w w w. s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Antinociceptive
and
antiulcer
activities
of
Pycnanthus
angolensis
Margaret
Oluwatoyin
Sofidiya
∗,
Adepero
Olubukola
Awolesi
DepartmentofPharmacognosy,FacultyofPharmacy,UniversityofLagos,Nigeria
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received20September2014 Accepted13May2015 Availableonline16June2015
Keywords:
Pycnanthusangolensis
Myristicaceae Ethanolicextract Antinociceptive Antiulcer
a
b
s
t
r
a
c
t
Pycnanthusangolensis(Welw)Warb.,Myristicaceae,isusedinNigeriafolkmedicinetotreatcomplaints suchastoothache,headache,sorethroat,ulcersandwounds.Theaimofthestudywastoinvestigate theantinociceptiveandantiulceractivitiesofthestembarkextractofPycnanthusangolensis.Acute tox-icitywasconductedwithasingleoraldoseof5g/kg.Antinociceptiveactivitywasevaluatedinacetic acid-inducedwrithing,formalinandtailimmersiontestsinmicewhileantiulceractivitywasevaluated inethanolandindomethacin-inducedmodelsinrats.Inaceticacid-inducedwrithingtest,theextract (50,100and150mg/kg,p.o.),significantlyreducedthenumberofwrithes(46.75%,57.28%and75.69%) respectively,comparedtocontrol.Theextractsignificantly(p<0.001)reducedthetimespentinlicking thehindpawatbothphases,informalintest.Intailimmersiontest,significantantinociceptiveeffect wasonlyobservedwiththedoseof150mg/kg,withpeakeffectat90min(43.38%).Thereisnosignificant changeinthespontaneouslocomotoractivityofanimalsintheopenfield.Theextractpreventedthe gas-triculcerationcausedbyethanolandindomethacintreatmentscomparedtocontrol.Theresultsshowed thatP.angolensisextractpossessesantinociceptiveandantiulceractivitiessupportingthetraditionaluse forrelievingpainandulcers.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Painhasbeenofficiallydefinedasanunpleasantsensoryand emotionalexperience associatedwithactual orpotentialtissue damageordescribedintermsofsuchdamage(IASP,1994).Itis adisablingaccompanimentwhichinmanycasesrepresentsthe onlysymptomforthediagnosisofseveraldiseases.Inrelieveof pain,classicalanalgesicdrugsnotablyopiatesand non-steroidal anti-inflammatorydrugs (NSAID)are used(García etal., 2011). TheuseofthesedrugsespeciallyNSAIDis consideredtobethe majorriskfactoringastriculcers.Inthiscontext,focusonplant researchhasincreasedworldwideandseveralstudieshadshowed immensepotentialofmedicinalplantsasalternativeremediesin themanagementofpainandgastriculcers.
Pycnanthusangolensis(Welw)Warb.,Myristicaceae,commonly knownas ‘Africannutmeg’ or ‘false nutmeg’, isused in a vari-etyofherbalrecipesforthetreatmentofchestpain, headache, various skin diseases and gastrointestinal ailments in Nigeria
(Omobuwajoetal.,1992;Oladimejietal.,2006).Theplantcontains
avarietyofchemicalconstituents,suchasallantoin(Pristaetal., 1960),kombicacid(Loketal.,1983),isoflavones-7,4′
-dimethoxy-2′ hydroxylisoflavoneand2′-hydroxyformononetin(Omobuwajo
∗ Correspondingauthor.
E-mail:msofidiya@unilag.edu.ng(M.O.Sofidiya).
etal.,1992),dihydroguaiaceticacid(Njokuetal.,1997), pycnanthu-quinoneAandB(Fortetal.,2000)andpycnanolideAandB(Onocha
andAli,2011).
BiologicalactivitiesofP.angolensisreportedinliteratureinclude antihelmintic (Gbolade and Adeyemi, 2008), anticancer (Njoku etal.,1997),antihyperglycemic(Luoetal.,1999;Fortetal.,2000), cytotoxicityandantileishmanialactivities(Onochaetal.,2008).The planthasalsobeenscreenedbyanumberofauthorsformalariaand antimicrobialactivities,withprovenefficacy(Ancolioetal.,2002;
Oladimejietal.,2006;Abrantesetal.,2008;OnochaandOtunla,
2010).Agyareetal.(2009),inanattempttoinvestigatethe
antiul-cerpotentialoftheplantscreenedthestembarkextractagainst
Helicobacterpylori.Asidethesereportsandtothebestofour knowl-edge,thepharmacologicaleffectoftheplantonpainfulprocesses andgastrointestinalsystemhasnotbeenreported.Therefore,the objectiveofthestudywastoinvestigatetheantinociceptiveand antiulcer properties of theethanolic extract of P. angolensis in experimentalanimalmodels.
