Research paper
Lipid transfer protein isolated from noni seeds displays antibacterial
activity
in vitro
and improves survival in lethal sepsis induced by CLP
in mice
Adson A. Souza
a, Andrea S. Costa
a, Dy
ely C.O. Campos
a, Andressa H.M. Batista
b,
Gleilton W.P. Sales
b, N
adia A.P. Nogueira
b, Keila M.M. Alves
c,
Andrelina Noronha Coelho-de-Souza
d, Herm
ogenes D. Oliveira
a,*aDepartment of Biochemistry and Molecular Biology, Federal University of Ceara, Campus do Pici Prof. Prisco Bezerra, 60440-900, Fortaleza, CE, Brazil bDepartment of Clinical and Toxicological Analysis, Faculty of Pharmacy, Dentistry and Nursing, Federal University of Ceara, 60431-372, Fortaleza, CE,
Brazil
c
Center for Laboratory Veterinary Medicine of Fortaleza - LAFORVET, 60135-130, Fortaleza, CE, Brazil
dLaboratory of Experimental Physiology, Superior Institute of Biomedical Sciences, State University of Ceara, 60714-903, Fortaleza, CE, Brazil
a r t i c l e
i n f o
Article history:
Received 21 September 2017 Accepted 20 March 2018 Available online 22 March 2018
Keywords: Morinda citrifoliaL. Lipid transfer protein Antimicrobial activity Sepsis
a b s t r a c t
In the present study, we aimed to evaluate the antibacterial activity of a lipid transfer protein isolated fromMorinda citrifoliaL. seeds, namedMcLTP1, and to investigate its effect in the cecal ligation and
puncture (CLP) mouse sepsis model. Antimicrobial assays revealed that McLTP1 (12.5e800mg/mL)
significantly reduced Staphylococcus aureus (ATCC 6538P and ATCC 14458) and Staphylococcus
epi-dermidis(ATCC 12228) planktonic growth, reaching maximal inhibition of approximately 50% and 98%,
respectively. Furthermore,McLTP1inhibited biofilm formation of bothS. aureusstrains, achieving
per-centages ranging from 39.1% to 69.1% (200e800mg/mL) for ATCC 6538P and 34.4%e63% (12.5e800mg/
mL) for ATCC 14458. A synergistic interaction between McLTP1and oxacillin against S. aureus and
S. epidermidiswas also observed, as determined by fractional inhibitory concentration indices of 0.18 and
0.38, respectively.McLTP1showed no significant inhibitory effect against Gram-negative bacteria. In the
in vivoexperiments, sepsis was lethal to 83% of the animals, 72 h after CLP. In contrast, 100% of the
animals treated withMcLTP1(8 mg/kg) before (intraperitoneal injection or oral dose) or after (oral dose)
CLP were still alive 3 days later. In addition, oral or intraperitoneal administration ofMcLTP1(8 mg/kg)
significantly reduced the body weight loss, fever, leukocytosis, organ damage, and the level of infl
am-matory serum cytokines induced by sepsis. In conclusion,McLTP1could be exploited for its antimicrobial
properties, and can be considered a potential therapeutic candidate for the management of clinical sepsis.
©2018 Elsevier B.V. and Société Française de Biochimie et Biologie Moléculaire (SFBBM). All rights
reserved.
1. Introduction
The emergence of microbial resistance is one of the greatest public health problems of this century. According to recent esti-mates, by 2050, this threat will generate 300 million premature deaths, costing the global economy approximately $100 trillion
[1,2]. Due to microbial resistance to drugs and the lack of new
an-tibiotics, an increase in infectious diseases’severity and the risk of
complications associated with bacterial infections has been
observed [2]. In the case of severe infections, which ultimately lead
to sepsis, this health state is characterized by systemic
Abbreviations:AMP, antimicrobial peptide; LTP, lipid transfer protein;McLTP1, LTP isolated fromMorinda citrifoliaL. seeds; FICI, fractional inhibitory concentration index; CLP, cecal ligation and puncture; IL, interleukin; TNF-a, tumor necrosis factor-a; MCP-1, monocyte chemotactic peptide 1; IFN-g, interferon-g; CBA, cyto-metric bead array; MIC, minimum inhibitory concentration; BHI, brain heart infu-sion; TSB, tryptic soy broth.
*Corresponding author.
E-mail address:hermogenes@ufc.br(H.D. Oliveira).
Contents lists available atScienceDirect
Biochimie
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / b i o c h i
https://doi.org/10.1016/j.biochi.2018.03.011
inflammatory response syndrome (SIRS), affecting the patient's
entire body and resulting in high morbidity and mortality [3e6].
Unfortunately, there has been a drop in the number of new
antibiotics introduced to the market in recent decades [7]. The
challenges of finding new antimicrobial agents with alternative
bacterial targets, lowerfinancial returns compared to those of other
disease therapies, and heavier regulatory guidelines are factors
involved in this decline [7,8]. Because of this crisis of antibiotic
development and the absence of drugs approved for the treatment of sepsis, research into novel approaches and alternative sources of
bioactive compounds has been greatly stimulated [6,8].
Plant antimicrobial peptides (AMPs) have emerged as promising
molecules with therapeutic potential in human health [9]. They are
small (usually 2e9 kDa), basic, and cysteine-rich molecules, with
disulfide bridges in their internal structures that generate highly
stable globular conformations [9,10]. According to sequence
ho-mologies, pattern distributions of disulfide bridges, and
three-dimensional structures, AMPs are divided into nine classes: thio-nins, defensins, shepherdins, cyclotides, snakins, heveins, knottins,
Ib-AMPs, and non-specific lipid transfer proteins (nsLTPs) [9e11].
