresponsetoRTX.
Funding
ThisstudywassupportedbytheFundodeApoioàPesquisae Eventos(FIPE),fromtheHospitaldeClínicasofthecityofPorto Alegre,Brazil,linkedtotheGroupforResearchPostgraduate Studies.ThelaboratoryProdutosRocheQuímicose Farmacêu-ticosS.A.,Brazil,contributedwiththedonationofrituximab forusein10patientsthroughaprotocolattheinitiativeofthe investigator,withoutinterferenceinthedesign,analysis,and preparationofthestudy.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. ArnettFC,EdworthySM,BlochDA,McShaneDJ,FriesJF, CooperNS,etal.TheAmericanRheumatismAssociation 1987revisedcriteriafortheclassificationofrheumatoid arthritis.ArthritisRheum.1988;31:315–24.
2. GoronzyJJ,WeyandCM.Rheumatoidarthritis.ImmunolRev. 2005;204:55–73.
3. OkrojM,HeinegardD,HolmdahlR,BlomAM.Rheumatoid arthritisandthecomplementsystem.AnnMed.
2007;39:517–30.
4. KimDD,SongWC.Membranecomplementregulatory proteins.ClinImmunol.2006;118:127–36.
5. LeandroMJ,CambridgeG,EhrensteinMR,EdwardsJC. ReconstitutionofperipheralbloodBcellsafterdepletionwith rituximabinpatientswithrheumatoidarthritis.Arthritis Rheum.2006;54:613–20.
6. DassS,RawstronAC,VitalEM,HenshawK,McGonagleD, EmeryP.HighlysensitiveBcellanalysispredictsresponseto rituximabtherapyinrheumatoidarthritis.ArthritisRheum. 2008;58:2993–9.
7. NakouM,KatsikasG,SidiropoulosP,BertsiasG,Papadimitraki E,RaptopoulouA,etal.Rituximabtherapyreducesactivated Bcellsinboththeperipheralbloodandbonemarrowof patientswithrheumatoidarthritis:depletionofmemoryB cellscorrelateswithclinicalresponse.ArthritisResTher. 2009;11:R131.
8. VosK,ThurlingsRM,WijbrandtsCA,vanSchaardenburgD, GerlagDM,TakPP.Earlyeffectsofrituximabonthesynovial cellinfiltrateinpatientswithrheumatoidarthritis.Arthritis Rheum.2007;56:772–8.
9. ThurlingsRM,VosK,WijbrandtsCA,ZwindermanAH,Gerlag DM,TakPP.Synovialtissueresponsetorituximab:
mechanismofactionandidentificationofbiomarkersof response.AnnRheumDis.2008;67:917–25.
10.KavanaughA,RosengrenS,LeeSJ,HammakerD,FiresteinGS, KalunianK,etal.Assessmentofrituximab’s
immunomodulatorysynovialeffects(ARISEtrial).1:clinical andsynovialbiomarkerresults.AnnRheumDis.
2008;67:402–8.
11.GurcanHM,KeskinDB,SternJN,NitzbergMA,ShekhaniH, AhmedAR.Areviewofthecurrentuseofrituximabin autoimmunediseases.IntImmunopharmacol.2009;9:10–25.
12.AtzeniF,DoriaA,TurielM,Sarzi-PuttiniP.Whatistheroleof rituximabinthetreatmentofrheumatoidarthritis.
AutoimmunRev.2007;6:553–8.
13.TakPP,RigbyW,Rubbert-RothA,PeterfyC,vanVollenhoven RF,StohlW,etal.Sustainedinhibitionofprogressivejoint damagewithrituximabplusmethotrexateinearlyactive rheumatoidarthritis:2-yearresultsfromtherandomised controlledtrialIMAGE.AnnRheumDis.2012;71:351–7.
14.TakPP,RigbyWF,Rubbert-RothA,PeterfyCG,van
VollenhovenRF,StohlW,etal.Inhibitionofjointdamageand improvedclinicaloutcomeswithrituximabplus
methotrexateinearlyactiverheumatoidarthritis:theIMAGE trial.AnnRheumDis.2011;70:39–46.
15.EdwardsJC,SzczepanskiL,SzechinskiJ,
Filipowicz-SosnowskaA,EmeryP,CloseDR,etal.Efficacyof B-cell-targetedtherapywithrituximabinpatientswith rheumatoidarthritis.NEnglJMed.2004;350:2572–81.
16.EmeryP,DeodharA,RigbyWF,IsaacsJD,CombeB,Racewicz AJ,etal.Efficacyandsafetyofdifferentdosesandretreatment ofrituximab:arandomised,placebo-controlledtrialin patientswhoarebiologicalnaivewithactiverheumatoid arthritisandaninadequateresponsetomethotrexate(Study EvaluatingRituximab’sEfficacyinMTXInadequate
Responders[SERENE]).AnnRheumDis.2010;69:1629–35.