Materialsandmethods
Plantmaterial
ThestembarkofPycnanthusangolensis(Welw)Warb., Myris-ticaceae, was collected from Olokemeji forest, Ibadan (7◦25′N,
3◦31′E),OyoState,NigeriainFebruary,2011andauthenticatedby
http://dx.doi.org/10.1016/j.bjp.2015.05.004
Mr.T.K.OdewoattheherbariumunitoftheDepartmentofBotany, UniversityofLagos,Lagos,Nigeria.Voucherspecimen(LUH4588) wasdepositedintheherbarium.
Extractionprocedure
Thestembarkwascutintopiecesanddriedatroom tempera-tureforthreeweeks.Theairdriedmaterial(500g)waspowdered usingChristyandNorriis8′LabMillingMachine(serialNo.50158)
andextractedtwicebymacerationwith95%ethanol(3l)atroom temperaturefor48h.Thecombinedethanolicextractwasfiltered withdouble-layeredmuslinclothandconcentratedonawaterbath at45◦Ctoyieldareddish-brownsolid(1.32%,w/w).
Animals
MaleAlbinoWistarrats(130–150g)wereobtainedfroma pri-vatevendor,ResearchEnterprise,Ibadan,Nigeria.Theanimalswere maintainedunderstandardlaboratoryconditionsinthe Labora-toryAnimalCentreoftheCollegeofMedicine,UniversityofLagos, Lagos,Nigeria.Theanimalswerekeptincagesattheroom temper-atureandfedwithfoodandwateradlibitum.Theywereallowed toacclimatizefortwoweeksbeforethecommencementof exper-imentalprocedures.Theexperimentalproceduresadoptedwere inaccordancewiththeprovisionsoftheExperimentationEthics CommitteeonAnimalUseoftheCollegeofMedicine,Universityof Lagos,Lagos,Nigeria(CM/COM/08/VOL.XXV)andtheUnitedStates NationalAcademyofSciencesGuidefortheCareandUseof Labo-ratoryAnimals(NIH,1985).
Acutetoxicity
Theacutetoxicity studyof P.angolensiswasperformedin a singleoraldoseadministrationof5000mg/kgtosevenmalemice
(Jaijoyetal.,2010).Micewerefastedfor12hbeforethe
adminis-trationoftheextract.Behavioralparametersincludingconvulsion, hyperactivity,sedation,grooming,increasedordecreased respira-tionwereobservedfor,overaperiodofsevendays.
Antinociceptivestudies
Aceticacid-inducedwrithingtest
The acetic acid-induced writhing test was done as previ-ouslydescribedbyArslanetal.,2010.Theextract(50,100and 150mg/kg),distilledwater(10ml/kg)andacetylsalicylicacid(ASA, 100mg/kg),wereadministeredorallytoeach groupofmice1h beforeintraperitoneal injectionwith0.6%acetic acid(10ml/kg, body weight) to induce writhings. The number of abdominal constrictionswithstretchingofthehindlimbswascounted cumu-lativelyoveraperiodof30min.Thepercentageofinhibitionofthe numberofwrithingswasthencalculated.
Formalintest
ThemethodusedwasaspreviouslydescribedbyGomesetal.
(2007),withslightmodification.Twentymicrolitersofformalin
(1%,v/v)wasinjectedsubcutaneouslyintotherighthindpawof mice.Thetime(insecond)spentinlickingandbitingoftheinjected pawwastakenasanindicatorofpainresponse.Responseswere measuredfor5min(firstphase) and15–30min(secondphase) afterformalininjection.Theextract(50,100and150mg/kg,p.o.), distilledwater(10ml/kg,p.o.)andmorphine(3mg/kg,s.c.)were administered60and30min,respectively,beforeformalin injec-tion.
Tailimmersiontest
Priortotheexperiment,theanimalswerescreenedfora sen-sitivitytest.Themousewasgentlyhandledandonethirdofthe tailwasimmersedintoawaterbathsetat55±1◦C(Agraharietal.,
2010).Themicewhichwithdrewthetailwithin5swereselected foractivity.Eachanimalservedasitscontrol.Thereactiontime forthesamegroupwastakenatintervals60,90,120,and150min afteralatencyperiodoflhfollowingtheoraladministrationof theextractatthedoseof50,100and150mg/kg.Controlgroups receivedmorphine(3mg/kg,s.c.)anddistilledwater(10ml/kg,p.o.) respectively.Acutoffperiodof10swasobserved,toavoiddamage tothetail.