Although several studies have shown that AMPs present broad
spectrumin vitroactivities against bacteria and fungi [9e11], these
activities have not been explored extensively throughin vivo
ex-periments (e.g., in strategies involving systemic infectious diseases, such as sepsis).
Recently, an antimicrobial peptide belonging to the lipid
trans-fer protein class, namedMcLTP1, was isolated fromMorinda citrifolia
L. (noni) seeds (UniProtKB accession number: C0HJH5). This LTP was shown to be thermostable and resistant to pepsin, trypsin, and chymotrypsin digestion; it was capable of displaying potent
anal-gesic and anti-inflammatory activities when orally administered to
mice [12].
The aim of this study was to evaluate thein vitroandin vivo
antibacterial properties ofMcLTP1, with the latter involving both
prophylactic and therapeutic interventions in a mouse sepsis
model. Because the actual efficacy of McLTP1 in vivo may be
different from thatin vitrodue to interactions with components of
the human/animal body, performing bothin vitroandin vivoassays
provided a more complete understanding of the peptide’s activity.
The outcomes will demonstrate that these molecules have poten-tial to address burgeoning antimicrobial resistance issues.
2. Materials and methods
2.1. Plant material and purification of McLTP1
M. citrifoliaL. var.citrifoliaseeds were obtained from EMBRAPA
Agroindustria Tropical, Fortaleza, Ce, Brazil. After extraction from
defatted noni seedflour,McLTP1was purified using a combination
of trichloroacetic acid precipitation and size exclusion
chroma-tography, as previously published by Campos et al. [12]. Samples
obtained from gel filtration and containingMcLTP1 were
lyophi-lized and stored at 20C for further use in biological assays. The
purity of McLTP1 was assessed by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), with a 15%
poly-acrylamide gel under non-reducing conditions [13]. The proteins
were visualized with Coomassie Brilliant Blue R-250 staining.
McLTP1samples used in biological assays were prepared based on
soluble total protein concentrations estimated by the method of Bradford [14].
2.2. Reagents and culture media
Gentamicin and oxacillin were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). The antimicrobial agents were diluted
according to the Clinical and Laboratory Standards Institute (CLSI)
guidelines [15]. The culture media, Brain Heart Infusion broth (BHI)
and Tryptic soy broth (TSB), were obtained from Himedia®
(Mumbai, Maharashtra, India). Crystal violet, which was used to
stain bacterial biofilms, was purchased from Laborclin LTDA
(Pin-hais, PR, Brazil). Other solvents and chemicals were analytical grade and obtained from local suppliers.
2.3. Antibacterial activity
2.3.1. Bacterial strains
Three Gram-negative bacteria strains (Escherichia coli ATCC
10536,Pseudomonas aeruginosaATCC 902, andKlebsiella
pneumo-niaeATCC 10031) and three Gram-positive strains (Staphylococcus
epidermidisATCC 12228,Staphylococcus aureus ATCC 6538P, and
ATCC 14458) were tested for their susceptibility to McLTP1. To
prepare bacterial cell suspensions for antimicrobial activity assays,
all strains were inoculated in BHI liquid medium at 37C for 24 h. A
saline inoculum suspension of each culture was then prepared to a
final concentration of 106CFU/mL.
2.3.2. Antibacterial susceptibility test
The antibacterial susceptibility test was performed in triplicate in 96-well culture plates in BHI medium, based on the
micro-dilution broth method described previously [15].McLTP1was
dis-solved in sterile distilled water,filtered using 0.22
m
m membranes,and tested at 12.5e800
m
g/mL. Gentamicin and oxacillin (positivecontrols) were also prepared in sterile distilled water and two-fold
serially diluted from 0.048 to 50
m
g/mL. Minimum inhibitorycon-centrations (MICs) were assessed visually after an incubation
period of 24 h (37C) and were recorded as the lowest sample
concentration at which there was no growth. To assess cell growth, the absorbance was measured at 620 nm (A620), using a microplate reader (Bio Tek, Winooski, VT, USA). Controls without antibiotics or
McLTP1were also measured. BHI broth containing only the
anti-microbial drugs at the concentrations tested was used as a blank. The planktonic culture turbidity measured in the absence of drugs was considered 100% growth, and was used as a reference to
determine the percent growth inhibition of theMcLTP1-treated
cultures.
2.3.3. Effect of McLTP1on staphylococcal biofilm formation
The effect ofMcLTP1 on S. aureusbiofilm formation was
per-formed in triplicate using polystyrene flat-bottomed microtiter
plates, as previously reported [16].S.aureusinocula (strains ATCC
14458 and ATCC 6538P) were cultured in TSB supplemented with
1% (w/v) glucose to afinal concentration of 106CFU/mL and
incu-bated withMcLTP1(12.5e800
m
g/mL) at 37C for 24 h. After theincubation period, the plate wells were washed with sterile saline (0.85% NaCl) to remove unattached cells, while the adherent cells
werefixed for 15 min with 99% methanol. Then, the adherent
bio-films were stained with 2% (w/v) crystal violet solution for 15 min.
The excess crystal violet was removed by washing with ultrapure water, and the plate was air-dried. Finally, the crystal violet was dissolved by adding acetic acid 33% (v/v) with shaking in an orbital
shaker for 15 min. Biofilm formation was determined by measuring
the absorbance at 570 nm (A570) using a microplate reader. The
percent inhibition was calculated, taking theA570of non-treated
controls as 100% [17].
2.3.4. Combination effect of antibiotics with McLTP1
Chequerboard titration was performed using McLTP1
(100e400
m
g/mL) and the standard drugs, gentamicin and oxacillin,at sub-MIC concentrations (MIC/16eMIC/2).S.aureus(ATCC 14458
determine the fractional inhibitory concentration index (FICI),
ac-cording to a previously published method [18]. The inoculum size
and the culture conditions were the same as those used for the
antibacterial susceptibility test. FICI values0.5 were considered
synergistic (SYN), 0.5<FICI4.0 indifferent (IND), and >4.0,
antagonistic (ANT) [19].