17.ThurlingsRM,VosK,GerlagDM,TakPP.Disease
activity-guidedrituximabtherapyinrheumatoidarthritis:the effectsofre-treatmentininitialnonrespondersversusinitial responders.ArthritisRheum.2008;58:3657–64.
18.EmeryP,FleischmannR,Filipowicz-SosnowskaA,
SchechtmanJ,SzczepanskiL,KavanaughA,etal.Theefficacy andsafetyofrituximabinpatientswithactiverheumatoid arthritisdespitemethotrexatetreatment:resultsofaphase IIBrandomized,double-blind,placebo-controlled,
dose-rangingtrial.ArthritisRheum.2006;54:1390–400.
19.CohenSB,EmeryP,GreenwaldMW,DougadosM,FurieRA, GenoveseMC,etal.Rituximabforrheumatoidarthritis refractorytoanti-tumornecrosisfactortherapy:resultsofa multicenter,randomized,double-blind,placebo-controlled, phaseIIItrialevaluatingprimaryefficacyandsafetyat twenty-fourweeks.ArthritisRheum.2006;54:2793–806.
20.IsaacsJD,FerraccioliG.Theneedforpersonalisedmedicine forrheumatoidarthritis.AnnRheumDis.2011;70:4–7.
21.GolayJ,ZaffaroniL,VaccariT,LazzariM,BorleriGM, BernasconiS,etal.BiologicresponseofBlymphomacellsto anti-CD20monoclonalantibodyrituximabinvitro:CD55and CD59regulatecomplement-mediatedcelllysis.Blood. 2000;95:3900–8.
22.GolayJ,LazzariM,FacchinettiV,BernasconiS,BorleriG, BarbuiT,etal.CD20levelsdeterminetheinvitro
susceptibilitytorituximabandcomplementofB-cellchronic lymphocyticleukemia:furtherregulationbyCD55andCD59. Blood.2001;98:3383–9.
23.TeruiY,SakuraiT,MishimaY,SugimuraN,SasaokaC,Kojima K,etal.Blockadeofbulkylymphoma-associatedCD55 expressionbyRNAinterferenceovercomesresistanceto complement-dependentcytotoxicitywithrituximab.Cancer Sci.2006;97:72–9.
25.MotaLMH,CruzBA,BrenolCV,PereiraIA,Rezende-FronzaLS, BertoloMB,etal.Diretrizesparaotratamentodaartrite reumatoide.RevBrasReumatol.2013;53:158–83.
26.HochbergMC,ChangRW,DwoshI,LindseyS,PincusT,Wolfe F.TheAmericanCollegeofRheumatology1991revised criteriafortheclassificationofglobalfunctionalstatusin rheumatoidarthritis.ArthritisRheum.1992;35:
498–502.
27.FelsonDT,AndersonJJ,BoersM,BombardierC,FurstD, GoldsmithC,etal.,AmericanCollegeofRheumatology. Preliminarydefinitionofimprovementinrheumatoid arthritis.ArthritisRheum.1995;38:727–35.
28.AlegrettiAP,MucenicT,MerzoniJ,FaulhaberGA,SillaLM, XavierRM.ExpressionofCD55andCD59onperipheralblood cellsfromsystemiclupuserythematosus(SLE)patients.Cell Immunol.2010;265:127–32.
29.DzietczeniaJ,WrobelT,MazurG,PorebaR,JazwiecB, KuliczkowskiK.Expressionofcomplementregulatory proteins:CD46,CD55,andCD59andresponsetorituximabin
patientswithCD20+non-Hodgkin’slymphoma.MedOncol. 2010;27:743–6.
30.MacorP,TripodoC,ZorzetS,PiovanE,BossiF,MarzariR,etal. Invivotargetingofhumanneutralizingantibodiesagainst CD55andCD59tolymphomacellsincreasestheantitumor activityofrituximab.CancerRes.2007;67:10556–63.
31.HuW,GeX,YouT,XuT,ZhangJ,WuG,etal.HumanCD59 inhibitorsensitizesrituximab-resistantlymphomacellsto complement-mediatedcytolysis.CancerRes.
2011;71:2298–307.
32.ZillerF,MacorP,BullaR,SblatteroD,MarzariR,TedescoF. Controllingcomplementresistanceincancerbyusinghuman monoclonalantibodiesthatneutralize
complement-regulatoryproteinsCD55andCD59.EurJ Immunol.2005;35:2175–83.