Open-fieldtest
Toevaluatetheeffectoftheextractonspontaneous locomo-toractivity,miceweresubjectedtoopen-fieldtestasreportedby
De Mattoset al.(2007).Groupsofmice(n=5)receivedvehicle,
diazepamorextract(50,100and150mg/kg,p.o.)1hbeforethe test.Eachanimalwasplacedinthemiddleoftheopenfieldfor a5minsessionduringwhichthefollowingparameterswere reg-istered:crossing(thenumberofsquarescrossedwithallpaws), rearing(risingonhindpaws)andtimeofimmobility.
Antiulcerstudies
Ethanol-inducedgastriculcermodel
TheexperimentwasdoneasdescribedbyAkindeleetal.(2012), withslightmodification.Fastedmaleratsweredistributedintofive groupsconsistingofsevenanimalseach.GroupIservedascontrol andreceiveddistilled water(10ml/kg,p.o.);groupsII,IIIandIV receivedtheextractorallyatdosesof50,100and150mg/kg,body weight,respectively.GroupVreceivedmisoprostol(100g/kg,p.o)
whichservedasthestandarddrug.Gastriculcerwasinducedinthe ratsbyadministrationoflmlofabsoluteethanol.Animalswere thensacrificed1hafterbycervicaldislocation.Thestomachswere removed,cut along thegreater curvature,washed withnormal saline(0.9%),andulcerindexscoredusingMagistrettiscoringscale
(Magistrettietal.,1988).Gastrictissuesamplesfromeachgroupof
ethanolinducedmodelwerefixedin10%formalin.Then,the for-malinfixedspecimenswereembeddedinparaffinandsectioned (3–5cm)andfurtherstainedwithhematoxylinandeosindye.The sectionswereevaluatedbylightmicroscopyandphotographed.
Indomethacin-inducedgastriculcermodel
The experiment was performed as described by Singh and
Majumdar(1999).MaleWistarrats(130–150g)fastedfor24hwere
treatedorallywiththeextract(50,100and150mg/kg)or omepra-zole(40mg/kg). Distilledwater wasused in thecontrol group. Onehourlater,theanimalswereadministeredwithindomethacin (40mg/kg,p.o.).After6hofindomethacinadministration,animals weresacrificed,andtheirstomachsremoved,cutalongthegreater curvature,washedwithnormalsalineandulcerindexscored.
Statisticalanalysis
Theresultswerepresentedasthemean±S.E.M.Statistical sig-nificancebetweengroupswascalculatedbyanalysisofvariance (ANOVA),followedbyTukey’sorBonferroni’stest.
Control
50 100 150
ASA (100 mg/kg) 0
50 100 150
Extract (mg/kg, p.o.)
***
***
***
***
No of writhings
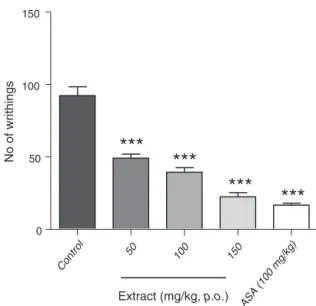
Fig.1.EffectofPycnanthusangolensisonaceticacid-inducedwrithinginmice.Each columnrepresentsthemean±S.E.M.(n=7).Datawereanalyzedusingone-way ANOVAfollowedbyTukey’smultiplecomparisontest***p<0.001,comparedwith control.
Results
Acutetoxicitystudy
AsingleoraladministrationofP.angolensisextract(5000mg/kg) didnotproduceanyvisiblesignsorsymptomsoftoxicity,abnormal behaviorandmortalityafter24handsevendaysofobservation.
Antinociceptivestudies
Writhingtest
Theresultoftheaceticinduced-writhinginmiceispresented inFig.1.Theextractdemonstratedadosedependentreductionin aceticacid-inducedwrithinginmice.Theaveragenoofwrithes forthiseffectwas49.14±2.82, 39.43±3.13,22.43±2.84which correspondsto46.75%,57.28%,75.69%inhibitionfor50,100and 150mg/kg, respectively. The effect was statistically significant
(p<0.001) relative tocontrol. Thestandard drug,acetylsalicylic acid(ASA,100mg/kg)showedareductioninthenowritheswith percentinhibitionof81.89,comparedtocontrol.
Formalintest
The effect of ethanolic extract of P. angolensis on formalin inducedpaininmiceisshowninTable1.Theextractina dose dependentmanner reduced the time spent in licking the hind pawat both phases. In thefirst phase, the extractat the dose of50, 100and 150mg/kg reducedthelickingtime with inhibi-tionpercentof35.06,44.47and 60.55,respectively.Thesecond phase showed a markedreduction in the averagelicking time (82.11±5.30,68.17±5.47and47.28±3.30s)atthedoseof50,100 and150mg/kg,respectively.Theaveragelickingtimeinthesecond phasewasrelativelylowerthanthatofthefirstphase.Thestandard reference,morphine,producedahigherinhibitionthantheextract atbothphases.