2.4. Effect of McLTP1on lethal sepsis induced by cecal ligation and
puncture (CLP) in mice
2.4.1. Animals
In this study, male Swiss albino mice (45 days old; 35 g) were used. The animals were housed in polypropylene cages under
standard environmental conditions (24±1C, humidity 45
e65%,
and 12 h light/dark cycle) and received food and waterad libitum.
All animals were treated in compliance with the ethical standards established by the National Guidelines for the Use of Experimental Animals of Brazil and by the Directive 2010/63/EU of the European Parliament and of the Council of the European Union. The experi-mental protocols were approved by the Committee for the Ethical
Use of Animals of the Federal University of Ceara (CEUA-UFC no.
108/2016).
2.4.2. Sepsis model
The experimental induction of sepsis was performed using the
CLP model, as outlined previously [20]. In brief, before the surgery,
all mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) solution. Under aseptic conditions, a laparotomy was performed with a median longitudinal incision of approximately 1.5 cm, allowing full expo-sure of the cecum. A non-obstructive ligation with cotton thread 4-0 was placed in the region immediately below the ileocecal valve, followed by a single perforation with an 18-gauge needle. After perforation, to allow leakage of a small amount of fecal content, the cecum was softly compressed and then returned to the peritoneal cavity. Sham-operated mice were submitted to the same surgical procedure, without ligation and perforation of the cecum.
Subse-quently, the abdomen was closed in two layers. Forfluid
replace-ment, isotonic saline (37C, 4% of body weight) was administered
subcutaneously, immediately after the surgical procedure. Then, all animals were returned to their cages, with free access to food and water, and were observed over 72 h.
2.4.3. Experimental design
The mice were randomized intofive groups (n¼8 per group). 1)
Sham operated, 2) sepsis induction but receiving only sterile saline (0.15 M NaCl), 3) sepsis induction and receiving a single
intraperi-toneal dose ofMcLTP1 (8 mg/kg) 1 h before surgery (prophylactic
effect of intraperitonealMcLTP1), 4) sepsis induction and receiving
a single oral dose ofMcLTP1 (8 mg/kg) 2 h before surgery
(pro-phylactic effect of oral McLTP1), and 5) sepsis induction and
receiving oral McLTP1 (8 mg/kg) immediately after surgery, and
then every 6 h over a 72-h period (therapeutic effect of oral
McLTP1). The survival, body weights, and rectal temperatures of the
animals were monitored every 12 h for 72 h after the surgery.
2.4.4. Total and differential blood cell counts
Blood was collected aseptically under anesthesia from the retro-orbital plexus at time intervals of 24 and 48 h. The blood was assayed with an automatic hematology analyzer BC-2800 (Shenz-hen Mindray Bio-Medical Electronics Co., S(Shenz-henz(Shenz-hen, Guangdong, China) to determine the white blood cell, granulocyte, and lymphocyte counts.
2.4.5. Histopathologic analysis
Seventy-two hours after CLP, all animals were euthanized with an overdose of anesthetic (300 mg/kg ketamine and 30 mg/kg xylazine). Tissue samples of the liver and superior lobe of the right lung were removed for histopathologic analysis. The organs were
fixed in phosphate-buffered saline (PBS) containing 4%
para-formaldehyde, and were then embedded in paraffin. A microtome
was used to produce sections that were mounted on slides,
deparaffinized, and then stained with hematoxylin and eosin. The
following parameters were evaluated in the stained tissue sections:
vascular congestion, hemorrhage, and cellular infiltration,
accord-ing to the followaccord-ing scores: 0, absent; 1, weak; 2, moderate; and 3,
strong, as described by Zhou et al. [21]. Histological assessment was
performed randomly and blindly under a light microscope (Nikon E200).
2.4.6. Quantification of cytokines by the cytometric bead array (CBA) technique
Blood samples from mice in group #2 (pretreatment with saline
1 h before CLP; n¼8), group #3 (intraperitoneal pretreatment with
McLTP11 h before CLP; n¼8), and group #5 (oral post-treatment
with McLTP1 immediately after CLP; n¼8) were collected from
the retro-orbital plexus 12 h after sepsis induction. Thereafter,
levels of tumor necrosis factor-
a
(TNF-a
), interferon-g
(IFN-g
),interleukin (IL)-6, IL-10, IL-12 p70, and monocyte chemotactic protein 1 (MCP-1) were measured from the serum samples by CBA
technique. Cytokine quantification was performed using a mouse
inflammatory cytokine kit (Becton Dickinson Biosciences, San Jose,
CA, EUA), following the manufacturer’s protocol. Data were
analyzed using FCAP Array software (BD Biosciences).
2.5. Statistical analysis
Experimental data are expressed as means±standard
de-viations. Significant differences among means were evaluated in
GraphPad Prism®
5 software (GraphPad Prism, Inc., San Diego, CA, USA) using one-way analysis of variance (ANOVA), followed by
Tukey’s post hoc tests. In all comparisons, significant differences
were considered atp<0.05.
3. Results
3.1. Antibacterial activity of McLTP1
3.1.1. Effect on planktonic cells and biofilm formation
McLTP1had a significant influence on the planktonic growth of
the threeStaphylococcusstrains tested (Fig. 1).McLTP1could inhibit
~50% of the planktonic growth (p<0.05) ofS.aureusstrains, ATCC
6538P and ATCC 14458, at 12.5 and 200
m
g/mL, respectively. Theobserved effect did not occur in a concentration-dependent manner for both strains. In contrast to that observed for the
S. aureus strains, McLTP1 inhibited S. epidermidis (ATCC 12228)
planktonic growth by ~20% at a concentration range of
12.5e200
m
g/mL (p<0.05), reaching a maximal inhibition of 98% at800
m
g/mL. Despite the bacterial antiplanktonic activity displayedby the antimicrobial peptide, it was not possible to calculate MIC,
because 800
m
g/mL McLTP1 (i.e., the maximum concentrationevaluated) was not able to inhibit the bacterial growth of the tested strains completely. Unlike the effects observed against the
Gram-positive bacteria,McLTP1did not inhibit the growth of the
Gram-negative bacterial strains at any tested concentration (data not shown).