Tailimmersion
TheresultofthetailimmersiontestwithethanolicextractofP. angolensisispresentedinTable2.Theeffectoftheextractwasnot dosedependentatalldosestested.However,significant antinoci-ceptiveeffect(p<0.01andp<0.001)wasobservedwiththedoseof 150mg/kg,at60,90,and120min,comparedtocontrol.Thepeak effect(43.38%)wasat90min.Morphineexhibitedamaximumpeak ofanalgesiceffect(94.11%)at60minafteradministration.
Openfieldtest
Micetreatedwiththeextract(50,100and150mg/kg,p.o.)did notproducesignificanteffectonthenumberofsquarecrossings, rearing,numberoffecalbolusesandimmobilitywhencompared tothecontrolgroup(Table3).However,diazepamsignificantly (p<0.05)decreasedthelocomotoractivitycomparedtocontrol.
Antiulceractivity
Ethanol-inducedulcerinrats
Thetreatmentofratswithethanolproducedextensivegastric lesionsin the glandularmucosa of stomachin control animals
(Figs.2and3).Ontheotherhand,thepretreatmentoftheanimals
Table1
EffectofPycnanthusangolensisonformalininducedhindpawlickinginmice.
Treatment Dose(mg/kg) 5min 15–30min
Extract 50 85.94±5.31(35.06%)*** 82.11
±5.30(37.43%)***
100 73.12±3.26(44.74%)*** 68.17±5.47(48.05%)***
150 52.19±2.71(60.55%)*** 47.28±3.30(63.97%)***
Morphine 3 0.00±0.00(100%)*** 0.14±0.05(99.92%)***
Control 10ml/kg 132.33±12.91 131.22±14.74
Allvaluesgiveninmean±S.E.M.(n=7).Datawereanalyzedusingtwo-wayANOVAfollowedbyBonferroni’stest.
***p<0.001,whencomparedwithcontrol.
Table2
EffectofPycnanthusangolensisonnociceptiveresponsesintailimmersiontestinmice.
Treatment Dose(mg/kg) Latencyperiod(min)
0 60 90 120 150
Extract 50 3.36±0.34 7.51±1.96(24.95%) 7.37±1.53(24.07%) 6.15±1.22(16.74%) 4.44±0.71(6.91%) 100 3.84±0.41 5.84±0.66(12.39%) 6.12±0.39(14.12%) 5.08±0.38(7.71%) 3.62±0.5(−1.31%) 150 3.03±0.44 8.43±1.99(31.84%)** 10.39±2.00(43.38%)*** 7.73±1.30(27.69%)** 5.78±1.08(6.24%)
Morphine 3 3.64±0.47 19.04±0.51(94.11%)*** 17.50±1.04(84.74%)*** 16.74±1.14(80.07%)*** 14.01±1.35(63.36%)***
Control 10ml/kg 2.66±0.52 2.86±0.46 2.57±0.38 2.46±0.36 2.10±0.28
Allvaluesgiveninmean±S.E.M.(n=7).Datawereanalyzedusingatwo-wayANOVAfollowedbyBonferoni’stest.
** p<0.01.
Table3
EffectofPycnanthusangolensisonmiceinopenfieldtest.
Treatment Dose(mg/kg) Numberofsquarecrossing Numberofcentersquarecrossing Numberofrearing Immobility(s)
Control – 66.40±8.80 1.80±0.58 19.80±2.82 76.60±14.42
Extract 50 62.20±5.14 2.20±0.49 18.00±1.67 35.20±9.46
100 64.20±2.06 1.80±0.49 16.40±3.39 40.00±8.96
150 58.20±5.45 2.40±0.51 13.80±1.56 103.20±11.59
Diazepam 1 33.60±9.14* 0.80
±0.37 1.00±0.45*** 198.60
±9.45***
Dataaremean±S.E.M.(n=5).Datawereanalyzedusingone-wayANOVAfollowedbyTukey’smultiplecomparisontest.
***p<0.001.
*p<0.05comparedwithcontrol.
Control
50 100 150
Misoprostol (100 µg/kg)
0 5 10 15
***
***
***
***
Extract (mg/kg, p.o.)
Ulcer score
Fig.2. EffectofPycnanthusangolensisinethanolinducedulcerinrats.Each col-umnrepresentsthemean±S.E.M.(n=7).Datawereanalyzedusingone-wayANOVA followedbyTukey’smultiplecomparisontest***p<0.001,comparedwithcontrol.
with P. angolensis extract (50, 100 and 150mg/kg) exhibited significant(p<0.001)reductionofgastriclesionwithpercentage protectionof47.58%,67.74%and79.26%,respectively.
Theeffect oftheextract at150mg/kg dosewascomparable tothegrouptreatedwithmisoprostol.Histologicalobservationof stomachsectionsofthecontrolgroupshowedcongestednecrotic mucosalsurfaceandinfiltrationofacuteinflammatorycellsintothe
mucosal(Fig.3).However,stomachtissuesofratspretreatedwith thedoseof50,100,and150mg/kgofP.angolensisextract demon-stratedmildlesionsofthemucosaandmildeffectsofhemorrhage, inadosedependentmanner.
Indomethacininducedulcerinrats
The antiulcereffect of ethanolicextract of P.angolensis was alsoassessed in indomethacininducedulcermodel in ratsand theresultispresentedinFig.4.Theextractproducedasignificant dose-dependentreductioninthegastriclesionwithulcerindexof 2.70±0.10,2.60±0.21and2.40±0.56for50,100and150mg/kg, respectively.Omeprazoleusedasreference,producedapercentage protectionof71.93%.
Discussion
Thepresentstudywasundertakentoevaluatethe antinocicep-tiveandantiulcer activitiesofP.angolensis.Theantinociceptive effectoftheextractwasevaluatedonthreeclassicalnociception modelsinmice:aceticacid-inducedwrithingtest,formalintest andtailimmersiontest,whichareusefulmethodsforscreening prospectiveantinociceptivecompoundsorplantextracts.
Intheacutetoxicitystudy,theethanolicextractofP.angolensis, atthedoseof5g/kgexhibitednosignsoftoxicity,indicatingthat theextractmighthaveareasonablylowtoxicityprofile,andcould beregardedasrelativelysafeatthetesteddose(Gregoryetal., 2009).However,furthertoxicity studies;sub-acute and chronic toxicitytestsarenecessaryinordertodeterminethelong-term effectsoftheextract.
The acetic acid-induced abdominal constriction is a visceral painmodel employedasa screeningtoolfor theassessmentof
Fig.3. Histologicalexamination(20×magnifications)oftheethanol-inducedgastricmucosaldamageinrats.(AandA(i))controlgroup;(BandB(i))50mg/kg;(CandC(i)) 100mg/kg;(DandD(i))150mg/kg;(EandE(i))misoprostol(100g/kg).Blackarrow:congested,necroticmucosalsurface.Bluearrow:mildmucosaldamage.Redarrow:
Control
50 100 150
Omeprazole (40 mg/kg). 0
2 4 6 8
Extract (mg/kg, p.o.)
*
**
**
***
Ulcer score
Fig.4. EffectofPycnanthusangolensisonindomethacininducedulcer.Each col-umnrepresentsthemean±S.E.M.(n=5).Datawereanalyzedusingone-wayANOVA followedbyTukey’sMultipleComparisonTest,p<0.05,comparedwithcontrol.
antinociceptive or anti-inflammatory activity of new analgesic agent(DeSouzaetal.,2009).Aceticacidcausesalgesicresponse whichinvolvestheinterperitonealliberationofseveralmediators suchasneurotransmittersandneuromodulators,kinnis,histamine, acetylcholine,substance P and prostaglandins.These mediators increasevascularpermeability,reducethethresholdofthe noci-ceptionandstimulatethenervousterminalofnociceptivefibers
(Gorzalczanyetal.,2011;Pinheiroetal.,2012).Inthismodel,the
extractexhibiteda significantand dosedependentreduction in theaveragenumberofwrithesinmice,comparedtocontrol.This impliesthattheextracthadperipheralanalgesiceffectwhichmay beassociatedwiththereductionoftheliberationofthese inflam-matorymediatorsintotheperitonealcavityorbydirectblockage ofitsreceptorsresultinginanantinociceptiveeffect(Loganayaki etal.,2012).
Thesubcutaneousinjectionofformalininthemiceinducesa biphasicnociceptiveresponse(Ramirezetal.,2010).Thefirstphase whichcorrespondstotheneurogenicpainiscausedbythedirect effectofformalinonthesensoryC-fibers,andfollowedbythe sec-ondphaseortheinflammatoryphasethatisassociatedwiththe developmentofaninflammatoryresponseandthereleaseof noci-ceptivemediatorssuchasprostaglandins,serotoninandbradykinin intheperipheraltissuesandfromfunctionalchangesinthespinal dorsalhorn(Reynosoetal.,2013).Theextractsignificantly inhib-ited both phases of the formalin-induced pain, indicating that theextracthasantinociceptiveeffectinbothphases,significantly attenuatingthepainresponse.