The antibiofilm effects ofMcLTP1 at increasing concentrations
(12.5e800
m
g/mL) onS.aureus(ATCC 14458 and ATCC 6538P) aremL), McLTP1 could diminish biofilm formation of both strains,
achieving inhibition of 39.1e69.1% for ATCC 6538P and ~63% for
ATCC 14458. At concentrations below 200
m
g/mL McLTP1, onlyATCC 14458 was susceptible, demonstrating a significant
reduc-tion (p<0.05) in biofilm formation activity, ranging from 34.4% (at
12.5
m
g/mL) to 55.6% (at 100m
g/mL).3.1.2. Effect of combination of antibiotics with McLTP1
Synergistic interactions were observed betweenMcLTP1and
oxacillin for the three bacterial strains, with indexes (FICI) ranging
from 0.18 to 0.38, as shown inTable 1. No synergism was observed
against the three bacterial strains with the cotreatment ofMcLTP1
and gentamicin at any concentration tested.
Fig. 1.Effect ofMcLTP1on planktonic growth ofStaphylococcusspp.S. aureus(ATCC 14458 and ATCC 6538P) andS. epidermidis(ATCC 12228). Absorbances (620 nm) were measured after 24 h at 37C in the absence or in the presence ofMcLTP1. *p<0.05
compared to control group (One-way ANOVA followed by Tukey's multiple com-parison test).
Fig. 2.Antibiofilm formation activity ofMcLTP1againstStaphylococcus aureusstrains. Data are expressed as the means of biofilm production percentage (±SD) of tripli-cates. *Indicates significant difference between untreated and treated bacteria cells (p<0.05; ANOVA Tukey’s post hoc test).
Fig. 3.Survival rates of animals with sepsis subjected toMcLTP1treatment. The animals were pretreated (i.p. and p.o.) or post-treated (p.o.) withMcLTP1(8 mg/kg), and survival assessed over 72 h. Data are expressed as the percentage survival of the animals (mean±SD for each group, n¼8). #Significantly different (p<0.05; ANOVA Tukey’s post hoc test) from CLP group. Note: since similar survival rates were observed among sham operated group andMcLTP1pre and post-treated groups, the lines overlap.
Table 2
Antipyretic activity ofMcLTP1on lethal sepsis induced by CLP in mice. Treatment Dose (mg/kg) Rectal temperature (C)
Normal After CLP
12 h 24 h 36 h 48 h 72 h
Vehicle _ 34.1±0.5 39.4±0.3 39.2±0.2 40.2±0.5 38.8±0.6 38.2±0.8
McLTP1pre-ip 8 mg/kg e 35.1±0.2# 34.1±0.6# 35.1±0.7# 34.0±0.4# 35.0±0.2#
McLTP1pre-p.o. 8 mg/kg e 37.8±0.4# 36.1±0.1# 35.5±0.1# 35.6±0.3# 35.5±0.6#
McLTP1post-p.o. 8 mg/kg e 34.1±0.1# 33.7±0.5# 34.1±0.7# 34.0±0.6# 34.0±0.1# Data are expressed as mean±S.D (n¼8/per group). ANOVA Tukey’s post hoc test (#p<0.05vs. vehicle).
3.2. Effect of McLTP1on lethal sepsis induced by CLP in mice
3.2.1. Effects on survival, body weight, and rectal temperature As expected, the sham-operated group had a survival rate of 100% during the observation period. In contrast, the vehicle group
had a survival rate reduction of 50% within thefirst 24 h, decreasing
to 17% after 72 h. Both pretreatment (oral and intraperitoneal) and
post-treatment withMcLTP1(8 mg/kg) promoted a significant level
of protection, with 100% of the mice surviving 72 h after CLP (Fig. 3).
Before the surgery, all animals weighed 35±0.1 g. In the
sham-operated group, the mice maintained their weight throughout the
experiment. However, a significant weight loss was observed in the
vehicle group, with the weights of these mice reaching 26±0.1 g
72 h post-CLP. After three days, the groups pretreated orally or
intraperitoneally and post-treated orally with McLTP1 (8 mg/kg)
weighed 36±0.1, 33±0.1, and 34±0.1 g, respectively, which was
similar to that observed in the sham group (34±0.1 g).
Table 2shows the antipyretic effect mediated byMcLTP1 treat-ments in the lethal sepsis induced by CLP. The mice of the vehicle
group developed fever within thefirst 12 h after surgery. Fever was
also observed in the mice of the group orally pretreated with
McLTP1 by 12 h; however, it normalized afterward (72 h,
35.5±0.6C). Both McLTP1 (8 mg/kg) pretreatment
(intraperito-neal) and post-treatment (oral) presented a potent antipyretic ac-tivity, normalizing the body temperature of the mice even 12 h after sepsis induction.