Thetailimmersionmodelisusedtodetermineacutepainand itisausefultesttodifferentiatecentralopioidlikeanalgesicsfrom peripheralanalgesics (Le Barset al.,2001).The antinociceptive effectoftheextractinthismodelwasverylow.Moreover, com-parisonoftheeffectoftheextractwiththatofmorphineshowed thattheextractwasaweakeropioidreceptoragonist.
Theopenfieldtestwasusedtoexcludethepossibilitythatthe anti-nociceptiveactionofextractcouldberelatedtononspecific disturbancesinthelocomotoractivityoftheanimals(Gonc¸alves etal.,2013).Theextractatalldosestestedcausednosignificant changeinthelocomotorcoordinationactivityoftheanimals.The resultstherefore, eliminate thepossibilityoflocomotor impair-mentintheantinociceptiveactivityoftheextract.
Gastric mucosa damage as a result of treatment with non-steroidalanti-inflammatorydrugsisrecognizedasmostserious
adversereactiontothisclassofcompoundsandhasbeenthe stim-ulusbehindmuchoftheresearchaimedatdevelopingnewand effectivegastroprotectivecompounds.Hence,theantiulcer activ-ityofethanolicextractofP.angolensisonthemucosaloftheratwas evaluatedusingethanolandindomethacininducedmodels.
Ethanolinducedulcersareduetodirectnecrotizingeffectof ethanolongastricmucosa(Dharmanietal.,2004).Studiessuggest thattheethanoldamagetothegastrointestinalmucosastartswith microvascularinjury,namelydisruptionofthevascular endothe-liumresultinginincreasedvascularpermeability,edemaformation andepitheliallifting(Szaboetal.,1995).Henceacytoprotective agent,which increasesmucussecretion,willbeeffectiveinthis model.Cytoprotectionhasbeenconsideredtobeduetothe capac-ityofsomecompounds/drugstoinduceprostaglandinproduction, whichinturnsimulatesmucusandbicarbonatesynthesis(Robert
etal.,1983andDeshpandeetal.,2003).Inthisstudy,theextract
atthedoseof50,100and150mg/kgexhibitedadose-dependent gastro-protectiveactivity.Theeffectatthedoseof150mg/kgwas comparable tothatof misoprostol.The abilityof theextractto reducetheethanolinducedgastriculcermaybeanindicationof itscytoprotectiveactivity.
TofurtherconfirmthecytoprotectiveeffectofP.angolensis,the extractwasevaluatedagainstindomethacininducedulcermodel. Indomethacin,anon-steroidalanti-inflammatorydrug,isknown to cause ulcer by inhibiting prostaglandin synthetase through cyclooxygenasepathway(Oyagietal.,2010).Prostaglandins func-tions to protect the stomach from injury by stimulating the secretionofbicarbonateandmucus,maintainingmucosalblood flow andregulatingmucosalturnover andrepair (Hiruma-Lima et al., 2006). The extract significantly reduced gastric mucosal damagecomparedtocontrol.Theabilityoftheextracttoreduce indomethacininducedgastriculcerfurthersupports cytoprotec-tiveeffectandsuggeststhepossiblemobilizationandinvolvement ofprostaglandinsintheantiulcereffectoftheextract.
Inconclusion,theresultsobtainedinthis studyshowedthat
P.angolensis bark extract possesses antinociceptive and antiul-ceractivities,therebyprovidingpharmacologicalreferenceforthe traditionalusesoftheplantinNigeriafolkmedicine.The mecha-nisminvolvedwasnotelucidatedinthepresentstudyandneed furtherinvestigations.Moreover,activityguidedisolationofthe compoundsresponsiblefortheseactivitiesneedtobecarriedout.
Authors’contributions
AOA contributed in collecting plant sample and identifica-tion,runningthelaboratoryworkandanalysisofthedata.MOS designedthestudy,supervisedthelaboratoryworkandwrotethe manuscript.Alltheauthorshave read thefinal manuscript and approvedsubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsacknowledgethetechnicalassistanceofMuyiwaS. FageyinboandSeunNoreen.
References
Abrantes,M.,Mil-Homens,T.,Duarte,N.,Lopes,D.,Cravo,P.,Madureira,M., Fer-rira,M.J.,2008.AntiplasmodialactivityoflignansandextractsfromPycnanthus angolensis.PlantaMed.74,1408–1412.
Agyare,C.,Asase,A.,Lechtenberg,M.,Niehues,M.,Deters,A.,Hensen,A.,2009. Anethnopharmacologicaluseofmedicinalplantsusedforwoundhealingin Bosomtwi-Atwima-Kwanwomaarea,Ghana.J.Ethnopharmacol.125,393–403. Akindele,A.J.,Aigbe,F.R.,Olowe,J.A.,Oduntan,B.O.,Adeyemi,O.O.,2012. Gastro-protectiveeffectsofDAS-77(aphytomedicine)inulcermodelsinrats.Trop.J. Pharm.Res.11,783–791.
Ancolio,C.,Azas,N.,Mahiou,V.,Ollivier,E.,DiGiorgio,C.,Keita,A.,Timon-David,P., Balansard,G.,2002.Antimalarialactivityofextractsandalkaloidsisolatedfrom sixplantsusedintraditionalmedicineinMaliandSaoTome.Phytother.Res.16, 646–649.
Arslan,R.,NurcanBektas,N.,Ozturk,Y.,2010.Antinociceptiveactivityofmethanol extractoffruitsofCapparisovatainmice.J.Ethnopharmacol.131,28–32. DeMattos,E.S.,Frederico,M.J.S.,Colle,T.D.,dePieri,D.V.,Peters,R.R.,Piovezan,A.P.,
2007.EvaluationofantinociceptiveactivityofCaseariasylvestrisandpossible mechanismofaction.J.Ethnopharmacol.112,1–6.
DeSouza, M.M.,Pereira,M.A.,Ardenghi,J.V.,Mora,T.C.,Bresciani,L.F.,Yunes, R.A.,DelleMonache,F.,Cechinel-Filho,V.,2009.Filiceneobtainedfrom Adi-antumcuneatuminteractswiththecholinergic,dopaminergic,glutamatergic, GABAergic,andtachykinergicsystemstoexertantinociceptiveeffectinmice. Pharmacol.Biochem.Behav.93,40–46.
Deshpande,S.S.,Shah,G.B.,Parmar,N.S.,2003.AntiulceractivityofTephrosia pur-pureainrats.IndianJ.Pharmacol.35,168–172.
Dharmani,P.,Kuchibhotla,V.K.,Maurya,R.,Srivastava,S.,Sharma,S.,Palit,G.,2004. Evaluationofanti-ulcerogenicandulcer-healingpropertiesofOcimumsanctum Linn.J.Ethnopharmacol.93,197–206.
Fort,D.M.,Ubillas,R.P.,Mendez,C.D.,Jolad,S.D.,Inman,W.D.,Canney,J.R.,etal.,2000. Novelantihyperglycemicterpenoid-quinonesfromPycnanthusangolensis.J.Org. Chem.65,6534–6539.
García,P.G.,García,S.E.,Martínez,G.I.,Scior,T.R.F.,Salvador,J.L.,Martínez,P.M.M., DelRío,R.E.,2011.AnalgesiceffectofleafextractfromAgeratinaglabratainthe hotplatetest.Rev.Bras.Farmacogn.21,928–935.
Gbolade,A.A.,Adeyemi,A.A.,2008.Investigationofinvitroanthelmintic activ-itiesofPycnanthusangolensisandSphenocentrumjollyanum.Fitoterapia79, 220–222.
Gomes,N.M.,Rezende,C.M.,Fontes,S.P.,Matheus,M.E.,Fernandes,P.D.,2007. Anti-nociceptiveactivityofAmazoniancopaibaoils.J.Ethnopharmacol.109, 468–492.
Gonc¸alves,G.M.,Marinho,D.G.,Almanc¸a,C.C.J.,BrunoGuimarãesMarinho,B.G., 2013.Antinociceptiveandanti-oedematogenicpropertiesofthe hydroethano-licextractofSidastrummicranthumleavesinmice.Rev.Bras.Farmacogn.23, 836–843.
Gorzalczany,S.,Marrassini,C.,Mi ˜no,J.,Acevedo,C.,Ferraro,G.,2011. Antinocicep-tiveactivityofethanolicextractandisolatedcompoundsofUrticacircularis.J. Ethnopharmacol.134,733–738.
Gregory,M.,Vithalrao,K.P.,Franklin,G.,Kalaichelavan,V.,2009.Anti-ulcer(ulcer preventive)activityofFicusarnottianaMiq.(Moraceae)leafmethanolicextract. Am.J.Pharmacol.Toxicol.4,89–93.
Hiruma-Lima,C.A.,Santos,L.C.,Kushima,H.,Pellizzon,C.H.,Silveira,G.G., Vas-concelos,P.C.,etal.,2006.Qualeagrandiflora,aBrazilianCerradomedicinal plant presents an important antiulcer activity. J. Ethnopharmacol. 104, 207–214.
IASPTaskForceonTaxonomy,1994.PartIII.Painterms:acurrentlistwithdefinitions andnotesonusage.In:Merskey,H.,Bogduk,N.(Eds.),ClassificationofChronic Pain.IASPPress,Seattle,pp.209–214.