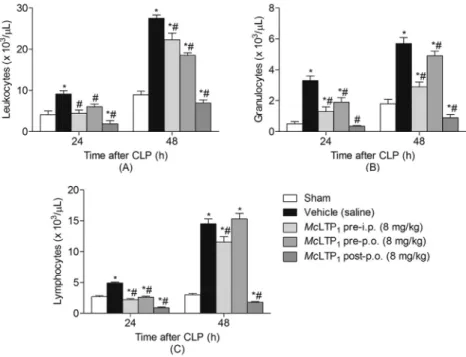
3.2.2. Hematological alterations
Fig. 4shows the hematological parameters of the mice with sepsis induced by the CLP method. The vehicle group presented
with leukocytosis in thefirst 24 h after induction of sepsis, and this
condition persisted over the next 48 h of the experiment. On the Fig. 5.Effect ofMcLTP1on lung and liver organ damage in septic animals. (A) Representative images from the different experimental groups are presented (magnification400). (B) Histopathologic injury scores were graded from 0 (normal) to 3 (severe), according to the tissues damage of 8 animals per group. * and # indicate significant differences compared with the sham and vehicle (saline) group, respectively (p<0.05; ANOVA Tukey’s post hoc test).
contrary, the prophylactic and therapeutic treatments withMcLTP1
(8 mg/kg) significantly reduced leukocytosis compared to that of
the vehicle control throughout this time.
3.2.3. Histological analysis
The severity of histopathologic changes and lesion scores are
shown inFig. 5. CLP induced lung and liver histopathological
al-terations, including congestion, inflammatory cell infiltration,
ne-crosis, and degeneration. All treatments with McLTP1 (8 mg/kg)
markedly attenuated these pathological changes by both routes (intraperitoneal and oral), especially in the pretreated mice, which showed tissue structures similar to those observed in the sham-operated group.
3.2.4. Production of cytokines
Cytokine levels were increased in the septic animals
(CLPþvehicle), as observed with IL-6, IL-10, MCP-1, and TNF-
a
levels (Fig. 6). In contrast, both pretreatment and post-treatment
withMcLTP1(intraperitoneal and oral, respectively) could reduce
(p<0.05) systemic production of all the aforementioned cytokines.
IFN-
g
and IL-12 p70 were detected at low levels (<10 pg/mL), andthere were no significant differences amongMcLTP1treated groups
and the vehicle group.
4. Discussion
AMPs have been described as components of plant innate im-mune mechanisms involved in the responses to pathogen attacks
[10]. Due to their antimicrobial abilities, AMPs have been proposed
as promising tools in human health and agribusiness applications
[9]. Some classes of the AMP family, such as LTP members, have
been the focus of antimicrobial studiesin vitro, particularly against
phytopathogenic microorganisms. However, their effects on human
pathogens andin vivoapplications have remained unclear.
Pharmacological studies have shown that McLTP1 displays
antinociceptive and anti-inflammatory activities by intraperitoneal
or oral routes, demonstrating its potential use as a therapeutic
agent [12]. Despite this peptide belonging to the AMP family and
the potential biological effect observed forMcLTP1, its antibacterial
activity has not been assessed until now. Therefore, we explored
the properties ofMcLTP1 on bacterial species of clinical interest
in vitro, and we investigated its protective effect on mice with sepsis
induced by CLP, via prophylactic and therapeutic treatments.
In our experiments, we found thatMcLTP1inhibited the growth
of Gram-positive microorganisms (Staphylococcusspp.), but did not
show antimicrobial effects on E. coli, P. aeruginosa, and
K. pneumoniae. These results are similarly to those of a study on an
LTP isolated fromPhaseolus mungoseeds, which could inhibit the
growth ofS. aureusbut was not able to inhibit the Gram-negative
Salmonella typhimuriumused in that study [22]. Most plant LTPs
show specificity against a particular range of microorganisms and
exhibit varied efficacy. Differences in the lipid composition of their
cell membranes are believed to be involved in the selective toxicity
of plant LTPs against pathogens [23]. Beyond this, the literature
shows that the outer cell wall of Gram-negative bacteria can confer low permeability to antibacterial molecules, diminishing their susceptibility to some antimicrobial agents compared to that of
Gram-positive microorganisms [24].
The action mechanism of the antimicrobial effects ofMcLTP1has
not been investigated in this work. However, based on the mode of action described for the antimicrobial activity of other LTPs, it is reasonable to assume that this peptide could act as a per-meabilizing membrane agent. The membrane pore-forming ca-pacity of LTPs occurs through electrostatic interactions between cationic amino acid residues present in the peptide structure and
membrane lipids, with no direct relationship to their ability to bind
and transfer lipids [10,11,23]. Reductions in the antimicrobial
ac-tivity of plant LTPs in the presence of high ionic-strength solutions, and a weaker effect observed for LTP isoforms with a smaller number of basic amino acid residues, support the mechanism of
action involving membrane targeting [23].
In addition to displaying bacterial antiplanktonic activity,
McLTP1was able to diminishS.aureusbiofilm formation.S.aureusis
a versatile and highly virulent pathogen involved in clinical
in-fections. It is commonly associated with strains producing biofilms,
which are difficult to treat, leading to bacteremia and metastatic
infections that are strongly associated with high morbidity and
mortality. Reductions in biofilm formation capacity by
staphylo-cocci represent decreased virulence of these bacteria, imparting greater susceptibility to body defenses and antimicrobials drugs
[25,26]. Thus, thesefindings reinforce the potential application of
McLTP1in clinical settings, since it can act against both planktonic
cells and biofilm formation.
Drug interaction strategies are alternatives being tested to overcome increases in microbial resistance. Particularly, a
prom-ising synergistic effect was found between McLTP1 and oxacillin
against S. aureusand S. epidermidis. Apart from re-establishing
microorganism sensitivity to antimicrobial drugs in clinical prac-tice, combination therapies can also lead to a reduction in thera-peutic doses and treatment durations, which are important to diminish adverse effects and minimize the recurrence of microbial resistance [8,27].
Concerning the mechanism of interaction between antibacterial drugs, several studies have reported that those with closely related mechanisms exhibit synergistic effects more readily than drug
combinations with an entirely different mode of action [28].