Jaijoy,K.,Soonthornchareonnon,N.,Lertprasertsuke,N.,Panthong,A., Sireerata-wong,S.,2010.Acuteandchronicoraltoxicityofstandardizedwaterextract fromthefruitofPhyllanthusemblicaLinn.Int.J.Appl.Res.Nat.Prod.3,48–58. LeBars,D.,Gozariu,M.,Cadden,S.W.,2001.Animalmodelsofnociception.
Pharma-col.Rev.53,597–652.
Loganayaki,N.,Siddhuraju,P.,Manian,S.,2012.Antioxidant,anti-inflammatoryand anti-nociceptiveeffectsofAmmanniabacciferaL.(Lythracceae),afolklore medic-inalplant.J.Ethnopharmacol.140,230–233.
Lok, C.M., Groenewegen, A., Stromk, J.B.A., Ward, J.P., 1983. Kombic acid, a hydroquininepolyisoprenoiccarboxylicacidfromPycnanthuskomboseedfat. Phytochemistry22,1973–1976.
Luo,J.,Cheung,E.M.,Clark,J.P.,Issai,J.,1999.Novelterpenoid–typequinnones isolatedfromPycnanthusangolensisofpotentialutilityinthetreatmentoftype 2diabetes.J.Pharmacol.Exp.Ther.288,529–534.
Magistretti,M.J.,Conti,M.,Cristoni,A.,1988.Antiulceractivityofananthocyanidin fromVaccinummyrtillus.Arzneim.Forsch.DrugRes.38,686–690.
NIH,1985.GuidefortheUseofLaboratoryAnimalsDHHS,PHS.NIHPublicationNo 85-23,rev.ed.NIH.
Njoku,C.J.,Hopp,D.C.,Alli,F.,Asuzu,I.U.,McLaughlin,J.L.,1997.Dihydroguaiaretic acid:abioactivecomponentofthestembarkofPycnanthusangolensis.Planta Med.63,580–581.
Oladimeji,O.H.,Ubulom,P.M.E.,Igboasoiyi,A.C.,Ndukwe,K.,Nia,R.,2006.Some biologicalactivitiesofPycnanthusangolensis(Welw.)Warb.J.Pharm.Biores.3, 49–55.
Omobuwajo,O.R.,Adesanya,S.A.,Babalola,G.O.,1992.Isoflavonoidsfrom Pycnan-thusangolensisandBaphianitida.Phytochemistry31,1013–1014.
Onocha,P.A.,Ali,M.S.,2011.PycnanolideAandB.Newlignanlactonesfromthe leavesofPycnanthusangolensis(Myristicaceae).Res.J.Phytochem.5,136–145. Onocha,P.A., Otunla,E.O.,2010.Biologicalactivities ofextractsofPycnanthus
angolensis(Welw.)Warb.Arch.Appl.Sci.Res.2,186–190.
Onocha,P.A.,Ajaiyeoba,E.O.,Ali,M.S.,2008.Invitroantileishamanias,phytoand cytotoxicityofPycnanthusangolensismethanolicextract.J.Med.Sci.2,178–181. Oyagi,A.,Ogawa,K.,Kakino,M.,Hara,H.,2010.Protectiveeffectsofa gastrointesti-nalagentcontainingKoreanredginsengongastriculcermodelsinmice.BMC ComplementAltern.Med.10,45.
Pinheiro,M.M.,Boykin,F.,DiasFernades,P.,2012.AntinociceptiveeffectofOrbignya speciosaBabassuleaves,evidencesforinvolvementofapigenin.J.LifeSci.10, 1013–1016.
Prista,L.N.,Alves,A.C.,Araujo,M.F.C.,1960.Allantoininsomeplantsusedinthe treatmentofwounds.GarciaOrta8,315–326.
Ramirez,M.R.,Guterres,L.,Odila,E.D.,Micheli,R.D.,Henriques,A.T.,De-Souza,M.M., Barros,D.M.,2010.Preliminarystudiesontheantinociceptiveactivityof Vac-cinumasheiberryinexperimentalanimalsmodels.J.Med.Food13,336–352. Reynoso,M.A.,Vera,N.,Aristimu ˜no,M.E.,Daud,A.,SánchezRiera,A.,2013.
Antinoci-ceptiveactivityoffruitsextractsand“arrope”ofGeoffroeadecorticans(cha ˜nar). J.Ethnopharmacol.145,355–362.
Robert,A.,Nezamis,J.E.,Lancaster,C.,Davis,J.P.,Field,S.O.,Hanchar,A.J.,1983.Mild irritantspreventgastricnecrosisthroughadaptativecytoprotectionmediated byprostaglandins.Am.J.Physiol.245,113–121.
Singh,S.,Majumdar,D.K.,1999.Evaluationofthegastricantiulceractivityoffixed oilofOcimumsanctum(HolyBasil).J.Ethnopharmacol.65,13–19.