Moreover, it has been proposed that the pore-forming capacity of AMPs can also enhance the activity of bacterial peptidoglycan hy-drolases. This event compromises the integrity of the peptido-glycan layer, increasing the access of antibiotics that affect cell wall
synthesis (e.g.
b
-lactams), and resulting in a more pronouncedantimicrobial effect [28,29]. A mechanism such as this might
explain the positive interaction observed between McLTP1 and
oxacillin.
In order to study the protective effect ofMcLTP1in severe sepsis,
we used the CLP experimental model. This model has been widely used to study the complexity of sepsis pathophysiology and to search for new therapeutic agents. Over the past 45 years, since its
establishment in 1970 by Chadry et al. [20], it has been considered
the‘gold standard’in sepsis studies, because it most closely mimics
the progress of the syndrome in humans [30,31].
In an attempt to observe the prophylactic effect ofMcLTP1, mice
were pretreated with the peptide by intraperitoneal injection and by oral delivery. However, this pretreatment is not conducive to clinical settings. Therefore, a therapeutic group involving
contin-uous oral administration ofMcLTP1to the mice after sepsis
induc-tion was also included.
In the present study,McLTP1(8 mg/kg) displayed a potent
pro-tective effect against sepsis induced by CLP in mice. Treatment with the peptide completely prevented the lethality frequently observed in severe sepsis that is caused by systemic and pathological changes, such as alterations in water and food consumption, loss of body weight, hyperpyrexia, leukocytosis, and organ damage
[32e35]. Due to an imbalance in the release of proinflammatory
and anti-inflammatory mediators, sepsis is commonly described as
a“cytokine storm.”Among the proinflammatory mediators,
upre-gulation of TNF-
a
, IL-1b
, IL-6, and IL-8 has been strongly associatedwith the harmful effects of sepsis. In general, the CLP model pro-vides an exacerbated release of these mediators, leading to the
McLTP1 administration was observed with pretreatment (intra-peritoneal and oral) and especially post-treatment (oral), which
supports the potential prophylactic and therapeutic benefits of
McLTP1in the management of sepsis in the clinical setting.
Sepsis induced by CLP consists of an endogenous polymicrobial infection, promoted through perforation of the cecum, which al-lows leakage of gut bacteria into the intraperitoneal cavity, gener-ating peritonitis and leading to sepsis. Thus, high blood bacterial counts are observed ~20 h after CLP, consisting mainly of
Gram-negative bacteria, such asEnterococcus cloacae,E. coli,Proteus
mir-abilis, andAlcaligenes faecalis[36]. BecauseMcLTP1did not display
efficacy against Gram-negative bacteriain vitro, includingE. coli, we
propose that its protective effect is related to the anti-inflammatory
properties of this peptide rather than its direct effect on
microor-ganisms. Using classical models of inflammation, Campos et al. [37]
have shown that McLTP1 is able to reduce the levels of
pro-inflammatory cytokines (i.e., TNF-
a
, IL-1, and IL-6) significantlyand increase the levels of IL-10, an anti-inflammatory cytokine, by
both intraperitoneal and oral routes (8 mg/kg).
In order to determine whether cytokine modulation could be
involved in the beneficial effects ofMcLTP1against sepsis, cytokine
(TNF-
a
, IFN-g
, IL-6, IL-10, IL-12 p70, and MCP-1) levels werequantified from serum samples of CLP-induced septic mice by the
CBA technique.McLTP1administration was able to downregulate
the proinflammatory cytokines, TNF-
a
, IL-6, and MCP-1, which wasconsistent with the results found earlier with the paw edema
in-flammatory model [37]. According to other investigators [5,33],
during the early phase of sepsis, TNF-
a
and IL-1 are the mainini-tiators of septic responses, released predominantly from activated macrophages within 30 min after the infectious stimulus. Once in the systemic circulation, these cytokines stimulate different target cells, such as endothelial cells, which upregulate expression of adhesion molecules and enhance integrin adhesiveness to
neu-trophils, leading to excessive extravasation into tissues. TNF-
a
andIL-1 are also responsible for amplifying later proinflammatory
cytokine production (e.g., IL-6 and IL-8). Elevated IL-6 blood levels are associated with mediation of the acute phase response in sepsis that involves fever, leukocytosis, and multiple organ failure. Moreover, IL-6 is an indicator of sepsis severity because it can be found at high levels for an extended period.
Several studies have also shown elevation of chemokines
(macrophage migration inhibitory factoreMIF; MCP-1) in the host
response to pathogens in sepsis [38,39]. MCP-1 also acts as a
proinflammatory mediator in sepsis, involved in the recruitment of
mononuclear cells and neutrophils. Therapies targeting MCP-1 synthesis have been shown to reduce lung and liver neutrophil
infiltration and to improve mouse survival during CLP-induced
sepsis [39]. Therefore, refined regulation in the cytokine and
che-mokine profiles plays a pivotal role in eliminating invading
path-ogens and attenuating excessive tissue-damaging inflammation to
improve sepsis outcomes [5].
Regarding IL-10 production,McLTP1administration
(intraperi-toneal and oral) significantly reduced the level of this
anti-inflammatory mediator compared to that in the control mice,
which differed from previous results where an increase in IL-10
production was found [37]. Despite the involvement of IL-10 in
the suppression of proinflammatory cytokines (e.g., TNF-
a
, 1,IL-6, and IFN-
g
), its role in sepsis is controversial. Although IL-10protected mice from lethal endotoxemia in another experimental sepsis model induced by lipopolysaccharide administration, its
inhibition 12 h after CLP markedly improved survival [5].
Although Gram-negative bacteria were originally described as the major causative organisms for sepsis, recent epidemiological data have revealed that septicemia due to Gram-positive bacteria
has become very common in the last 25 years, withStaphylococcus
spp. frequently involved in such cases [3,32]. SinceMcLTP1displays
antimicrobial activity against Gram-positive bacteria (S.aureusand
S. epidermidis), we hypothesized that, in systemic infections
involving these organisms,McLTP1could exert anti-inflammatory
and antimicrobial activity, making it even more effective in the management of sepsis. However, further investigations are needed to support this hypothesis.
In conclusion, the data presented herein extend the antibacterial
properties displayed by the LTP group of AMPs.McLTP1was active
not only against the planktonic growth of Gram-positive bacteria,
but also against biofilm formation. To the best of our knowledge,
this is thefirst report that describes an LTP with protective effects
against polymicrobial sepsis by alleviating organ damage. The current investigation provides a novel candidate for the potential prevention and treatment of sepsis.
Conflicts of interest
The authors have declared that there is no conflict of interest,
includingfinancial, personal or any other relationships with other
people or organisations.
Acknowledgements
The authors thank the Brazilian institutions CNPq (National
Council for Scientific and Technological Development; grant
num-ber 482507/2013-6), FUNCAP (Ceara Research Foundation) and UFC
(Federal University of Ceara), for physical installation andfinancial
support of this research.
References
[1] C. Arias, B. Murray, A new antibiotic and the evolution of resistance, N Engl J Med 372 (2015) 1168e1170,https://doi.org/10.1056/NEJMcibr1500292.A. [2] C. Llor, L. Bjerrum, Antimicrobial resistance: risk associated with antibiotic
overuse and initiatives to reduce the problem, Ther Adv Drug Saf 5 (2014) 229e241,https://doi.org/10.1177/2042098614554919.
[3] G. Ramachandran, Gram-positive and gram-negative bacterial toxins in sepsis: a brief review, Virulence 5 (2013) 213e218,https://doi.org/10.4161/ viru.27024.
[4] C.T. Scoparo, L.M. de Souza, Y.D. Rattmann, E.C. Kiatkoski, N. Dartora, M. Iacomini, The protective effect of green and black teas (Camellia sinensis) and their identified compounds against murine sepsis, Food Res Int 83 (2016) 102e111,https://doi.org/10.1016/j.foodres.2016.02.011.
[5] W. Schulte, J. Bernhagen, R. Bucala, Cytokines in sepsis: potent immunor-egulators and potential therapeutic targetsdan updated view, Mediators Inflamm 2013 (2013) 1e17.https://doi.org/10.1155/2013/165974. [6] C.S. Deutschman, K.J. Tracey, Sepsis: current dogma and new perspectives,
Immunity 40 (2014) 463e475,https://doi.org/10.1016/j.immuni.2014.04.001. [7] P. Fernandes, The global challenge of new classes of antibacterial agents: an industry perspective, Curr Opin Pharmacol 24 (2015) 7e11,https://doi.org/ 10.1016/j.coph.2015.06.003.
[8] B. Spellberg, J. Bartlett, R. Wunderink, D.N. Gilbert, Novel approaches are needed to develop tomorrow's antibacterial therapies, Am J Respir Crit Care Med 191 (2015) 135e140,https://doi.org/10.1164/rccm.201410-1894OE. [9] E. De Souza C^andido, M.H.E. Silva Cardoso, D.A. Sousa, J.C. Viana, N.G. De
Oliveira-Júnior, V. Miranda, O.L. Franco, The use of versatile plant antimicro-bial peptides in agribusiness and human health, Peptides 55 (2014) 65e78, https://doi.org/10.1016/j.peptides.2014.02.003.
[10] A. Yili, V. Maksimov, Q.-L. Ma, Y.-H. Gao, O. Veshkurova, S. Salikhov, H.A. Aisa, Antimicrobial peptides from the plants, J Pharm Pharmacol 2 (2014) 627e641, https://doi.org/10.17265/2328-2150/2014.11.001.
[11] R. Nawrot, J. Barylski, G. Nowicki, J. Broniarczyk, W. Buchwald, A. Go zdzicka-Jozefiak, Plant antimicrobial peptides, Folia Microbiol (Praha) 59 (2014) 181e196,https://doi.org/10.1007/s12223-013-0280-4.
[12] D.C.O. Campos, A.S. Costa, A.D.R. Lima, F.D.A. Silva, M.D.P. Lobo, A.C.O. Monteiro-Moreira, R.A. Moreira, L.K. Leal, D. Miron, I.M. Vasconcelos, H.D. Oliveira, First isolation and antinociceptive activity of a lipid transfer protein from noni (Morinda citrifolia) seeds, Int J Biol Macromol 86 (2016) 71e79,https://doi.org/10.1016/j.ijbiomac.2016.01.029.
[13] U.K. Laemmli, Cleavage of structural proteins during assembly of head of Bacteriophage-T4, Nature 227 (1970) 680e685, https://doi.org/10.1038/ 227680a0.
Biochem 72 (1976) 248e254,https://doi.org/10.1016/0003-2697(76)90527-3. [15] CLSI, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, seventh ed., CLSI, United States, 2006. [16] S. Stepanovic, D. Vukovic, I. Dakic, B. Savic, M. Svabic-Vlahovic, A modified microtiter-plate test for quantification of staphylococcal biofilm formation, J Microbiol Methods 40 (2000) 175e179, https://doi.org/10.1016/S0167-7012(00)00122-6.
[17] Y. Liu, L. Wang, X. Zhou, S. Hu, S. Zhang, H. Wu, Effect of the antimicrobial decapeptide KSL on the growth of oral pathogens and Streptococcus mutans biofilm, Int J Antimicrob Agents 37 (2011) 33e38,https://doi.org/10.1016/ j.ijantimicag.2010.08.014.
[18] B. Liu, H. Huang, Z. Yang, B. Liu, S. Gou, C. Zhong, X. Han, Y. Zhang, J. Ni, R. Wang, Design of novel antimicrobial peptide dimer analogues with enhanced antimicrobial activityin vitroandin vivoby intermolecular triazole bridge strategy, Peptides 88 (2017) 115e125, https://doi.org/10.1016/ j.peptides.2016.12.016.
[19] F.C. Odds, Synergy, antagonism, and what the chequerboard puts between them, J Antimicrob Chemother 52 (2003) 1, https://doi.org/10.1093/jac/ dkg301.
[20] K.A. Wichterman, A.E. Baue, I.H. Chaudry, Sepsis and septic shockda review of laboratory models and a proposal, J Surg Res 29 (1980) 189e201,https:// doi.org/10.1016/0022-4804(80)90037-2.
[21] L. Zhou, M. Gao, Z. Xiao, J. Zhang, X. Li, A. Wang, Protective effect of astax-anthin against multiple organ injury in a rat model of sepsis, J Surg Res 195 (2015) 559e567,https://doi.org/10.1016/j.jss.2015.02.026.
[22] S.Y. Wang, J.H. Wu, T.B. Ng, X.Y. Ye, P.F. Rao, A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean, Peptides 25 (2004) 1235e1242, https://doi.org/10.1016/ j.peptides.2004.06.004.
[23] E.I. Finkina, D.N. Melnikova, I.V. Bogdanov, T.V. Ovchinnikova, Lipid transfer proteins as components of the plant innate immune system: structure, functions, and applications, Acta Naturae 8 (2016) 847e861.
[24] D.J. Farrell, M. Robbins, W. Rhys-Williams, W.G. Love,In vitroactivity of XF-73, a novel antibacterial agent, against antibiotic-sensitive and -resistant Gram-positive and Gram-negative bacterial species, Int J Antimicrob Agents 35 (2010) 531e536,https://doi.org/10.1016/j.ijantimicag.2010.02.008. [25] G.R. Corey, Staphylococcus aureus bloodstream infections: definitions and
treatment, Clin Infect Dis 48 (2009) S254eS259, https://doi.org/10.1086/ 598186.
[26] J.M. Yarwood, P.M. Schlievert, Quorum sensing in Staphylococcus infections, J Clin Invest 112 (2003) 1620e1625,https://doi.org/10.1172/JCI200320442. [27] H. Choi, D.G. Lee, Synergistic effect of antimicrobial peptide arenicin-1 in
combination with antibiotics against pathogenic bacteria, Res Microbiol 163
(2012) 479e486,https://doi.org/10.1016/j.resmic.2012.06.001.
[28] X. Wu, Z. Li, X. Li, Y. Tian, Y. Fan, C. Yu, B. Zhou, Y. Liu, R. Xiang, L. Yang, Synergistic effects of antimicrobial peptide DP7 combined with antibiotics against multidrug-resistant bacteria, Drug Des Devel Ther 11 (2017) 939e946, https://doi.org/10.2147/DDDT.S107195.
[29] J. Amani, K.A. Barjini, M.M. Moghaddam, A. Asadi,In vitrosynergistic effect of the CM11 antimicrobial peptide in combination with common antibiotics against clinical isolates of six species of multidrug-resistant pathogenic bac-teria, Protein Pept Lett 22 (2015) 940e951, https://doi.org/10.2174/ 0929866522666150728115439.
[30] G. Schabbauer, Polymicrobial sepsis models: CLP versus CASP, Drug Discov Today Dis Model 9 (2012) e17ee21, https://doi.org/10.1016/ j.ddmod.2011.10.002.
[31] L. Dejager, I. Pinheiro, E. Dejonckheere, C. Libert, Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19 (2011) 198e208,https://doi.org/10.1016/j.tim.2011.01.001.
[32] E.S. Van Amersfoort, T.J. Van Berkel, J. Kuiper, Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock, Clin Microbiol Rev 16 (2003) 379e414,https://doi.org/10.1128/CMR.16.3.379.
[33] N. Yun, C.H. Lee, S.M. Lee, Protective effect of Aloe vera on polymicrobial sepsis in mice, Food Chem Toxicol 47 (2009) 1341e1348,https://doi.org/ 10.1016/j.fct.2009.03.013.
[34] S.L. Zanotti-Cavazzoni, R.D. Goldfarb, Animal models of sepsis, Crit Care Clin 25 (2009) 703e719,https://doi.org/10.1016/j.ccc.2009.08.005.
[35] M. Sagy, Y. Al-Qaqaa, P. Kim, Definitions and pathophysiology of sepsis, Curr Probl Pediatr Adolesc Health Care 43 (2013) 260e263, https://doi.org/ 10.1016/j.cppeds.2013.10.001.
[36] S. Parida, T.U. Singh, R. Thangamalai, C.E. Narasimha Reddy, M. Panigrahi, K. Kandasamy, V. Singh, S.K. Mishra, Daidzein pretreatment improves survival in mouse model of sepsis, J Surg Res 197 (2015) 363e373,https://doi.org/ 10.1016/j.jss.2015.03.059.
[37] D.C.O. Campos, A.S. Costa, P.B. Luz, P.M.G. Soares, N.M.N. Alencar, H.D. Oliveira, Morinda citrifolia lipid transfer protein 1 exhibits anti-inflammatory activity by modulation of pro- and anti-inflammatory cytokines, Int J Biol Macromol 103 (2017) 1121e1129,https://doi.org/10.1016/j.ijbiomac.2017.05.148. [38] R.D. Ramnath, S.W. Ng, A. Guglielmotti, M. Bhatia, Role of MCP-1 in
endo-toxemia and sepsis, Int Immunopharmacol 8 (2008) 810e818,https://doi.org/ 10.1016/j.intimp.2008.01.033.